-
PDF
- Split View
-
Views
-
Cite
Cite
Marine Dekervel, Nicolas Henry, Massimo Torreggiani, Lise-Marie Pouteau, Jean-Paul Imiela, Chloé Mellaza, Anne-Sophie Garnier, Amaury Dujardin, Marine Asfar, Alexandra Ducancelle, Axelle Paquin, Sophie Blanchi, Virginie Besson, Giorgina Barbara Piccoli, Jean-François Augusto, Humoral response to a third injection of BNT162b2 vaccine in patients on maintenance haemodialysis, Clinical Kidney Journal, Volume 14, Issue 11, November 2021, Pages 2349–2355, https://doi.org/10.1093/ckj/sfab152
Close - Share Icon Share
Abstract
Humoral response against sudden acute respiratory syndrome coronavirus 2 (SARS-CoV-2) after two doses of BNT162b2 (Pfizer-BioNTech) has been proven to be less intense in maintenance dialysis patients as compared with healthy subjects, leading the French authorities to recommend a third injection in this population. Here we investigated the response to the third injection in two cohorts of haemodialysis (HD) patients.
Data from two prospective observational cohorts were collected. In the first (‘systematic’) cohort, patients from two HD centres (n = 66) received a third injection of BNT162b2, regardless of the response after two injections. In the second (‘conditional’) cohort, the injection was only prescribed to patients (n = 34) with no or low response to the previous two doses. In both cohorts, the third dose was injected 1–2 months after the second dose. Serology was performed after the second and third doses to assess anti-Spike immunoglobulin G (S IgG) antibody titre.
In the systematic cohort, anti-S IgG was found in 83.3 and 92.4% of patients after the second and third doses of BNT162b2, respectively. In this cohort, 6/11 (54.5%) and 20/21 (95.2%) patients switched from non-responder to low responder and from low responder to high responder, respectively. In low and high responders to two doses, 50/55 (90.9%) at least doubled their anti-S IgG titre. Similar trends were observed in the conditional cohort.
In maintenance HD patients, humoral response against SARS-CoV-2 was boosted after a third dose of BNT162b2, allowing seroconversion in more than half of non-responders. These data may support an intensified vaccination protocol with a third dose of BNT162b2 in dialysis patients.
INTRODUCTION
Coronavirus disease 2019 (COVID-19) is associated with very high morbidity and mortality in patients on maintenance dialysis [1, 2]. Consequently, patients on maintenance dialysis were prioritized in vaccination programmes worldwide [3]. While morbidity and mortality are high both on haemodialysis (HD) and peritoneal dialysis, HD patients combine the challenges of a fragile clinical status with the need to perform treatment in often crowded settings, at risk for contamination. Based on the French Renal Epidemiology and Information Network registry, since the beginning of the pandemic, the estimated cumulative prevalence of coronavirus infection in dialysis patients is 15%; in these patients the mortality rate is ∼19% [4].
Recently, several studies have reported on immunogenicity of COVID-19 vaccines in maintenance HD patients, mainly of BNT162b2 (Pfizer-BioNtech) [5–19]. In these reports, immunoglobulin G (IgG) directed against the receptor-binding domain of the S1 subunit of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) developed in 82.0–96% of patients after two doses of SARS-CoV-2 messenger RNA (mRNA) vaccine. However, the intensity of the humoral response was lower as compared with the one observed in healthy subjects, suggesting that the protection conferred by the vaccine may be lower in uraemic patients [5, 14–16, 18–20]. The factors associated with low or no antibody response to SARS-CoV-2 mRNA vaccine are the same as those associated with the nutritional status and inflammation commonly observed in this population: older age, lymphopaenia, low serum albumin and the need for high iron sucrose supplementation [5, 8, 9, 20]. In keeping with incomplete protection, severe and even fatal reinfections with COVID-19 have been reported in dialysis patients who had already experienced COVID-19 or had completed the vaccine cycle [16, 21].
