-
PDF
- Split View
-
Views
-
Cite
Cite
William R Short, Matty M Zimmerman, Ola Mohamed, Lynne M Mofenson, Safety Data Timelines for Pregnant Individuals With HIV on Antiretroviral Therapy, Clinical Infectious Diseases, Volume 79, Issue 6, 15 December 2024, Pages 1472–1474, https://doi.org/10.1093/cid/ciae249
Close - Share Icon Share
Abstract
Antiretrovirals are often approved by the Food and Drug Administration without sufficient safety data regarding their use in pregnancy. To quantify this delay, we calculated the interval from the approval date to their inclusion in the Antiretroviral Pregnancy Registry prospective analysis (≥200 first-trimester exposures); the median delay was 6 years.
Antiretroviral therapy (ART) is essential for the prevention of perinatal human immunodeficiency virus (HIV) transmission and it is recommended for all persons with HIV [1]. Antiretroviral therapy should be started as soon as possible and preferably prior to conception [2]; however, the most vulnerable period for the teratogenic effects of drugs is early first trimester, before most are aware that they are pregnant [3]. There are often minimal data available to make recommendations about the safety of antiretrovirals (ARVs) in pregnancy, given that pregnancy is often an exclusion criterion from ARV clinical trials [4]. One source of data evaluating the potential for teratogenicity is animal studies; however, the predictive value for an effect in humans is often conflicting or unknown [5]. Data on outcomes after exposure to new ARV agents come from case reports, cohort studies, billing claims [6], or registries.
The Antiretroviral Pregnancy Registry (APR) is an international, observational, pregnancy exposure-type registry designed to monitor prenatal exposure to ARVs and identify any significant teratogenic effects. Crucially, the APR relies on voluntary reporting from healthcare providers. In the United States, approximately 3525 individuals with HIV give birth annually [7] and the APR receives an estimated 1000 reports annually [8]. The primary objective of our study was to determine the years of delay between when an ARV receives Food and Drug Administration (FDA) approval to having a sufficient number of reports in the APR's published safety data to enable comparison with US population–based overall birth defect data; with 200 first-trimester exposures, a 2-fold increase in overall birth defects can be detected [9].
METHODS
Data Source
The APR is the oldest ongoing pregnancy exposure registry, established in 1989 as the Zidovudine (ZDV) in Pregnancy Registry to monitor for birth defects for anyone exposed to ZDV during pregnancy [8]. The registry has expanded to include all marketed ARVs used for the treatment of HIV and includes drugs used for hepatitis B and pre-exposure prophylaxis. The APR receives voluntary and confidential reports submitted either prospectively (the participant is registered in the APR prior to the outcome of the pregnancy being known), retrospectively (registration and outcome are received at the same time), or through clinical studies. The APR has been diligently gathering data on ART exposures since its inception. Regular interim reports, published semi-annually, provide updated birth defect information. The report is publicly available via the APR website, www.APRegistry.com. Anyone interested in the safety of ARV drugs can access the report.
Data Collection
We used the APR's published Interim report, through July 2023, for our data collection. We searched Appendix A: Prevalence of Birth Defects, which contains the prevalence of birth defects, 95% exact CIs, and raw numbers for ARV drugs that have exceeded the threshold of N ≥200 first-trimester–exposed live births and the report date. We documented the reporting date when each ARV drug reached the 200-exposure threshold necessary for inclusion in the APR's primary analysis. To determine the FDA approval for each drug, we searched the package insert and collected the initial FDA approval date.
Analysis
The APR uses a predefined analytic method and criteria to provide an early signal of teratogenicity associated with prenatal use of the drugs monitored through the registry. The primary analysis evaluates the prevalence of defects among people with initial exposure in the first trimester compared with those with initial exposure in the second and third trimester. For inclusion in the primary analysis, a minimum of 200 first-trimester exposures are necessary to detect a 2-fold increased risk of overall defects, with 80% statistical power and a type I error rate of 5%. This threshold is established in comparison to the population-based expected rate for the overall birth defects prevalence of 3%. To detect a 1.5-fold increase in overall birth defects and a 2-fold increase in the risk of birth defects in the more common classes, cardiovascular and genitourinary systems, 1000 first-trimester exposures are required. For specific defects, the power to detect an increased risk varies on the frequency of the defect in the population. For rare defects such as neural tube defects, occurring in less than 1 in 1000 individuals, a sample size of 2000 is required to detect a 3-fold increased risk [9].
The duration from FDA approval of the individual drug until it met the inclusion threshold was calculated, determining the years of delay for each drug in the analysis. For the descriptive analysis, we used medians and interquartile ranges (IQRs). Statistical analyses were performed with Stata version 17.0 (StataCorp, College Station, TX, USA).
For ARVs included in the primary analysis, we proceeded to assess whether they reached subsequent thresholds of either 1000 or 2000 first-trimester–exposed live births. Once again, we computed descriptive statistics (median and IQR) and the length of time from FDA approval until the drug became part of the analysis. This meticulous approach ensures a comprehensive understanding of the timeline and thresholds associated with each ARV in our analysis.
