-
PDF
- Split View
-
Views
-
Cite
Cite
Guillaume Jeantet, Vincent Pernin, Vincent Brunot, Arnaud Roccabianca, Anouk Macombe, Ilan Szwarc, Kada Klouche, Chantal Loirat, Georges Mourad, Véronique Frémeaux-Bacchi, Moglie Le Quintrec, Successful treatment of a Streptococcus pneumoniae-associated haemolytic uraemic syndrome by eculizumab, Clinical Kidney Journal, Volume 12, Issue 1, February 2019, Pages 106–109, https://doi.org/10.1093/ckj/sfy019
Close - Share Icon Share
Abstract
Haemolytic uraemic syndrome (HUS) is a rare complication of invasive infection by Streptococcus pneumoniae (SP-HUS), especially in adults. Here we report an unusual case of a 53-year-old man presenting SP-HUS with severe multivisceral involvement. After failure of supportive care and plasma exchanges, eculizumab (anti-C5 antibody) resulted in a favourable outcome.
INTRODUCTION
Haemolytic uraemic syndrome (HUS) is a known complication of Shiga toxin-producing Escherichia coli (STEC) and Streptococcus pneumoniae infections (SP-HUS) and is characterized by microangiopathic haemolytic anaemia, thrombocytopenia and renal failure [1]. SP-HUS is a rare disease, especially in adults, and is associated with a higher mortality than HUS due to other aetiologies [2]. Extra-renal complications are frequent including pancreatitis, purpura fulminans, cholecystitis, thrombosis and hearing loss.
SP-HUS is due to the S. pneumoniae neuraminidase activity that removes neuraminic acid from erythrocytes, platelets and glomerular endothelial cell (EC) membranes, leading to the exposure of Thomsen-Friedenreich (T)-cryptantigen [3]. As IgM antibodies to T-antigen are naturally present in human serum, the T-antigen–anti-T IgM interaction results in an agglutination of red blood cells, haemolysis with positive direct Coombs’ test, platelet aggregation, microvascular thrombosis and glomerular endothelial injuries [4]. Here, we report a case of SP-HUS in an adult with severe renal and extra-renal manifestations, improved by eculizumab (ECZ), a monoclonal humanized anti-C5 antibody.
CASE REPORT
The patient was a 53-year-old man, with known hypertension, type 2 diabetes mellitus and alcohol abuse. Four days after a massive bout of alcohol intake followed by liquid diarrhoea and self-medication by non-steroidal anti-inflammatory drugs (NSAIDs) (ibuprofen), he presented with febrile respiratory distress associated with diffuse cutaneous marbling justifying emergency admission in a critical care unit. On first examination, blood pressure was preserved (mean blood pressure 70–80 mmHg) and oxygen saturation was 83% without oxygen therapy. The patient was confused and the marbling involved all four members with purpuric lesions on the extremities. He remained oligo-anuric despite the initiation of massive fluid infusion.
Laboratory investigations showed acute renal failure with serum creatinine at 4.7 mg/dL and blood urea nitrogen at 8.4 mmol/L, severe inflammation with C reactive protein 300 mg/L, a procalcitonin at 195 mg/L and metabolic acidosis (pH 7.24, bicarbonataemia at 14 mmol/L) due to hyperlactataemia (6 mmol/L). Liver enzymes glutamyl-oxaloacetate-transferase (TGO)/glutamyl-pyruvate-transferase (TGP) were 1.5–2 N. Blood count showed anaemia (9.6 g/dL), subnormal platelet count (130 G/L) and hyperleucocytosis (24 G/L), associated with increased lactate dehydrogenase (800 UI/L) and free bilirubin (25 mmol/L) plasma levels. Haptoglobinaemia was normal at 1.3 g/L (normal range 0.5–3) and schizocytosis was 2%. Prothrombin time was 15 s (normal range 11–13). Fibrinogen (6.1 g/L) and troponin (618 ng/mL, normal <14 ng/mL) levels were high.
Chest X-ray showed pneumonia of the lower right lobe and echocardiography demonstrated global left ventricle hypokinesia with an ejection fraction of 20%.
Probabilist antibiotherapy (ceftriaxone/levofloxacin) and haemodialysis were initiated within hours following admission for severe pneumonia complicated by acute renal failure and myocarditis. Pneumoccocal infection was confirmed by positive urinary pneumococcal antigenuria while blood cultures were negative.
After 2 days of supportive treatment and antibiotherapy, the patient was still oligo-anuric, confused and cutaneous necrotic lesions had appeared on extremities, legs, hands, thorax and nose (Figure 1A–C). Skin biopsy in the necrotic zone showed fibrin thrombi and epidermolysis. Haemoglobinaemia dropped from 9.6 g/dL to 8.2 g/dL and platelets from 130 G/L to 60 G/L. Schizocytes rose to 4% and haptoglobin decreased to < 0.1 g/L.