On these bases, in April 2021, the French authorities recommended a third dose of SARS-CoV-2 mRNA vaccine in patients on renal replacement therapy [22]. At the time of the present report, France is the only country in which such a choice has been made. While this policy has a clear rationale, and the follow-up time is too short to allow an analysis of the efficacy of the risk of developing the disease, timing is crucial in the fight against COVID-19 and we considered that data on the effect of the third vaccine dose could be helpful in defining further vaccination strategies. Therefore this study aimed to analyse the humoral response induced by the third dose of BNT162b2 vaccine in maintenance HD patients.
MATERIALS AND METHODS
Study design
This study gathered data from two HD centres (CHU Angers and CH Laval) where a third dose of BNT162b2 was proposed to all patients, unless they had previously developed COVID-19, regardless of the humoral response they had developed after two doses (‘systematic’ cohort), and from a larger HD centre (CH Le Mans) in which the third BNT162b2 dose was administered only to patients with no or low response after two injections (‘conditional’ cohort). All patients received two doses of BNT162b2 vaccine between February 2021 and April 2021. As per the suggested policy, the third dose was administrated at least 1 month after the second dose.
Clinical data, dialysis schedules and data on vaccine tolerance were obtained from medical records. The primary endpoint was the response provided after the third dose of vaccine compared with the anti-Spike IgG (S IgG) antibody titre measured after the second BNT162b2 dose.
Anti-S-IgG antibody determination
In the systematic cohort (CHU Angers and CH Laval), anti-S IgG antibodies were measured at least 3 weeks after the second (and before the third BNT162b2 injection) and at least 3 weeks after the third BNT162b2 injection. Anti-S IgG antibodies were determined using the SARS-CoV-2 IgG II Quant assay on an I2000SR analyser (Abbott Laboraotires, Abbott Park, IL, USA). In this assay, a value of anti-S IgG antibodies >50 AUs was considered the cut-off for positivity (according to the manufacturer’s instructions).
In the conditional cohort (CH Le Mans), anti-S IgG antibodies were measured 3 weeks after the second and third BNT162b2 injection using the Elecsys anti-SARS-CoV-2 S assay (Roche Diagnostics, Rotkreuz, Switzerland). In this assay, a value of anti-S IgG antibodies >0.8 U/mL was considered the cut-off for positivity (according to the manufacturer’s instructions).
Definitions
Groups were defined based on the level of anti-S IgG antibodies measured after the second vaccine dose: non-responders, low responders and high responders in the systematic cohort and non-responders and low responders in the conditional cohort. Anti-S IgG cut-off points were based on the available tests: in the systematic cohort, non-responders were defined as levels <50 AU/mL, low responders with levels between 50 and 1000 AU/mL and high responders with levels ≥1000 AU/mL. In the conditional cohort, non-responders were defined as anti-S IgG levels <0.8 U/mL, low responders with levels <80 U/mL and high responders with levels ≥80 U/mL. The value of 80 U/mL was chosen according to a recent study that examined the efficacy of passive antibody therapy in COVID-19 with convalescent plasma using the same method of anti-S IgG quantification [23].
Statistical analysis
Descriptive data are reported as median and interquartile range (IQR) for continuous variable and as number of patients and percentages for categorical variables. Continuous variables were compared using the Mann–Whitney U test or the Wilcoxon test and categorical variables were compared using the chi-squared test or Fisher’s exact test. Response was evaluated in absolute terms, as the percentage increase and a switch between responder categories.
The primary composite endpoint was defined as the percentage of patients switching from no- to low-responder status, from low- to high-responder status or doubling their anti-S IgG titre.
Results were considered statistically significant if the P-value was <0.05. SPSS Statistics for Mac version 22 (IBM, Armonk, NY, USA) and GraphPad Prism for Mac what version? (GraphPad Software, San Diego, CA, USA) were used for the statistical analyses.
Ethical issues
The study was conducted in accordance with the Declaration of Helsinki. The protocol was approved by the local ethics committee of Angers University Hospital (CE 2021-112). All patients accepted the whole vaccination schedule and gave agreed to the use of their medical data.