RESULTS
There were 23 283 prospective reports with outcomes reported to the APR through 31 July 2023. As of this report, 22 ARVs met the threshold of 200 exposures, with a median length of time from FDA approval to inclusion in the APR prospective analysis of 6 years (IQR = 5–7) (Figure 1). Zidovudine and lamivudine (LAM) have reached the threshold but were not included in this analysis because the APR evaluated classes of ARVs as opposed to individual drugs prior to 2001.
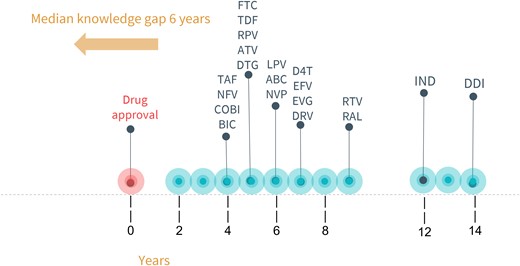
Time-to-first-published pregnancy safety data in the APR. Years between US Food and Drug Administration approval and inclusion in APR's primary analysis (n = 200 first-trimester exposures). Abbreviations: ABC, abacavir; ATV, atazanavir; BIC, bictegravir; COBI, cobicistat; D4T, stavudine; DDI, didanosine; DRV, darunavir; DTG, dolutegravir; EFV, efavirenz; EVG, elvitegravir; FTC, emtricitabine; IND, indinavir; LPV, lopinavir; NFV, nelfinavir; NVP, nevirapine; RAL, raltegravir; RPV, rilpivirine; RTV, ritonavir; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate. Adapted from A.C., Clin Infect Dis 2019;69(7):1255. Colbers, A (figured adapted from this and no permission needed).
For abacavir, atazanavir, efavirenz, emtricitabine, LAM, lopinavir, nelfinavir, nevirapine, ritonavir, tenofovir disoproxil fumarate (TDF), ZDV, and tenofovir alafenamide, sufficient numbers of first-trimester exposures have been monitored to detect at least a 1.5-fold increase in the risk of overall birth defects and a 2-fold increase in the risk of birth defects in the common classes—cardiovascular and genitourinary systems. The median time to inclusion from approval to reaching 1000 first-trimester exposures in the APR was 12.5 years (IQR = 9.5–15.5 years). For rare defects, only ZDV, LAM, TDF, emtricitabine, and ritonavir have enough exposures to date. The median time to inclusion was 13 years (IQR = 13–17 years).
There are several ARVs for which we continue to have no or limited data in pregnancy despite their widespread use among nonpregnant individuals, including doravirine and cabotegravir. Additionally, little or no data in pregnancy are available for ARVs used for heavily treatment-experienced HIV, including lenacapravir, fostemsavir, ibalizumab, and maraviroc.
DISCUSSION
In the United States an estimated 82% of women of childbearing age with HIV have had at least 1 unplanned pregnancy [10]. Having more timely and complete data on contemporary ARVs can help clinicians more confidently prescribe the ARVs they believe to be ideal for an individual patient of reproductive potential, can identify ARVs with toxicity issues, and can also ease 1 concern of women during pregnancy.
Postmarketing surveillance data of ARV use in pregnancy play a critical role in monitoring the safety of these medications [11]. Our study highlights that, despite having a well-established registry in place, there are still significant delays in the data becoming available after drug approval. Consequently, numerous ARV-exposed pregnancies transpire without accompanying safety data, potentially endangering infants.
Cabotegravir (CAB) in combination with rilpivirine (RPV) is the first and only complete long-acting regimen approved for the treatment of HIV in 2021 [12]. Clinical trials established that monthly or every-2-month regimens of long-acting CAB + RPV demonstrated noninferior efficacy compared with daily oral ART [13, 14]. Patient-reported outcomes have demonstrated patient satisfaction [15], and in another study, 90% of participants who switched to the injectable preferred the complete long-acting regimen to daily pills [16]. As of 31 July 2023, only 17 first-trimester exposures of CAB have been reported to the APR [8], limiting our ability to draw any conclusions regarding safety.
Despite awareness activities conducted by members of the APR's Advisory Committee, there continues to be underreporting. Some strategies to boost reporting may include ongoing provider education and training, providing incentives to providers, establishing collaboration and partnerships with professional organizations that can help broaden outreach efforts and facilitate access to a large number of healthcare providers, and advocating for legislative or policy measures that mandate reporting.
The success of the APR relies on broad participation of healthcare providers to prospectively register all patients who have ARV exposure and provide follow-up information so that we continue to collect prospective, nonbiased data on new ARVs, and so it does not take years to allow use in pregnancy after a drug is approved and widely used in nonpregnant individuals. We request that this serves as a wake-up call and a call to action to commence reporting all ARV exposures.
References
Author notes
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.




