-
PDF
- Split View
-
Views
-
Cite
Cite
Ahmet Burak Dirim, Seda Safak, Berk Andac, Nurana Garayeva, Erol Demir, Ayse Serra Artan, Yasemin Ozluk, Isin Kilicaslan, Ozgur Akin Oto, Savas Ozturk, Halil Yazici, Minimal change disease following vaccination with CoronaVac, Clinical Kidney Journal, Volume 14, Issue 10, October 2021, Pages 2268–2269, https://doi.org/10.1093/ckj/sfab123
Close - Share Icon Share
CoronaVac (Sinovac) is an inactivated severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine, and it was effective in Phase 1/2 trials [1, 2]. Phase 3 trials are ongoing, and no publications are available yet [3]. No serious adverse events were detected in adults older than 60 years in the Phase 1/2 trial [2]. Herein, we report a patient who developed nephrotic syndrome after the first dose of CoronaVac. Renal biopsy revealed minimal change disease (MCD), and the patient was successfully treated with methylprednisolone.
A 65-year-old male was admitted to the clinic 17 days after the first dose of CoronaVac with generalized oedema and 20 kg weight gain. The first symptoms developed a week after the CoronaVac administration. Previous medical history was remarkable for well-controlled type 2 diabetes mellitus and Hashimoto thyroiditis for 12 years. Medications were metformin 2 g/day, dapagliflozin 10 mg/day, vildagliptin 100 mg/day and levothyroxine 150 μg/day. Physical examination revealed bilateral pitting pretibial oedema and ascites. Laboratory results showed severe hypoalbuminaemia with an 11.9 g/g spot urine protein to creatinine ratio (UPCR) (Table 1). A renal biopsy was performed due to nephrotic syndrome, and it consisted of 17 glomeruli with a normal appearance. No segmental or global sclerotic lesion could be detected despite serial sections in the corticomedullary junction. Mild interstitial fibrosis, tubular atrophy and moderate arterial intimal fibrosis were detected. Immunofluorescence and Congo staining were negative. MCD was diagnosed, and 1 mg/kg/day methylprednisolone was started. Also, intravenous 120 mg/day furosemide was added. As serum creatinine level increased to 2.1 mg/dL, the dosage of furosemide was reduced. After 12 days of corticosteroid treatment, UPCR declined to 0.33 g/g, and serum albumin increased to 3.01 g/dL (Figure 1). A 12-week steroid tapering regimen was prescribed, and diuretic treatment was discontinued due to reaching the basal weight.
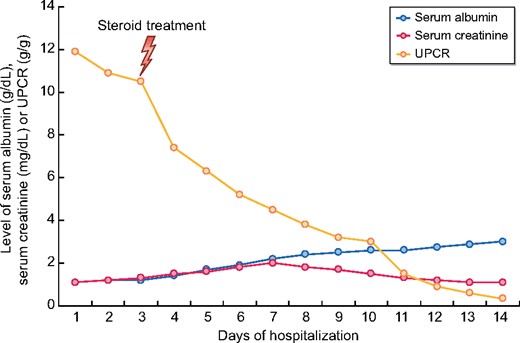
The course of the serum albumin (g/dL), UPCR (g/g) and serum creatinine (mg/dL) during follow-up.
Laboratory data of the patient at admission (bold values indicate abnormal results)
| Laboratory data (normal range) . | Results . |
|---|---|
| Haemoglobin (g/dL) (13–17) | 15.3 |
| Serum creatinine (mg/dL) (0.6–1.3) | 1 |
| Serum albumin (g/dL) (3.5–5.5) | 1.1 |
| UPCR (g/g) | 11.9 |
| HbA1c (%) (<6.5) | 6.9 |
| LDL cholesterol (mg/dL) (100–130) | 413 |
| HDL cholesterol (mg/dL) (>40) | 41 |
| TG (mg/dL) (<150) | 241 |
| TSH (mIU/L) (0.3–4.2) | 9.3 |
| Free T4 (pmol/L) (12–22) | 17.24 |
| Serum-free light chain ratio (0.26–1.65) | 0.3 |
| Serum/urine immunofixation | Negative for monoclonality |
| C3 (mg/dL) (90–180) | 129 |
| C4 (mg/dL) (10–40) | 40.7 |
| ANA/ENA panel/anti-dsDNA/ HBsAg/anti-HCV/anti-HIV | Negative |
| Laboratory data (normal range) . | Results . |
|---|---|
| Haemoglobin (g/dL) (13–17) | 15.3 |
| Serum creatinine (mg/dL) (0.6–1.3) | 1 |
| Serum albumin (g/dL) (3.5–5.5) | 1.1 |
| UPCR (g/g) | 11.9 |
| HbA1c (%) (<6.5) | 6.9 |
| LDL cholesterol (mg/dL) (100–130) | 413 |
| HDL cholesterol (mg/dL) (>40) | 41 |
| TG (mg/dL) (<150) | 241 |
| TSH (mIU/L) (0.3–4.2) | 9.3 |
| Free T4 (pmol/L) (12–22) | 17.24 |
| Serum-free light chain ratio (0.26–1.65) | 0.3 |
| Serum/urine immunofixation | Negative for monoclonality |
| C3 (mg/dL) (90–180) | 129 |
| C4 (mg/dL) (10–40) | 40.7 |
| ANA/ENA panel/anti-dsDNA/ HBsAg/anti-HCV/anti-HIV | Negative |
ANA, antinuclear antibody; anti-dsDNA, anti-double-stranded DNA antibody; ENA, extractable nuclear antibodies; HBsAg, hepatitis B surface antigen; HCV, hepatitis C virus; HDL, high-density lipoprotein; HIV, human immunodeficiency virus; LDL, low-density lipoprotein; TG, triglyceride; TSH, thyroid-stimulating hormone.
