-
PDF
- Split View
-
Views
-
Cite
Cite
Kaleb H Wolfe, Virginia M Pierce, Romney M Humphries, How New Regulation of Laboratory-Developed Antimicrobial Susceptibility Tests Will Affect Infectious Diseases Clinical Practice, Clinical Infectious Diseases, Volume 78, Issue 5, 15 May 2024, Pages 1140–1147, https://doi.org/10.1093/cid/ciae075
Close - Share Icon Share
Abstract
Antimicrobial resistance (AMR) affects 2.8 million Americans annually. AMR is identified through antimicrobial susceptibility testing (AST), but current and proposed regulatory policies from the United States Food and Drug Administration (FDA) jeopardize the future availability of AST for many microorganisms. Devices that perform AST must be cleared by the FDA using their susceptibility test interpretive criteria, also known as breakpoints. The FDA list of breakpoints is relatively short. Today, laboratories supplement FDA breakpoints using breakpoints published by the Clinical and Laboratory Standards Institute, using legacy devices and laboratory-developed tests (LDTs). FDA proposes to regulate LDTs, and with no FDA breakpoints for many drug–bug combinations, the risk is loss of AST for key clinical indications and stifling innovation in technology development. Effective solutions require collaboration between manufacturers, infectious diseases clinicians, pharmacists, laboratories, and the FDA.
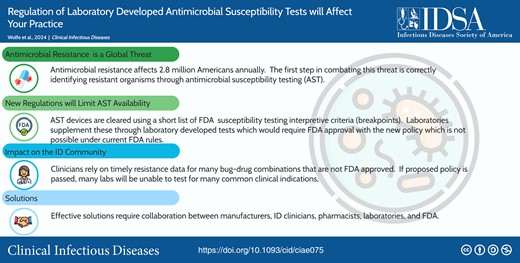
This graphical abstract is also available at Tidbit: https://tidbitapp.io/tidbits/regulation-of-laboratory-developed-antimicrobial-susceptibility-tests-will-affect-your-practice
Antimicrobial resistance (AMR) affects 2.8 million patients in the United States (US) and is estimated to cause, or to be associated with, the death of nearly 5 million people globally, annually [1]. Central to combating AMR is identifying antibiotic-resistant organisms through laboratory-performed antimicrobial susceptibility testing (AST). Availability of AST is at serious risk in the US due to regulatory policies that have eroded access and innovation for AST devices.
BACKGROUND ON ANTIMICROBIAL SUSCEPTIBILITY TESTS
The most widely used approach to AST is the in vitro minimum inhibitory concentration (MIC) test [2]. MICs are interpreted using clinical breakpoints, that is, values that categorize an organism's measured MIC as susceptible, susceptible-dose dependent, intermediate, or resistant. These categories are specific to each organism-antimicrobial combination and are based on population-level microbiological, pharmacokinetic/pharmacodynamic, and clinical studies to predict the likelihood of a successful clinical outcome, if antibiotic dosing typical for the site of infection is used [3]. In the US, 2 primary organizations establish and publish clinical breakpoints: the US Food and Drug Administration’s (FDA) Center for Drug Evaluation and Research (CDER) [4] and the Clinical and Laboratory Standards Institute (CLSI) [5]. CDER approves antibiotics for human use with organism-specific indications. For an organism to be listed in the indications for use and assigned a breakpoint, the clinical outcomes for that species treated with the antibiotic require evaluation in clinical trials. CDER allows additional in vitro activity claims in the drug label for organisms relevant to the indication, provided 90% of recent clinical isolates are susceptible to the antibiotic [6]. Generally, 10–15 microorganisms are granted CDER indications for a given antibiotic. However, a myriad of microorganisms cause infections among the increasingly complex, immunocompromised patients cared for in US hospitals, so it is inevitable that many infections will not fit into this short list of indications.
As part of the 21st Century Cures Act, CDER can defer to breakpoints established by a recognized standards development organization that meets statutory requirements (ie, CLSI). Today, >220 differences exist between CLSI and CDER published breakpoints, 173 of which occur where CLSI has a breakpoint published in the M100 standard that CDER has not recognized and has no breakpoint. This tally does not include any of the breakpoints listed in the CLSI M45 guideline for fastidious or infrequently isolated bacteria such as Abiotrophia/Granulicatella and Vibrio spp, among many others [4]. The most clinically important disconnects between CDER and CLSI breakpoints are listed in Table 1, and a full listing is available from CLSI (https://clsi.org/meetings/ast/breakpoints-in-use-toolkit/). It is estimated that 35%–43% of antibiotic prescriptions in intensive care unit patients are off-label [29, 30], something CDER considers the practice of medicine and therefore does not limit or control.
