-
PDF
- Split View
-
Views
-
Cite
Cite
Dominique Bertrand, Mélanie Hanoy, Stéphane Edet, Veronique Lemée, Mouad Hamzaoui, Charlotte Laurent, Lebourg Ludivine, Isabelle Etienne, Mathilde Lemoine, Dorian Nezam, Sophie Candon, Jean-Christophe Plantier, Frank Le Roy, Dominique Guerrot, Antibody response to SARS-CoV-2 mRNA BNT162b2 vaccine in kidney transplant recipients and in-centre and satellite centre haemodialysis patients, Clinical Kidney Journal, Volume 14, Issue 9, September 2021, Pages 2127–2128, https://doi.org/10.1093/ckj/sfab100
Close - Share Icon Share
Pandemic severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been particularly deleterious in patients with end-stage renal disease (ESRD) [1, 2]. Emerging data report a low rate of immunization after two doses of messenger RNA (mRNA) in kidney transplantation [3], but higher rates in dialysis patients [4]. We report here the humoural response to two injections of mRNA BNT162b2 vaccine in subpopulations of ESRD patients.
Between 15 January and 30 April 2021, 505 kidney transplant recipients (KTRs) and 62 in-centre haemodialysis patients (HDPs) followed in Rouen University Hospital and 59 HDPs from our medical satellite facility received two injections of Pfizer SARS-CoV-2 mRNA BNT162b2 vaccine 21 days apart. Among them, 235 KTRs and all HDPs were examined for humoural immune response after a mean time of 34.6 days [interquartile range (IQR) 22.5–62.2] following the second dose. No patient included in this cohort had a previous history of SARS-CoV-2 infection. The anti-SARS-CoV-2 post-vaccination antibody response against the spike protein was assessed using the ARCHITECT immunoglobulin G (IgG) II Quant test (Abbott Laboratories, Abbott Park, IL, USA), with titres of >50 arbitrary units per milliliter (AU/mL) being considered as positive. This retrospective study was submitted for approval by the Rouen Centre Institutional Review Board (CERNI No. E2021-37).
Among the 235 KTRs (mean age 60.8 ± 15.1 years, sex 146 males/89 females), 65 (27.7%) developed anti-SARS-CoV-2 antibodies. The median antibody titre in responders was 292 AU/mL (IQR 127–861) (Figure 1). After having received two doses of vaccine, eight KTRs developed coronavirus disease 2019 (COVID-19), three of them with a severe infection and one needed intensive care unit admission [median time after second dose 52.5 days (IQR 32.5–74.2)]. All had negative antibodies after two injections. Characteristics of the 235 KTRs according to the immunosuppressive regimen are described in Table 1. KTRs under belatacept therapy had a lower rate of seroconversion (4.2%).
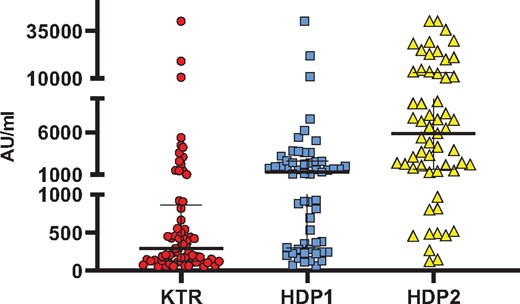
SARS-CoV-2 anti-spike antibody response following a second injection of SARS-CoV-2 mRNA BNT162b2 vaccine in responders. Titres of serum IgG are shown in the samplings of 235 KTRs and 62 in-centre HDPs (HDP1) and 59 HDPs in a satellite centre (HDP2). The median and IQR are shown. Median titres of antibodies are significantly higher in HDP1 compared with HDP2 (P < 0.0001), in HDP2 compared with KTRs (P < 0.0001) and in HDP1 compared with KTRs (P = 0.001). Non‐parametric Mann–Whitney tests were used to compare median titres between groups with StatView version 5.0 (SAS Institute, Cary, NC, USA).
Baseline characteristics of KTRs examined according to the immunosuppression regimen
| Characteristics . | TAC + MMF . | TAC without MMF or no TAC/no bela . | Bela-based immunosuppression . |
|---|---|---|---|
| (n = 113) . | (n = 74) . | (n = 48) . | |
| Sex (male/female), n/n | 66/47 | 46/28 | 34/14 |
| Age (years), median (IQR) | 57.8 (45.2–70.5) | 65.5 (57.9–75.2) | 66.6 (53.6–74.1) |
| Time between kidney transplant and vaccination (years), median (IQR) | 4.1 (1.4–8.3) | 13.9 (5.5–20.9) | 5.8 (2.1–7.2) |
| TAC, n (%) | 113 (100) | 20 (27) | – |
| MMF, n (%) | 113 (100) | – | 42 (87.5) |
| AZA, n (%) | – | 14 (18.9) | 4 (8.3) |
| Ciclosporine, n (%) | – | 39 (52.7) | – |
| mTor I, n (%) | – | 12 (16.2) | |
| Bela, n (%) | – | – | 48 (100) |
| Steroids, n (%) | 54 (47.8) | 33 (44.6) | 32 (66.7) |
| Anti-SARS-CoV-2 antibodies, n (%) | 23 (20.3) | 40 (54) | 2 (4.2) |
| Characteristics . | TAC + MMF . | TAC without MMF or no TAC/no bela . | Bela-based immunosuppression . |
|---|---|---|---|
| (n = 113) . | (n = 74) . | (n = 48) . | |
| Sex (male/female), n/n | 66/47 | 46/28 | 34/14 |
| Age (years), median (IQR) | 57.8 (45.2–70.5) | 65.5 (57.9–75.2) | 66.6 (53.6–74.1) |
| Time between kidney transplant and vaccination (years), median (IQR) | 4.1 (1.4–8.3) | 13.9 (5.5–20.9) | 5.8 (2.1–7.2) |
| TAC, n (%) | 113 (100) | 20 (27) | – |
| MMF, n (%) | 113 (100) | – | 42 (87.5) |
| AZA, n (%) | – | 14 (18.9) | 4 (8.3) |
| Ciclosporine, n (%) | – | 39 (52.7) | – |
| mTor I, n (%) | – | 12 (16.2) | |
| Bela, n (%) | – | – | 48 (100) |
| Steroids, n (%) | 54 (47.8) | 33 (44.6) | 32 (66.7) |
| Anti-SARS-CoV-2 antibodies, n (%) | 23 (20.3) | 40 (54) | 2 (4.2) |
TAC, tacrolimus; bela, belatacept; MMF, mycophenolate mofetil; AZA, azathioprine; mTor, mechanistic target of rapamycin.