RESULTS
Study populations
The study flowchart is presented in Figure 1. In the systematic cohort study, 75 patients received two BNT162b2 doses. Four patients refused the third BNT162b2 injection and one developed COVID-19 after the second dose of vaccine. Thus 70 patients received the third dose. In four cases, anti-S IgG antibody titre determination was not available (two transferred to another dialysis centre and two died). Lastly, 66 patients in this cohort had anti-S IgG antibody titre determination after the third injection that could be analysed.
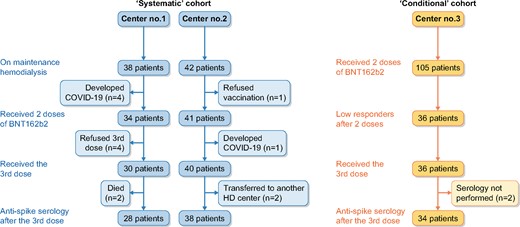
Study flowchart. The study was composed of two prospective observational cohorts. In the systematic cohort (left panel), all patients from two dialysis centres received a third dose of BNT162b2 regardless of the anti-S IgG titre measured after the second dose. In the conditional cohort (right panel), the third dose of BNT162b2 was given to patients who did not develop or developed a low anti-S IgG titre after the second dose.
In the conditional cohort, 36 patients (of 105 vaccinated patients) with low or non-detectable antibodies after two BNT162b2 doses received a third dose. Two patients without serology after the third dose were excluded. Thus 34 patients could be analysed in the conditional cohort. The characteristics of patients are detailed in Table 1.
Characteristics of patients included in the systematic and conditional cohorts
| Variables . | Systematic cohort (n = 66) . | Conditional cohort (n = 34) . |
|---|---|---|
| Age (years), median (IQR) | 73.3 (55–83) | 76.6 (70–85) |
| Gender (male/female), n (%) | 39/27 (59.1/40.9) | 18/16 (55.9/47.1) |
| Dry weight (kg), median (IQR) | 69.3 (60–82) | 66 (60–78) |
| BMI (kg/m2), median (IQR) | 25.6 (22–29) | 25.0 (22–29) |
| Obesity (BMI >30 kg/m2), n (%) | 12 (18.2) | 6 (17.6) |
| Dialysis vintage (months), median (IQR) | 29 (15–61) | 42.8 (16–84.5) |
| Dialysis access (AVF/catheter), n (%) | 55/11 (83.3/16.7) | 22/12 (64.7/35.3) |
| Primary kidney disease, n (%) | ||
| Hypertensive kidney disease | 13 (19.7) | 7 (20.6) |
| Diabetic nephropathy | 13 (19.7) | 9 (26.5) |
| ADPKD | 2 (3.0) | 2 (5.9) |
| Glomerulonephritis | 12 (19.4) | 2 (5.5) |
| Myeloma | 4 (6.1) | – |
| Other | 13 (19.7) | 12 (35.3) |
| Unknown | 9 (13.6) | 2 (5.9) |
| Diabetes mellitus, n (%) | 17 (25.8) | 15 (44.1) |
| Past kidney transplantation, n (%) | 9 (13.6) | 2 (5.9) |
| Waiting for a kidney transplant, n (%) | 11 (16.7) | 6 (17.6) |
| On immunosuppressive drugs, n (%) | 9 (13.6) | 3 (8.8) |
| On steroids, n (%) | 10 (15.2) | 3 (8.8) |
