-
PDF
- Split View
-
Views
-
Cite
Cite
Ana García-Prieto, José Carlos de la Flor, Elisabet Coll, Elena Iglesias, Javier Reque, Francisco Valga, Expanded hemodialysis: what’s up, Doc?, Clinical Kidney Journal, Volume 16, Issue 7, July 2023, Pages 1071–1080, https://doi.org/10.1093/ckj/sfad033
Close - Share Icon Share
ABSTRACT
In recent years there has been an increasing interest in expanded hemodialysis (HDx), an emerging renal replacement therapy based on the use of medium cut-off membranes (MCO). Thanks to the internal architecture of these types of membranes, with a higher pore size and smaller fiber inner diameter to favor internal filtration rate, they can increase the removal of larger middle molecules in conventional hemodialysis. Secondarily, several reports suggest that this therapy potentially improve the outcomes for end-stage renal disease patients. However, HDx has not been defined yet and the characteristics of MCO membranes are not well stablished. The aim of this narrative review is to define HDx and summarize the dialyzers that have been used so far to perform this therapy, collect the evidence available on its efficacy and clinical outcomes compared with other hemodialysis techniques and settle the bases for its optimal prescription.
Lay Summary
The aim of this narrative review is to define expanded hemodialysis and summarize the dialyzers that have been used so far to perform this therapy, collect the evidence available on its efficacy and clinical outcomes compared with other hemodialysis techniques, and settle the bases for its optimal prescription.
INTRODUCTION
The advent of dialysis has improved both survival and quality of life for millions of people with end-stage chronic kidney disease (CKD) [1]. Despite the progressive improvement of its technical aspects (Fig. 1), there are still opportunities for improvement on its slow road to resemble the glomerular membrane of the kidney.
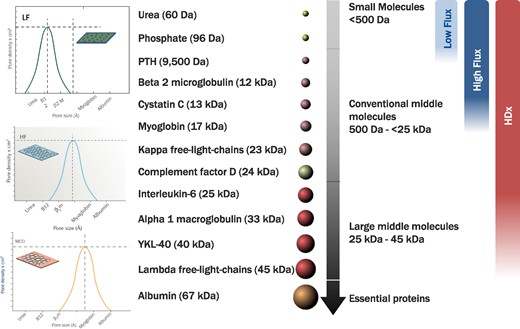
Effect of low flux HD, HF-HD and HDx on different UT in relation to pore size of the different dialyzers.
In this regard, the development of high-flux (HF) polyethersulfone dialyzers [2] significantly improved the quality of dialysis. More recently, the use of these membranes in online hemodiafiltration (OL-HDF), by allowing the combination of convection and diffusion, improved the spectrum of uremic toxins that can be purified. In addition, when using high convective volumes (>23 L/session) in post-dilution OL-HDF, patient survival improved [3]. However, there are some limitations to achieving these high convective volumes such as the need for higher vascular access flows, special monitors and lines or ultrapure water which have limited its application [4].
In recent years, along with the development of new technologies in the manufacture of hemodialysis (HD) membranes, emphasis has been placed on the need to achieve greater convective capacity without significant albumin loss in order to enhance the clearance of middle molecular weight uremic toxins (UT). Therefore, the classic classification of HD membranes based on in vitro ultrafiltration coefficient [5, 6] is being relegated to the background as it fails to include the most recent membranes, defined as medium cut-off (MCO), which thanks to their innovative structure with a higher pore size and enhanced internal filtration rate allow the removal of higher molecular weight UT. It is in this context that the concept of “expanded hemodialysis” (HDx) arises as a technique that combines diffusive and convective transport inside a MCO dialyzer [7], allowing higher clearance rates without the need for the fluid exchange that would be needed in HDF [8].
However, the term “expanded hemodialysis” has not been well defined yet and there are still controversies about which dialyzers should be considered as MCO. Moreover, other membranes called super high-flux (SHF) dialyzers have also been developed and seem to behave in a similar way to MCO dialyzers, allowing higher clearance of middle molecular weight UT in HD. The aim of this narrative review is to define HDx and summarize the dialyzers that have been used so far to perform this therapy, collect the evidence available on its efficacy and clinical outcomes compared with other HD techniques, and settle the bases for its optimal prescription.
DEFINITION OF EXPANDED HEMODIALYSIS AND TECHNICAL CHARACTERISTICS OF MEDIUM CUT-OFF DIALYZERS
“Expanded hemodialysis” was a term first described by Ronco [9] in 2017 as “a treatment where diffusion and convection are conveniently combined inside a hollow fiber dialyzer equipped with a High-Retention Onset (HRO) membrane.” Although the term was initially described for commercial purposes, it has then been generalized to the use of MCO membranes in HD. Thus, the main benefits of this therapy are based on the use of this type of dialyzers, the MCO membrane Theranova® being the first one to be commercialized and proven in HDx [10]. However, some other dialyzers have been recently described and tested in HDx with good results [11, 12].
To better understand the studies conducted by Maduell et al. [11] and Belmouaz et al. [12], which compare different types of MCO dialyzers for the removal of UT, it is necessary to be aware that the classification of dialyzers differs between countries (Table 1). In Europe, dialyzers are classified into five categories, as low-flux, high-flux, medium cut-off, super high-flux/super flux/protein leaking and high cut-off membranes. This classification is based on in vitro ultrafiltration coefficient, albumin sieving coefficient, β2-microglobulin sieving coefficient and clearance with a blood flow rate of 200–400 mL/min. In Japan, dialyzers are classified into five types based on the β2-microglobulin clearance at a blood flow rate of 200 mL/min and a dialysate flow rate of 500 mL/min: type I are classified as low-flux dialyzers (<10 mL/min clearance); type II and III as HF dialyzers (≥10 to <30 mL/min and ≥30 to <50 mL/min clearance, respectively); and type IV and V as SHF dialyzers (≥50 to <70 mL/min and ≥70 mL/min clearance, respectively) [13]. Unfortunately, this information is not always provided by manufacturers so it is sometimes a challenge to classify the different dialyzers.