Baseline characteristics of KTRs examined according to the immunosuppression regimen
| Characteristics . | TAC + MMF . | TAC without MMF or no TAC/no bela . | Bela-based immunosuppression . |
|---|---|---|---|
| (n = 113) . | (n = 74) . | (n = 48) . | |
| Sex (male/female), n/n | 66/47 | 46/28 | 34/14 |
| Age (years), median (IQR) | 57.8 (45.2–70.5) | 65.5 (57.9–75.2) | 66.6 (53.6–74.1) |
| Time between kidney transplant and vaccination (years), median (IQR) | 4.1 (1.4–8.3) | 13.9 (5.5–20.9) | 5.8 (2.1–7.2) |
| TAC, n (%) | 113 (100) | 20 (27) | – |
| MMF, n (%) | 113 (100) | – | 42 (87.5) |
| AZA, n (%) | – | 14 (18.9) | 4 (8.3) |
| Ciclosporine, n (%) | – | 39 (52.7) | – |
| mTor I, n (%) | – | 12 (16.2) | |
| Bela, n (%) | – | – | 48 (100) |
| Steroids, n (%) | 54 (47.8) | 33 (44.6) | 32 (66.7) |
| Anti-SARS-CoV-2 antibodies, n (%) | 23 (20.3) | 40 (54) | 2 (4.2) |
| Characteristics . | TAC + MMF . | TAC without MMF or no TAC/no bela . | Bela-based immunosuppression . |
|---|---|---|---|
| (n = 113) . | (n = 74) . | (n = 48) . | |
| Sex (male/female), n/n | 66/47 | 46/28 | 34/14 |
| Age (years), median (IQR) | 57.8 (45.2–70.5) | 65.5 (57.9–75.2) | 66.6 (53.6–74.1) |
| Time between kidney transplant and vaccination (years), median (IQR) | 4.1 (1.4–8.3) | 13.9 (5.5–20.9) | 5.8 (2.1–7.2) |
| TAC, n (%) | 113 (100) | 20 (27) | – |
| MMF, n (%) | 113 (100) | – | 42 (87.5) |
| AZA, n (%) | – | 14 (18.9) | 4 (8.3) |
| Ciclosporine, n (%) | – | 39 (52.7) | – |
| mTor I, n (%) | – | 12 (16.2) | |
| Bela, n (%) | – | – | 48 (100) |
| Steroids, n (%) | 54 (47.8) | 33 (44.6) | 32 (66.7) |
| Anti-SARS-CoV-2 antibodies, n (%) | 23 (20.3) | 40 (54) | 2 (4.2) |
TAC, tacrolimus; bela, belatacept; MMF, mycophenolate mofetil; AZA, azathioprine; mTor, mechanistic target of rapamycin.
Among the 62 in-centre HDPs (mean age 73.3 ± 13.8 years, sex 31 males/31 females), 57 (91.9%) developed antibodies. The median titre in responders was 1554 AU/mL (IQR 307–2586) (Figure 1). One patient, with negative serology, developed severe COVID-19 4 days after the second injection. Among the 59 HDPs in a medical satellite facility (mean age 64.3 ± 16.6 years, sex 34 males/25 females), 57 (96.6%) developed antibodies. The median titre in responders was 5893 AU/mL (IQR 1617–13111) (Figure 1). None of them developed SARS-CoV-2 infection during follow-up (mean 44.5 ± 8.6 days).
Our results confirm the low rate of immunization in KTRs after two doses of mRNA vaccine, as recently reported by others. Boyarsky et al. [3] reported a rate of immunization of 54% in 658 solid organ recipients. The immune response is largely influenced by immunosuppression regimen and is particularly low with the usual association of tacrolimus and mycophenolate mofetil [3] and with belatacept [5]. However, the occurrence of severe infections after vaccination in KTR, reported in our study and by others [6] is worrying. Our results also confirm [4] the high degree of seroconversion in HDPs, although with lower titres of antibodies compared with the general population. A strong correlation between neutralizing antibody levels and protection from symptomatic COVID-19 after vaccination is now reported [7]. We report for the first time, reassuring data for HDPs in a medical satellite facility. These patients are younger and have fewer comorbidities than in-centre HDPs.
In conclusion, the mRNA BNT162b2 vaccine seems efficient in HDPs, particularly in HDPs in medical satellite facilities, indicating that vaccination should be highly recommended in these patients, and overall in waitlisted patients for kidney transplantation. In contrast, the low seroconversion rate observed in KTRs is worrying. In seronegative patients, a third dose of vaccine might trigger a humoural response. However, in patients failing to generate any response, should immunizing household members and close contacts be the priority?
CONFLICT OF INTEREST STATEMENT
The authors declare that the results presented in this article have not been published previously in whole or part. None of the authors declare any conflicts of interest.





Comments