Selected Clinical and Laboratory Standards Institute M100a Breakpoints Not Recognized by the Center for Drug Evaluation and Research
| Organism . | Antimicrobial . | Resistance Rates in US by 2023 CLSI Breakpoints [Ref] . | Professional Societies That Recommend as Treatment Option [Ref] . |
|---|---|---|---|
| Acinetobacter spp | Cefepime | 37% [7, 8] | UpToDate: Considered first-line therapy by expert opinion for susceptible isolates. Resistance contributes to the case definition of MDR and XDR isolates [9] |
| Polymyxin B/Colistin | 8%–22% [7, 8] | IDSA: In combination with at least 1 other agent for treatment of carbapenem-resistant Acinetobacter baumannii if MIC ≤2 µg/mL [10] | |
| Streptococcus pyogenes | Azithromycin | 35.1% [11] | IDSA: Treatment of acute group A Streptococcus pharyngitis in patients allergic to penicillin [12] |
| Streptococcus agalactiae | Azithromycin | 60.0% [11] | ACOG: Alternative treatment for preterm prelabor rupture of membranes in pregnant women with documented β-lactam allergy [13] |
| Burkholderia cepacia complex | All antimicrobials | Ceftazidime: 5.0% Meropenem: 5.9% Levofloxacin: 18.8% Minocycline: 5.0% SXT: 6.9% [8] | None but routinely performed for the care of patients with cystic fibrosis |
| Salmonella | Azithromycin | 5.9% (typhoidal); 1.7% (nontyphoidal) [14] | IDSA: Treatment of bloody diarrhea based on local susceptibility patterns [15] American Academy of Family Physicians: First-line therapy for infectious diarrhea, if treatment is indicated [16] |
| Shigella | Azithromycin | 28.3% [14] | IDSA: Treatment of bloody diarrhea based on local susceptibility patterns [15] |
| Enterobacterales, including Escherichia coli and Klebsiella pneumoniae | Cefazolin as a surrogate for oral cephalosporins for treatment of uncomplicated UTI | No data, but anticipated to be ≥10%, based on ESBL rates | UpToDate: Second-line agent for treatment of uncomplicated cystitis |
| Enterococcus faecium | Daptomycin | 0.4% [17] | American Heart Association: Endocarditis caused by enterococci resistant to penicillin, aminoglycosides, and vancomycin [18] European Society of Cardiology: Endocarditis (in combination with ampicillin) for vancomycin-resistant enterococci [19] |
| Neisseria gonorrhoeae | Azithromycin | 5.8% [20] | CDC: In combination with gentamicin if ceftriaxone is not available [21] |
| Neisseria meningitidis | Ciprofloxacin, levofloxacin | Low, but of concern [22] | CDC: Chemoprophylaxis for close contacts of infected individuals [23, 24] |
| Non-Enterobacterales (includes Pseudomonas spp excluding P. aeruginosa, such as Pseudomonas putida) | All antimicrobials | No data—varies substantially by genus | None, although antibiotic treatment is indicated if causing an infection |
| Staphylococcus spp | Doxycycline SXT | <5% <5% | IDSA: Empiric therapy of mild diabetic foot infections if a β-lactam allergy is present or if high risk for MRSA and moderate to severe infections if MRSA risk factors present [25]; SSTI with surrounding cellulitis [26]; osteomyelitis (in combination with rifampin) [27] |
| Rifampin | <5% | IDSA (in combination with another agent): MRSA prosthetic valve endocarditis; MRSA osteomyelitis; MRSA device-related osteoarticular infections; MRSA meningitis; MRSA brain abscess, subdural empyema, spinal epidural abscess; MRSA septic thrombosis of cavernous or dural venous sinus; MRSA bacteremia vancomycin treatment failures [27] | |
| Stenotrophomonas maltophilia | Cefiderocol | 0% [28] | IDSA: Use any of these antibiotics, in combination with 1 other agent with activity, for infections caused by S. maltophilia [10] |
| Levofloxacin | 10.1% [28] | ||
| Minocycline | 0.0% [28] | ||
| SXT | 2.1% [28] |
| Organism . | Antimicrobial . | Resistance Rates in US by 2023 CLSI Breakpoints [Ref] . | Professional Societies That Recommend as Treatment Option [Ref] . |
|---|---|---|---|
| Acinetobacter spp | Cefepime | 37% [7, 8] | UpToDate: Considered first-line therapy by expert opinion for susceptible isolates. Resistance contributes to the case definition of MDR and XDR isolates [9] |
| Polymyxin B/Colistin | 8%–22% [7, 8] | IDSA: In combination with at least 1 other agent for treatment of carbapenem-resistant Acinetobacter baumannii if MIC ≤2 µg/mL [10] | |
| Streptococcus pyogenes | Azithromycin | 35.1% [11] | IDSA: Treatment of acute group A Streptococcus pharyngitis in patients allergic to penicillin [12] |
| Streptococcus agalactiae | Azithromycin | 60.0% [11] | ACOG: Alternative treatment for preterm prelabor rupture of membranes in pregnant women with documented β-lactam allergy [13] |
| Burkholderia cepacia complex | All antimicrobials | Ceftazidime: 5.0% Meropenem: 5.9% Levofloxacin: 18.8% Minocycline: 5.0% SXT: 6.9% [8] | None but routinely performed for the care of patients with cystic fibrosis |
| Salmonella | Azithromycin | 5.9% (typhoidal); 1.7% (nontyphoidal) [14] | IDSA: Treatment of bloody diarrhea based on local susceptibility patterns [15] American Academy of Family Physicians: First-line therapy for infectious diarrhea, if treatment is indicated [16] |
| Shigella | Azithromycin | 28.3% [14] | IDSA: Treatment of bloody diarrhea based on local susceptibility patterns [15] |
| Enterobacterales, including Escherichia coli and Klebsiella pneumoniae | Cefazolin as a surrogate for oral cephalosporins for treatment of uncomplicated UTI | No data, but anticipated to be ≥10%, based on ESBL rates | UpToDate: Second-line agent for treatment of uncomplicated cystitis |
| Enterococcus faecium | Daptomycin | 0.4% [17] | American Heart Association: Endocarditis caused by enterococci resistant to penicillin, aminoglycosides, and vancomycin [18] European Society of Cardiology: Endocarditis (in combination with ampicillin) for vancomycin-resistant enterococci [19] |
| Neisseria gonorrhoeae | Azithromycin | 5.8% [20] | CDC: In combination with gentamicin if ceftriaxone is not available [21] |
| Neisseria meningitidis | Ciprofloxacin, levofloxacin | Low, but of concern [22] | CDC: Chemoprophylaxis for close contacts of infected individuals [23, 24] |
| Non-Enterobacterales (includes Pseudomonas spp excluding P. aeruginosa, such as Pseudomonas putida) | All antimicrobials | No data—varies substantially by genus | None, although antibiotic treatment is indicated if causing an infection |
| Staphylococcus spp | Doxycycline SXT | <5% <5% | IDSA: Empiric therapy of mild diabetic foot infections if a β-lactam allergy is present or if high risk for MRSA and moderate to severe infections if MRSA risk factors present [25]; SSTI with surrounding cellulitis [26]; osteomyelitis (in combination with rifampin) [27] |
| Rifampin | <5% | IDSA (in combination with another agent): MRSA prosthetic valve endocarditis; MRSA osteomyelitis; MRSA device-related osteoarticular infections; MRSA meningitis; MRSA brain abscess, subdural empyema, spinal epidural abscess; MRSA septic thrombosis of cavernous or dural venous sinus; MRSA bacteremia vancomycin treatment failures [27] | |
| Stenotrophomonas maltophilia | Cefiderocol | 0% [28] | IDSA: Use any of these antibiotics, in combination with 1 other agent with activity, for infections caused by S. maltophilia [10] |
| Levofloxacin | 10.1% [28] | ||
| Minocycline | 0.0% [28] | ||
| SXT | 2.1% [28] |
Abbreviations: ACOG, American College of Obstetricians and Gynecologists; CDC, Centers for Disease Control and Prevention; CLSI, Clinical and Laboratory Standards Institute; ESBL, extended-spectrum β-lactamase; IDSA, Infectious Diseases Society of America; MDR, multidrug-resistant; MIC, minimum inhibitory concentration; MRSA, methicillin-resistant Staphylococcus aureus; SSTI, skin and soft tissue infection; SXT, trimethoprim-sulfamethoxazole; UTI, urinary tract infection; XDR, extensively drug-resistant.
aAdditional breakpoints published in M45 are not recognized by the Center for Drug Evaluation and Research.
