-
PDF
- Split View
-
Views
-
Cite
Cite
Vivien Wai Yun Lai, David Griffin, Jillian Lau, Catriona McLean, Christina Chang, Shalini Bigwood, James Hare, Orla Morrissey, A Confusing Collapse: Case of an Intracranial Mass Mimicker in a Cardiac Transplant Recipient, Clinical Infectious Diseases, Volume 78, Issue 2, 15 February 2024, Pages 484–487, https://doi.org/10.1093/cid/ciad697
Close - Share Icon Share
A 56-year-old Caucasian retired powerplant operator from regional Australia was admitted after an unwitnessed collapse with head-strike at home. The collapse occurred suddenly without any preceding symptoms with no associated neurological or cardiac symptoms. However, he reported an 18-month history of intermittent drenching night sweats without weight loss.
This occurred in the context of an orthotopic heart transplantation for dilated ischemic cardiomyopathy 8 years prior, for which he was immunosuppressed with cyclosporine 100 mg twice daily, everolimus 1.25 mg twice daily, and mycophenolate mofetil 1.5 g twice daily. At transplantation, both donor and recipient were positive for cytomegalovirus (CMV) (D+/R+), while mismatched for Epstein-Barr virus (EBV) (D+/R−). He was immunoglobulin G (IgG) positive for Toxoplasma pre-transplant and was on trimethoprim/sulfamethoxazole prophylaxis.
Other past history included paroxysmal atrial fibrillation treated with sotalol and warfarin; hypertension and a right cerebellar stroke 5 years prior without residual deficit.
On examination, he was afebrile (36.8°C), with a blood pressure of 142/89, heart rate 98 beats/minute, respiratory rate 18 breaths/minute, oxygen saturation of 97% on room air and Glasgow Coma Scale 15. His full neurological examination was normal. He had no hepatosplenomegaly or lymphadenopathy. His electrocardiogram was in sinus rhythm with no ischaemic changes or ectopics.
Laboratory investigations revealed lymphopenia (0.67 × 109/L) but normal total white cell count, liver function tests, renal function, and C-reactive protein of <1 mg/L. Cyclosporine and everolimus levels were in the therapeutic range. Quantiferon Gold and serum cryptococcal antigen were negative.
Non-contrast computerized tomography (CT) demonstrated an area of hypoattenuation in the left anterior temporal lobe (Figure 1A). Contrast-enhanced magnetic resonance imaging (MRI) demonstrated a 15 × 8 mm enhancing lesion at this site (Figure 1B), with moderate perilesional oedema (Figure 1C). Further subcentimetre enhancing foci were demonstrated in the left caudate head and the right external capsule (Figure 1D).
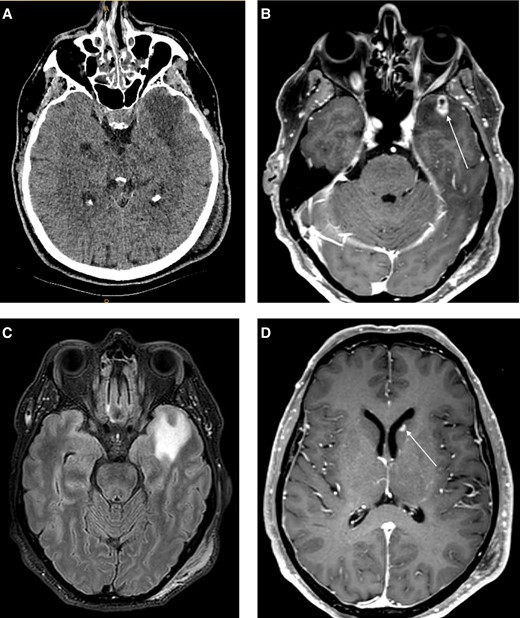
A, Non-contrast CT demonstrated an area of hypoattenuation with mild mass effect in the left anterior temporal lobe. B, MRI brain, T1 post contrast sequence demonstrated a 15 mm enhancing lesion in the left anterior temporal lobe with small area of central non-enhancement. C, FLAIR sequence showing moderate perilesional oedema. D, T1 post contrast sequence depicting a smaller enhancing lesion in the left caudate head. Abbreviations: CT, computerized tomography; MRI, magnetic resonance imaging.
