-
PDF
- Split View
-
Views
-
Cite
Cite
Gaël Ensergueix, Nicolas Pallet, Dominique Joly, Charlène Levi, Sophie Chauvet, Claire Trivin, Jean-Francois Augusto, Rémi Boudet, Hail Aboudagga, Guy Touchard, Dominique Nochy, Marie Essig, Eric Thervet, Hélène Lazareth, Alexandre Karras, Ifosfamide nephrotoxicity in adult patients, Clinical Kidney Journal, Volume 13, Issue 4, August 2020, Pages 660–665, https://doi.org/10.1093/ckj/sfz183
Close - Share Icon Share
Abstract
Ifosfamide, a widely prescribed antineoplasic agent, is frequently associated with kidney dysfunction. Its nephrotoxicity is well documented in children, but data are lacking in adult patients.
The aim of this retrospective study was to describe the clinical, biological and histological characteristics of ifosfamide nephrotoxicity.
We report 34 patients (median age: 41 years) admitted in six French nephrology departments for kidney failure and/or tubular dysfunction. Fifteen patients (44.1%) received cisplatin as part of their chemotherapy. In 6 patients (17.7%), ifosfamide nephrotoxicity was revealed by a proximal tubular dysfunction (PTD), in 5 patients (14.4%) by an acute kidney injury (AKI), in 6 patients (17.7%) by a chronic kidney disease (CKD) and in 17 patients (49.7%) by an association of PTD and AKI. Fourteen renal biopsies (41.2%) were performed and revealed acute tubular necrosis (85.7%), vacuolation (78.6%) and nuclear atypias (71.4%) of renal epithelial cells, interstitial inflammation (71.4%) and fibrosis (57.1%). Electron microscopy showed mitochondrial enlargement and dysmorphic changes suggestive of mitochondrial toxicity. Ten patients (29.4%) progressed to Stage 5 CKD, six (17.6%) required haemodialysis and six patients died during a median follow-up period of 31 months. Risk factors for Stage 5 CKD were age and cisplatin co-administration.
INTRODUCTION
Ifosfamide is a commonly used alkylating chemotherapeutic agent of the oxaphosphorines family that is often combined with other drugs, such as cisplatin, for the treatment of sarcomas [1, 2], testicular tumours [3] and some refractory lymphomas [4], both in children and in adult patients.
Even though the first reported patients with ifosfamide nephrotoxicity were adults [5], most recent studies concern paediatric populations, and comprehensive data on adult patients are lacking. According to these studies, ∼30% of the children treated by ifosfamide will consequently develop a chronic kidney disease (CKD) [6]. Nevertheless, the reported prevalence of nephrotoxicity ranges from 15% to 60% [7], according to the definition of kidney injury, the duration of follow-up and the therapeutic protocols that have been used in each series. The spectrum of nephrotoxic phenotypes depends on the type, the severity and the reversibility of the kidney injury, as well as the time to onset, after administration of the treatment. The most frequent renal symptom is proximal tubular dysfunction (PTD) [8, 9], but acute kidney injury (AKI) often occurs, possibly as a consequence of renal thioredoxin reductase activity inhibition [10], and can progress to CKD. In some rare cases, ifosfamide may be responsible for hypokalaemic distal tubular acidosis or nephrogenic diabetes insipidus [11]. The aim of this study was to describe the clinical and histological features of the ifosfamide-associated nephrotoxicity in an adult population.
MATERIALS AND METHODS
A multicentric observational retrospective study was conducted in collaboration with six French nephrology departments. Adult patients who received ifosfamide were included in this study if they had renal failure (AKI and/or CKD) and/or tubular dysfunctions defined by at least two concurrent anomalies among: a hypokalaemic proximal tubular acidosis, a uric acid fractional excretion (UAFE) >10%, a ratio of tubular maximum reabsorption of phosphate (TmPO42-) to estimated glomerular filtration rate (eGFR), TmPO42-/eGFR <0.80, a low molecular weight proteinuria, an aminoaciduria and/or a normoglycaemic glycosuria, all occurring after the prescription of an ifosfamide-based chemotherapy regimen.
AKI was defined according to the 2012 Kidney Disease Improving Global Outcomes (KDIGO) guidelines (peak serum creatinine ×1.5 baseline value or delta creatinine >26.5 μmol/L), and CKD was defined by a chronic renal dysfunction, confirmed by an eGFR <60 mL/min/1.73 m2, by using the Modification of Diet in Renal Disease (MDRD) formula. Proteinuria was defined by a urine protein-to-creatinine ratio >300 mg/g.