| (A) Classification of HD membranes in Europe . | ||||
|---|---|---|---|---|
| . | . | β2-microglobulin . | . | |
| Category . | UF coefficient (mL/h/mmHg/m2)a . | Sieving coefficienta . | Clearance (mL/min)b . | Albumin sieving coefficienta . |
| Low flux | <12 | <10 | 0 | |
| High flux | 14–40 | <0.7–0.8 | 20–80 | <0.01 |
| MCO | 40–60 | 0.99 | >80 | <0.01 |
| Protein-leaking | >40 | 0.9–1 | >80 | 0.01–0.03 |
| High cut-off | 40–60 | 1 | <0.2 | |
| (B) Classification of HD membranes in Japan | ||||
| β2-microglobulin | ||||
| Class type | Category | clearance (mL/min)c | ||
| I | Low flux | <10 | ||
| II | <30 | |||
| III | <50 | |||
| High flux | ||||
| IV | <70 | |||
| V | >70 | |||
| Super high flux | ||||
| (A) Classification of HD membranes in Europe . | ||||
|---|---|---|---|---|
| . | . | β2-microglobulin . | . | |
| Category . | UF coefficient (mL/h/mmHg/m2)a . | Sieving coefficienta . | Clearance (mL/min)b . | Albumin sieving coefficienta . |
| Low flux | <12 | <10 | 0 | |
| High flux | 14–40 | <0.7–0.8 | 20–80 | <0.01 |
| MCO | 40–60 | 0.99 | >80 | <0.01 |
| Protein-leaking | >40 | 0.9–1 | >80 | 0.01–0.03 |
| High cut-off | 40–60 | 1 | <0.2 | |
| (B) Classification of HD membranes in Japan | ||||
| β2-microglobulin | ||||
| Class type | Category | clearance (mL/min)c | ||
| I | Low flux | <10 | ||
| II | <30 | |||
| III | <50 | |||
| High flux | ||||
| IV | <70 | |||
| V | >70 | |||
| Super high flux | ||||
In vitro.
For conventional HD with with a blood flow rate of 200 to 400 mL/min.
For conventional HD at a blood flow rate of 200 mL/min and a dialysate flow rate of 500 mL/min.
| (A) Classification of HD membranes in Europe . | ||||
|---|---|---|---|---|
| . | . | β2-microglobulin . | . | |
| Category . | UF coefficient (mL/h/mmHg/m2)a . | Sieving coefficienta . | Clearance (mL/min)b . | Albumin sieving coefficienta . |
| Low flux | <12 | <10 | 0 | |
| High flux | 14–40 | <0.7–0.8 | 20–80 | <0.01 |
| MCO | 40–60 | 0.99 | >80 | <0.01 |
| Protein-leaking | >40 | 0.9–1 | >80 | 0.01–0.03 |
| High cut-off | 40–60 | 1 | <0.2 | |
| (B) Classification of HD membranes in Japan | ||||
| β2-microglobulin | ||||
| Class type | Category | clearance (mL/min)c | ||
| I | Low flux | <10 | ||
| II | <30 | |||
| III | <50 | |||
| High flux | ||||
| IV | <70 | |||
| V | >70 | |||
| Super high flux | ||||
| (A) Classification of HD membranes in Europe . | ||||
|---|---|---|---|---|
| . | . | β2-microglobulin . | . | |
| Category . | UF coefficient (mL/h/mmHg/m2)a . | Sieving coefficienta . | Clearance (mL/min)b . | Albumin sieving coefficienta . |
| Low flux | <12 | <10 | 0 | |
| High flux | 14–40 | <0.7–0.8 | 20–80 | <0.01 |
| MCO | 40–60 | 0.99 | >80 | <0.01 |
| Protein-leaking | >40 | 0.9–1 | >80 | 0.01–0.03 |
| High cut-off | 40–60 | 1 | <0.2 | |
| (B) Classification of HD membranes in Japan | ||||
| β2-microglobulin | ||||
| Class type | Category | clearance (mL/min)c | ||
| I | Low flux | <10 | ||
| II | <30 | |||
| III | <50 | |||
| High flux | ||||
| IV | <70 | |||
| V | >70 | |||
| Super high flux | ||||
In vitro.
For conventional HD with with a blood flow rate of 200 to 400 mL/min.
For conventional HD at a blood flow rate of 200 mL/min and a dialysate flow rate of 500 mL/min.
As has been previously mentioned, Theranova® was the first MCO membrane to be commercialized and proven in HDx and thus, most of the evidence available on HDx comes from studies performed with Theranova®. Although the main characteristics of MCO membranes are not yet well established, they can be summarized as follow:
They have an innovative design characterized by the presence of larger and more homogeneous pores with a reduced pore-size dispersion [14]. The highly selective and controlled porosity results in an adequate adjustment between the molecular weight retention onset and molecular weight cut-off of the membranes, allowing greater removal of the larger middle molecules UT (25–58 kDa) without significant albumin losses [14–17] (Fig. 1).
Increased internal filtration rate favored by the reduced internal diameter of the fibers of some MCO dialyzers or the increased fiber length in others that enhances the convective volume inside the dialyzer [10]. The primary mechanism of HDx is still diffusion [18]; however, changes in membrane design also allow an increase in internal filtration leading to additional convective transport which has been proved and quantified in various theoretical models [10, 17, 19].
Currently, seven dialyzers are commercialized and have been used in HDx, the characteristics of which are summarized in Table 2. The results of the few studies available so far that compare these dialyzers will be summarized in the following sections.