| Serum albumin (g/L), median (IQR) | 36.8 (34–38) | 34.5 (33–38) |
| C-reactive protein (mg/L), median (IQR) | 4.0 (3.2–8.9) | 6.0 (3.0–10.8) |
| Blood lymphocyte count (g/L), median (IQR) | 0.92 (0.70–1.20) | 0.95 (0.68–1.23) |
| Haemoglobin level (g/dL), median (IQR) | 11.4 (10.6–11.9) | 11.0 (10.2–11.7) |
| Darbepoietin (µg/week), median (IQR) | 20 (10–50) | 20 (10–53) |
| Blood ferritin level (µg/L), median (IQR) | 302 (196–470) | 416 (306–537) |
| Iron dose (mg/week), median (IQR) | 50 (25–100) | 100 (0–100) |
| HBV status, n (%) | ||
| HBV infection | 3 (4.5) | 2 (5.9) |
| HBV vaccination | 40 (60.6) | 28 (82.4) |
| Anti-HBV >10 UI/L | 23 (34.8) | 11 (32.4) |
| Variables . | Systematic cohort (n = 66) . | Conditional cohort (n = 34) . |
|---|---|---|
| Age (years), median (IQR) | 73.3 (55–83) | 76.6 (70–85) |
| Gender (male/female), n (%) | 39/27 (59.1/40.9) | 18/16 (55.9/47.1) |
| Dry weight (kg), median (IQR) | 69.3 (60–82) | 66 (60–78) |
| BMI (kg/m2), median (IQR) | 25.6 (22–29) | 25.0 (22–29) |
| Obesity (BMI >30 kg/m2), n (%) | 12 (18.2) | 6 (17.6) |
| Dialysis vintage (months), median (IQR) | 29 (15–61) | 42.8 (16–84.5) |
| Dialysis access (AVF/catheter), n (%) | 55/11 (83.3/16.7) | 22/12 (64.7/35.3) |
| Primary kidney disease, n (%) | ||
| Hypertensive kidney disease | 13 (19.7) | 7 (20.6) |
| Diabetic nephropathy | 13 (19.7) | 9 (26.5) |
| ADPKD | 2 (3.0) | 2 (5.9) |
| Glomerulonephritis | 12 (19.4) | 2 (5.5) |
| Myeloma | 4 (6.1) | – |
| Other | 13 (19.7) | 12 (35.3) |
| Unknown | 9 (13.6) | 2 (5.9) |
| Diabetes mellitus, n (%) | 17 (25.8) | 15 (44.1) |
| Past kidney transplantation, n (%) | 9 (13.6) | 2 (5.9) |
| Waiting for a kidney transplant, n (%) | 11 (16.7) | 6 (17.6) |
| On immunosuppressive drugs, n (%) | 9 (13.6) | 3 (8.8) |
| On steroids, n (%) | 10 (15.2) | 3 (8.8) |
| Serum albumin (g/L), median (IQR) | 36.8 (34–38) | 34.5 (33–38) |
| C-reactive protein (mg/L), median (IQR) | 4.0 (3.2–8.9) | 6.0 (3.0–10.8) |
| Blood lymphocyte count (g/L), median (IQR) | 0.92 (0.70–1.20) | 0.95 (0.68–1.23) |
| Haemoglobin level (g/dL), median (IQR) | 11.4 (10.6–11.9) | 11.0 (10.2–11.7) |
| Darbepoietin (µg/week), median (IQR) | 20 (10–50) | 20 (10–53) |
| Blood ferritin level (µg/L), median (IQR) | 302 (196–470) | 416 (306–537) |
| Iron dose (mg/week), median (IQR) | 50 (25–100) | 100 (0–100) |
| HBV status, n (%) | ||
| HBV infection | 3 (4.5) | 2 (5.9) |
| HBV vaccination | 40 (60.6) | 28 (82.4) |
| Anti-HBV >10 UI/L | 23 (34.8) | 11 (32.4) |
Characteristics of patients included in the systematic and conditional cohorts