Selected Clinical and Laboratory Standards Institute M100a Breakpoints Not Recognized by the Center for Drug Evaluation and Research
| Organism . | Antimicrobial . | Resistance Rates in US by 2023 CLSI Breakpoints [Ref] . | Professional Societies That Recommend as Treatment Option [Ref] . |
|---|---|---|---|
| Acinetobacter spp | Cefepime | 37% [7, 8] | UpToDate: Considered first-line therapy by expert opinion for susceptible isolates. Resistance contributes to the case definition of MDR and XDR isolates [9] |
| Polymyxin B/Colistin | 8%–22% [7, 8] | IDSA: In combination with at least 1 other agent for treatment of carbapenem-resistant Acinetobacter baumannii if MIC ≤2 µg/mL [10] | |
| Streptococcus pyogenes | Azithromycin | 35.1% [11] | IDSA: Treatment of acute group A Streptococcus pharyngitis in patients allergic to penicillin [12] |
| Streptococcus agalactiae | Azithromycin | 60.0% [11] | ACOG: Alternative treatment for preterm prelabor rupture of membranes in pregnant women with documented β-lactam allergy [13] |
| Burkholderia cepacia complex | All antimicrobials | Ceftazidime: 5.0% Meropenem: 5.9% Levofloxacin: 18.8% Minocycline: 5.0% SXT: 6.9% [8] | None but routinely performed for the care of patients with cystic fibrosis |
| Salmonella | Azithromycin | 5.9% (typhoidal); 1.7% (nontyphoidal) [14] | IDSA: Treatment of bloody diarrhea based on local susceptibility patterns [15] American Academy of Family Physicians: First-line therapy for infectious diarrhea, if treatment is indicated [16] |
| Shigella | Azithromycin | 28.3% [14] | IDSA: Treatment of bloody diarrhea based on local susceptibility patterns [15] |
| Enterobacterales, including Escherichia coli and Klebsiella pneumoniae | Cefazolin as a surrogate for oral cephalosporins for treatment of uncomplicated UTI | No data, but anticipated to be ≥10%, based on ESBL rates | UpToDate: Second-line agent for treatment of uncomplicated cystitis |
| Enterococcus faecium | Daptomycin | 0.4% [17] | American Heart Association: Endocarditis caused by enterococci resistant to penicillin, aminoglycosides, and vancomycin [18] European Society of Cardiology: Endocarditis (in combination with ampicillin) for vancomycin-resistant enterococci [19] |
| Neisseria gonorrhoeae | Azithromycin | 5.8% [20] | CDC: In combination with gentamicin if ceftriaxone is not available [21] |
| Neisseria meningitidis | Ciprofloxacin, levofloxacin | Low, but of concern [22] | CDC: Chemoprophylaxis for close contacts of infected individuals [23, 24] |
| Non-Enterobacterales (includes Pseudomonas spp excluding P. aeruginosa, such as Pseudomonas putida) | All antimicrobials | No data—varies substantially by genus | None, although antibiotic treatment is indicated if causing an infection |
| Staphylococcus spp | Doxycycline SXT | <5% <5% | IDSA: Empiric therapy of mild diabetic foot infections if a β-lactam allergy is present or if high risk for MRSA and moderate to severe infections if MRSA risk factors present [25]; SSTI with surrounding cellulitis [26]; osteomyelitis (in combination with rifampin) [27] |
| Rifampin | <5% | IDSA (in combination with another agent): MRSA prosthetic valve endocarditis; MRSA osteomyelitis; MRSA device-related osteoarticular infections; MRSA meningitis; MRSA brain abscess, subdural empyema, spinal epidural abscess; MRSA septic thrombosis of cavernous or dural venous sinus; MRSA bacteremia vancomycin treatment failures [27] | |
| Stenotrophomonas maltophilia | Cefiderocol | 0% [28] | IDSA: Use any of these antibiotics, in combination with 1 other agent with activity, for infections caused by S. maltophilia [10] |
| Levofloxacin | 10.1% [28] | ||
| Minocycline | 0.0% [28] | ||
| SXT | 2.1% [28] |
| Organism . | Antimicrobial . | Resistance Rates in US by 2023 CLSI Breakpoints [Ref] . | Professional Societies That Recommend as Treatment Option [Ref] . |
|---|---|---|---|
| Acinetobacter spp | Cefepime | 37% [7, 8] | UpToDate: Considered first-line therapy by expert opinion for susceptible isolates. Resistance contributes to the case definition of MDR and XDR isolates [9] |
| Polymyxin B/Colistin | 8%–22% [7, 8] | IDSA: In combination with at least 1 other agent for treatment of carbapenem-resistant Acinetobacter baumannii if MIC ≤2 µg/mL [10] | |
| Streptococcus pyogenes | Azithromycin | 35.1% [11] | IDSA: Treatment of acute group A Streptococcus pharyngitis in patients allergic to penicillin [12] |
| Streptococcus agalactiae | Azithromycin | 60.0% [11] | ACOG: Alternative treatment for preterm prelabor rupture of membranes in pregnant women with documented β-lactam allergy [13] |
| Burkholderia cepacia complex | All antimicrobials | Ceftazidime: 5.0% Meropenem: 5.9% Levofloxacin: 18.8% Minocycline: 5.0% SXT: 6.9% [8] | None but routinely performed for the care of patients with cystic fibrosis |
| Salmonella | Azithromycin | 5.9% (typhoidal); 1.7% (nontyphoidal) [14] | IDSA: Treatment of bloody diarrhea based on local susceptibility patterns [15] American Academy of Family Physicians: First-line therapy for infectious diarrhea, if treatment is indicated [16] |
| Shigella | Azithromycin | 28.3% [14] | IDSA: Treatment of bloody diarrhea based on local susceptibility patterns [15] |
| Enterobacterales, including Escherichia coli and Klebsiella pneumoniae | Cefazolin as a surrogate for oral cephalosporins for treatment of uncomplicated UTI | No data, but anticipated to be ≥10%, based on ESBL rates | UpToDate: Second-line agent for treatment of uncomplicated cystitis |
| Enterococcus faecium | Daptomycin | 0.4% [17] | American Heart Association: Endocarditis caused by enterococci resistant to penicillin, aminoglycosides, and vancomycin [18] European Society of Cardiology: Endocarditis (in combination with ampicillin) for vancomycin-resistant enterococci [19] |
| Neisseria gonorrhoeae | Azithromycin | 5.8% [20] | CDC: In combination with gentamicin if ceftriaxone is not available [21] |
| Neisseria meningitidis | Ciprofloxacin, levofloxacin | Low, but of concern [22] | CDC: Chemoprophylaxis for close contacts of infected individuals [23, 24] |