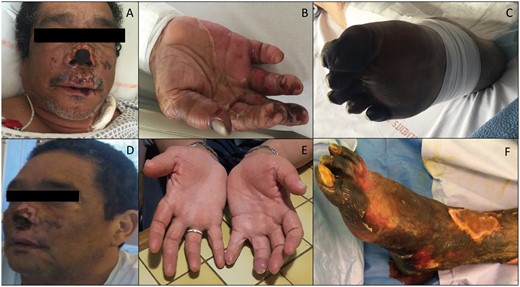
(A–C) Skin damage to the face (A), hands (B) and feet (C) of the acute phase of SP-HUS. (D, E and F) The respective evolution at 2 months with partial destruction of the nose wing cartilages (D), complete recovery on the hands (E) and severe necrosis of the legs (F), which led to trans-tibial amputation at 3 months.
As the situation worsened, plasmatic exchanges (PE) were initiated with plasma substitution. After 4 days of PE, renal function and haemolysis parameters remained unchanged, with persistence of anuria, haptoglobin < 0.1 g/L, severe thrombopenia (32 G/L) and schizocytosis (11%) (Figure 2). The skin lesions rapidly worsened with necrosis extension to the roots of members and nose wings. Confusion and obnubilation persisted. Only cardiac parameters improved with troponin-Ultrasensible decreasing to 106 ng/mL and improved left ventricular function (cardiac ejection fraction 30–35%).
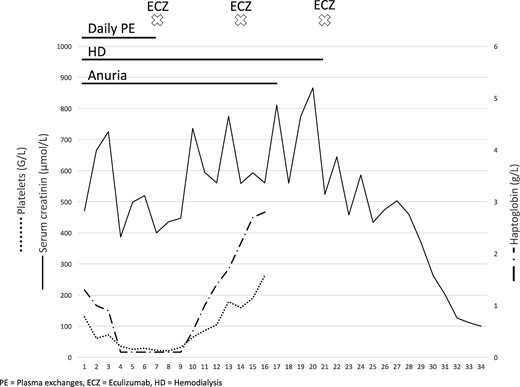
Chronological evolution of treatments and clinico-biological parameters.
As severe HUS was uncontrolled under PE, we decided to introduce ECZ treatment with a first dose of 1200 mg then 900 mg weekly for three doses, according to a slightly modified schedule from that recommended for atypical HUS.
Three days after the first injection, mental confusion regressed and a rise in platelet count (48 G/L) and haptoglobin plasma level (0.4 g/L) was observed. Platelets were >150 G/L on Day 6. Diuresis resumed on Day 10 and renal function improved gradually allowing dialysis withdrawal on Day 14. Renal function had fully recovered with a plasma creatinine level at 0.8 mg/mL by Day 25 (Figure 2).
ECZ therapy was maintained at a dose of 1200 mg twice monthly after the first month.
The skin lesions on the nose and hands healed without sequelae (Figure 1D and E). Unfortunately, the legs and feet (more seriously affected) were infected (Figure 1F) and a trans-tibial bilateral amputation was necessary 3 months after the onset of the disease.
Investigations into the cause of thrombotic microangiopathy (TMA) found a normal ADAMTS13 activity. There was no monoclonal gammapathy. The human immunodeficiency virus (HIV), hepatitis C, Parvovirus B19 and cytomegalovirus serologies were negatives. Stool culture was negative for STEC and PCR on the stools showed negative for shigatoxin genes. Direct and indirect Coombs’ tests were positive. Plasma levels of complement factors were normal with C3: 824 mg/L (normal: 660–1250); C4: 119 mg/L (normal: 93–180); CH50: 145% (normal: 50–150%); and normal activity of complement alternative pathway (CAP) regulators [factor I (CFI) 106% (normal: 70–130); factor H (CFH) 101% (normal: 65–140); CD46 leucocyte expression 13, 6% (normal: 13–19)]. Plasmatic C5b9 was also normal [63 ng/mL (normal: < 420 ng/mL)]. Screening for anti-CFH antibodies was negative. Genetic screening showed no variants of complement genes complement factor H (CFH), complement factor I (CFI), C3, factor B, membrane cofactor protein (MCP or CD46) or thrombomodulin (THBD).
ECZ was discontinued after a total duration of 6 months. One year later, all biological parameters were normal, including renal function with a serum creatinine at 0.89 mg/dL.
DISCUSSION
We report an unusual case of SP-HUS associated with severe renal and extra-renal manifestations in an adult, which was successfully treated by ECZ.