Laboratory data of the patient at admission (bold values indicate abnormal results)
| Laboratory data (normal range) . | Results . |
|---|---|
| Haemoglobin (g/dL) (13–17) | 15.3 |
| Serum creatinine (mg/dL) (0.6–1.3) | 1 |
| Serum albumin (g/dL) (3.5–5.5) | 1.1 |
| UPCR (g/g) | 11.9 |
| HbA1c (%) (<6.5) | 6.9 |
| LDL cholesterol (mg/dL) (100–130) | 413 |
| HDL cholesterol (mg/dL) (>40) | 41 |
| TG (mg/dL) (<150) | 241 |
| TSH (mIU/L) (0.3–4.2) | 9.3 |
| Free T4 (pmol/L) (12–22) | 17.24 |
| Serum-free light chain ratio (0.26–1.65) | 0.3 |
| Serum/urine immunofixation | Negative for monoclonality |
| C3 (mg/dL) (90–180) | 129 |
| C4 (mg/dL) (10–40) | 40.7 |
| ANA/ENA panel/anti-dsDNA/ HBsAg/anti-HCV/anti-HIV | Negative |
| Laboratory data (normal range) . | Results . |
|---|---|
| Haemoglobin (g/dL) (13–17) | 15.3 |
| Serum creatinine (mg/dL) (0.6–1.3) | 1 |
| Serum albumin (g/dL) (3.5–5.5) | 1.1 |
| UPCR (g/g) | 11.9 |
| HbA1c (%) (<6.5) | 6.9 |
| LDL cholesterol (mg/dL) (100–130) | 413 |
| HDL cholesterol (mg/dL) (>40) | 41 |
| TG (mg/dL) (<150) | 241 |
| TSH (mIU/L) (0.3–4.2) | 9.3 |
| Free T4 (pmol/L) (12–22) | 17.24 |
| Serum-free light chain ratio (0.26–1.65) | 0.3 |
| Serum/urine immunofixation | Negative for monoclonality |
| C3 (mg/dL) (90–180) | 129 |
| C4 (mg/dL) (10–40) | 40.7 |
| ANA/ENA panel/anti-dsDNA/ HBsAg/anti-HCV/anti-HIV | Negative |
ANA, antinuclear antibody; anti-dsDNA, anti-double-stranded DNA antibody; ENA, extractable nuclear antibodies; HBsAg, hepatitis B surface antigen; HCV, hepatitis C virus; HDL, high-density lipoprotein; HIV, human immunodeficiency virus; LDL, low-density lipoprotein; TG, triglyceride; TSH, thyroid-stimulating hormone.
The pathogenesis of MCD is still not well known. However, dysregulation in T-cell-mediated immunity is thought to be the main culprit. In particular, increased type-2 T helper cell activity causes cytokine release and the formation of a permeability factor, which has been hypothesized in the pathogenesis of MCD. Medications such as d-penicillamine, infections, autoimmune diseases and malignancies could be causative factors [4]. Also, allergens, bee stings and vaccines could trigger MCD [5].
MCD is not uncommon following the administration of vaccines. Development of MCD has been reported after influenza, hepatitis B, pneumococcal, measles and tetanus–diphtheria–poliomyelitis vaccines [6]. A recent publication reported a patient with MCD following the Pfizer-BioNTech coronavirus disease 2019 (COVID-19) vaccine [7]. Symptoms started 4 days after the vaccination in that case, and proteinuria was resolved after 2 weeks of 80 mg/day prednisone, similar to our patient.
To the best of our knowledge, CoronaVac-associated new-onset nephrotic syndrome has not been reported before. On the other hand, COVID-19 vaccines may cause a flare in patients with glomerulonephritis. Consequently, individuals should be monitored carefully for side effects after vaccinations.
ETHICAL APPROVAL
This article does not contain any studies with human participants or animals performed by any of the authors.
PATIENT CONSENT
Written informed consent was obtained from the patient.
FUNDING
None.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.





Comments