| Non-Enterobacterales (includes Pseudomonas spp excluding P. aeruginosa, such as Pseudomonas putida) | All antimicrobials | No data—varies substantially by genus | None, although antibiotic treatment is indicated if causing an infection |
| Staphylococcus spp | Doxycycline SXT | <5% <5% | IDSA: Empiric therapy of mild diabetic foot infections if a β-lactam allergy is present or if high risk for MRSA and moderate to severe infections if MRSA risk factors present [25]; SSTI with surrounding cellulitis [26]; osteomyelitis (in combination with rifampin) [27] |
| Rifampin | <5% | IDSA (in combination with another agent): MRSA prosthetic valve endocarditis; MRSA osteomyelitis; MRSA device-related osteoarticular infections; MRSA meningitis; MRSA brain abscess, subdural empyema, spinal epidural abscess; MRSA septic thrombosis of cavernous or dural venous sinus; MRSA bacteremia vancomycin treatment failures [27] | |
| Stenotrophomonas maltophilia | Cefiderocol | 0% [28] | IDSA: Use any of these antibiotics, in combination with 1 other agent with activity, for infections caused by S. maltophilia [10] |
| Levofloxacin | 10.1% [28] | ||
| Minocycline | 0.0% [28] | ||
| SXT | 2.1% [28] |
Abbreviations: ACOG, American College of Obstetricians and Gynecologists; CDC, Centers for Disease Control and Prevention; CLSI, Clinical and Laboratory Standards Institute; ESBL, extended-spectrum β-lactamase; IDSA, Infectious Diseases Society of America; MDR, multidrug-resistant; MIC, minimum inhibitory concentration; MRSA, methicillin-resistant Staphylococcus aureus; SSTI, skin and soft tissue infection; SXT, trimethoprim-sulfamethoxazole; UTI, urinary tract infection; XDR, extensively drug-resistant.
aAdditional breakpoints published in M45 are not recognized by the Center for Drug Evaluation and Research.
AST devices must be cleared by FDA prior to marketing, and the problem with breakpoints comes into play as FDA will only approve AST devices that include MICs interpreted with CDER breakpoints, not those that report MICs alone [31]. If CDER does not have a published breakpoint, no regulatory pathway exists to obtain clearance for an AST. Today, the impact of this is largely invisible to clinicians, as AST devices cleared by FDA prior to 2008 were cleared with CLSI breakpoints from the time of clearance [31]. It is critical to note that combinations tested on these “legacy” AST devices cannot be updated by the manufacturer if there is no CDER breakpoint. Furthermore, if a manufacturer updates its AST device for a given antibiotic to better detect contemporary AMR mechanisms or to incorporate newly published CDER breakpoints, they may lose claims for organisms that do not have recognized CDER breakpoints for that antibiotic. For example, many manufacturers lost claims for meropenem testing of various non-Enterobacterales gram-negative bacteria (eg, non-aeruginosa Pseudomonas) when breakpoints were updated for Enterobacterales and Pseudomonas aeruginosa. As such, it is inevitable that laboratories will lose the ability to perform AST for many antibiotics and organisms that are taken for granted today as manufacturers lose claims for their devices.
In absence of FDA-approved options, many modern diagnostics for infectious diseases are developed by individual laboratories. These “laboratory-developed tests” (LDTs) are not reviewed by the FDA but undergo robust analytical (and sometimes clinical) analysis by the laboratory prior to use. The FDA recently released draft rules proposing regulation of LDTs [32]. This move has the potential to limit access to tests (see summary here: https://www.idsociety.org/science-speaks-blog/2023/fda-proposal-threatens-access-to-critical-id-diagnostic-tests/#/+/0/publishedDate_na_dt/desc/) and has important consequences for AST. Because FDA does not clear ASTs for off-label indications, FDA regulation of LDTs would eliminate AST for off-label organisms, an important mitigator to the risk of off-label antibiotic prescribing. This would completely strip the ability of clinical laboratories to test these antibiotics against unclaimed microorganisms, as it would require submission to FDA for approval, which is an impossibility without an CDER breakpoint.
CLINICAL VIGNETTES DEMONSTRATING THE IMPACT OF THE BREAKPOINT DILEMMA
Daptomycin for Enterococcus faecium
A 55-year-old man with history of aortic stenosis presents to hospital with new-onset left lower quadrant (LLQ) abdominal pain. Imaging reveals free air under the diaphragm, and an emergent exploratory laparotomy identifies perforation in the descending colon. He undergoes hemicolectomy with colostomy. Postoperatively, he develops progressively worsening LLQ abdominal pain and fevers. Four of 4 blood cultures grow vancomycin-resistant Enterococcus faecium. Echocardiography confirms endocarditis. He is transitioned to daptomycin, but 2 days later, daptomycin MIC >8 µg/mL is reported by the laboratory using an LDT, indicating resistance by CLSI breakpoints.
Daptomycin is approved by CDER for complicated skin and soft tissue infections (SSTIs) due to staphylococci, streptococci, and vancomycin-susceptible Enterococcus faecalis at 4 mg/kg/day, as well as for bacteremia, including endocarditis, due to methicillin-resistant Staphylococcus aureus (MRSA) at 6 mg/kg/day [33, 34]. There is no CDER-approved E. faecium indication for daptomycin. Nevertheless, high-dose daptomycin is standard for the treatment of E. faecium infections [35]. The Infectious Diseases Society of America (IDSA) guidelines recommend >6 mg/kg/day daptomycin for bacteremia/endocarditis due to ampicillin- and vancomycin-resistant Enterococcus [34]. Daptomycin 10–12 mg/kg daily is recommended in the American Heart Association endocarditis guidelines for treatment of enterococci resistant to penicillin, aminoglycosides, and vancomycin [18], and daptomycin is recommended in combination with ampicillin by the European Society of Cardiology for this infection [19]. It is preferred therapy for endocarditis caused by vancomycin-resistant enterococci listed on UpToDate [36] and the Sanford Guide [37].
