-
PDF
- Split View
-
Views
-
Cite
Cite
Kotaro Sasaki, Sajal Kumar, Mario E. Chenal, Roberto F. Nicosia, Intraglomerular micrometastasis of squamous cell carcinoma, Clinical Kidney Journal, Volume 5, Issue 4, August 2012, Pages 292–296, https://doi.org/10.1093/ckj/sfs051
Close - Share Icon Share
Abstract
Intraglomerular metastasis is a rare manifestation of disseminated malignancies. We present here a case of intraglomerular metastatic carcinoma diagnosed as an incidental finding on a kidney biopsy in a 62-year-old male presenting with acute renal failure and metastatic penile squamous cell carcinoma. A proliferative lesion composed of highly atypical epithelial cells was found within a capillary loop and adjacent urinary space of an isolated glomerulus, which was immunoreactive for markers of squamous cell carcinoma. This case is a reminder that circulating cancer cells can occasionally lodge in glomeruli and appear as micrometastasis in kidney biopsies performed for the evaluation of renal dysfunction.
Background
Metastases of solid tumors of the kidney are occasionally seen in terminally ill patients with disseminated cancer. Renal involvement by metastatic cancer ranges from 3 to 8% based on autopsy series [1, 2]. Secondary renal neoplasms are typically identified in the tubulointerstitial compartment, whereas intraglomerular seeding by metastatic cells is seldom observed. Existing literature on intraglomerular metastasis is primarily based on autopsy findings [3–13], and only rarely has this lesion been identified in kidney biopsies [14–16].
We report here a case of metastatic intraglomerular carcinoma of penile origin diagnosed on a needle-core biopsy in a patient with acute renal failure. In addition to the acute tubulointerstitial nephritis responsible for the patient's renal dysfunction, we found a microscopic focus of metastatic intraglomerular carcinoma which mimicked a focally crescentic glomerulonephritis. Immunohistochemical studies confirmed the neoplastic nature of these cells and established the diagnosis of metastasis. Our experience with this case is a reminder that micrometastatic carcinoma should be included in the differential diagnosis of atypical glomerular crescents.
Case report
A 62-year-old man with type 2 diabetes mellitus and hypertension presented with generalized weakness. The patient had undergone partial penectomy 5 months earlier for a mass, which was diagnosed histologically as squamous cell carcinoma, moderately to well differentiated, condylomatous subtype. The neoplasm involved the entire glans and distal part of the urethra, with up to 1.5 cm depth of invasion (Stage T3). No lymph node dissection was performed at the time of surgery. Physical examination showed the trace edema of lower extremities but was otherwise unremarkable. Laboratory data on admission were significant for hypercalcemia with a serum calcium of 13.4 mg/dL (3.35 mmol/L) and increased serum creatinine at 2.3 mg/dL (203.3 μmol/L). Urinalysis showed pH 5, 2+ proteinuria and granular casts (2–4/low-power field). A computed tomographic scan revealed lymphadenopathy in mediastinum, inguinal regions and external iliac lymph nodes. Bone scintigraphy showed increased uptake in the skull, ribs, shoulders, vertebral bones and left sacroiliac joint, consistent with metastasis. The hypercalcemia was treated with intravenous fluid and bisphosphonate, with significant improvement. However, the patient's serum creatinine rapidly increased to 8.1 mg/dL (716 μmol/L) in 2 weeks. Nephrology was consulted and a kidney biopsy was performed.
Microscopic examination showed acute interstitial nephritis characterized by diffuse interstitial edema and infiltration by mononuclear and polymorphonuclear leukocytes including neutrophils. The interstitial inflammatory changes were associated with widespread acute tubular injury. The glomeruli showed features of diabetic nephropathy including nodular mesangial sclerosis. One of the glomeruli was partially obliterated by a proliferative epithelial lesion with crescent-like features involving the capillary tuft and adjacent urinary space (Figure 1A and B). Epithelial cells comprising this lesion were cytologically atypical and showed feature suggestive of squamous differentiation including the eosinophilic cytoplasm, distinct cell borders and occasional intercellular bridges. Many of these cells had large irregular nuclei, and prominent eosinophilic nucleoli (Figure 1A and B). Occasional mitotic figures and apoptotic bodies were also identified (Figure 1A). The glomerular capillary involved with this lesion was markedly distended and completely filled by atypical epithelial cells. A few of these cells were also identified in the adjacent proximal tubules. A review of the partial penectomy specimen, previously diagnosed as squamous cell carcinoma, showed a strong similarity between the cancer cells of the primary neoplasm and the atypical epithelial cells found in the kidney biopsy (data not shown). Taken together with the squamous features of the intraglomerular epithelial cells, this finding was most suggestive of glomerular involvement by metastatic squamous cell carcinoma of the penile origin.