| Variables . | Systematic cohort (n = 66) . | Conditional cohort (n = 34) . |
|---|---|---|
| Age (years), median (IQR) | 73.3 (55–83) | 76.6 (70–85) |
| Gender (male/female), n (%) | 39/27 (59.1/40.9) | 18/16 (55.9/47.1) |
| Dry weight (kg), median (IQR) | 69.3 (60–82) | 66 (60–78) |
| BMI (kg/m2), median (IQR) | 25.6 (22–29) | 25.0 (22–29) |
| Obesity (BMI >30 kg/m2), n (%) | 12 (18.2) | 6 (17.6) |
| Dialysis vintage (months), median (IQR) | 29 (15–61) | 42.8 (16–84.5) |
| Dialysis access (AVF/catheter), n (%) | 55/11 (83.3/16.7) | 22/12 (64.7/35.3) |
| Primary kidney disease, n (%) | ||
| Hypertensive kidney disease | 13 (19.7) | 7 (20.6) |
| Diabetic nephropathy | 13 (19.7) | 9 (26.5) |
| ADPKD | 2 (3.0) | 2 (5.9) |
| Glomerulonephritis | 12 (19.4) | 2 (5.5) |
| Myeloma | 4 (6.1) | – |
| Other | 13 (19.7) | 12 (35.3) |
| Unknown | 9 (13.6) | 2 (5.9) |
| Diabetes mellitus, n (%) | 17 (25.8) | 15 (44.1) |
| Past kidney transplantation, n (%) | 9 (13.6) | 2 (5.9) |
| Waiting for a kidney transplant, n (%) | 11 (16.7) | 6 (17.6) |
| On immunosuppressive drugs, n (%) | 9 (13.6) | 3 (8.8) |
| On steroids, n (%) | 10 (15.2) | 3 (8.8) |
| Serum albumin (g/L), median (IQR) | 36.8 (34–38) | 34.5 (33–38) |
| C-reactive protein (mg/L), median (IQR) | 4.0 (3.2–8.9) | 6.0 (3.0–10.8) |
| Blood lymphocyte count (g/L), median (IQR) | 0.92 (0.70–1.20) | 0.95 (0.68–1.23) |
| Haemoglobin level (g/dL), median (IQR) | 11.4 (10.6–11.9) | 11.0 (10.2–11.7) |
| Darbepoietin (µg/week), median (IQR) | 20 (10–50) | 20 (10–53) |
| Blood ferritin level (µg/L), median (IQR) | 302 (196–470) | 416 (306–537) |
| Iron dose (mg/week), median (IQR) | 50 (25–100) | 100 (0–100) |
| HBV status, n (%) | ||
| HBV infection | 3 (4.5) | 2 (5.9) |
| HBV vaccination | 40 (60.6) | 28 (82.4) |
| Anti-HBV >10 UI/L | 23 (34.8) | 11 (32.4) |
| Variables . | Systematic cohort (n = 66) . | Conditional cohort (n = 34) . |
|---|---|---|
| Age (years), median (IQR) | 73.3 (55–83) | 76.6 (70–85) |
| Gender (male/female), n (%) | 39/27 (59.1/40.9) | 18/16 (55.9/47.1) |
| Dry weight (kg), median (IQR) | 69.3 (60–82) | 66 (60–78) |
| BMI (kg/m2), median (IQR) | 25.6 (22–29) | 25.0 (22–29) |
| Obesity (BMI >30 kg/m2), n (%) | 12 (18.2) | 6 (17.6) |
| Dialysis vintage (months), median (IQR) | 29 (15–61) | 42.8 (16–84.5) |
| Dialysis access (AVF/catheter), n (%) | 55/11 (83.3/16.7) | 22/12 (64.7/35.3) |
| Primary kidney disease, n (%) | ||
| Hypertensive kidney disease | 13 (19.7) | 7 (20.6) |
| Diabetic nephropathy | 13 (19.7) | 9 (26.5) |
| ADPKD | 2 (3.0) | 2 (5.9) |
| Glomerulonephritis | 12 (19.4) | 2 (5.5) |
| Myeloma | 4 (6.1) | – |