In 2019, CLSI updated Enterococcus daptomycin breakpoints to include E. faecium–specific, susceptible-dose-dependent breakpoint of ≤4 ug/mL, based on 8–12 mg/kg/day [33]. CDER rejected these breakpoints since this dose and organism are off-label [38], meaning no AST can obtain FDA clearance for daptomycin against E. faecium, including LDTs with the proposed rule. Most laboratories perform daptomycin testing for E. faecium using LDTs, meaning this testing would go away if the LDT rule is implemented. Importantly, daptomycin-resistant E. faecium (MIC >4 µg/mL) is common in some US hospitals, particularly among patients previously treated with daptomycin [39]. Daptomycin resistance can emerge rapidly during therapy [40–42], reinforcing need for routine AST of this agent for E. faecium.
Doxycycline and Trimethoprim-Sulfamethoxazole for Staphylococcus aureus
A 49-year-old man presents to urgent care with complaint of pain and spreading erythema on his right forearm. His temperature is 38.0°C (100.4°F), and a 3-cm fluctuant mass on his forearm with surrounding erythema and tenderness is observed. An incision and drainage (I&D) is performed and pus is sent for culture. He is prescribed 10 days of trimethoprim-sulfamethoxazole (SXT). Cultures reveal MRSA, which is reported as susceptible to SXT by CLSI breakpoints through an LDT.
Cellulitis and cutaneous abscess place significant burden to outpatient healthcare systems, and up to 5% of these infections lead to hospitalization [43]. The most common causes of these SSTIs are Streptococcus pyogenes and S. aureus [26]. I&D remains the most important intervention in purulent SSTI, and antibiotics are needed in cases of local or systemic spread. IDSA's 2014 practice guidelines for the treatment of SSTI recommend culture and AST and, if prescribed, antibiotics targeted toward MRSA [26]. Recommended oral agents include SXT, doxycycline, clindamycin, and linezolid. The latter both have CDER-recognized breakpoints [4] but are rarely used for SSTI due to cost, high resistance rates, side effects, and drug interactions. After decades of use, SXT and doxycycline continue to be prescribed for outpatient treatment of purulent SSTI [44, 45]. Among ambulatory care visits for SSTIs in the US during 2011–2016, an estimated 7.5 million prescriptions for SXT and 4.5 million prescriptions for tetracyclines were given, representing 17.4% and 10.6% of all antibiotics prescribed in these visits, respectively [46]. Staphylococcus aureus SXT and doxycycline resistances rates remain <5% in the US [47]. However, data from electronic health record databases and the National Healthcare Safety Network demonstrate that resistance rates have increased, most dramatically for community-onset MRSA infections. In some regions of the US, >10% of MRSA from SSTI cases were SXT resistant by CLSI breakpoints [48], indicating a clear need to confirm susceptibility to inform definitive therapy.
In addition to the treatment of SSTI, doxycycline, and SXT are options for treatment of osteomyelitis caused by MRSA, when combined with rifampin, as informed by AST [27]. There are no CDER-approved breakpoints to Staphylococcus spp for any of these antibiotics, meaning no tests, including LDTs, can achieve FDA clearance for this combination [4]. For the many laboratories not using a legacy device, the ability to test for resistance of S. aureus to SXT and doxycycline would go away.
Stenotrophomonas maltophilia
A 10-year-old girl with history of severe aplastic anemia and allogeneic hematopoietic stem cell transplant presents to clinic with fever, tachypnea, and oxygen saturation of 90% on ambient air. Breath sounds are diminished on the left lower lung field. She is transferred to the emergency department. Chest X-ray confirms left lower lobe pneumonia. Blood and sputum cultures are collected, and she is started on empiric cefepime and vancomycin. Blood cultures reveal Stenotrophomonas maltophilia.
Stenotrophomonas maltophilia is an emerging nosocomial threat, especially in immunocompromised hosts and those with underlying lung disease [49]. Stenotrophomonas maltophilia is intrinsically resistant to most antibiotics, due to complex resistance mechanisms that includes an L1 metallo-β-lactamase and an L2 serine β-lactamase, which combined render most β-lactams ineffective [49, 50]. IDSA recommends combination therapy with 2 of any active agent (SXT, minocycline, levofloxacin, cefiderocol) at least until clinical improvement is noted, or combination ceftazidime-avibactam and aztreonam if intolerance or inactivity to the other agents is identified [10]. IDSA specifically recommends against treatment with ceftazidime as monotherapy or in combination with anything other than avibactam and aztreonam [10]. CLSI breakpoints for S. maltophilia, which include SXT, levofloxacin, minocycline, and cefiderocol, are used by laboratories today for AST of by LDTs. CLSI deleted ceftazidime breakpoints following recent review, and in vitro studies demonstrate ceftazidime is unable to inhibit S. maltophilia growth at CDER-approved concentrations [51]. Paradoxically, the only CDER breakpoint for S. maltophilia is for ceftazidime [4], which is specifically contraindicated by IDSA. Without LDTs, no AST for S. maltophilia could be performed, leaving clinicians in the dark when treating this extensively resistant organism.
IMPACT ON PATIENT MANAGEMENT
Many physicians are unaware of the breakpoint dilemma as laboratories have applied strategies to prevent these regulatory hurdles from impacting their ability to perform AST. Two approaches are used by laboratories to this end: (1) use obsolete breakpoints that are >2 decades out of date (legacy devices) or (2) develop an LDT, generally via modification of an existing FDA-cleared test to extend testing to unclaimed organisms.
Laboratories with access to legacy devices cleared by FDA prior to 2008 can test organisms against antimicrobials without CDER breakpoints, using historical CLSI breakpoints. The downside of this practice is that the manufacturer cannot update these systems to adapt to the ever-evolving spectrum of AMR mechanisms or updated breakpoints, as they would lose claims for off-label organisms. As such, these tests are unlikely to perform well when faced with contemporary microorganisms and resistance mechanisms. On the other hand, development of LDTs requires the laboratory to take on the task of establishing the analytical performance of the test. Manual tests, such as gradient diffusion or disk diffusion, are subject to FDA regulation and using these manual tests with organisms without CDER breakpoints is considered a modification of the test—in other words, an LDT. The FDA-proposed regulation of LDTs [32] includes modified FDA-cleared tests, eliminating the second strategy, as there is no regulatory pathway to submit an AST to FDA without CDER breakpoints. In this case, clearance of the LDT by FDA would not be granted, effectively eliminating all avenues of testing these agents.
This dilemma has substantial impact on public health. Nearly all Centers for Disease Control and Prevention (CDC)–defined antibiotic resistance threats (Table 2) cannot be identified and/or treated without use of LDTs. Only 3 of the 21 antibiotic resistance threats (Clostridioides difficile, extended-spectrum β-lactamase–producing Enterobacterales, and drug-resistant Salmonella enterica serotype Typhi) have CDER-recognized breakpoints for the primary agents recommended by IDSA and/or CDC for treatment. The FDA proposed rule would threaten action against antimicrobial resistance in the US, as hospital facilities and public health departments would not be able to identify these AMR threats, potentially leading to increase in spread and dialing back years of progress on this front.
