-
PDF
- Split View
-
Views
-
Cite
Cite
Ramón Peces, Rocío Mena, Carlos Peces, Emilio Cuesta, Pablo Lapunzina, Rafael Selgas, Julián Nevado, Birth of two healthy girls following preimplantation genetic diagnosis and gestational surrogacy in a rapidly progressive autosomal dominant polycystic kidney disease case using tolvaptan, Clinical Kidney Journal, Volume 14, Issue 8, August 2021, Pages 1987–1989, https://doi.org/10.1093/ckj/sfab082
Close - Share Icon Share
Autosomal dominant polycystic kidney disease (ADPKD) is the most common monogenetic hereditary kidney disease, mainly resulting from mutations in PKD1 and PKD2 [1]. The growth of renal cysts ends in renal failure in the majority of affected individuals, as 50% lead to end-stage renal disease (ESRD). The early clinical manifestations of ADPKD are seen at <30 years of age, and kidney growth rates before adulthood significantly define patients with severe disease [1]. Only tolvaptan has been approved as a specific treatment to slow progression for patients with rapidly progressive disease, highlighting the need to identify such cases [2]. Women with ADPKD, besides the 50% risk of transmission to their offspring, have an increased risk of maternal and fetal complications in pregnancy [3]. Preimplantation genetic diagnosis (PGD) provides an alternative for avoiding pregnancy and the birth of children with ADPKD in at-risk couples. Gestational surrogacy (GS) represents another alternative in affected women who wish to have a biological child and avoid severe obstetric complications.
We report a Caucasian woman who had been diagnosed with ADPKD at 13 years of age when she presented with arterial hypertension and ultrasonography with bilaterally enlarged cystic kidneys. There was no family history of ADPKD. At age 15, the patient experienced an episode of hematuria. At age 28, she was classified as Mayo Class 1E and the prediction of future estimated glomerular filtration rateds (eGFRs) determined ESRD at age 45 years. Her genotype showed a heterozygous nonsense mutation of PKD1 and the patient was classified as PROPKD (8 points) with a genetic score of 3 points (Figure 1A). She was married, but without offspring. Her husband was 30 years old and had normal seminal parameters. At age 29 the couple decided to undergo PGD and GS in the USA (GS is not legal in Spain) in June 2014, with subsequent selection of two healthy embryos for uterine transfer. One embryo was transferred into the surrogate host, resulting in pregnancy and a healthy girl delivered by vaginal delivery at term in April 2015 (Figure 1D). The other embryo was cryopreserved.
At age 32 her eGFR decreased and her total kidney volume (TKV) increased (annual increase of 16%) (Figure 1B and C). In addition, the patient showed a greater TKV and annual rate of TKV and a lower eGFR when compared with three different ADPKD control cohorts (Figure 1C). In 2017 her eGFR decreased (annual decrease of 12.5%) and treatment with tolvaptan was initiated in October 2017 (Figure 1B). Her eGFR remained stable during the following 3 years. In May 2019 the couple decided to use the embryo that was cryopreserved in 2014 and underwent a second GS (in the same host), resulting in a healthy girl by vaginal delivery at term in January 2020.
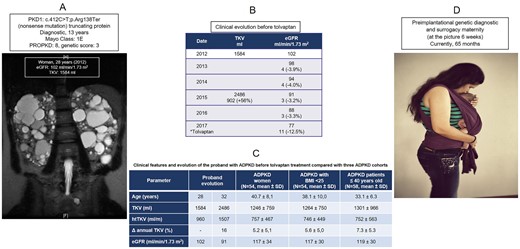
A) Coronal T2-weighted MRI scan of the abdomen in the proband at the age of 28 years showing a total kidney volume (TKV) of 1584 ml and TKV adjusted by height (htTKV) of 960 ml/m. Combined imaging, eGFR and genotype findings. B) Clinical evolution of the proband before tolvaptan. C) Clinical evolution of the proband compared with three ADPKD cohorts. D) Result of PGD and GS.
Although PGD has become available worldwide as an alternative to prenatal diagnosis for many different genetic conditions, only a few cases of ADPKD have been reported (Table 1) [4–11], whereas GS has not been performed until now in ADPKD. PGD is allowed in many European countries, including Spain; the allowed indications for PGD vary by country [9–11]. GS is accepted in 16 of 43 European countries, but public funding systems are extremely variable. The development of methods to identify patients whose renal function is likely to decline rapidly and treatments altering the natural history will affect attitudes in ADPKD. Thus the implementation of genetic analysis would be helpful in the early identification of such patients who would likely progress and probably would benefit the most from therapy. Since currently only two treatments are considered effective for ADPKD—tolvaptan to slow disease progression and PGD—our data suggest that the combination of both genotyping, imaging predictions and the trajectory of eGFR should be implemented to identify rapidly progressive patients before considering a treatment option (tolvaptan and/or PGD with or without GS). Although costly, the expense of PGD with or without GS is significantly less than paying for a lifetime of treatment for ADPKD. This case has shown that integral treatment should be offered to these patients.