| Membrane . | Brand . | Membrane polymer . | Inner diametera (µm) . | Wall thicknessa (µm) . | Available Surface areasa (m²) . | UF coefficienta (mL/h/mmHg/m²) . | Myoglobin sieving coefficient . | β2-microglobulin sieving coefficient . | Albumin sieving coefficient . | Sterilization . |
|---|---|---|---|---|---|---|---|---|---|---|
| Phylter | Medtronic | Polyphenylene | 200 | 30 | 1.7 (Phylter 17 SD) | 31.2 | 0.7 | 0.93 | <0.02 | Steam |
| Vie | Asahi | Polysulfone | 185 | 45 | 1.8 (Vie 18X) | 49 | 0.8 | 0.9 | <0.01 | Gamma radiation |
| 2.1 (Vie 21X) | ||||||||||
| Elisio | Nipro | Polyethersulfone | 200 | 40 | 1.9 (Elisio 19HX) | 39 | 0.86 | 1 | 0.0024 | Gamma radiation |
| 2.1 (Elisio 21HX) | ||||||||||
| Theranova | Baxter | Polyarylethersulfone | 180 | 35 | 1.7 (Theranova 400) | 28.5 | 0.9 | 1 | 0.008 | Steam |
| 2 (Theranova 500) | ||||||||||
| FDY | Nikkiso | Polyester polymer alloy | 210 | 30 | 2.1 (FDY 210 GW) | 30.5 | ND | 0.94 | ND | Gamma radiation |
| Membrane . | Brand . | Membrane polymer . | Inner diametera (µm) . | Wall thicknessa (µm) . | Available Surface areasa (m²) . | UF coefficienta (mL/h/mmHg/m²) . | Myoglobin sieving coefficient . | β2-microglobulin sieving coefficient . | Albumin sieving coefficient . | Sterilization . |
|---|---|---|---|---|---|---|---|---|---|---|
| Phylter | Medtronic | Polyphenylene | 200 | 30 | 1.7 (Phylter 17 SD) | 31.2 | 0.7 | 0.93 | <0.02 | Steam |
| Vie | Asahi | Polysulfone | 185 | 45 | 1.8 (Vie 18X) | 49 | 0.8 | 0.9 | <0.01 | Gamma radiation |
| 2.1 (Vie 21X) | ||||||||||
| Elisio | Nipro | Polyethersulfone | 200 | 40 | 1.9 (Elisio 19HX) | 39 | 0.86 | 1 | 0.0024 | Gamma radiation |
| 2.1 (Elisio 21HX) | ||||||||||
| Theranova | Baxter | Polyarylethersulfone | 180 | 35 | 1.7 (Theranova 400) | 28.5 | 0.9 | 1 | 0.008 | Steam |
| 2 (Theranova 500) | ||||||||||
| FDY | Nikkiso | Polyester polymer alloy | 210 | 30 | 2.1 (FDY 210 GW) | 30.5 | ND | 0.94 | ND | Gamma radiation |
aAccording to manufacturer's instruction for use.
ND: non-determined.
| Membrane . | Brand . | Membrane polymer . | Inner diametera (µm) . | Wall thicknessa (µm) . | Available Surface areasa (m²) . | UF coefficienta (mL/h/mmHg/m²) . | Myoglobin sieving coefficient . | β2-microglobulin sieving coefficient . | Albumin sieving coefficient . | Sterilization . |
|---|---|---|---|---|---|---|---|---|---|---|
| Phylter | Medtronic | Polyphenylene | 200 | 30 | 1.7 (Phylter 17 SD) | 31.2 | 0.7 | 0.93 | <0.02 | Steam |
| Vie | Asahi | Polysulfone | 185 | 45 | 1.8 (Vie 18X) | 49 | 0.8 | 0.9 | <0.01 | Gamma radiation |
| 2.1 (Vie 21X) | ||||||||||
| Elisio | Nipro | Polyethersulfone | 200 | 40 | 1.9 (Elisio 19HX) | 39 | 0.86 | 1 | 0.0024 | Gamma radiation |
| 2.1 (Elisio 21HX) | ||||||||||
| Theranova | Baxter | Polyarylethersulfone | 180 | 35 | 1.7 (Theranova 400) | 28.5 | 0.9 | 1 | 0.008 | Steam |
| 2 (Theranova 500) | ||||||||||
| FDY | Nikkiso | Polyester polymer alloy | 210 | 30 | 2.1 (FDY 210 GW) | 30.5 | ND | 0.94 | ND | Gamma radiation |
| Membrane . | Brand . | Membrane polymer . | Inner diametera (µm) . | Wall thicknessa (µm) . | Available Surface areasa (m²) . | UF coefficienta (mL/h/mmHg/m²) . | Myoglobin sieving coefficient . | β2-microglobulin sieving coefficient . | Albumin sieving coefficient . | Sterilization . |
|---|---|---|---|---|---|---|---|---|---|---|
| Phylter | Medtronic | Polyphenylene | 200 | 30 | 1.7 (Phylter 17 SD) | 31.2 | 0.7 | 0.93 | <0.02 | Steam |
| Vie | Asahi | Polysulfone | 185 | 45 | 1.8 (Vie 18X) | 49 | 0.8 | 0.9 | <0.01 | Gamma radiation |
| 2.1 (Vie 21X) | ||||||||||
| Elisio | Nipro | Polyethersulfone | 200 | 40 | 1.9 (Elisio 19HX) | 39 | 0.86 | 1 | 0.0024 | Gamma radiation |
| 2.1 (Elisio 21HX) | ||||||||||
| Theranova | Baxter | Polyarylethersulfone | 180 | 35 | 1.7 (Theranova 400) | 28.5 | 0.9 | 1 | 0.008 | Steam |
| 2 (Theranova 500) | ||||||||||
| FDY | Nikkiso | Polyester polymer alloy | 210 | 30 | 2.1 (FDY 210 GW) | 30.5 | ND | 0.94 | ND | Gamma radiation |
aAccording to manufacturer's instruction for use.
ND: non-determined.
EFFECTS OF EXPANDED HEMODIALYSIS COMPARED WITH OTHER DIALYSIS TECHNICS
HDx targets one of the major shortcomings of current extracorporeal dialysis techniques, that is, the inability to adequately remove large middle molecules [16]. In comparison with HF membranes, HRO membranes have significantly increased clearance rates of middle molecules with molecular weights >15 kDa. There are multiple molecules above that molecular weight whose concentrations are elevated in patients with end-stage CKD, which in general can be classified as: cytokines, adipokines, growth factors, and other hormones and immune-mediated molecules [15].