HUS secondary to invasive pneumococcal infection is rare and mostly described in children: the annual incidence of SP-HUS in 2009 was 0.06 cases per 100 000 children (<18 years) in the USA and even rarer in adults [5].
To the best of our knowledge, only nine adult cases have been reported in the literature [6–12]. In our case, SP-HUS was diagnosed from the clinical triad of HUS in a 53-year-old man who presented S. pneumoniae infection. Although self-medication by NSAIDs is known to induce TMA [13], the association of concomitant pneumonia with a positive S. pneumoniae urinary antigen test and positive direct Coombs’ test strongly suggested the involvement of S. pneumoniae.
In eight of the nine reported cases, renal function improved completely or partially with disease resolution. None of the patients had extra-renal lesions, such as cardiac or cerebral lesions, or skin involvement. TMA is responsible for EC injury leading to organ failure. Although TMA in HUS cases predominantly affects the renal microvasculature, extra-renal manifestations are observed in 20% of complement-mediated HUS including involvement of the central nervous system, cardiovascular system, lungs, skin, skeletal muscle and gastrointestinal tract. There are a number of case reports describing cardiac complications in shigatoxin-HUS as well. In contrast, gangrenous finger lesions have mainly been reported in the few cases of patients with complement alterations such as pathogenic variants in complement genes or with anti-Factor H antibodies. In our case, additional investigation including plasma level dosage of ADAMTS13, C3, CFH, CFI and MCP, were normal. Antibodies to CFH were undetectable, and mutation testing of six complement genes CFH, CFI, MCP, FB, C3 and THBD, involved in complement-mediated HUS, gave negative results.
Prompt initiation of antibiotherapy by third-generation cephalosporin along with supportive intensive care is the recommended treatment of SP-HUS. Since plasma brings anti-Thomsen-Friedenreich (T) antibodies, which may enhance T-anti-T agglutination and worsen the HUS course, plasma infusion and unwashed red blood cells or platelets are usually avoided, as long as agglutination tests are positive.
The deleterious role of PE in SP-HUS, however, remains controversial. PE might be beneficial by removing pathogenic neuraminidase. Five of the nine previously reported cases of adult patients received PE therapy with favourable outcome. In our patient, although started very early, no evidence of improvement was observed under PE. As part of TMA is linked to complement overactivation and conventional treatment was not effective, we decided to use ECZ as second-line treatment.
Eculizumab (ECZ, Soliris; Alexion Pharmaceuticals) is a humanized monoclonal antibody that binds to the complement protein C5, preventing cleavage of C5 to C5a and C5b, thereby inhibiting the generation of the terminal complement complex C5b-9. It is widely used to treat atypical HUS, mostly due to hereditary (complement factors variants) or acquired (anti-CFH antibodies) CAP dysregulation [14, 15]. To date, only one case of SP-HUS treated by ECZ has been reported [16], in a 21-month-old girl. ECZ was started after 7 days of peritoneal dialysis without prior PE. Treatment was successful with complete haemolysis regression and improvement of renal function. No positive biomarkers of CAP activation and no complement variant were found. ECZ was stopped after four doses.
In our case, the effectiveness of ECZ was striking, resulting in reversal of most skin injuries, cerebral involvement and recovery of normal renal function by Day 25. Efficiency of ECZ suggest that the infection may cause uncontrolled complement activation leading to renal failure and extra-renal manifestations.
The formation of a T-antigen–antibody complex could be responsible for restoring classical complement pathway consumption. In samples taken from patients in the acute phase of SP-HUS, complement components C4, C3 of the classical pathway were found to be decreased, indicating severe activation and complement consumption, although most of these parameters normalized with remission [11]. Pathogenic variants in CFH and CFI have been reported in patients with SP-HUS suggesting that the regulation dysfunction of the CAP may be involved in the pathophysiology [11]. In an in vitro model, Johnson et al. showed a surprising increase in deposition of CFH on the EC surface after exposure to human serum and neuraminidase [17]. It has been hypothesized that neuraminidase alters the cell surface polyanions, including sialic acid, and impairs the interaction of CFH and C3b with polyanions, inducing a transient dysfunction of CFH. Furthermore, sepsis related to S. pneumoniae infection also contributes to EC dysfunction.
We report the first case of adult SP-HUS successfully treated by ECZ. ECZ could provide a new therapeutic weapon for the most serious forms of SP-HUS. Further studies are required to document the benefit of complement blockade therapy in severe forms of SP-HUS.
CONFLICT OF INTEREST STATEMENT
V.F.-B. has received lecture, consultancy and travel honoraria from Alexion Pharmaceuticals, Inc. M.L.Q. and C.L. has received consulting fees from Alexion Pharmaceuticals, Inc. Results presented in this article have not been published previously in whole or part.





Comments