A left temporal craniotomy and excision of lesion was performed which revealed a nodular lesion with abnormal consistency. Histopathology demonstrated a lymphohistiocytic inflammatory reaction with focal necrosis (Figure 2A), with no evidence of malignancy or granulomata. Bacterial and fungal cultures were negative, as were immunoperoxidase stains for CMV. Epstein-Barr encoding region (EBER) in situ hybridization (ISH) is shown in Figure 2B.
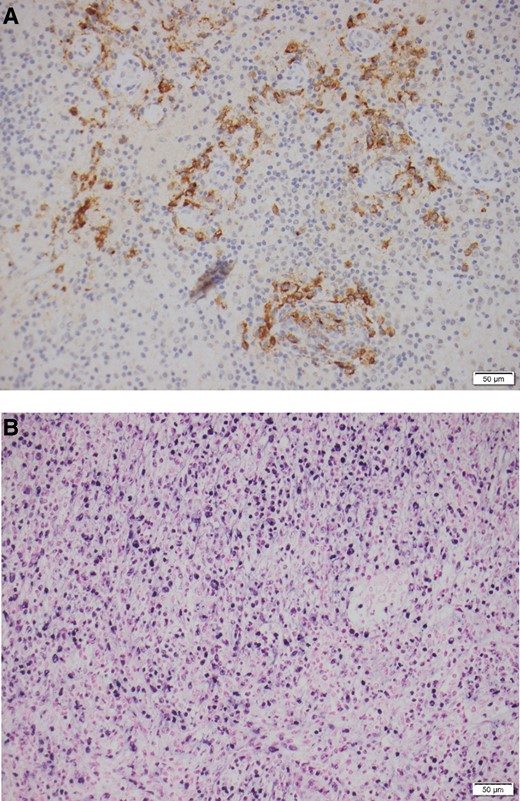
A, Histopathology of temporal mass lesion, showing lymphohistiocytic inflammatory reaction with focal necrosis. B, EBER ISH of the temporal mass lesion. Abbreviations: EBER, Epstein-Barr encoding region; ISH, in situ hybridization.
What is your diagnosis?
Diagnosis: EBV encephalitis mimicking a mass lesion,
EBER ISH showed moderately strong immunoreactivity of the histiocytic cytoplasm lining the cystic area (Figure 2B). This was also present in adjacent tissues in the peri-vascular space. The histopathology was consistent with a localised immunoreaction to EBV in keeping with EBV encephalitis, although the mass-like lesion was unusual. EBV serum viral load was also elevated at 6.5 × 105 copies/mL.
EBV is a lymphotropic herpesvirus, most commonly causing infectious mononucleosis in children and adolescents. Seroprevalence by adulthood is around 90%–95% [1]. Central nervous system (CNS) involvement includes encephalitis, typically presenting with altered mental state, confusion, fever, headaches, and occasionally seizures, aseptic meningitis, transverse myelitis, Guillain-Barré syndrome, facial nerve palsy, peripheral myeloradiculitis, and optic neuritis. EBV is responsible for 2%–6% of acute encephalitis in children [2, 3] and is often self-limiting however the incidence in adults is rare.
MRI brain findings in EBV encephalitis are variable and non-specific. Imaging can be normal [4]. T2 hyperintensity in the basal ganglia, thalamus and cortex can be seen, as well as in the white matter, brain stem, and corpus callosum [4]. Diffusion restriction and meningeal enhancement may be seen [5]. Mass-like lesions as in this case are rare [5].
In the immunocompromised patient, other differential diagnoses of CNS lesions include: infection (especially toxoplasma, tuberculosis, CMV, cryptococcus, nocardia, and non-tuberculous mycobacterium), progressive multifocal leukoencephalopathy, and malignancy, including primary CNS lymphoma and post-transplant lymphoproliferative disorder (PTLD), for which EBV is also implicated. In our patient, these disorders were excluded by clinical, biochemical, microbiological, radiological, and histopathological investigations, with negative toxoplasma immunoperoxidase, bacterial, fungal, CMV, and mycobacterial stains. PTLD was excluded as there were no atypical lymphocytes on histopathology.
Current treatment of EBV encephalitis relies on principles for treating CNS viral infections due to a paucity of studies reported for EBV. In the few case reports, most patients are treated with intravenous ganciclovir and oral valganciclovir [6, 7, 8]. Foscarnet [7, 9] and bruvudine [9] have been utilised for treatment-resistant cases.