| Other | 13 (19.7) | 12 (35.3) |
| Unknown | 9 (13.6) | 2 (5.9) |
| Diabetes mellitus, n (%) | 17 (25.8) | 15 (44.1) |
| Past kidney transplantation, n (%) | 9 (13.6) | 2 (5.9) |
| Waiting for a kidney transplant, n (%) | 11 (16.7) | 6 (17.6) |
| On immunosuppressive drugs, n (%) | 9 (13.6) | 3 (8.8) |
| On steroids, n (%) | 10 (15.2) | 3 (8.8) |
| Serum albumin (g/L), median (IQR) | 36.8 (34–38) | 34.5 (33–38) |
| C-reactive protein (mg/L), median (IQR) | 4.0 (3.2–8.9) | 6.0 (3.0–10.8) |
| Blood lymphocyte count (g/L), median (IQR) | 0.92 (0.70–1.20) | 0.95 (0.68–1.23) |
| Haemoglobin level (g/dL), median (IQR) | 11.4 (10.6–11.9) | 11.0 (10.2–11.7) |
| Darbepoietin (µg/week), median (IQR) | 20 (10–50) | 20 (10–53) |
| Blood ferritin level (µg/L), median (IQR) | 302 (196–470) | 416 (306–537) |
| Iron dose (mg/week), median (IQR) | 50 (25–100) | 100 (0–100) |
| HBV status, n (%) | ||
| HBV infection | 3 (4.5) | 2 (5.9) |
| HBV vaccination | 40 (60.6) | 28 (82.4) |
| Anti-HBV >10 UI/L | 23 (34.8) | 11 (32.4) |
Anti-SARS-CoV-2 humoral response following the third dose of BNT162b2 vaccine in the systematic cohort
At baseline, anti-S-IgG titres were measured after a median of 55 days (IQR 44–55) from the second vaccine dose. The median level of anti-S IgG after two doses was 1056 UI/mL (IQR 173–2832) (Figure 2A). No, low and high responses were observed in 11 (16.7%), 21 (31.8%) and 34 (51.5%) patients, respectively (Figure 3A). Thus, overall, an antibody response developed in 83.3% of patients after two doses of BNT162b2.
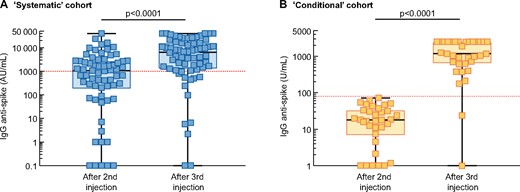
Anti-S IgG titre after the second and third BNT162b2 doses in the (A) systematic and (B) conditional cohorts. Each patient is represented by a square. The red dashed line represents the cut-off level for high response to vaccine (anti-S IgG antibody >1000 AU/mL in the systematic cohort and >80 U/mL in the conditional cohort). Comparisons were made using the Mann–Whitney U test. Logarithmic scale.
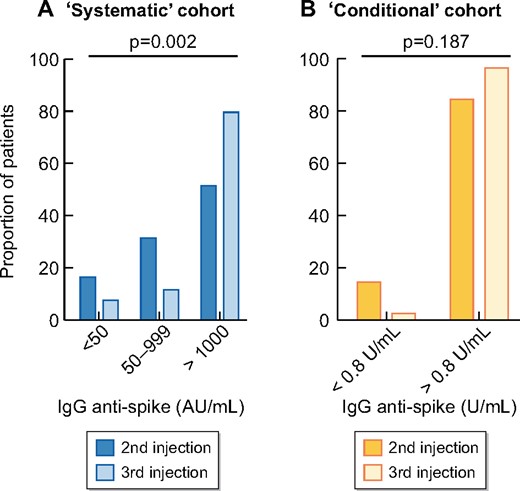
Percentage of patients according to response status after the second and third dose of BNT162b2 in the (A) systematic and (B) conditional cohorts.
The third vaccine dose was administered after a median delay of 60 days (IQR 55–97) from the second dose. Anti-S IgG titres were measured after a median of 28 days (IQR 20–28) from the third vaccine dose. The median anti-S IgG titre significantly increased after the third dose (6464 versus 1056; P < 0.0001; Figure 2A). After the third dose, 61/66 (92.4%) patients were responders (anti-S IgG >50 UI/mL; Figure 3A).