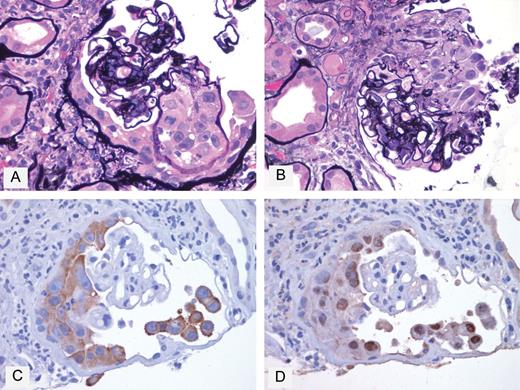
Intraglomerular micrometastasis of squamous cell carcinoma. (A) Cluster of neoplastic cells within a markedly distended capillary loop and adjacent urinary space. Neoplastic cells have abundant eosinophilic cytoplasm, distinct cell borders and large nuclei with prominent nucleoli. Mitotic figure and apoptotic bodies are seen (Jones' silver stain ×60). (B) A different level section of the same glomerulus shows neoplastic cells with polygonal to elongated shapes within the urinary space (Jones' silver stain ×60). (C) Immunoperoxidase stain for CK5 shows diffuse cytoplasmic positivity of neoplastic cells (×60). (D) Immunoperoxidase stain for P63 specifically highlights the nuclei of neoplastic cells (×60).
Immunohistochemical studies were performed to confirm the squamous differentiation and neoplastic nature of the intraglomerular proliferative epithelial lesion. The atypical intraglomerular epithelial cells were positive for cytokeratin (CK) 5 (EP1601Y, Cell Marque; Figure 1C) and p63 (BC4A4, Cell Marque; Figure 1D), markers of squamous cell carcinoma [17, 18], and negative for WT1 (6F-H2, Dako, podocyte marker), CK8 (35bH11, Cell Marque, parietal epithelial cell marker) and CD31 (JC/70A, Dako, endothelial marker) (data not shown). Atypical cells with similar cytologic and immunoreactive features were identified within the urinary space of an additional glomerulus (data not shown).
Based on the histologic and immunohistochemical findings, the lesion was diagnosed as intraglomerular metastatic squamous cell carcinoma of penile origin. The patient's clinical condition deteriorated despite supportive care, including dialysis, to the extent that systemic chemotherapy could not be administered. The patient was kept on palliative care until his demise 3 weeks after the kidney biopsy. No autopsy was performed.
Discussion
Penile carcinoma is a rare malignancy in the USA and Europe, whereas it is more common in African and South American countries [19]. The vast majority of malignant penile tumors are squamous cell carcinomas [20]. Penile squamous cell carcinoma includes low-grade tumors such as warty and papillary carcinomas, and basaloid and sarcomatoid carcinomas which have a worse prognosis [20]. Warty variants of squamous cell carcinoma can occasionally behave aggressively and metastasize as seen in our case [20]. Overall, systemic dissemination occurs in one-fourth of the patients [21]. According to an autopsy study by Chaux et al. [22], kidneys are the fifth most common site of metastasis of penile squamous cell carcinoma following the liver, lungs, lymph nodes and heart. However, intraglomerular metastasis of penile carcinoma, to our knowledge, has never been reported, particularly on the kidney biopsy.
Intraglomerular metastases occur in 6–7% cases of metastatic carcinoma of the kidney [6, 8, 10], but the existing literature is primarily based on autopsy studies. Clinicopathologic findings in cases of intraglomerular metastasis, including our patient, are summarized in Table 1 [3–16, 23, 24]. Reported cases include 34 patients ranging from 14 to 88 years old with a mean age of 57. There is a slight male predilection with a male-to-female ratio of 1.8:1. Nine out of 18 (50%) patients had proteinuria, including one case of nephrotic syndrome [11]. Seven out of 19 (37%) patients had hematuria [14]. Four out of 11 (36%) patients, including ours, presented with elevated serum creatinine. Renal insufficiency in our patient, however, was most likely due to acute interstitial nephritis and not metastatic cancer. Thirteen cases (50%) had macroscopic and/or radiographic evidence of kidney metastasis. The vast majority of patients presented with multiple distant metastases. The most common cancers associated with intraglomerular metastases were lung (eight cases, 29%) and breast (four cases, 14%). The vast majority of cases were diagnosed on autopsy and only four cases were identified on needle-core biopsies. The prognosis is dismal in cases diagnosed on the kidney biopsy, as all patients died within 3 months with a mean survival of 28 weeks. Our patient expired 3 weeks following the biopsy procedure.