UAFE was calculated using the formula: (urinary uric acid×serum creatinine)/(plasmatic uric acid×urinary creatinine) and TmPO42−/eGFR was calculated using the Bijvoet nomogram, taking into account phosphataemia and phosphate reabsorption rate (PRR): 1−[(plasmatic creatinine×urine phosphate)/(urine creatinine*plasmatic phosphate)].
Clinical, biological and histological data were collected on Day 0, corresponding to the first day of the ifosfamide administration, and after 1, 6 and 12 months following ifosfamide initiation, as well as at the last follow-up.
JMP® version 13.2.1 software was used to calculate means and standard deviation or standard error of mean (SEM), percentages, medians and interquartile range (IQR) and to apply statistical analyses and association analyses (Chi-square).
RESULTS
A total of 34 patients have been explored between 1995 and 2016 in six French nephrology departments. The demographic characteristics of the patients are presented in Table 1. The male to female ratio was 21:13 and the median age at treatment initiation was 46 years (range 18–75). Sarcoma was the most common indication for ifosfamide treatment (91.2%). The median cumulative dose of ifosfamide was 32.5 g/m2 (range 6–102). Fifteen patients (44.1%) received cisplatin treatment in addition to ifosfamide chemotherapy. There was no other nephrotoxic drug found. Acrolein urotoxicity was prevented by hydration and administration of 2-mercaptoethanesulfonic acid (Uromitexan) for all patients.
| Patient characteristics . | Overall . | Overall . | Ifosfamide . | Overall . | Ifosfamide + cisplatin . | P-value . |
|---|---|---|---|---|---|---|
| . | n = 34 . | n . | . | n . | . | . |
| General characteristics | ||||||
| Age at diagnosis [mean (range)], years | 44.9 (18–75) | 19 | 39.5 (17.75– 62.25) | 15 | 47 (28–75) | NS |
| Male, n (%) | 21 (61.7) | 19 | 13 (68.4) | 15 | 8 (53.3) | NS |
| Comorbidities | ||||||
| Hypertension, n (%) | 8 (23.5) | 18 | 6 (33.3) | 15 | 2 (13.3) | NS |
| Diabetes mellitus, n (%) | 1 (2.9) | 18 | 1 (5.5) | 15 | 0 (0) | NS |
| Cancer history | ||||||
| Type of cancer | ||||||
| Sarcoma, n (%) | 31 (91.2) | 18 | 16 (88.8) | 15 | 13 (86.7) | NS |
| Carcinoma, n (%) | 2 (5.9) | 18 | 1 (5.5) | 15 | 2 (13.3) | NS |
| Germinal tumour, n (%) | 1 (2.9) | 18 | 1 (5.5) | 15 | 0 (0) | NS |
| Treatment | ||||||
| Cisplatin, n (%) | 15 (44.1) | 19 | 0 (0) | 15 | 15 (100) | – |
| Ifosfamide cumulative dose (mean ± SEM), mg/m2 | – | 6 | 59 800 ± 17 700 | 10 | 30 780 ± 8395 | – |
| Use of uromitexan, n (%) | – | 10 | 10 (52.6) | 12 | 12 (80) | – |
| Patient characteristics . | Overall . | Overall . | Ifosfamide . | Overall . | Ifosfamide + cisplatin . | P-value . |
|---|---|---|---|---|---|---|
| . | n = 34 . | n . | . | n . | . | . |
| General characteristics | ||||||
| Age at diagnosis [mean (range)], years | 44.9 (18–75) | 19 | 39.5 (17.75– 62.25) | 15 | 47 (28–75) | NS |
| Male, n (%) | 21 (61.7) | 19 | 13 (68.4) | 15 | 8 (53.3) | NS |
| Comorbidities | ||||||
| Hypertension, n (%) | 8 (23.5) | 18 | 6 (33.3) | 15 | 2 (13.3) | NS |
| Diabetes mellitus, n (%) | 1 (2.9) | 18 | 1 (5.5) | 15 | 0 (0) | NS |
| Cancer history | ||||||
| Type of cancer | ||||||
| Sarcoma, n (%) | 31 (91.2) | 18 | 16 (88.8) | 15 | 13 (86.7) | NS |
| Carcinoma, n (%) | 2 (5.9) | 18 | 1 (5.5) | 15 | 2 (13.3) | NS |
| Germinal tumour, n (%) | 1 (2.9) | 18 | 1 (5.5) | 15 | 0 (0) | NS |
| Treatment | ||||||
| Cisplatin, n (%) | 15 (44.1) | 19 | 0 (0) | 15 | 15 (100) | – |
| Ifosfamide cumulative dose (mean ± SEM), mg/m2 | – | 6 | 59 800 ± 17 700 | 10 | 30 780 ± 8395 | – |
| Use of uromitexan, n (%) | – | 10 | 10 (52.6) | 12 | 12 (80) | – |
NS, not significant.