Gaps in US Food and Drug Administration–Recognized Antimicrobial Susceptibility Test Interpretive Criteria for US Centers for Disease Control and Prevention–Identified Antimicrobial Resistance Threats
| Threat Level . | Antibiotic Without FDA Breakpoints . | Current Testing Method and Gaps Imposed by FDA Proposed Rule . |
|---|---|---|
| Urgent threat | ||
| Carbapenem-resistant Acinetobacter | Polymyxins | Currently tested using an LDT. No testing possible under FDA proposed rule. |
| Candida auris | All antifungal agents | Currently tested using an LDT and interpreted using epidemiological cutoff values available from CDC and CLSI. No testing possible under FDA proposed rule. |
| Carbapenem-resistant Enterobacterales | Carbapenems | FDA breakpoints available for some members of the Enterobacterales group, but not all. Key exceptions include meropenem for Klebsiella aerogenes (ie, no FDA indication for meropenem against K. aerogenes). Currently tested using legacy devices or LDTs. Significantly reduced testing available under FDA proposed rule. |
| Drug-resistant Neisseria gonorrhoeae | Azithromycin | FDA rejected CLSI breakpoints. Testing not routinely performed outside public health laboratories, which use LDTs. No testing possible under FDA proposed rule. |
| Serious threat | ||
| Drug-resistant Campylobacter | All antimicrobial agents | Testing performed using LDTs with CLSI M45 breakpoints. No testing possible under FDA proposed rule. |
| Drug-resistant Candida | Amphotericin B | Testing performed using LDT that yields and MIC. No testing possible under FDA proposed rule. |
| VRE | Daptomycin | Testing performed using LDTs. No testing possible under FDA proposed rule. |
| MDR Pseudomonas | Cefiderocol | Testing performed using FDA breakpoints, which are more conservative than CLSI. Testing possible under proposed rule, but with significantly reduced susceptibility rates. |
| Drug-resistant nontyphoidal Salmonella | Azithromycin | Testing performed using LDTs. No testing possible under FDA proposed rule. |
| Drug-resistant Shigella | Azithromycin | Testing performed using LDTs. No testing possible under FDA proposed rule. |
| Drug-resistant Salmonella Typhi | Azithromycin | Testing performed using LDTs. No testing possible under FDA proposed rule. |
| MRSA | SXT, doxycycline | Testing performed using legacy devices or LDTs. No testing possible under FDA proposed rule. |
| Drug-resistant Streptococcus pneumoniae | Cefepime, vancomycin | Testing performed using legacy devices or LDTs. No testing possible under FDA proposed rule. |
| Drug-resistant tuberculosis | Isoniazid, rifampin, ethambutol, pyrazinamide | Testing performed using LDTs. No testing possible under FDA proposed rule. |
| Concerning threat | ||
| Erythromycin-resistant group A Streptococcus | Azithromycin | Testing performed using legacy devices or LDTs. No testing possible under FDA proposed rule. |
| Clindamycin-resistant group B Streptococcus | Azithromycin | Testing performed using legacy devices or LDTs. No testing possible under FDA proposed rule. |
| Watch list | ||
| Azole-resistant Aspergillus | Azole agents | Testing performed using LDTs. No testing possible under FDA proposed rule. |
| Drug-resistant Mycoplasma genitalium | All antimicrobials | Testing performed using LDTs. No testing possible under FDA proposed rule. |
| Drug-resistant Bordetella pertussis | All antimicrobials | Testing performed using LDTs. No testing possible under FDA proposed rule. |
| Threat Level . | Antibiotic Without FDA Breakpoints . | Current Testing Method and Gaps Imposed by FDA Proposed Rule . |
|---|---|---|
| Urgent threat | ||
| Carbapenem-resistant Acinetobacter | Polymyxins | Currently tested using an LDT. No testing possible under FDA proposed rule. |
| Candida auris | All antifungal agents | Currently tested using an LDT and interpreted using epidemiological cutoff values available from CDC and CLSI. No testing possible under FDA proposed rule. |
| Carbapenem-resistant Enterobacterales | Carbapenems | FDA breakpoints available for some members of the Enterobacterales group, but not all. Key exceptions include meropenem for Klebsiella aerogenes (ie, no FDA indication for meropenem against K. aerogenes). Currently tested using legacy devices or LDTs. Significantly reduced testing available under FDA proposed rule. |
| Drug-resistant Neisseria gonorrhoeae | Azithromycin | FDA rejected CLSI breakpoints. Testing not routinely performed outside public health laboratories, which use LDTs. No testing possible under FDA proposed rule. |
| Serious threat | ||
| Drug-resistant Campylobacter | All antimicrobial agents | Testing performed using LDTs with CLSI M45 breakpoints. No testing possible under FDA proposed rule. |
| Drug-resistant Candida | Amphotericin B | Testing performed using LDT that yields and MIC. No testing possible under FDA proposed rule. |
| VRE | Daptomycin | Testing performed using LDTs. No testing possible under FDA proposed rule. |
| MDR Pseudomonas | Cefiderocol | Testing performed using FDA breakpoints, which are more conservative than CLSI. Testing possible under proposed rule, but with significantly reduced susceptibility rates. |
| Drug-resistant nontyphoidal Salmonella | Azithromycin | Testing performed using LDTs. No testing possible under FDA proposed rule. |
| Drug-resistant Shigella | Azithromycin | Testing performed using LDTs. No testing possible under FDA proposed rule. |
| Drug-resistant Salmonella Typhi | Azithromycin | Testing performed using LDTs. No testing possible under FDA proposed rule. |
| MRSA | SXT, doxycycline | Testing performed using legacy devices or LDTs. No testing possible under FDA proposed rule. |
| Drug-resistant Streptococcus pneumoniae | Cefepime, vancomycin | Testing performed using legacy devices or LDTs. No testing possible under FDA proposed rule. |
| Drug-resistant tuberculosis | Isoniazid, rifampin, ethambutol, pyrazinamide | Testing performed using LDTs. No testing possible under FDA proposed rule. |
| Concerning threat | ||
| Erythromycin-resistant group A Streptococcus | Azithromycin | Testing performed using legacy devices or LDTs. No testing possible under FDA proposed rule. |
| Clindamycin-resistant group B Streptococcus | Azithromycin | Testing performed using legacy devices or LDTs. No testing possible under FDA proposed rule. |
| Watch list | ||
| Azole-resistant Aspergillus | Azole agents | Testing performed using LDTs. No testing possible under FDA proposed rule. |
| Drug-resistant Mycoplasma genitalium | All antimicrobials | Testing performed using LDTs. No testing possible under FDA proposed rule. |
| Drug-resistant Bordetella pertussis | All antimicrobials | Testing performed using LDTs. No testing possible under FDA proposed rule. |
Abbreviations: CDC, Centers for Disease Control and Prevention; CLSI, Clinical and Laboratory Standards Institute; FDA, US Food and Drug Administration; LDT, laboratory-developed test; MDR, multidrug-resistant; MIC, minimum inhibitory concentration; MRSA, methicillin-resistant Staphylococcus aureus; SXT, trimethoprim-sulfamethoxazole; VRE, vancomycin-resistant enterococci.