| Author(s) . | Year . | n . | Age . | Sex . | Primary malignancy (site) . | Diagnostic source . | Macroscopic or radiographic evidence of kidney metastases . | Other sites of metastases . | Time of death after the diagnosis . | Proteinuria . | Hematuria . | Serum creatinine . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lauterburg [12] | 1917 | 5 | 38 | F | Lung | Autopsy | Yes | Adrenals, liver, lung, lymph nodes, bone, thyroid | NA | NA | NA | NA |
| NA | F | Lung | Autopsy | Yes | None | NA | NA | NA | NA | |||
| 48 | F | Lung | Autopsy | Yes | Brain, lymph nodes | NA | NA | NA | NA | |||
| 57 | F | Breast | Autopsy | Yes | Adrenals, liver, lungs, lymph nodes, bones | NA | NA | NA | NA | |||
| NA | NA | Breast | Nephrectomy | NA | NA | NA | NA | NA | NA | |||
| Mariconda [23] | 1927 | 1 | 69 | F | Melanoma, skin | Autopsy | No | Adrenal capsule, bladder, heart, lymph nodes, liver, spleen, stomach | NA | NA | Yes (gross) | NA |
| Wuketich [4] | 1960 | 1 | 50 | F | Melanoma, skin | Autopsy | Yes | Brain, esophagus, intestines, liver, lungs, myocardium, skin, stomach, spleen | NA | Yes | Yes | NA |
| Galloway and Ray [5] | 1964 | 1 | 48 | M | Renal cell ca, kidney | Autopsy | NA | Adrenal, brain, heart, liver, lung | NA | No | No | Elevated (8.9 mg/dL) |
| Ross [9] | 1966 | 1 | 68 | M | Squamous cell ca, lung | Autopsy | Yes | Lymph nodes | NA | NA | NA | NA |
| Wagle et al. [10] | 1975 | 5 | NA | NA | NA | Autopsy | NA | NA | NA | NA | NA | NA |
| Datta [3] | 1978 | 1 | 60 | M | Adeno ca, lung | Autopsy | No | Adrenal, liver, Lymph nodes | NA | NA | NA | NA |
| Belghiti et al. [16] | 1984 | 2 | 45 | M | Ca, NA | Needle biopsy | No | Bone | 3 months | Yes (1.5–2 g/day) | Yes (1/hpf) | Normal |
| 58 | F | Adeno ca, ovary | Autopsy | No | Adrenals, liver, lungs, lymph nodes, pericardium, pleura | NA | No | No | NA | |||
| Toth [6] | 1987 | 7 | 61 | M | Epidermoid ca, bronchus | Autopsy | No | Bones, lymph nodes | NA | Yes | No | NA |
| 56 | M | Squamous cell ca, esophagus | Autopsy | No | Lungs, lymph nodes, pancreas | NA | Yes | No | NA | |||
| 69 | M | Oat cell ca, bronchus | Autopsy | Yes | Adrenal, brain, Lymph nodes, pancreas | NA | No | No | NA | |||
| 67 | M | Epidermoid ca, bronchus | Autopsy | Yes | Lungs, lymph nodes | NA | No | No | NA | |||
| 50 | M | Tubular ca, breast | Autopsy | Yes | Epidermis, liver, lymph nodes | NA | No | No | NA | |||
| 58 | F | Ductal ca, breast | Autopsy | Yes | Liver, lymph nodes | NA | No | No | NA | |||
| 70 | M | Adeno ca, pancreas | Autopsy | Yes | Lungs, lymph nodes | NA | No | No | NA | |||
| Sarma and Simmons [13] | 1989 | 1 | 53 | M | Papillary ca, thyroid | Autopsy | No | Adrenals, bones, liver, lungs, lymph nodes, mediastinum, omentum, prostate | NA | Yes (trace) | Yes (trace) | Normal |
| Melato et al. [7] | 1991 | 1 | 85 | M | Adenosquamous ca, lung | Autopsy | No | Liver | NA | NA | NA | Normal |
| Carr et al. [24] | 1994 | 1 | 52 | F | Renal cell ca, kidney | Nephrectomy | NA | Bone | 6 weeks | NA | NA | NA |
| Nomura et al. [11] | 1995 | 1 | 88 | M | Adeno ca, lung | Autopsy | No | NA | NA | Yes (7.8 g/day) | Yes (5–10/hpf) | Elevated (1.9 mg/dL) |
| Sridevi et al. [8] | 1999 | 3 | 47 | M | Squamous cell ca, lung | Autopsy | Yes | Adrenals, brain | NA | No | No | Normal |