| Patient characteristics . | Overall . | Overall . | Ifosfamide . | Overall . | Ifosfamide + cisplatin . | P-value . |
|---|---|---|---|---|---|---|
| . | n = 34 . | n . | . | n . | . | . |
| General characteristics | ||||||
| Age at diagnosis [mean (range)], years | 44.9 (18–75) | 19 | 39.5 (17.75– 62.25) | 15 | 47 (28–75) | NS |
| Male, n (%) | 21 (61.7) | 19 | 13 (68.4) | 15 | 8 (53.3) | NS |
| Comorbidities | ||||||
| Hypertension, n (%) | 8 (23.5) | 18 | 6 (33.3) | 15 | 2 (13.3) | NS |
| Diabetes mellitus, n (%) | 1 (2.9) | 18 | 1 (5.5) | 15 | 0 (0) | NS |
| Cancer history | ||||||
| Type of cancer | ||||||
| Sarcoma, n (%) | 31 (91.2) | 18 | 16 (88.8) | 15 | 13 (86.7) | NS |
| Carcinoma, n (%) | 2 (5.9) | 18 | 1 (5.5) | 15 | 2 (13.3) | NS |
| Germinal tumour, n (%) | 1 (2.9) | 18 | 1 (5.5) | 15 | 0 (0) | NS |
| Treatment | ||||||
| Cisplatin, n (%) | 15 (44.1) | 19 | 0 (0) | 15 | 15 (100) | – |
| Ifosfamide cumulative dose (mean ± SEM), mg/m2 | – | 6 | 59 800 ± 17 700 | 10 | 30 780 ± 8395 | – |
| Use of uromitexan, n (%) | – | 10 | 10 (52.6) | 12 | 12 (80) | – |
| Patient characteristics . | Overall . | Overall . | Ifosfamide . | Overall . | Ifosfamide + cisplatin . | P-value . |
|---|---|---|---|---|---|---|
| . | n = 34 . | n . | . | n . | . | . |
| General characteristics | ||||||
| Age at diagnosis [mean (range)], years | 44.9 (18–75) | 19 | 39.5 (17.75– 62.25) | 15 | 47 (28–75) | NS |
| Male, n (%) | 21 (61.7) | 19 | 13 (68.4) | 15 | 8 (53.3) | NS |
| Comorbidities | ||||||
| Hypertension, n (%) | 8 (23.5) | 18 | 6 (33.3) | 15 | 2 (13.3) | NS |
| Diabetes mellitus, n (%) | 1 (2.9) | 18 | 1 (5.5) | 15 | 0 (0) | NS |
| Cancer history | ||||||
| Type of cancer | ||||||
| Sarcoma, n (%) | 31 (91.2) | 18 | 16 (88.8) | 15 | 13 (86.7) | NS |
| Carcinoma, n (%) | 2 (5.9) | 18 | 1 (5.5) | 15 | 2 (13.3) | NS |
| Germinal tumour, n (%) | 1 (2.9) | 18 | 1 (5.5) | 15 | 0 (0) | NS |
| Treatment | ||||||
| Cisplatin, n (%) | 15 (44.1) | 19 | 0 (0) | 15 | 15 (100) | – |
| Ifosfamide cumulative dose (mean ± SEM), mg/m2 | – | 6 | 59 800 ± 17 700 | 10 | 30 780 ± 8395 | – |
| Use of uromitexan, n (%) | – | 10 | 10 (52.6) | 12 | 12 (80) | – |
NS, not significant.
Renal diseases associated with ifosfamide
Ifosfamide nephrotoxicity was characterized by an isolated PTD in 6 patients (17.7%), among which 4 (11.8%) received ifosfamide alone and 2 (5.9%) ifosfamide and cisplatin; an isolated AKI in 5 patients (14.7%), among which 1 (2.9%) received ifosfamide and 4 (11.8%) ifosfamide in association with cisplatin; or a PTD complicated by AKI in 17 patients (50%), among which 10 (29.4%) received ifosfamide and 7 (20.6%) ifosfamide and cisplatin. In six cases (17.7.%), among which four (11.8%) were only treated by ifosfamide and two (5.9%) by ifosfamide and cisplatin (Table 2), ifosfamide toxicity was suspected by the diagnosis of delayed-onset CKD, identified after a mean duration of 16.4 months following chemotherapy initiation. These results indicate that the spectrum of nephrotoxicity patterns associated with ifosfamide is wide, with a high prevalence of PTD and CKD, whereas AKI associated with ifosfamide occurs less frequently.