Expanded hemodialysis compared with high-flux hemodialysis
Although there are many studies that compare the efficacy of HDx with HF-HD, the best evidence comes from the work performed by Weiner et al. This safety study included 172 patients on maintenance HD who were randomized to receive dialysis with either a MCO dialyzer (Theranova 400®) or an HF dialyzer (Elisio-17H®) over 24 weeks of treatment. The reduction ratio (RR) for the removal of λ free light chains (λFLC) was significantly higher in the HDx group compared with the HF-HD group after 4 weeks (39% versus 20%) and 24 weeks (33% versus 17%; both P = .001). Among secondary endpoints, the HDx group demonstrated significantly larger RR at 4 and 24 weeks for complement factor D, κ free light chains (κFLC), tumor necrosis factor-alpha (TNF-α) and β2-microglobulin (P = .001 for all) [20].
Also, the Trial Evaluating Mid Cut-Off Value Membrane Clearance of Albumin and Light Chains in Hemodialysis Patients (REMOVAL-HD), a nonrandomized study in which 89 patients in Australia and New Zealand received 24 weeks of treatment with HDx, showed a reduction in free light chains after 2 weeks into HDx [λFLC: Δ –9.1 mg/L, 95% confidence interval (CI) –14.4 to –3.7; κFLC: Δ –5.7 mg/L, 95% CI –9.8 to –1.6] and was sustained for the rest of the study intervention. Both free light chains increased after the cessation of MCO dialyzer use [21].
In a recently published meta-analysis of 18 trials including a total of 853 patients on maintenance HD, HDx increased the RR of β2-microglobulin (–6.28%, 95% CI 0.83, 1.73, P = .02), λFLC (–15.86%, 95% CI 6.96, 24.76, P = .0005) and κFLC (–22.42%, 95% CI 17.95, 26.88, P = .0001) compared with HF-HD [22]. Similar results have been described in other meta-analysis performed by Hung et al. [23], where the RR of β2-microglobulin was significantly higher for the MCO group compared with HF-HD (P < .0001), and the RR for κFLC and λFLC were also decreased after the use of MCO membranes (P < .0001 and P = .02, respectively). However, the RR for interleukin-6 (IL-6) did not differ between groups (P = .07). In the five randomized controlled trial (RCTs) included, only one employed MCO-Ci 400® instead of Theranova® 400 or Theranova® 500. If we exclude this study, the albumin levels would show no difference between groups [mean difference –0.09 g/L (–0.21, 0.04), P = .19]. In 2022, Yang et al. [24] performed a meta-analysis that included nine studies and a total of 529 patients where they found that serum levels of urea, creatinine, β2-microglobulin, κFLC and λFLC were not significantly different between MCO and HF dialyzers. However, RR of β2-microglobulin, κFLC and λFLC were greater for MCO than HF dialyzers. Moreover, MCO dialyzers reduce TNF-α serum levels better than HF-HD. Subgroup analysis stratified by study design indicated that in RCTs, albumin levels were lower in the MCO than the HF dialyzers group, but there was no difference in non-RCT subgroup. In another meta-analysis performed by Kandi et al. [25] which included 26 studies (10 RCT and 16 non RCT with 1883 patients), they found that MCO dialyzers increase the clearance of a wide range of large middle molecules and likely reduce inflammatory mediators with a concomitant transient reduction in serum albumin concentration. For example, MCO dialyzers increased the RR of TNF-α by 7.7% (95% CI 4.7, 10.6) and reduced predialysis TNF-α serum levels by −0.48 (95% CI −0.91, −0.04). However, MCO dialyzers had little to no effect on predialysis IL-6 plasma concentrations. On the other hand, MCO dialysis reduced mRNA expression of TNF-α and IL-6 in peripheral leukocytes by −15% (95% CI −19.6, −10.4) and −8.8% (95% CI −10.2, −7.4), respectively. Table 3 summarizes the currently available meta-analyses evaluating the efficacy of HDx versus HF-HD.
Principal meta-analyses designed to assess the effect of MCO dialysers in HD compared with HF-HD.
| Meta-analysis . | Studies included . | N . | Comparison . | Primary outcomes . | Secondary outcomes . | Results . |
|---|---|---|---|---|---|---|
| Hung et al. [23] | 5 RCTs | 328 | HD with MCO membranes vs HF-HD | RR of β2-microglobulin, κFLC, λFLC and IL-6 | Serum albumin levels | Superior clearance of β2-microglobulin (P < .0001), κFLC (P < .0001) and λFLC (P = .02) with MCO dialysers; no differences in serum IL-6 levels Albumin loss was observed in MCO group (P = .04) Higher reduction in serum albumin in one study |
| Yang et al. [24] | 9 (6 RCTs, 3 non-RCTs) | 529 | HD with MCO membranes vs HF-HD | RR of β2-microglobulin, κFLC and λFLC Levels of β2-microglobulin, κFLC, λFLC, IL-6, TNF-α and albumin | Superior clearance of β2-microglobulin (P < .00001), κFLC (P < .00001) and λFLC (P < .00001) with MCO dialysers No difference in serum levels of β2-microglobulin, κFLC, λFLC and IL-6 between groups Reduced serum levels of TNF-α (P = .005) and albumin (P = .02) with MCO dialysers | |
| Kandi et al. [25] | 26 (10 RCTs, 16 non-RCTs) | 1883 | HD with MCO membranes vs HF-HD | RR of β2-microglobulin, myoglobin, TNF-α, κFLC and λFLC Levels of β2-microglobulin, κFLC, λFLC, IL-6, TNF-α and albumin Albumin removal | Superior clearance (SMD >2) and reduced serum levels (SMD >0.5) of β2-microglobulin, myoglobin, κFLC and λFLC Increased RR of TNF-α by 7.7% (95% CI 4.7, 10.6) and reduced predialysis TNF-α by SMD −0.48 Albumin removal was 2.31 g per session (95% CI 2.79, 1.83) with a reduction in predialysis albumin of −0.12 g/dL (95% CI −0.16, −0.07) in the first 24 weeks, returning to normal after 24 weeks | |