| Study . | Country . | Genes . | Genetic analysis . | Couples . | Pregnancies . | Live births . |
|---|---|---|---|---|---|---|
| Verlinsky et al., 2004 [4] | USA | ADPKD | Microsatellite markers | 1 | 1 | 2 (twins) |
| Kuliev, 2005 | 4 | 4 | 4 | |||
| De Rycke et al., 2005 [5] | Belgium | PKD1 | Microsatellite markers | 4 | 2 | 2 |
Li et al., 2017 [6] Zhou et al., 2018 [7] | China | PKD1 | NGS | 3 | 3 | 3 |
| Murphy et al., 2018 [8] | USA | PKD1 PKD2 | Microsatellite markers and NGS | 8 | 4 | 4 |
| Berckmoes et al., 2019 [9] | Belgium | PKD1 PKD2 | Microsatellite markers and NGS | 36 | 21 | 16 |
| Mir Pardo et al., 2020 [10] | Spain | ADPKD | NGS | 74 | ND | ND |
| Snoek et al., 2020 [11] | Netherlands | PKD1 PKD2 | NGS | 37 | ND | ND |
| This report* | Spain | PKD1 | NGS | 1 | 2 | 2 |
| Total | 168 | 37 | 33 |
| Study . | Country . | Genes . | Genetic analysis . | Couples . | Pregnancies . | Live births . |
|---|---|---|---|---|---|---|
| Verlinsky et al., 2004 [4] | USA | ADPKD | Microsatellite markers | 1 | 1 | 2 (twins) |
| Kuliev, 2005 | 4 | 4 | 4 | |||
| De Rycke et al., 2005 [5] | Belgium | PKD1 | Microsatellite markers | 4 | 2 | 2 |
Li et al., 2017 [6] Zhou et al., 2018 [7] | China | PKD1 | NGS | 3 | 3 | 3 |
| Murphy et al., 2018 [8] | USA | PKD1 PKD2 | Microsatellite markers and NGS | 8 | 4 | 4 |
| Berckmoes et al., 2019 [9] | Belgium | PKD1 PKD2 | Microsatellite markers and NGS | 36 | 21 | 16 |
| Mir Pardo et al., 2020 [10] | Spain | ADPKD | NGS | 74 | ND | ND |
| Snoek et al., 2020 [11] | Netherlands | PKD1 PKD2 | NGS | 37 | ND | ND |
| This report* | Spain | PKD1 | NGS | 1 | 2 | 2 |
| Total | 168 | 37 | 33 |
ND, not determined; NGS, next-generation sequencing;
PGD and GS.
| Study . | Country . | Genes . | Genetic analysis . | Couples . | Pregnancies . | Live births . |
|---|---|---|---|---|---|---|
| Verlinsky et al., 2004 [4] | USA | ADPKD | Microsatellite markers | 1 | 1 | 2 (twins) |
| Kuliev, 2005 | 4 | 4 | 4 | |||
| De Rycke et al., 2005 [5] | Belgium | PKD1 | Microsatellite markers | 4 | 2 | 2 |
Li et al., 2017 [6] Zhou et al., 2018 [7] | China | PKD1 | NGS | 3 | 3 | 3 |
| Murphy et al., 2018 [8] | USA | PKD1 PKD2 | Microsatellite markers and NGS | 8 | 4 | 4 |
| Berckmoes et al., 2019 [9] | Belgium | PKD1 PKD2 | Microsatellite markers and NGS | 36 | 21 | 16 |
| Mir Pardo et al., 2020 [10] | Spain | ADPKD | NGS | 74 | ND | ND |
| Snoek et al., 2020 [11] | Netherlands | PKD1 PKD2 | NGS | 37 | ND | ND |
| This report* | Spain | PKD1 | NGS | 1 | 2 | 2 |
| Total | 168 | 37 | 33 |
| Study . | Country . | Genes . | Genetic analysis . | Couples . | Pregnancies . | Live births . |
|---|---|---|---|---|---|---|
| Verlinsky et al., 2004 [4] | USA | ADPKD | Microsatellite markers | 1 | 1 | 2 (twins) |
| Kuliev, 2005 | 4 | 4 | 4 | |||
| De Rycke et al., 2005 [5] | Belgium | PKD1 | Microsatellite markers | 4 | 2 | 2 |
Li et al., 2017 [6] Zhou et al., 2018 [7] | China | PKD1 | NGS | 3 | 3 | 3 |
| Murphy et al., 2018 [8] | USA | PKD1 PKD2 | Microsatellite markers and NGS | 8 | 4 | 4 |
| Berckmoes et al., 2019 [9] | Belgium | PKD1 PKD2 | Microsatellite markers and NGS | 36 | 21 | 16 |
| Mir Pardo et al., 2020 [10] | Spain | ADPKD | NGS | 74 | ND | ND |
| Snoek et al., 2020 [11] | Netherlands | PKD1 PKD2 | NGS | 37 | ND | ND |
| This report* | Spain | PKD1 | NGS | 1 | 2 | 2 |
| Total | 168 | 37 | 33 |
ND, not determined; NGS, next-generation sequencing;
PGD and GS.
PATIENT CONSENT
Written informed consents were obtained from the patients for publication of this article and any accompanying images.
FUNDING
This study was financed in part by the Carlos III Institute of Health, the Ministry of Science and Innovation (EC08/00236) and the Programme for Intensifying Research Activities (IdiPAZ) to R.P. and J.N. ISCIII RETIC REDINREN RD16/0009 FEDER FUNDS.
CONFLICT OF INTEREST STATEMENT
R.P. was an investigator for an Osuka-sponsored study investigating tolvaptan in the treatment of ADPKD (REPRISE). The rest of authors declare no conflicts of interest.
ACKNOWLEDGEMENTS
The authors thank the affected individual and her family who contributed to this study. The study was conducted following the Declaration of Helsinki.





Comments