| Renal presentation . | Overall, n = 34 . | Ifosfamide, n = 19 . | Ifosfamide + cisplatin, n = 15 . |
|---|---|---|---|
| Pure PTD, n (%) | 6 (17.7) | 4 (11.8) | 2 (5.9) |
| Pure AKI, n (%) | 5 (14.7) | 1 (2.9) | 4 (11.8) |
| PTD + AKI, n (%) | 17 (50.0) | 10 (29.4) | 7 (20.6) |
| CKD, n (%) | 6 (17.7) | 4 (11.8) | 2 (5.9) |
| Renal presentation . | Overall, n = 34 . | Ifosfamide, n = 19 . | Ifosfamide + cisplatin, n = 15 . |
|---|---|---|---|
| Pure PTD, n (%) | 6 (17.7) | 4 (11.8) | 2 (5.9) |
| Pure AKI, n (%) | 5 (14.7) | 1 (2.9) | 4 (11.8) |
| PTD + AKI, n (%) | 17 (50.0) | 10 (29.4) | 7 (20.6) |
| CKD, n (%) | 6 (17.7) | 4 (11.8) | 2 (5.9) |
| Renal presentation . | Overall, n = 34 . | Ifosfamide, n = 19 . | Ifosfamide + cisplatin, n = 15 . |
|---|---|---|---|
| Pure PTD, n (%) | 6 (17.7) | 4 (11.8) | 2 (5.9) |
| Pure AKI, n (%) | 5 (14.7) | 1 (2.9) | 4 (11.8) |
| PTD + AKI, n (%) | 17 (50.0) | 10 (29.4) | 7 (20.6) |
| CKD, n (%) | 6 (17.7) | 4 (11.8) | 2 (5.9) |
| Renal presentation . | Overall, n = 34 . | Ifosfamide, n = 19 . | Ifosfamide + cisplatin, n = 15 . |
|---|---|---|---|
| Pure PTD, n (%) | 6 (17.7) | 4 (11.8) | 2 (5.9) |
| Pure AKI, n (%) | 5 (14.7) | 1 (2.9) | 4 (11.8) |
| PTD + AKI, n (%) | 17 (50.0) | 10 (29.4) | 7 (20.6) |
| CKD, n (%) | 6 (17.7) | 4 (11.8) | 2 (5.9) |
Among patients with a PTD (Table 3), the main anomalies were, in order of frequency, hypokalaemia (20/23 patients), metabolic acidosis (12/23), hypophosphataemia (14/23), low molecular weight proteinuria (10/23), TmPO42−/eGFR <0.80 (9/23), hypouricaemia (9/23) and UAFE >10% (5/23). These results indicate that, among individuals with PTD associated with ifosfamide, the addition of cisplatin seems to increase the metabolic alterations reflecting the PTD.
| Proximal dysfunction features . | Overall, n = 23 . | Overall, n . | Ifosfamide . | Overall, n . | Ifosfamide + cisplatin . | P-value . |
|---|---|---|---|---|---|---|
| Hypokalaemia, n (%) | 20 (87.0) | 14 | 11 (78.6) | 9 | 9 (100) | NS |
| Metabolic acidosis, n (%) | 12 (52.2) | 12 | 6 (50) | 8 | 6 (75) | NS |
| Hypophosphataemia, n (%) | 14 (61.0) | 14 | 8 (57.1) | 8 | 6 (75) | NS |
| TmPO4²−/eGFR < 0.80, n (%) | 9 (39.1) | 6 | 6 (100) | 3 | 3 (100) | NS |
| Hypouricaemia, n (%) | 9 (39.1) | 11 | 5 (45.4) | 5 | 4 (80) | NS |
| UAFE > 10%, n (%) | 5 (21.7) | 3 | 3 (100) | 2 | 2 (100) | NS |
| Low weight proteinuria, n (%) | 10 (43.5) | 10 | 7 (70) | 6 | 3 (50) | NS |
| Proximal dysfunction features . | Overall, n = 23 . | Overall, n . | Ifosfamide . | Overall, n . | Ifosfamide + cisplatin . | P-value . |
|---|---|---|---|---|---|---|
| Hypokalaemia, n (%) | 20 (87.0) | 14 | 11 (78.6) | 9 | 9 (100) | NS |
| Metabolic acidosis, n (%) | 12 (52.2) | 12 | 6 (50) | 8 | 6 (75) | NS |
| Hypophosphataemia, n (%) | 14 (61.0) | 14 | 8 (57.1) | 8 | 6 (75) | NS |
| TmPO4²−/eGFR < 0.80, n (%) | 9 (39.1) | 6 | 6 (100) | 3 | 3 (100) | NS |
| Hypouricaemia, n (%) | 9 (39.1) | 11 | 5 (45.4) | 5 | 4 (80) | NS |
| UAFE > 10%, n (%) | 5 (21.7) | 3 | 3 (100) | 2 | 2 (100) | NS |
| Low weight proteinuria, n (%) | 10 (43.5) | 10 | 7 (70) | 6 | 3 (50) | NS |
NS, not significant.