| Kandi et al. [53] | 22 (6 RCTs, 16 non-RCTs) | 1811 | HD with MCO membranes vs HF-HD | QoL, pruritus, RLS and recovery time | All-cause mortality, SAEs, hospitalization, infection and ESA resistance | Improved: - QoL (MD: 16.7/100 points; 6.9, 26.4) - Pruritus (MD = −4.4; −7.1, −1.7) - RLS (odds ratio = 0.39; 0.29, 0.53) - Recovery time (MD = −420 min; −541, −299). Reduced: - Hospitalization (rate ratio = 0.48; 0.27, 0.84) - Hospitalization days (−1.5 days; 95% CI −2.22, −0.78) - Infection (rate ratio = 0.38; 0.17, 0.85) - ESAs resistance (−2.92 U/kg/week/g/L; 95% CI −4.25, −1.6) Little to no difference in mortality (risk difference = −0.4%; −2.8, 2.1) and SAEs (rate ratio = 0.63; 0.38, 1.04) |
| Meta-analysis . | Studies included . | N . | Comparison . | Primary outcomes . | Secondary outcomes . | Results . |
|---|---|---|---|---|---|---|
| Hung et al. [23] | 5 RCTs | 328 | HD with MCO membranes vs HF-HD | RR of β2-microglobulin, κFLC, λFLC and IL-6 | Serum albumin levels | Superior clearance of β2-microglobulin (P < .0001), κFLC (P < .0001) and λFLC (P = .02) with MCO dialysers; no differences in serum IL-6 levels Albumin loss was observed in MCO group (P = .04) Higher reduction in serum albumin in one study |
| Yang et al. [24] | 9 (6 RCTs, 3 non-RCTs) | 529 | HD with MCO membranes vs HF-HD | RR of β2-microglobulin, κFLC and λFLC Levels of β2-microglobulin, κFLC, λFLC, IL-6, TNF-α and albumin | Superior clearance of β2-microglobulin (P < .00001), κFLC (P < .00001) and λFLC (P < .00001) with MCO dialysers No difference in serum levels of β2-microglobulin, κFLC, λFLC and IL-6 between groups Reduced serum levels of TNF-α (P = .005) and albumin (P = .02) with MCO dialysers | |
| Kandi et al. [25] | 26 (10 RCTs, 16 non-RCTs) | 1883 | HD with MCO membranes vs HF-HD | RR of β2-microglobulin, myoglobin, TNF-α, κFLC and λFLC Levels of β2-microglobulin, κFLC, λFLC, IL-6, TNF-α and albumin Albumin removal | Superior clearance (SMD >2) and reduced serum levels (SMD >0.5) of β2-microglobulin, myoglobin, κFLC and λFLC Increased RR of TNF-α by 7.7% (95% CI 4.7, 10.6) and reduced predialysis TNF-α by SMD −0.48 Albumin removal was 2.31 g per session (95% CI 2.79, 1.83) with a reduction in predialysis albumin of −0.12 g/dL (95% CI −0.16, −0.07) in the first 24 weeks, returning to normal after 24 weeks | |
| Kandi et al. [53] | 22 (6 RCTs, 16 non-RCTs) | 1811 | HD with MCO membranes vs HF-HD | QoL, pruritus, RLS and recovery time | All-cause mortality, SAEs, hospitalization, infection and ESA resistance | Improved: - QoL (MD: 16.7/100 points; 6.9, 26.4) - Pruritus (MD = −4.4; −7.1, −1.7) - RLS (odds ratio = 0.39; 0.29, 0.53) - Recovery time (MD = −420 min; −541, −299). Reduced: - Hospitalization (rate ratio = 0.48; 0.27, 0.84) - Hospitalization days (−1.5 days; 95% CI −2.22, −0.78) - Infection (rate ratio = 0.38; 0.17, 0.85) - ESAs resistance (−2.92 U/kg/week/g/L; 95% CI −4.25, −1.6) Little to no difference in mortality (risk difference = −0.4%; −2.8, 2.1) and SAEs (rate ratio = 0.63; 0.38, 1.04) |
QoL: quality of life; SAEs: serious adverse events; SMD: standardized mean difference; MD: mean difference.
Principal meta-analyses designed to assess the effect of MCO dialysers in HD compared with HF-HD.
| Meta-analysis . | Studies included . | N . | Comparison . | Primary outcomes . | Secondary outcomes . | Results . |
|---|---|---|---|---|---|---|
| Hung et al. [23] | 5 RCTs | 328 | HD with MCO membranes vs HF-HD | RR of β2-microglobulin, κFLC, λFLC and IL-6 | Serum albumin levels | Superior clearance of β2-microglobulin (P < .0001), κFLC (P < .0001) and λFLC (P = .02) with MCO dialysers; no differences in serum IL-6 levels Albumin loss was observed in MCO group (P = .04) Higher reduction in serum albumin in one study |
| Yang et al. [24] | 9 (6 RCTs, 3 non-RCTs) | 529 | HD with MCO membranes vs HF-HD | RR of β2-microglobulin, κFLC and λFLC Levels of β2-microglobulin, κFLC, λFLC, IL-6, TNF-α and albumin | Superior clearance of β2-microglobulin (P < .00001), κFLC (P < .00001) and λFLC (P < .00001) with MCO dialysers No difference in serum levels of β2-microglobulin, κFLC, λFLC and IL-6 between groups Reduced serum levels of TNF-α (P = .005) and albumin (P = .02) with MCO dialysers | |
| Kandi et al. [25] | 26 (10 RCTs, 16 non-RCTs) | 1883 | HD with MCO membranes vs HF-HD | RR of β2-microglobulin, myoglobin, TNF-α, κFLC and λFLC Levels of β2-microglobulin, κFLC, λFLC, IL-6, TNF-α and albumin Albumin removal | Superior clearance (SMD >2) and reduced serum levels (SMD >0.5) of β2-microglobulin, myoglobin, κFLC and λFLC Increased RR of TNF-α by 7.7% (95% CI 4.7, 10.6) and reduced predialysis TNF-α by SMD −0.48 Albumin removal was 2.31 g per session (95% CI 2.79, 1.83) with a reduction in predialysis albumin of −0.12 g/dL (95% CI −0.16, −0.07) in the first 24 weeks, returning to normal after 24 weeks | |
| Kandi et al. [53] | 22 (6 RCTs, 16 non-RCTs) | 1811 | HD with MCO membranes vs HF-HD | QoL, pruritus, RLS and recovery time | All-cause mortality, SAEs, hospitalization, infection and ESA resistance | Improved: - QoL (MD: 16.7/100 points; 6.9, 26.4) - Pruritus (MD = −4.4; −7.1, −1.7) - RLS (odds ratio = 0.39; 0.29, 0.53) - Recovery time (MD = −420 min; −541, −299). Reduced: - Hospitalization (rate ratio = 0.48; 0.27, 0.84) - Hospitalization days (−1.5 days; 95% CI −2.22, −0.78) - Infection (rate ratio = 0.38; 0.17, 0.85) - ESAs resistance (−2.92 U/kg/week/g/L; 95% CI −4.25, −1.6) Little to no difference in mortality (risk difference = −0.4%; −2.8, 2.1) and SAEs (rate ratio = 0.63; 0.38, 1.04) |