| 42 | M | Adeno ca, pancreas | Autopsy | Yes | Adrenal, gallbladder, heart, Liver, lungs, lymph nodes | NA | Yes (trace) | No | Normal | |||
| 14 | M | Mesothelioma, lung | Autopsy | No | Adrenals, Liver, lung, lymph nodes | NA | NA | Normal | ||||
| Yokoi et al. [15] | 2001 | 1 | 60 | M | Adeno ca, pancreas | Needle biopsy/Autopsy | No | Epicardium, lungs, peritoneum, pleura | 46 days | Yes (0.4 g/day) | Yes (10–20/hpf) | Normal |
| Ozluk et al. [14] | 2011 | 1 | 64 | F | Melanoma, skin | Needle biopsy | No | None | NA | Yes (3.1 g/day) | Yes (4–5/hpf) | Elevated (2.8 mg/dL) |
| Current case | 2012 | 1 | 62 | M | Squamous cell ca, penis | Needle biopsy | No | Bones, liver, lungs, lymph nodes | 3 weeks | No | No | Elevated (5.0 mg/dL) |
| Author(s) . | Year . | n . | Age . | Sex . | Primary malignancy (site) . | Diagnostic source . | Macroscopic or radiographic evidence of kidney metastases . | Other sites of metastases . | Time of death after the diagnosis . | Proteinuria . | Hematuria . | Serum creatinine . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lauterburg [12] | 1917 | 5 | 38 | F | Lung | Autopsy | Yes | Adrenals, liver, lung, lymph nodes, bone, thyroid | NA | NA | NA | NA |
| NA | F | Lung | Autopsy | Yes | None | NA | NA | NA | NA | |||
| 48 | F | Lung | Autopsy | Yes | Brain, lymph nodes | NA | NA | NA | NA | |||
| 57 | F | Breast | Autopsy | Yes | Adrenals, liver, lungs, lymph nodes, bones | NA | NA | NA | NA | |||
| NA | NA | Breast | Nephrectomy | NA | NA | NA | NA | NA | NA | |||
| Mariconda [23] | 1927 | 1 | 69 | F | Melanoma, skin | Autopsy | No | Adrenal capsule, bladder, heart, lymph nodes, liver, spleen, stomach | NA | NA | Yes (gross) | NA |
| Wuketich [4] | 1960 | 1 | 50 | F | Melanoma, skin | Autopsy | Yes | Brain, esophagus, intestines, liver, lungs, myocardium, skin, stomach, spleen | NA | Yes | Yes | NA |
| Galloway and Ray [5] | 1964 | 1 | 48 | M | Renal cell ca, kidney | Autopsy | NA | Adrenal, brain, heart, liver, lung | NA | No | No | Elevated (8.9 mg/dL) |
| Ross [9] | 1966 | 1 | 68 | M | Squamous cell ca, lung | Autopsy | Yes | Lymph nodes | NA | NA | NA | NA |
| Wagle et al. [10] | 1975 | 5 | NA | NA | NA | Autopsy | NA | NA | NA | NA | NA | NA |
| Datta [3] | 1978 | 1 | 60 | M | Adeno ca, lung | Autopsy | No | Adrenal, liver, Lymph nodes | NA | NA | NA | NA |
| Belghiti et al. [16] | 1984 | 2 | 45 | M | Ca, NA | Needle biopsy | No | Bone | 3 months | Yes (1.5–2 g/day) | Yes (1/hpf) | Normal |
| 58 | F | Adeno ca, ovary | Autopsy | No | Adrenals, liver, lungs, lymph nodes, pericardium, pleura | NA | No | No | NA | |||
| Toth [6] | 1987 | 7 | 61 | M | Epidermoid ca, bronchus | Autopsy | No | Bones, lymph nodes | NA | Yes | No | NA |
| 56 | M | Squamous cell ca, esophagus | Autopsy | No | Lungs, lymph nodes, pancreas | NA | Yes | No | NA | |||
| 69 | M | Oat cell ca, bronchus | Autopsy | Yes | Adrenal, brain, Lymph nodes, pancreas | NA | No | No | NA | |||
| 67 | M | Epidermoid ca, bronchus | Autopsy | Yes | Lungs, lymph nodes | NA | No | No | NA | |||
| 50 | M | Tubular ca, breast | Autopsy | Yes | Epidermis, liver, lymph nodes | NA | No | No | NA | |||