| Proximal dysfunction features . | Overall, n = 23 . | Overall, n . | Ifosfamide . | Overall, n . | Ifosfamide + cisplatin . | P-value . |
|---|---|---|---|---|---|---|
| Hypokalaemia, n (%) | 20 (87.0) | 14 | 11 (78.6) | 9 | 9 (100) | NS |
| Metabolic acidosis, n (%) | 12 (52.2) | 12 | 6 (50) | 8 | 6 (75) | NS |
| Hypophosphataemia, n (%) | 14 (61.0) | 14 | 8 (57.1) | 8 | 6 (75) | NS |
| TmPO4²−/eGFR < 0.80, n (%) | 9 (39.1) | 6 | 6 (100) | 3 | 3 (100) | NS |
| Hypouricaemia, n (%) | 9 (39.1) | 11 | 5 (45.4) | 5 | 4 (80) | NS |
| UAFE > 10%, n (%) | 5 (21.7) | 3 | 3 (100) | 2 | 2 (100) | NS |
| Low weight proteinuria, n (%) | 10 (43.5) | 10 | 7 (70) | 6 | 3 (50) | NS |
| Proximal dysfunction features . | Overall, n = 23 . | Overall, n . | Ifosfamide . | Overall, n . | Ifosfamide + cisplatin . | P-value . |
|---|---|---|---|---|---|---|
| Hypokalaemia, n (%) | 20 (87.0) | 14 | 11 (78.6) | 9 | 9 (100) | NS |
| Metabolic acidosis, n (%) | 12 (52.2) | 12 | 6 (50) | 8 | 6 (75) | NS |
| Hypophosphataemia, n (%) | 14 (61.0) | 14 | 8 (57.1) | 8 | 6 (75) | NS |
| TmPO4²−/eGFR < 0.80, n (%) | 9 (39.1) | 6 | 6 (100) | 3 | 3 (100) | NS |
| Hypouricaemia, n (%) | 9 (39.1) | 11 | 5 (45.4) | 5 | 4 (80) | NS |
| UAFE > 10%, n (%) | 5 (21.7) | 3 | 3 (100) | 2 | 2 (100) | NS |
| Low weight proteinuria, n (%) | 10 (43.5) | 10 | 7 (70) | 6 | 3 (50) | NS |
NS, not significant.
Pathological findings
A kidney biopsy was performed in 14 cases (41.2%) (Table 4). Although seven of these patients (53.8%) did not receive cisplatin, we found no difference in type and extent of histology findings between the two groups of patients. Under light microscopy (Figure 1), acute tubular necrosis with denudation of proximal tubular epithelium was present in 12 biopsies (85.7%), vacuolation of epithelial cells in 11 biopsies (78.6%), nuclear atypia in 10 biopsies (71.4%), interstitial inflammation in 10 biopsies (71.4%) and interstitial fibrosis (IF) (>10%) in 8 biopsies (57.1%). Focal arteriolar hyalinosis was found in four biopsies (28.6%). Electron microscopy analysis was available in three cases and showed proximal tubular injury with thinning of proximal tubular epithelium, loss of brush bordure and vacuolation of epithelial cells. Moreover, severe mitochondrial abnormalities with irregular mitochondria and disappearance of some mitochondrial ridges were identified (Figure 2).