Solid organ transplant recipients who are EBV-seronegative before transplant, such as our patient, are recommended to undergo regular monitoring for EBV viraemia post-transplant, particularly given the opportunity to reduce immunosuppression in patients with EBV viraemia to reduce incidence of early PTLD. The role of antiviral prophylaxis for those who are EBV-seronegative but receiving organs from EBV-seropositive donors remains controversial and there is insufficient evidence to recommend any specific prophylactic regimen. A meta-analysis examining anti-viral prophylaxis reported no significant effect on incidence of PTLD across all types of solid organ transplants and age groups in high-risk EBV-naïve patients [10]. The role of antiviral prophylaxis for EBV encephalitis specifically has not been quantified.
Our patient was treated with 2 weeks of intravenous ganciclovir 5 mg/kg twice daily and a further 4 weeks of oral valganciclovir 900 mg twice daily. Immunosuppression was reduced to cyclosporine 80 mg twice daily and everolimus 0.75 mg twice daily, with mycophenolate mofetil and bactrim prophylaxis withheld due to neutropenia. On review, 6-weeks post beginning of treatment, EBV viral load was undetectable, so valganciclovir was reduced to 450 mg orally twice daily; however, our patient relapsed within 3 weeks with detectable EBV viraemia and fevers. A therapeutic dosing of valganciclovir 900 mg twice daily was recommenced, which has continued since May 2019 with good tolerability. Since then, further leucopenia resulted in everolimus being ceased and prednisolone 5 mg once daily commenced instead in May 2019; bactrim prophylaxis was restarted in August 2020; mycophenolate mofetil has not been recommenced and cyclosporine continues at 80 mg twice daily. The patient returned to baseline function with no neurological deficits since treatment. On imaging 1 year later, there was no residual enhancement in the left temporal resection cavity (Figure 3A). The smaller lesions had nearly completely resolved (Figure 3B). The optimal treatment duration and ongoing prophylaxis of EBV encephalitis remains unknown.
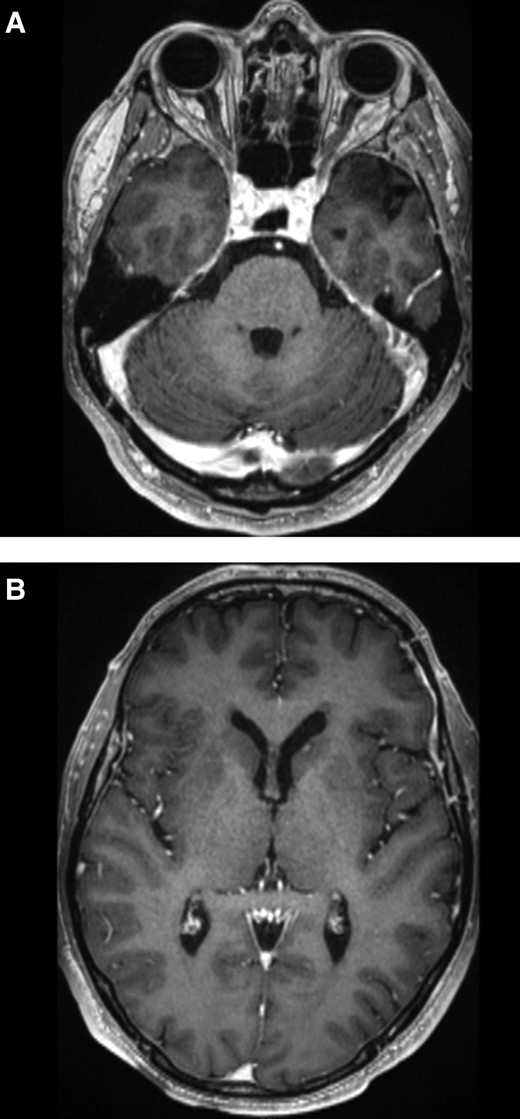
MRI brain post-treatment. A, T1 post contrast sequence demonstrating no residual enhancement at the resection site. B, T1 post contrast sequence demonstrating a tiny focus of enhancement in the left caudate head. Abbreviation: MRI, magnetic resonance imaging.
Notes
Financial support. None.
References
Author notes
Potential conflicts of interest. C. C. reports travel reimbursement for IDweek 2023; a role as Principal investigator at early phase clinical trial unit (Nucleus Network). J. L. reports Investigator initiated grant to conduct unrelated clinical research from Merck Sharpe & Dolme; consulting fees paid to institute Monash University from Gilead Sciences YES fellowship and ViiV Healthcare advisory board; Local flights and accommodation to attend World AIDS conference 2022 in Montreal and premeeting in San Francisco from Gilead Sciences Educational Grant. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.