| 58 | F | Ductal ca, breast | Autopsy | Yes | Liver, lymph nodes | NA | No | No | NA | |||
| 70 | M | Adeno ca, pancreas | Autopsy | Yes | Lungs, lymph nodes | NA | No | No | NA | |||
| Sarma and Simmons [13] | 1989 | 1 | 53 | M | Papillary ca, thyroid | Autopsy | No | Adrenals, bones, liver, lungs, lymph nodes, mediastinum, omentum, prostate | NA | Yes (trace) | Yes (trace) | Normal |
| Melato et al. [7] | 1991 | 1 | 85 | M | Adenosquamous ca, lung | Autopsy | No | Liver | NA | NA | NA | Normal |
| Carr et al. [24] | 1994 | 1 | 52 | F | Renal cell ca, kidney | Nephrectomy | NA | Bone | 6 weeks | NA | NA | NA |
| Nomura et al. [11] | 1995 | 1 | 88 | M | Adeno ca, lung | Autopsy | No | NA | NA | Yes (7.8 g/day) | Yes (5–10/hpf) | Elevated (1.9 mg/dL) |
| Sridevi et al. [8] | 1999 | 3 | 47 | M | Squamous cell ca, lung | Autopsy | Yes | Adrenals, brain | NA | No | No | Normal |
| 42 | M | Adeno ca, pancreas | Autopsy | Yes | Adrenal, gallbladder, heart, Liver, lungs, lymph nodes | NA | Yes (trace) | No | Normal | |||
| 14 | M | Mesothelioma, lung | Autopsy | No | Adrenals, Liver, lung, lymph nodes | NA | NA | Normal | ||||
| Yokoi et al. [15] | 2001 | 1 | 60 | M | Adeno ca, pancreas | Needle biopsy/Autopsy | No | Epicardium, lungs, peritoneum, pleura | 46 days | Yes (0.4 g/day) | Yes (10–20/hpf) | Normal |
| Ozluk et al. [14] | 2011 | 1 | 64 | F | Melanoma, skin | Needle biopsy | No | None | NA | Yes (3.1 g/day) | Yes (4–5/hpf) | Elevated (2.8 mg/dL) |
| Current case | 2012 | 1 | 62 | M | Squamous cell ca, penis | Needle biopsy | No | Bones, liver, lungs, lymph nodes | 3 weeks | No | No | Elevated (5.0 mg/dL) |
ca, carcinoma.
| Author(s) . | Year . | n . | Age . | Sex . | Primary malignancy (site) . | Diagnostic source . | Macroscopic or radiographic evidence of kidney metastases . | Other sites of metastases . | Time of death after the diagnosis . | Proteinuria . | Hematuria . | Serum creatinine . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lauterburg [12] | 1917 | 5 | 38 | F | Lung | Autopsy | Yes | Adrenals, liver, lung, lymph nodes, bone, thyroid | NA | NA | NA | NA |
| NA | F | Lung | Autopsy | Yes | None | NA | NA | NA | NA | |||
| 48 | F | Lung | Autopsy | Yes | Brain, lymph nodes | NA | NA | NA | NA | |||
| 57 | F | Breast | Autopsy | Yes | Adrenals, liver, lungs, lymph nodes, bones | NA | NA | NA | NA | |||
| NA | NA | Breast | Nephrectomy | NA | NA | NA | NA | NA | NA | |||
| Mariconda [23] | 1927 | 1 | 69 | F | Melanoma, skin | Autopsy | No | Adrenal capsule, bladder, heart, lymph nodes, liver, spleen, stomach | NA | NA | Yes (gross) | NA |
| Wuketich [4] | 1960 | 1 | 50 | F | Melanoma, skin | Autopsy | Yes | Brain, esophagus, intestines, liver, lungs, myocardium, skin, stomach, spleen | NA | Yes | Yes | NA |
| Galloway and Ray [5] | 1964 | 1 | 48 | M | Renal cell ca, kidney | Autopsy | NA | Adrenal, brain, heart, liver, lung | NA | No | No | Elevated (8.9 mg/dL) |
| Ross [9] | 1966 | 1 | 68 | M | Squamous cell ca, lung | Autopsy | Yes | Lymph nodes | NA | NA | NA | NA |
| Wagle et al. [10] | 1975 | 5 | NA | NA | NA | Autopsy | NA | NA | NA | NA | NA | NA |
| Datta [3] | 1978 | 1 | 60 | M | Adeno ca, lung | Autopsy | No | Adrenal, liver, Lymph nodes | NA | NA | NA | NA |