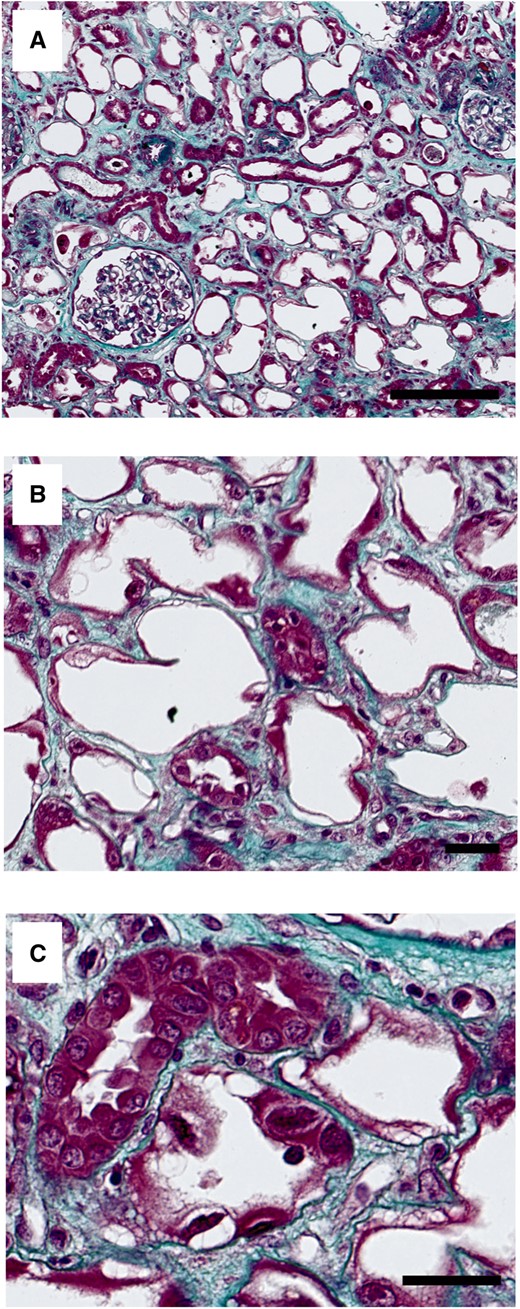
Masson’s trichrome staining of kidney biopsy showing (A) diffuse tubular denudation at low magnification (scale bar, 250 µm). At higher magnification, Masson’s staining showing (B) denudation of proximal tubular epithelium with loss of brush border (scale bar, 50 µm) and (C) vacuolization of epithelial cells and nuclear atypia (scale bar, 50 µm).
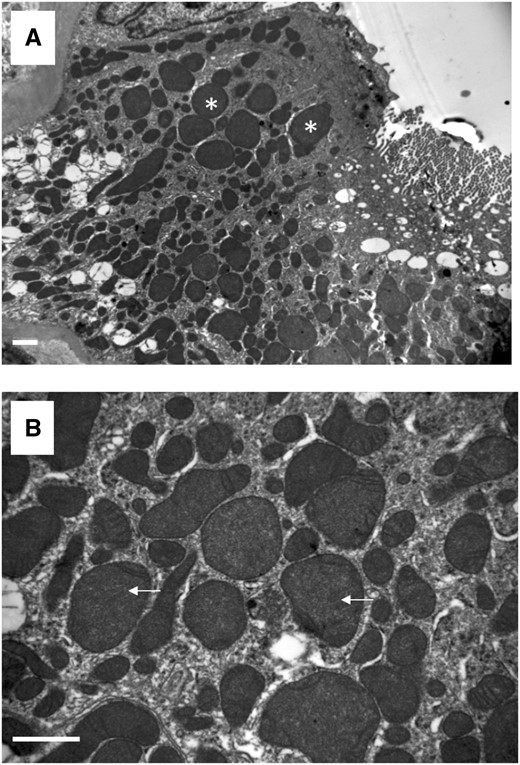
Representative images of transmission electron microscopy showing (A) enlarged and irregular mitochondria (white stars) and (B) disappearance of mitochondrial ridges (white arrows). Scale bar, 1 µm.
| Light microscopy features . | . |
|---|---|
| Denudation of proximal tubular epithelium, n (%) | 12 (85.7) |
| Vacuolation of epithelial cells, n (%) | 11 (78.6) |
| Nuclear atypia, n (%) | 10 (71.4) |
| Vascular hyalinosis, n (%) | 4 (28.6) |
| Tubulo-interstitial inflammation, n (%) | 10 (71.4) |
| TA/IF, n (%) | 8 (57.1) |
| Light microscopy features . | . |
|---|---|
| Denudation of proximal tubular epithelium, n (%) | 12 (85.7) |
| Vacuolation of epithelial cells, n (%) | 11 (78.6) |
| Nuclear atypia, n (%) | 10 (71.4) |
| Vascular hyalinosis, n (%) | 4 (28.6) |
| Tubulo-interstitial inflammation, n (%) | 10 (71.4) |
| TA/IF, n (%) | 8 (57.1) |
TA/IF, Tubular Atrophy/Interstitial Fibrosis.
| Light microscopy features . | . |
|---|---|
| Denudation of proximal tubular epithelium, n (%) | 12 (85.7) |
| Vacuolation of epithelial cells, n (%) | 11 (78.6) |
| Nuclear atypia, n (%) | 10 (71.4) |
| Vascular hyalinosis, n (%) | 4 (28.6) |
| Tubulo-interstitial inflammation, n (%) | 10 (71.4) |
| TA/IF, n (%) | 8 (57.1) |
| Light microscopy features . | . |
|---|---|
| Denudation of proximal tubular epithelium, n (%) | 12 (85.7) |
| Vacuolation of epithelial cells, n (%) | 11 (78.6) |
| Nuclear atypia, n (%) | 10 (71.4) |
| Vascular hyalinosis, n (%) | 4 (28.6) |
| Tubulo-interstitial inflammation, n (%) | 10 (71.4) |
| TA/IF, n (%) | 8 (57.1) |
TA/IF, Tubular Atrophy/Interstitial Fibrosis.