Gaps in US Food and Drug Administration–Recognized Antimicrobial Susceptibility Test Interpretive Criteria for US Centers for Disease Control and Prevention–Identified Antimicrobial Resistance Threats
| Threat Level . | Antibiotic Without FDA Breakpoints . | Current Testing Method and Gaps Imposed by FDA Proposed Rule . |
|---|---|---|
| Urgent threat | ||
| Carbapenem-resistant Acinetobacter | Polymyxins | Currently tested using an LDT. No testing possible under FDA proposed rule. |
| Candida auris | All antifungal agents | Currently tested using an LDT and interpreted using epidemiological cutoff values available from CDC and CLSI. No testing possible under FDA proposed rule. |
| Carbapenem-resistant Enterobacterales | Carbapenems | FDA breakpoints available for some members of the Enterobacterales group, but not all. Key exceptions include meropenem for Klebsiella aerogenes (ie, no FDA indication for meropenem against K. aerogenes). Currently tested using legacy devices or LDTs. Significantly reduced testing available under FDA proposed rule. |
| Drug-resistant Neisseria gonorrhoeae | Azithromycin | FDA rejected CLSI breakpoints. Testing not routinely performed outside public health laboratories, which use LDTs. No testing possible under FDA proposed rule. |
| Serious threat | ||
| Drug-resistant Campylobacter | All antimicrobial agents | Testing performed using LDTs with CLSI M45 breakpoints. No testing possible under FDA proposed rule. |
| Drug-resistant Candida | Amphotericin B | Testing performed using LDT that yields and MIC. No testing possible under FDA proposed rule. |
| VRE | Daptomycin | Testing performed using LDTs. No testing possible under FDA proposed rule. |
| MDR Pseudomonas | Cefiderocol | Testing performed using FDA breakpoints, which are more conservative than CLSI. Testing possible under proposed rule, but with significantly reduced susceptibility rates. |
| Drug-resistant nontyphoidal Salmonella | Azithromycin | Testing performed using LDTs. No testing possible under FDA proposed rule. |
| Drug-resistant Shigella | Azithromycin | Testing performed using LDTs. No testing possible under FDA proposed rule. |
| Drug-resistant Salmonella Typhi | Azithromycin | Testing performed using LDTs. No testing possible under FDA proposed rule. |
| MRSA | SXT, doxycycline | Testing performed using legacy devices or LDTs. No testing possible under FDA proposed rule. |
| Drug-resistant Streptococcus pneumoniae | Cefepime, vancomycin | Testing performed using legacy devices or LDTs. No testing possible under FDA proposed rule. |
| Drug-resistant tuberculosis | Isoniazid, rifampin, ethambutol, pyrazinamide | Testing performed using LDTs. No testing possible under FDA proposed rule. |
| Concerning threat | ||
| Erythromycin-resistant group A Streptococcus | Azithromycin | Testing performed using legacy devices or LDTs. No testing possible under FDA proposed rule. |
| Clindamycin-resistant group B Streptococcus | Azithromycin | Testing performed using legacy devices or LDTs. No testing possible under FDA proposed rule. |
| Watch list | ||
| Azole-resistant Aspergillus | Azole agents | Testing performed using LDTs. No testing possible under FDA proposed rule. |
| Drug-resistant Mycoplasma genitalium | All antimicrobials | Testing performed using LDTs. No testing possible under FDA proposed rule. |
| Drug-resistant Bordetella pertussis | All antimicrobials | Testing performed using LDTs. No testing possible under FDA proposed rule. |
| Threat Level . | Antibiotic Without FDA Breakpoints . | Current Testing Method and Gaps Imposed by FDA Proposed Rule . |
|---|---|---|
| Urgent threat | ||
| Carbapenem-resistant Acinetobacter | Polymyxins | Currently tested using an LDT. No testing possible under FDA proposed rule. |
| Candida auris | All antifungal agents | Currently tested using an LDT and interpreted using epidemiological cutoff values available from CDC and CLSI. No testing possible under FDA proposed rule. |
| Carbapenem-resistant Enterobacterales | Carbapenems | FDA breakpoints available for some members of the Enterobacterales group, but not all. Key exceptions include meropenem for Klebsiella aerogenes (ie, no FDA indication for meropenem against K. aerogenes). Currently tested using legacy devices or LDTs. Significantly reduced testing available under FDA proposed rule. |
| Drug-resistant Neisseria gonorrhoeae | Azithromycin | FDA rejected CLSI breakpoints. Testing not routinely performed outside public health laboratories, which use LDTs. No testing possible under FDA proposed rule. |
| Serious threat | ||
| Drug-resistant Campylobacter | All antimicrobial agents | Testing performed using LDTs with CLSI M45 breakpoints. No testing possible under FDA proposed rule. |
| Drug-resistant Candida | Amphotericin B | Testing performed using LDT that yields and MIC. No testing possible under FDA proposed rule. |
| VRE | Daptomycin | Testing performed using LDTs. No testing possible under FDA proposed rule. |
| MDR Pseudomonas | Cefiderocol | Testing performed using FDA breakpoints, which are more conservative than CLSI. Testing possible under proposed rule, but with significantly reduced susceptibility rates. |
| Drug-resistant nontyphoidal Salmonella | Azithromycin | Testing performed using LDTs. No testing possible under FDA proposed rule. |
| Drug-resistant Shigella | Azithromycin | Testing performed using LDTs. No testing possible under FDA proposed rule. |
| Drug-resistant Salmonella Typhi | Azithromycin | Testing performed using LDTs. No testing possible under FDA proposed rule. |
| MRSA | SXT, doxycycline | Testing performed using legacy devices or LDTs. No testing possible under FDA proposed rule. |
| Drug-resistant Streptococcus pneumoniae | Cefepime, vancomycin | Testing performed using legacy devices or LDTs. No testing possible under FDA proposed rule. |
| Drug-resistant tuberculosis | Isoniazid, rifampin, ethambutol, pyrazinamide | Testing performed using LDTs. No testing possible under FDA proposed rule. |
| Concerning threat | ||
| Erythromycin-resistant group A Streptococcus | Azithromycin | Testing performed using legacy devices or LDTs. No testing possible under FDA proposed rule. |
| Clindamycin-resistant group B Streptococcus | Azithromycin | Testing performed using legacy devices or LDTs. No testing possible under FDA proposed rule. |
| Watch list | ||
| Azole-resistant Aspergillus | Azole agents | Testing performed using LDTs. No testing possible under FDA proposed rule. |
| Drug-resistant Mycoplasma genitalium | All antimicrobials | Testing performed using LDTs. No testing possible under FDA proposed rule. |
| Drug-resistant Bordetella pertussis | All antimicrobials | Testing performed using LDTs. No testing possible under FDA proposed rule. |
Abbreviations: CDC, Centers for Disease Control and Prevention; CLSI, Clinical and Laboratory Standards Institute; FDA, US Food and Drug Administration; LDT, laboratory-developed test; MDR, multidrug-resistant; MIC, minimum inhibitory concentration; MRSA, methicillin-resistant Staphylococcus aureus; SXT, trimethoprim-sulfamethoxazole; VRE, vancomycin-resistant enterococci.