| Belghiti et al. [16] | 1984 | 2 | 45 | M | Ca, NA | Needle biopsy | No | Bone | 3 months | Yes (1.5–2 g/day) | Yes (1/hpf) | Normal |
| 58 | F | Adeno ca, ovary | Autopsy | No | Adrenals, liver, lungs, lymph nodes, pericardium, pleura | NA | No | No | NA | |||
| Toth [6] | 1987 | 7 | 61 | M | Epidermoid ca, bronchus | Autopsy | No | Bones, lymph nodes | NA | Yes | No | NA |
| 56 | M | Squamous cell ca, esophagus | Autopsy | No | Lungs, lymph nodes, pancreas | NA | Yes | No | NA | |||
| 69 | M | Oat cell ca, bronchus | Autopsy | Yes | Adrenal, brain, Lymph nodes, pancreas | NA | No | No | NA | |||
| 67 | M | Epidermoid ca, bronchus | Autopsy | Yes | Lungs, lymph nodes | NA | No | No | NA | |||
| 50 | M | Tubular ca, breast | Autopsy | Yes | Epidermis, liver, lymph nodes | NA | No | No | NA | |||
| 58 | F | Ductal ca, breast | Autopsy | Yes | Liver, lymph nodes | NA | No | No | NA | |||
| 70 | M | Adeno ca, pancreas | Autopsy | Yes | Lungs, lymph nodes | NA | No | No | NA | |||
| Sarma and Simmons [13] | 1989 | 1 | 53 | M | Papillary ca, thyroid | Autopsy | No | Adrenals, bones, liver, lungs, lymph nodes, mediastinum, omentum, prostate | NA | Yes (trace) | Yes (trace) | Normal |
| Melato et al. [7] | 1991 | 1 | 85 | M | Adenosquamous ca, lung | Autopsy | No | Liver | NA | NA | NA | Normal |
| Carr et al. [24] | 1994 | 1 | 52 | F | Renal cell ca, kidney | Nephrectomy | NA | Bone | 6 weeks | NA | NA | NA |
| Nomura et al. [11] | 1995 | 1 | 88 | M | Adeno ca, lung | Autopsy | No | NA | NA | Yes (7.8 g/day) | Yes (5–10/hpf) | Elevated (1.9 mg/dL) |
| Sridevi et al. [8] | 1999 | 3 | 47 | M | Squamous cell ca, lung | Autopsy | Yes | Adrenals, brain | NA | No | No | Normal |
| 42 | M | Adeno ca, pancreas | Autopsy | Yes | Adrenal, gallbladder, heart, Liver, lungs, lymph nodes | NA | Yes (trace) | No | Normal | |||
| 14 | M | Mesothelioma, lung | Autopsy | No | Adrenals, Liver, lung, lymph nodes | NA | NA | Normal | ||||
| Yokoi et al. [15] | 2001 | 1 | 60 | M | Adeno ca, pancreas | Needle biopsy/Autopsy | No | Epicardium, lungs, peritoneum, pleura | 46 days | Yes (0.4 g/day) | Yes (10–20/hpf) | Normal |
| Ozluk et al. [14] | 2011 | 1 | 64 | F | Melanoma, skin | Needle biopsy | No | None | NA | Yes (3.1 g/day) | Yes (4–5/hpf) | Elevated (2.8 mg/dL) |
| Current case | 2012 | 1 | 62 | M | Squamous cell ca, penis | Needle biopsy | No | Bones, liver, lungs, lymph nodes | 3 weeks | No | No | Elevated (5.0 mg/dL) |
| Author(s) . | Year . | n . | Age . | Sex . | Primary malignancy (site) . | Diagnostic source . | Macroscopic or radiographic evidence of kidney metastases . | Other sites of metastases . | Time of death after the diagnosis . | Proteinuria . | Hematuria . | Serum creatinine . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lauterburg [12] | 1917 | 5 | 38 | F | Lung | Autopsy | Yes | Adrenals, liver, lung, lymph nodes, bone, thyroid | NA | NA | NA | NA |
| NA | F | Lung | Autopsy | Yes | None | NA | NA | NA | NA | |||
| 48 | F | Lung | Autopsy | Yes | Brain, lymph nodes | NA | NA | NA | NA | |||
| 57 | F | Breast | Autopsy | Yes | Adrenals, liver, lungs, lymph nodes, bones | NA | NA | NA | NA | |||
| NA | NA | Breast | Nephrectomy | NA | NA | NA | NA | NA | NA | |||