| Meta-analysis . | Studies included . | N . | Comparison . | Primary outcomes . | Secondary outcomes . | Results . |
|---|---|---|---|---|---|---|
| Hung et al. [23] | 5 RCTs | 328 | HD with MCO membranes vs HF-HD | RR of β2-microglobulin, κFLC, λFLC and IL-6 | Serum albumin levels | Superior clearance of β2-microglobulin (P < .0001), κFLC (P < .0001) and λFLC (P = .02) with MCO dialysers; no differences in serum IL-6 levels Albumin loss was observed in MCO group (P = .04) Higher reduction in serum albumin in one study |
| Yang et al. [24] | 9 (6 RCTs, 3 non-RCTs) | 529 | HD with MCO membranes vs HF-HD | RR of β2-microglobulin, κFLC and λFLC Levels of β2-microglobulin, κFLC, λFLC, IL-6, TNF-α and albumin | Superior clearance of β2-microglobulin (P < .00001), κFLC (P < .00001) and λFLC (P < .00001) with MCO dialysers No difference in serum levels of β2-microglobulin, κFLC, λFLC and IL-6 between groups Reduced serum levels of TNF-α (P = .005) and albumin (P = .02) with MCO dialysers | |
| Kandi et al. [25] | 26 (10 RCTs, 16 non-RCTs) | 1883 | HD with MCO membranes vs HF-HD | RR of β2-microglobulin, myoglobin, TNF-α, κFLC and λFLC Levels of β2-microglobulin, κFLC, λFLC, IL-6, TNF-α and albumin Albumin removal | Superior clearance (SMD >2) and reduced serum levels (SMD >0.5) of β2-microglobulin, myoglobin, κFLC and λFLC Increased RR of TNF-α by 7.7% (95% CI 4.7, 10.6) and reduced predialysis TNF-α by SMD −0.48 Albumin removal was 2.31 g per session (95% CI 2.79, 1.83) with a reduction in predialysis albumin of −0.12 g/dL (95% CI −0.16, −0.07) in the first 24 weeks, returning to normal after 24 weeks | |
| Kandi et al. [53] | 22 (6 RCTs, 16 non-RCTs) | 1811 | HD with MCO membranes vs HF-HD | QoL, pruritus, RLS and recovery time | All-cause mortality, SAEs, hospitalization, infection and ESA resistance | Improved: - QoL (MD: 16.7/100 points; 6.9, 26.4) - Pruritus (MD = −4.4; −7.1, −1.7) - RLS (odds ratio = 0.39; 0.29, 0.53) - Recovery time (MD = −420 min; −541, −299). Reduced: - Hospitalization (rate ratio = 0.48; 0.27, 0.84) - Hospitalization days (−1.5 days; 95% CI −2.22, −0.78) - Infection (rate ratio = 0.38; 0.17, 0.85) - ESAs resistance (−2.92 U/kg/week/g/L; 95% CI −4.25, −1.6) Little to no difference in mortality (risk difference = −0.4%; −2.8, 2.1) and SAEs (rate ratio = 0.63; 0.38, 1.04) |
QoL: quality of life; SAEs: serious adverse events; SMD: standardized mean difference; MD: mean difference.
Expanded hemodialysis compared with online hemodiafiltration
There is a disparity in results when comparing the RR obtained with HDx with those in OL-HDF.
A recent prospective study conducted by Maduell et al. [11] included 23 patients who underwent six dialysis sessions: two sessions with the FX80 Cordiax® in HF-HD and OL-HDF, and four HDx sessions with the Phylther 17-SD®, Vie-18X®, Elisio HX19® and Theranova 400® MCO dialyzers. The differences in efficacy between the evaluated dialyzers were minimal in small molecules and even up to the β2-microglobulin's molecular size. The main differences were found in the RR of myoglobin, κFLC, prolactin, α1-microglobulin and λFLC, which were clearly superior with all four MCO dialyzers (with similar efficacy among them) compared with HF-HD, and slightly inferior to OL-HDF. Similar results were showed by García-Prieto et al. [26] in an observational study including 18 patients.
Some studies report better RR with HDx than with OL-HDF, for example the study conducted by Reque et al. [4] where no differences were found in the urea and β2-microglobulin RR. However, in the case of myoglobin, the RR with OL-HDF was 35% vs 60% with HDx (P < .001). Similarly, in the case of prolactin, the RR with OL-HDF was 45% and 61% with HDx (P < .001). This disparity in the results could be explained by different factors, but fundamentally by the volume of convective transport achieved which was 30 ± 4 L per session in the Maduell et al. study [11] and 23 ± 4 L in the Reque et al. study [4]. Kirsch et al. [10] also showed better results in RR of middle molecules with HDx compared with OL-HDF in an RCT including 39 patients, especially for large solutes.
SAFETY
Albumin losses
According to available evidence, albumin loss with MCO dialyzers is within the range observed in OL-HDF treatment with HF dialyzers [11, 27], below transperitoneal albumin losses seen in peritoneal dialysis [28], and less than a third of what has been reported for HD with high cut-off membranes [29].
Regarding serum albumin levels, in a clinical trial where patients on maintenance HD were randomized to receive dialysis with either an MCO dialyzer (Theranova® 400) or a HF dialyzer (Elisio-17H®) over 24 weeks of treatment both groups maintained similar predialysis serum albumin (4.3 ± 0.28 g/dL and 4.2 ± 0.3 g/dL, respectively) [21]. Similarly, Maduell et al. [30] reported an albumin loss of 2200 mg/session with the Theranova® 400 dialyzer, slightly less than the 2600 mg per session registered for the FX80® dialyzer with OL-HDF. The difference was not statistically significant.