The primary composite endpoint (defined as the cumulative percentage of patients switching from no- to low-responder status, from low- to high-responder status or doubling their anti-S IgG titre), was reached in 56 (84.8%) patients. In detail, 6/11 (54.5%) changed from no- to low-responder status, 20/21 (95.2%) from low- to high-responder status and 50/55 (90.9%) at least doubled their anti-S IgG titre. Figure 4 shows the kinetics of anti-S IgG titres between the second and third vaccine dose according to the response status after the second dose. A 13.1-fold (IQR 3.6–25.1) increase in anti-S IgG was observed in the low-responder group after the third dose and a 3.8-fold (IQR 2.5–7.4) increase was observed in the high-responder group (Figure 5A). Moreover, the anti-S IgG titres measured after the second and third vaccine dose were highly correlated in patients who were low or high responders after two doses (Figure 5B).
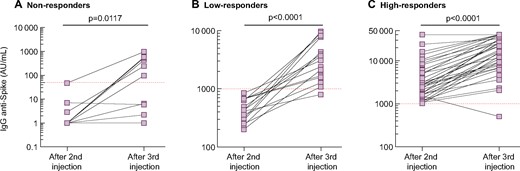
Kinetics of anti-S IgG titre between the second and third dose of BNT162b2 in the systematic cohort in (A) non-responders, (B) low reponders and (C) high responders. Each patient is represented by two squares with a connecting line. The red dashed line represents the cut-off value for test positivity. Comparisons were made using the Wilcoxon matched pairs ranked test. Logarithmic scale.

Anti-S IgG after the second and third dose of BNT162b2 in the systematic cohort. (A) The increase in anti-S IgG titre after the third dose. Logarithmic scale. (B) Correlation between the anti-S IgG titres measured after the second and third dose. Linear scale.
Anti-SARS-CoV-2 humoral response following the third dose of BNT162b2 in the conditional cohort
In the conditional cohort, anti-S IgG titre was measured at a median delay of 35.5 days (IQR 35–36) from the second dose of BNT162b2. The third dose was administered a median of 71 days (IQR 69–71) after the second dose.
The anti-S IgG titres increased from 17.8 U/mL (IQR 6.6–44.0) after the second dose to 1180 U/mL (IQR 627–2500) after the third dose (Figure 2B). Anti-S IgG titres increased by 67-fold (IQR 37–99) between the second and third vaccine doses in low responders. Four of five seronegative patients developed significant levels of anti-S IgG antibodies after the third dose. Following the third injection, 33/34 (97.1%) patients displayed anti-S IgG >0.8 U/mL versus 29/34 (85.3%) after two doses. The composite endpoint (defined as the cumulative percentage of patients switching from no- to low-responder status, from low- to high-responder status or doubling their anti-S IgG titre) was reached in 33 (97.1%) patients.
Tolerance to the third BNT162b2 dose and factors of no or low response to the third dose in the systematic cohort
In Centre 1, two patients died after the third vaccine dose administration (at 10 days and 3 weeks). Both patients had severe heart and peripheral vascular disease and their life expectancies were considered <1 year at HD initiation. Death was related to bacterial sepsis in both cases and considered unrelated to vaccine administration by the medical staff. No other serious side effects were observed in both the systematic and conditional cohorts following the third injection of BNT162b2. No other patient required hospitalization or needed urgent medical consultation.
In the systematic cohort, we compared characteristics of patients according to their response to the third dose. The only factor associated with no or low response after the third dose was steroid treatment (Supplementary data, Table S1). Patients without response to hepatitis B virus (HBV) vaccination tended to be more represented in non-responders to the third injection (Supplementary data, Table S1). In the subgroup analysis of patients withHBV vaccination, we did not observe any significant statistical differences in the response to the second or third BNT162b2 dose between those with (n = 20) and without (n = 20) anti-HBV antibodies (data not shown). Clinical characteristics of non-responders to the third dose of both cohorts are reported in Supplementary data, Table S2.