| Mariconda [23] | 1927 | 1 | 69 | F | Melanoma, skin | Autopsy | No | Adrenal capsule, bladder, heart, lymph nodes, liver, spleen, stomach | NA | NA | Yes (gross) | NA |
| Wuketich [4] | 1960 | 1 | 50 | F | Melanoma, skin | Autopsy | Yes | Brain, esophagus, intestines, liver, lungs, myocardium, skin, stomach, spleen | NA | Yes | Yes | NA |
| Galloway and Ray [5] | 1964 | 1 | 48 | M | Renal cell ca, kidney | Autopsy | NA | Adrenal, brain, heart, liver, lung | NA | No | No | Elevated (8.9 mg/dL) |
| Ross [9] | 1966 | 1 | 68 | M | Squamous cell ca, lung | Autopsy | Yes | Lymph nodes | NA | NA | NA | NA |
| Wagle et al. [10] | 1975 | 5 | NA | NA | NA | Autopsy | NA | NA | NA | NA | NA | NA |
| Datta [3] | 1978 | 1 | 60 | M | Adeno ca, lung | Autopsy | No | Adrenal, liver, Lymph nodes | NA | NA | NA | NA |
| Belghiti et al. [16] | 1984 | 2 | 45 | M | Ca, NA | Needle biopsy | No | Bone | 3 months | Yes (1.5–2 g/day) | Yes (1/hpf) | Normal |
| 58 | F | Adeno ca, ovary | Autopsy | No | Adrenals, liver, lungs, lymph nodes, pericardium, pleura | NA | No | No | NA | |||
| Toth [6] | 1987 | 7 | 61 | M | Epidermoid ca, bronchus | Autopsy | No | Bones, lymph nodes | NA | Yes | No | NA |
| 56 | M | Squamous cell ca, esophagus | Autopsy | No | Lungs, lymph nodes, pancreas | NA | Yes | No | NA | |||
| 69 | M | Oat cell ca, bronchus | Autopsy | Yes | Adrenal, brain, Lymph nodes, pancreas | NA | No | No | NA | |||
| 67 | M | Epidermoid ca, bronchus | Autopsy | Yes | Lungs, lymph nodes | NA | No | No | NA | |||
| 50 | M | Tubular ca, breast | Autopsy | Yes | Epidermis, liver, lymph nodes | NA | No | No | NA | |||
| 58 | F | Ductal ca, breast | Autopsy | Yes | Liver, lymph nodes | NA | No | No | NA | |||
| 70 | M | Adeno ca, pancreas | Autopsy | Yes | Lungs, lymph nodes | NA | No | No | NA | |||
| Sarma and Simmons [13] | 1989 | 1 | 53 | M | Papillary ca, thyroid | Autopsy | No | Adrenals, bones, liver, lungs, lymph nodes, mediastinum, omentum, prostate | NA | Yes (trace) | Yes (trace) | Normal |
| Melato et al. [7] | 1991 | 1 | 85 | M | Adenosquamous ca, lung | Autopsy | No | Liver | NA | NA | NA | Normal |
| Carr et al. [24] | 1994 | 1 | 52 | F | Renal cell ca, kidney | Nephrectomy | NA | Bone | 6 weeks | NA | NA | NA |
| Nomura et al. [11] | 1995 | 1 | 88 | M | Adeno ca, lung | Autopsy | No | NA | NA | Yes (7.8 g/day) | Yes (5–10/hpf) | Elevated (1.9 mg/dL) |
| Sridevi et al. [8] | 1999 | 3 | 47 | M | Squamous cell ca, lung | Autopsy | Yes | Adrenals, brain | NA | No | No | Normal |
| 42 | M | Adeno ca, pancreas | Autopsy | Yes | Adrenal, gallbladder, heart, Liver, lungs, lymph nodes | NA | Yes (trace) | No | Normal | |||
| 14 | M | Mesothelioma, lung | Autopsy | No | Adrenals, Liver, lung, lymph nodes | NA | NA | Normal | ||||
| Yokoi et al. [15] | 2001 | 1 | 60 | M | Adeno ca, pancreas | Needle biopsy/Autopsy | No | Epicardium, lungs, peritoneum, pleura | 46 days | Yes (0.4 g/day) | Yes (10–20/hpf) | Normal |
| Ozluk et al. [14] | 2011 | 1 | 64 | F | Melanoma, skin | Needle biopsy | No | None | NA | Yes (3.1 g/day) | Yes (4–5/hpf) | Elevated (2.8 mg/dL) |
| Current case | 2012 | 1 | 62 | M | Squamous cell ca, penis | Needle biopsy | No | Bones, liver, lungs, lymph nodes | 3 weeks | No | No | Elevated (5.0 mg/dL) |
ca, carcinoma.