Endotoxin and pyrogens
The elevated internal filtration achieved inside the MCO dialyzers could lead to a higher permeability for endotoxins and other bacterial degradation products potentially present in dialysis fluids. Nevertheless, none of the clinical studies carried out to date suggests an increase in inflammatory reactants in dialysis patients with these membranes. The main in vitro evidence comes from a study conducted by Schepers et al. [31] which showed that when using a 4-fold overload of endotoxin, larger pore membranes, MCO and HCO, are likely safe. Still, it must be guaranteed that water and dialysate comply with current regulations regarding biological contaminants.
Allergies
In the last decades of the previous century, acute dialysis reactions were common in HD patients. They were related to the use of bio-incompatible, complement-activating dialyzer membranes often combined with hypoxia-inducing acetate-containing dialysate, ethylene-oxide (EtO) sterilization of dialyzers that caused immunoglobulin E–mediated hypersensitivity or exposure to polyacrylonitrile membranes that stimulated the production of bradykinin [32]. Nowadays, with biocompatible dialyzers being used, bicarbonate replacing acetate as a dialysate buffer and EtO sterilization abandoned, occasional acute dialyzer reactions are still reported [33]. The MCO and SHF membranes are synthetic membranes, so allergic reactions could occur as with other dialysis membranes. Nevertheless, as far as it is known, no allergies have been described with MCO membranes.
Concomitant medication
Because of its increased pore size, a higher drug clearance could be expected with HDx than HF-HD. In this way, Voigt et al. [34] studied the in vitro retention of different drugs of diverse molecular weights commonly prescribed for dialysis patients: erythropoietin (30 kDa), low-molecular-weight heparin (LMWH; 5 kDa), insulin (6 kDa) and vancomycin (1.5 kDa), comparing three dialysis modalities (HDx, HF-HD and OL-HDF). The selectivity of the MCO membrane for these drugs was comparable to that of the well-established HF dialyzers. Thus, switching from HF-HD to HDx should not require changes to the medication dosing or anticoagulation protocols of dialysis patients.
CLINICAL EFFICACY OF EXPANDED HEMODIALYSIS
Thanks to the higher removal of middle molecules in HDx which has been previously mentioned, there are some clinical situations where this therapy has been proved to be of special interest. It is important to point out that the benefits obtained in most of these situations have only been demonstrated so far with the first MCO dialyzer available, Theranova®. More studies are needed to confirm if these findings can be also achieved by the rest of MCO dialyzers that have been recently commercialized.
Patients with vascular disease
The higher prevalence of vascular disease in CKD patients is caused by traditional risk factors such as inflammation, oxidative stress and UT [35]. HDx increases the elimination of tryptophan and its metabolites (involved in cardiac toxicity); also it enhances the clearance of pentraxin-3 (related to endothelial disfunction) and improves the clearance of cytokines and proinflammatory proteins related to vascular calcification, showing a way to improve vascular outcomes in HD patients [36, 37]. Moreover, HDx decreases the procalcifying effect, quantified by calcium deposition and alkaline phosphatase and decreased calcification-associated proteins such as matrix Gla protein and osteoprotegerin [37].
Patients with poor anemia control
Anemia is a frequent complication of end-stage renal disease (ESRD) associated with increased morbidity and mortality rates [38]. It has multifactorial causes, including erythropoietin deficiency, uremia-related inhibition of erythropoiesis, inflammation and low dialysis adequacy. UT and associated chronic inflammation affect iron metabolism and interfere with the response to erythropoietic stimulating agents (ESA). The increase of hepcidin clearance could improve the anemia management in HD patients, improve the erythropoietin resistance index, and lower the need for erythropoietin, intravenous iron and blood transfusions [39]. Lim et al. showed that HDx reduced ESA resistance compared with HF-HD [40]. This suggests that HDx reduced inflammation, which may improve the iron metabolism and ESA response, probably due to a better removal of TNF-α. Moreover, Hadad-Arrascue et al. reported a trend for reduced ESA dose without a concomitant reduction in the hemoglobin level in the HDx group compared with those on the high-efficiency OL-HDF [41].
Patients with malnutrition
Malnutrition is a frequent and deleterious complication in HD patients. Several factors, including inflammation, retention of middle molecule UT and dialysis procedure, contribute to protein-energy wasting [42]. Belmouaz et al. showed that HDx improved the variation rate of lean tissue index and skeletal muscle index, presumably good surrogate markers of protein-energy wasting. Moreover, albumin levels remained stable after 12 months of HDx [43]. The increased clearance of leptin, obestatin, acyl-ghrelin and pro-inflammatory cytokines (related to decreased appetite in HD patients) could also improve the nutritional status of HD patients [44].
Patients with inflammation
In CKD, persistent systemic inflammation contributes to vascular disease, protein-energy wasting, depression, osteoporosis and frailty [45]. The effective removal of some cytokines and the reduction of IL-6 mRNA expression in peripheral leukocytes explain how HDx could improve the inflammatory state of HD patients [24].
A recent observational study with a large number of cases described changes in cytokine activation and compared responses and clinical outcomes among patients undergoing OL-HDF and HDx. Results showed that HDx provided a further decrease in serum TNF-α and β2-microglobulin levels compared with OL-HDF with lower mortality (18.2% vs 57.1%, respectively), probably related to the normalization of cytokine levels [46, 47].
In the same way, Catar et al. [48] found that uremic serum induces endothelial maladaptation and dysfunction through a TNF-α/sTNF-R1 signaling cascade and is transduced through AP1/c-FOS signaling, promoting maladaptive endothelial Vascular Endothelial Growth Factor (VEGF) expression and angiogenesis. Compared with HF-HD, HDx led to a reduction in proinflammatory mediators and reduced endothelial VEGF production and angiogenesis.
Moreover, Trojanowicz et al. [49] tested the impact of HCO, MCO and HF dialysis on leucocytic transcripts of angiotensin-converting enzymes (ACE and ACE2) showing that the use of membranes with high permeability eliminates a spectrum of mediators from circulation that affect the RAS components in leucocytes, especially ACE/ACE2.