Evolution
In all cases, ifosfamide therapy was discontinued after the diagnosis of the renal disease. The median follow-up duration between drug cessation and last follow-up was 31 months (range 2–245). Mean progression of renal dysfunction during follow-up is described in Figure 3A. Baseline median eGFR before initiation of chemotherapy was 67 mL/min/1.73 m2 (51–89 mL/min/1.73 m2). Median eGFR was 31 mL/min/1.7 m2 (7–89.5 mL/min/1.73 m2) 1 month after ifosfamide treatment initation, 38 mL/min/1.73 m2 (21–65.5 mL/min/1.73 m2) at 6 months and 35 mL/min/1.73 m2 (11–57 mL/min/1.73 m2) at the last follow-up. In 16 patients, renal dysfunction continued to deteriorate [delta eGFR −26.5 mL/min/1.73 m2 range (−4 to −109 mL/min/1.73 m2)] between the cessation of ifosfamide and the last follow-up (Figure 3B).
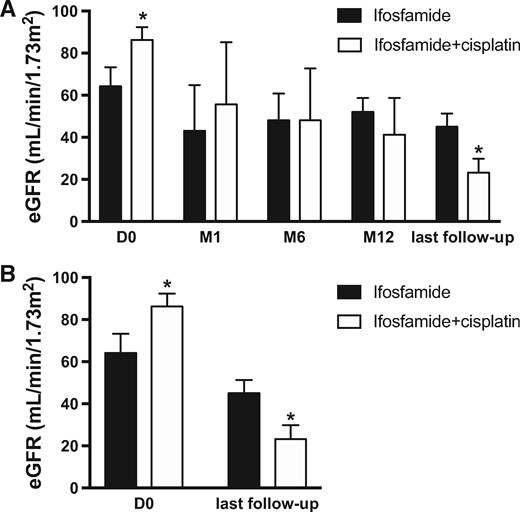
Comparison of eGFR evolution in patients receiving Ifosfamide or combination of Ifosfamide + Cisplatin using MDRD formula. (A) Global evolution of eGFR at start (D0) and after one, six, twelve months (M1, M6, M12) and at last follow-up (*P < 0.05). (B) Comparison of initial eGFR (D0) and eGFR at last follow-up between patients receiving Ifosfamide or Ifosfamide+cisplatin (*P < 0.05). D0, Day 0; M1, M6, M12, Months 1, 6, 12.
Five patients (14.7%) were treated with corticosteroids for inflammatory interstitial nephritis, with no significant improvement of renal function. At the end of follow-up, 15 patients (44.1%) had progressed to Stage 4 CKD and 10 patients (29.4%) to Stage 5 CKD. Overall, six patients required transient (n = 1) or definitive (n = 5) renal replacement therapy, after a median delay of 4.75 months (0.2–111 months) following the diagnosis of kidney disease. Six patients (17.6%) died from progression of the neoplastic disease during the follow-up period.
On univariate analysis, renal function (eGFR) at last follow-up was associated with age (P = 0.01) and cisplatin co-administration (P = 0.04). Interestingly, renal outcomes were not associated with the cumulative dose of ifosfamide (P = 0.28), the presence of proximal tubulopathy (P = 0.41) or eGFR at baseline (P = 0.38).
DISCUSSION
Our results indicate that ifosfamide promotes tubulo-interstitial injury that can be revealed mostly by an AKI or sometimes a progressive CKD diagnosed several months or years after ifosfamide administration. Ifosfamide also frequently promotes PTD along with a complete or incomplete Fanconi syndrome. Mitochondria could be frequently injured by ifosfamide, and mitochondrial dysfunction may play an important role in proximal tubular injury. However, our retrospective study has some limitations, such as the absence of comprehensive biological tubular data or the lack of follow-up of proximal tubulopathy outcomes. Moreover, the absence of a control group did not allow identification of risk factors of nephrotoxicity.
In our study, the association of ifosfamide and cisplatin appears more toxic than treatment with ifosfamide alone; 44% of patients in the cohort received ifosfamide in combination with cisplatin, which is usually associated with a tubulo-interstitial AKI but not with PTD. Cisplatin frequently affects magnesium reabsorption [12] and impairs ability to concentrate urine [13], suggesting a tubular injury affecting preferentially the Henle loop and the distal tubule, in contrast to ifosfamide. However, >50% of our patients did not receive cisplatin and had a PTD, suggesting ifosfamide may promote a specific pattern of nephrotoxicity. These results are consistent with those observed among children treated with ifosfamide [14], in whom PTD is a common complication that can sometimes persist for several years after the initial treatment. Moreover, it has been shown that adult survivors of childhood cancer have a residual tubular dysfunction, characterized by an increase of urine β2 microglobulin/creatinine ratio, when ifosfamide cumulative doses are >16 g/m2 [15].