Although intraglomerular metastases are rarely seen on the kidney biopsy, their occurrence may pose significant diagnostic challenges. Histologically, two types of intraglomerular metastasis have been reported, intracapillary and extracapillary [14]. Intracapillary metastatic carcinoma must be distinguished from intravascular large B cell lymphoma, formerly known as angioendotheliomatosis [25, 26]. This can be accomplished by staining for markers of leukocyte/lymphoid differentiation, such as CD45 and CD20 [25, 26], which are expressed by lymphomatous cells but not by carcinoma cells. In the extracapillary type of intraglomerular metastasis, neoplastic cells occupy Bowman's spaces, occasionally extending into proximal tubules [14]. The histologic differential diagnosis in this case includes crescentic glomerulonephritis and tubular metaplasia. Distinction between neoplastic involvement of the urinary space and crescentic proliferation or tubular metaplasia of the parietal epithelium is relatively straightforward since cellular crescents and metaplastic lesions do not show frank nuclear atypia (nuclear enlargement, hyperchromasia, irregular chromatin distribution, prominent nucleoli). Mitotic activity is an additional feature of metastatic carcinoma, but crescents may also exhibit mitotic figures. In addition, metastatic cancer cells adhere to and cover the parietal epithelium, as seen in our case, while metaplastic tubular epithelial cells characteristically replace it [6]. Definite evidence of neoplastic involvement of the urinary space is ultimately obtained by immunohistochemistry. In our case, we were able to unequivocally establish the diagnosis of intraglomerular metastatic squamous cell carcinoma by immunostaining the biopsy for P63 and CK5, highly specific and sensitive markers of squamous cell carcinoma [17, 18]. Immunoperoxidase staining with antibodies directed against these markers specifically highlighted the neoplastic cells (Figure 1C and D).
The mechanisms responsible for the intraglomerular trapping and growth of metastatic cancer cells are unclear. As seen in our patient, the majority of reported cases show glomerulocentric distribution of the cancer cells with little involvement of the tubulointerstitium. The predominant pattern is intracapillary, with some cases also showing extracapillary involvement. The route of this form of metastatic spread is clearly hematogenous. The entrapment of circulating neoplastic cells within glomerular capillaries is followed by lysis of the glomerular capillary walls and spread of the cancer cells through the urinary stream. The seeding of circulating neoplastic cells in the glomerular capillaries may be mediated by mechanical factors (reduced blood flow, filtration pressure, size of tumor cells or tumor emboli), paracrine/trophic stimuli and adhesive interactions between neoplastic cells and endothelial cells. Given the terminal nature of the renal arterial tree and the topography of glomeruli which are interposed between two arteriolar vessels, one may argue that hematogenous renal metastases should always originate from glomerular tumor emboli which would subsequently grow and spread into the tubulointerstitial compartment [27]. According to autopsy studies [6, 8, 10], however, renal metastases predominantly involve the renal stroma and capsule and are rarely seen within glomeruli. This would suggest that glomerular capillaries are a minor route for the intrarenal spread of metastatic cancer. It remains unclear, however, why glomeruli are often spared by the metastatic process given their upstream location in relation to the peritubular capillary plexus through which metastatic cancer cells eventually invade the peritubular interstitium.
In conclusion, we have presented a rare case of intraglomerular metastasis detected as an incidental finding in a medical renal biopsy performed for evaluation of renal failure. This is, to our knowledge, the first case of intraglomerular metastasis from penile squamous cell carcinoma. Awareness of this rare finding is critical for accurate histopathologic diagnosis in patients presenting with concurrent renal dysfunction and metastatic cancer disease. Intraglomerular metastases are distinguished from reactive proliferative lesions of the parietal epithelium based on their cytologic atypia and immunoreactivity for specific cell markers. The prognosis in these cases is extremely poor as intraglomerular metastases are characteristically found in association with widely disseminated malignancy.
Conflict of interest statement
None declared.
Acknowledgements
We are indebted to Dr Tri Q. Nguyen for translating journal articles from the German literature. We thank Dr Charles E. Alpers, MD, for critical comments on the manuscript and Drs Kelly Smith, Behzad Najafian and Larry True for helpful advice and discussion.





Comments