IMPACT ON INNOVATION
The breakpoint dilemma also stifles innovation for novel AST devices. The 2 primary AST devices used by clinical laboratories in the US are the Vitek 2 (bioMérieux, Durham, North Carolina) and Microscan Walkaway Plus (Beckman Coulter Diagnostics, Sacramento, California), which were first cleared in 2004 and 2007, respectively [52]. These devices are at a substantial market advantage over new technology as they continue to market legacied tests approved by FDA prior to 2008. Newer devices (eg, PhenoTest Blood Culture, Accelerate Diagnostics, Tucson, Arizona; or Next Generation Phenotyping [NGP] System, Selux, Boston, Massachusetts) are unable to test the same complement of organisms and antibiotics, requiring continued reliance by laboratories on legacy devices, which limits uptake of new devices as few laboratories can support multiple automated AST devices. As an example, Selux NGP, which achieved FDA clearance for its innovative AST device that yields results in 5.5 hours, was denied claims for testing SXT against S. aureus and coagulase-negative staphylococci due to the lack of CDER breakpoints (https://www.accessdata.fda.gov/cdrh_docs/pdf21/K211759.pdf). Furthermore, claims were denied for testing cefepime and meropenem against Klebsiella aerogenes and piperacillin-tazobactam against Enterobacter cloacae, K. aerogenes, Klebsiella pneumoniae, and Citrobacter freundii, among others, as these organisms are not listed under the indications for use in the drug's prescribing information (personal communication with Eric Stern to R. M. H.). As such, laboratories that desire to adopt this technology will require off-line alternative, legacy devices to test these combinations, which significantly increases laboratory complexity and cost and delays reporting of test results. Patients with infections caused by organisms without CDER breakpoints receive a lower standard of care than those patients with organisms claimed by the device, as they await traditional AST results.
Many LDTs have been standardized by CLSI, for example, a synergy test for the activity of ceftazidime-avibactam plus aztreonam, one of the very few treatment options available for gram-negative bacteria that produce metallo-β-lactamases. It is unclear how such standardized, non-FDA-cleared tests would fare in the face of LDT regulation, but most likely would require submission to FDA, something that may prove impossible without CDER breakpoints for the combinations.
CONCLUSIONS
The challenge of breakpoints is complex and largely underappreciated. Solutions to this challenge are pressing as the threat of AMR continues to affect an ever-expanding number of patients in the US. It is easy to suggest that requiring broader clinical claims for antibiotics during development is the solution. This is not realistic. Under the present market model, antibiotic developers struggle to recover costs for antibiotic development and often fail [53, 54]. Given the staggering number of microorganisms that may cause serious infections (particularly in immunocompromised hosts), it is simply not feasible to demonstrate in clinical trials that patients infected with these organisms respond favorably to a given antibiotic, as is currently required by CDER to assign an indication for the organism. In addition, the antibiotics discussed herein are now generic, meaning there is no sponsor to support studies for further claims with CDER. One might also suggest that test developers, who profit from AST device sales, should be the party to take on the burden of ensuring clearance for their tests. However, just like laboratories, diagnostic manufacturers cannot obtain FDA clearance of devices without CDER breakpoints. Solutions will require collaboration between manufacturers, clinicians, pharmacists, pharmaceutical industry, laboratorians, and the FDA and are likely multifaceted. As an example, clearance by FDA of commercial AST devices for MIC accuracy alone could allow laboratories to apply CLSI breakpoints to those MICs, and to submit any remaining LDTs (eg, where no commercial AST is available) for clearance should the FDA proposed rule proceed. This would ensure accuracy of test results (MIC) while allowing interpretation according to the most up-to-date breakpoints, as published by CLSI. This would also remove the requirement for resubmission to FDA by commercial AST developers when breakpoints are updated by CDER/CLSI, leading to a much shorter time to implementation by clinical laboratories (ie, as soon as published) [55]. Alternatively, broad recognition of CLSI breakpoints by CDER, particularly those listed in Table 1, would enable test development and clearance. The majority of clinical laboratories will be unable to submit data to FDA for LDT clearance of ASTs, meaning AST will be relegated to large reference laboratories with turnaround times that are not conducive to management of acute infections. As such, rapid submission of these tests by AST manufacturers to FDA is required via this pathway. A third option is a carve-out for AST from LDT regulation by FDA, which would allow the status quo to remain. However, it must be reinforced that this last option is not preferred, as most laboratories rely on commercial AST devices, which would remain outdated without CDER breakpoints for the types of antibiotic claims discussed herein. We call on FDA to engage with the infectious diseases community on these issues, with an eye to rapid resolution.
References
Author notes
Potential conflicts of interest. R. M. H. reports investigator-initiated grants from bioMérieux, consulting fees from Selux and bioMérieux, lecture honoraria from bioMérieux, voting membership of the Clinical and Laboratory Standards Institute (CLSI) Subcommittee on Antimicrobial Susceptibility Testing (AST), and Accelerate Diagnostics stock. V. M. P. reports participation and voting membership of the CLSI Subcommittee on AST. K. H. W. reports no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.