DISCUSSION
In this study we report on the humoral response after a third BNT162b2 injection in maintenance HD patients. The main result is that the third dose of vaccine allows boosting the humoral response to SARS-CoV-2 in this population. Importantly, the third dose allowed seroconversion in about half of the cases without antibody response after the conventional vaccination schedule. Even more importantly, the third BNT162b2 dose was well tolerated and we did not observe any serious side effects in our patients.
The best way to prescribe the third vaccine dose is not established, and both a systematic approach, revaccinating all patients accepting it, and a conditional one, in which vaccination is proposed in case with low response or when antibody titres decrease, are reasonable and feasible. Here, two participating centres chose the first option, while a larger one chose the second.
In both cohorts, patients with low response significantly increased their antibody levels. Furthermore, in the systematic cohort, a further increase in the response was also observed in high responders. Thus the third challenge allowed consolidating the humoral response against SARS-CoV-2. In the conditional cohort, all but two patients (94%) achieved high response after the third dose.
This observation is of interest given that antibody titres are described as significantly lower in HD patients after vaccination as compared with healthy subjects [5, 8, 14, 15] and that a lower antibody response is associated with a lower ability to neutralize SARS-CoV-2 in vitro [14]. Additionally, a recent study demonstrated that a rapid decline of anti-SARS-CoV-2 antibodies occurs following natural immunization after COVID-19 in dialysis patients [24].
Recent studies have also shown that, unlike kidney transplant patients, most maintenance HD patients are able to mount a specific T-cell response against SARS-CoV-2 after two doses of BNT162b2 [25, 26]. However, in both populations, B-cell response appears defective, with reduced vaccine-induced specific memory B cells and plasmablasts as compared with healthy subjects. Moreover, a significant correlation between plasmablasts and anti-S IgG titres was observed [27]. Thus intensification of the vaccination protocol appears to be a reliable strategy for strengthening protection against SARS-CoV-2.
In the systematic cohort, the majority of patients who had detectable antibodies (90.9%) at least doubled their anti-S IgG titre, increasing the prevalence of high responders. Indeed, after the third dose, the median anti-S IgG titre was in the same range as that reported for healthy subjects who received a conventional vaccine schedule [5]. The same trend occurred in the conditional cohort.
In both the systematic and conditional cohorts, we observed that more than half of non-responders achieved seroconversion with a third BNT162b2 dose. However, anti-S IgG titres of these patients remained low in some patients, below the cut-off we defined as high-responder status (systematic cohort: <1000 UA/mL; conditional cohort: 80 U/mL). These observations are in line with a recent report showing seroconversion after the third dose in 5/12 non-responders to the two-dose protocol [11]. Based on these observations, it appears plausible that patients with no or low response despite three doses may still develop or improve the antibody response with supplemental doses.
Our study was not powered to study predictive factors of response to vaccination. However, as already reported, patients with steroid treatment were significantly more represented in the no or low responders to the third dose [11]. We also did not observe any significant difference in the response to a third BNT162b2 dose according to HBV vaccination status. However, once again, the number of patients was limited and does not allow us to provide definite conclusions.
Our study has several limitations, starting with its observational design and the small number of patients. Furthermore, we did not analyse the cellular response to vaccination, and the follow-up is still limited, thus we cannot conclude that the improved humoral response is also associated with clinical protection. Furthermore, anti-S IgG antibodies were assessed by two different tests, thus preventing precise comparison of responses. Within these limits, we hope that the data reported here may help in defining the future strategy for our patients.
In conclusion, our data show that a third dose of BNT162b2 is effective for increasing the humoral response against SARS-CoV-2 in maintenance HD patients and support considering a third dose of BNT162b2, at least in patients with no or low response to the conventional vaccine protocol.
SUPPLEMENTARY DATA
Supplementary data are available at ckj online.
CONFLICT OF INTEREST STATEMENT
None declared. The results presented in this article have not been published previously in whole or part.





Comments