Patients with uremic pruritus
About 40% of patients with ESRD experience pruritus. This symptom has been associated with poor quality of life, impaired sleep, depression and increased mortality [50]. Its pathogenesis remains unclear, but some contributing factors have been considered, including increased systemic inflammation, accumulation of large UT, abnormal serum parathyroid hormone, high phosphorus levels, an imbalance in opiate receptors and a neuropathic process [51]. Compared with HF-HD, HDx may improve self-reported outcomes such as pruritus, probably related to its higher clearance capacity [25, 40, 44].
Patients with restless leg syndrome
Restless legs syndrome (RLS) has a prevalence between 12% and 25% in dialysis patients. The etiology is not clear, but UT seem to play an important role because it tends to decrease after kidney transplantation [51]. Alarcon et al. [52] showed a significant 54.86% reduction in patients meeting the diagnostic criteria for RLS after 12 months of HDx compared with baseline with HF-HD. These results are in accordance with those observed in a recent meta-analysis performed by Kandi et al. [53] and suggest that the expanded clearance of large middle molecules in HDx may alleviate the development and impact of RLS.
Patients with fatigue and bad recovery after hemodialysis sessions
Post-dialysis fatigue is one of the most commonly occurring symptoms in patients on HD. It is not predicted by clinical measures, such as nutritional status or lab results or adequacy in dialysis. It appears to be associated with serum levels of IL-6 and TNF-α, supporting that inflammation could be involved in its pathogenesis [54, 55]. In a study performed by Bolton et al., sustained improvements in post-dialysis recovery time and perceived fatigue level were observed after a 12-month period after a switch from HF-HD or OL-HDF to HDx [56].
Patients with calciphylaxis
Calciphylaxis is a serious complication in HD patients. Some adipokines, including VEGF-A, have been related to mineralization of smooth muscle cells. HDx can remove adipokines involved in the mineralization of smooth muscle cells, and patients with calciphylaxis have shown a good evolution when thiosulphate and HDx are combined [57, 58].
HOW TO PERFORM EXPANDED HEMODIALYSIS
Patient selection
Any patient on HF-HD could benefit from HDx due to its ability to remove a broader spectrum of molecules, as previously reviewed.
Moreover, based on its capacity to increase the clearance of large middle molecules similar to OL-HDF but without its special needing and limitations, HDx could be used instead of OL-HDF in patients unable to achieve goals, where this technique has proved to be beneficial, such as in patients with difficulty in achieving targeted convective volume (>23 L/session) due to vascular access problems, hypercoagulability or hemoconcentration.
In home HD, which has been associated with better survival than in-center HD [59], but where due to logistical reasons OL-HDF cannot usually be offered, HDx could be an excellent option. As HDx removes larger UTs than HF-HD, it could be a promising solution for these patients; however, further evaluation of long-term outcomes in this group of patients is required [60].
Prescription
A dialysis machine with ultrafiltration control is required, but no replacement solution or elevated ultrafiltration rates are needed to perform the therapy. These dialysis machines do not require specific software or additional complex technology. Any of the dialyzers with the characteristics previously mentioned and summarized in Table 3 can be used. A blood flow of 300 mL/min or higher and dialysate flow of 500 mL/min or higher are recommended, but they have proved to be as efficient as HF-HD and OL-HDF in patients with lower blood flows [61]. Dialysate flow can be routinely prescribed in each dialysis unit for HF-HD, as there is no need for a different adjustment. Water purity is recommended.
Contraindications
MCO membranes have been proven to be safe and easy to use and they require no additional skills in clinical practice. However, two practical aspects deserve attention when delivering HDx therapy:
OL-HDF therapy with MCO membranes is contraindicated as it may favor the loss of albumin being able to reach 15 g per session.
Isolated ultrafiltration is not recommended with MCO membranes due to increased permeability of larger plasma proteins such as free hemoglobin. In dialysis patients’ plasma, free hemoglobin is usually present in low concentrations (up to 239 mg/dL) [62]. During isolated ultrafiltration, free hemoglobin is filtered and concentrated in the dialysis fluid compartment. This causes a reddish coloration of the ultrafiltrate which could activate the internal blood leak detector.
SAVINGS
HD is an expensive therapy in economic and ecological terms, owing to a high carbon footprint and significant consumption of natural sources, especially water.
HDx provides clinicians the possibility of performing HD with similar efficacy to OL HDF without increasing technical complexity and costs as it does not require high volumes of replacement fluid.
HDx provide clinicians the possibility of developing HD with similar efficacy to OL-HDF without increasing the technical complexity and cost of dialysis by requiring large volumes of replacement ultrapure dialysis fluid replacement without specialized equipment.
In this way, Sanabria et al. showed that switching a cohort of 81 patients on chronic HF-HD to HDx was associated with reducing medication use, including ESA and iron [63]. Ariza et al. found that the doses per patient of ESA, iron, insulin and hypertension medications, and hospitalization length measured by hospital day per patient-year were significantly lower for HDx patients compared with HF-HD patients [64]. Subsequently, Molano et al. [65] also showed a lower rate of hospitalization in patients undergoing HDx with MCO membranes. Finally, Blackowicz et al. [66], in a post hoc analysis of a RCT, found that the hospitalization rate decreased by 45% with HDx versus HF-HD.
However, most of these results are based on observational studies so these economic benefits attributable to HDx should be confirmed in cost-effectiveness studies.
CONCLUSIONS
In light of the previously revised results which point out that HDx allows enhancement of the elimination of higher molecular weight UT compared with HF-HD and similar to OL-HDF, the authors believe that HDx should be considered in every patient undergoing HD, especially those who cannot achieve higher convective volumes in OL-HDF. However, prospective randomized studies are needed in order to demonstrate whether this higher efficacy leads to fewer comorbidities and better survival in HD patients.
CONFLICT OF INTEREST STATEMENT
The authors declare no financial support for this review. A.G.-P. has received consultancy fees from Baxter and lecture fees from Baxter, Pfizer, AstraZeneca, Vifor and Kyowa Kirin. J.R., J.C.F., F.V., E.I. and E.C. have received consultancy fees from Baxter.
DATA AVAILABILITY STATEMENT
The data underlying this article are available in the article and in its online supplementary material.





Comments