Unlike other publications, a nephrotoxicity threshold was not found in our study. It is possible that the threshold of cumulative dose exposing patients to ifosfamide nephrotoxicity is <60 g/m2. Moreover, this threshold may depend upon the age of the patients or their genetic background, such as polymorphisms in genes coding for tubular transporter.
Ifosfamide penetrates the proximal tubular cells through the influx carrier human organic cation transporter 2 [16]. After internalization, ifosfamide is processed by two different metabolic pathways [17]: a 4-hydroxylation by P450 CYP3A4 and 2B6 [18] into an active nitrogen mustard that alkylates and damages DNA, and the urotoxic by-product acrolein [19], and a dechloroethylation step that produces chloroacetaldehyde (CAA), which is nephrotoxic [20, 21]. The mechanisms of CAA-induced nephrotoxicity are partially understood. CAA alters cellular energy metabolism by inhibiting oxidative phosphorylation at the level of mitochondrial respiratory chain complex I [8, 22], and increases cellular vulnerability to oxidative stress [21, 23, 24], and has a profibrotic effect [25]. Notably, coadministration of 2-mercaptoethanesulfonic acid prevents haemorrhagic cystitis and allows an increase of the administrated ifosfamide dose, but may facilitate ifosfamide nephrotoxicity. Histological descriptions of ifosfamide nephrotoxicity in adults are uncommon, and light microscopy analysis usually shows non-specific proximal tubular atrophy (TA), proximal tubular basement membrane denudation, proximal tubular cell vacuolation, nuclear atypia and a moderate monomorphic interstitial infiltrate [26, 27], which is consistent with our findings. Our ultrastructural analysis performed in only three patients showed mitochondrial changes may suggest specific toxicity, including irregular mitochondria, mitochondrial enlargement and disappearance of some mitochondrial ridges. This observation is compatible with the CAA nephrotoxicity mechanism. These findings are reminiscent of the nephrotoxicity of tenofovir, which targets proximal tubules and can induce mitochondrial alteration [28]. However, tenofovir nephrotoxicity is usually irreversible, which is not always the case for ifosfamide. Ifosfamide also induces nuclear atypia, which can result from the ifosfamide mustard alkylating effect. These atypia are similar to those produced upon viral infection, or during pseudo-caryomegalic nephropathy, which is a rare familial chronic tubule-interstitial nephritis [29]. Recently, Zhou et al. [30] have described the association of caryomegalic nephropathy with mutation in the Fanconi anaemia-associated nuclease 1 gene, which encodes a DNA repair enzyme.
In our series, 10 patients developed Stage 5 CKD, 6 required haemodialysis and 6 died. Only five still survived without CKD at the end of a mean follow-up of 41.8 months [(min–max) = (2–71)]. In line with this, in one study on adults treated by ifosfamide [31], the mean eGFR decreased from 81.5 to 57.9 mL/min/1.73 m2, with 53% of patients having eGFR <60 mL/min/1.73 m2 5 years after chemotherapy, although none of them progressed to Stage 5 CKD. In our study, all patients treated with corticosteroids developed CKD. This treatment, sometimes used in cases with significant interstitial inflammation, seems not to have any effect on ifosfamide nephrotoxicity and especially on tubular injury. There is no current strategy for preventing ifosfamide nephrotoxicity. N-acetylcysteine (NAC) prophylaxis has showed some beneficial effects, both in vitro and in vivo, in animal models of ifosfamide nephrotoxicity [32], but not in humans. Some reported cases of AKI occurring in children have suggested some benefits obtained by the use of intravenous NAC [33].
In conclusion, we have described the pattern of tubulo-interstitial injuries induced by ifosfamide in a cohort of 34 adult patients. Ifosfamide nephrotoxicity can occur in most cases by AKI immediately following administration, sometimes by CKD diagnosed several months or years after the chemotherapy, frequently associated with proximal tubulopathy. Severe CKD is frequent, especially in patients receiving ifosfamide–cisplatin combination. The histological and ultrastructural findings observed in our study suggest that the proximal tubular injury can be mediated by mitochondrial damage. Future studies are necessary to determine adult risk factors prior to the treatment and prophylactic therapies to decrease ifosfamide nephrotoxicity. Careful monitoring of tubular functions is important to ensure timely drug withdrawal or initiation of nephroprotective measures.
CONFLICT OF INTEREST STATEMENT
None declared.





Comments