-
PDF
- Split View
-
Views
-
Cite
Cite
Susannah Colt, Cole D Miller, Andrew Edielu, Emily L Webb, Patrice A Mawa, Hannah W Wu, Racheal Nakyesige, Edridah Muheki, Narcis Kabatereine, Amaya L Bustinduy, Jennifer F Friedman, Relationships Between Schistosoma mansoni Infection Intensity and Nutritional Status and Anemia Among Preschool-aged Children in Uganda, Clinical Infectious Diseases, Volume 78, Issue 1, 15 January 2024, Pages 90–93, https://doi.org/10.1093/cid/ciad470
Close - Share Icon Share
Abstract
In a cross-sectional analysis of 354 Ugandan children (age 12–48 months) infected with Schistosoma mansoni, we assessed relationships between infection intensity and nutritional morbidities. Higher intensity was associated with an increased risk for anemia (RR = 1.05, 95% confidence interval [CI] 1.01–1.10) yet not associated with risk for underweight, stunting, or wasting.
Schistosomiasis is a parasitic disease of the genus Schistosoma spp., with an estimated 779 million people worldwide at risk. Human transmission results from exposure to freshwater sources contaminated with larval forms (cercariae) of the parasite. In school-age children, Schistosoma mansoni infection can lead to undernutrition, anemia, impaired linear growth, and cognitive development, and in severe cases, hepatosplenic disease, periportal fibrosis, and death [1]. S. mansoni is thought to contribute to anemia due to both anemia of inflammation and occult blood loss causing iron deficiency [2–4]. Furthermore, treatment with praziquantel (PZQ) in school-age children and adolescents has been shown to improve schistosomiasis-related morbidities [5]. Although anemia and anthropometric undernutrition are well documented in school-age children and adolescents with schistosomiasis [1], our understanding for these morbidities in preschool-age children (PSAC) is very limited. This is largely due to perceived low infection burden in this younger age group due to limited contact with freshwater [6]. PSAC were often excluded from surveillance surveys and, until very recently, were also excluded from mass drug administration (MDA) treatment programs, leaving schistosome infections and associated morbidities untreated. Studies in recent years examining the prevalence and infection intensity of S. mansoni among PSAC suggest that these young children do, in fact, share the burden of schistosomiasis in endemic regions. In Uganda, recent surveys have identified S. mansoni infection in as many as 74.9% of PSAC with heavy infection intensities (≥ 400 eggs per gram of stool [EPG]) detected among 19.4% [7]. In this cross-sectional study, we examine relationships between S. mansoni infection intensity and nutritional morbidities among a sample of PSAC with S. mansoni infection in Uganda.
METHODS
Study Area and Population
This study examines the baseline data from the Praziquantel in Preschoolers (PIP) trial (NCT03640377), an ongoing National Institutes of Health (NIH)-funded phase II trial of optimal PZQ dosing for PSAC (12–48 months) with S. mansoni infection recruited from the Lake Albert region of Uganda. Details of the trial have been previously described [8]. Written, informed consent was obtained from the participant's parent or legal guardian. The study was approved by the Rhode Island Hospital Institutional Review Board (IRB), the ethics committee of The London School of Hygiene and Tropical Medicine, the Research and Ethics committee of the Uganda Virus Research Institute, the Uganda National Council for Science and Technology, and the Uganda National Drug Authority.
Data Collection
Demographics
Information was collected pertaining to age and sex as well as household information including maternal and paternal education level, drinking water source, toilet or latrine access, and electricity. Data were entered in tablets and managed using the REDCap platform. Using the household information, an aggregate socioeconomic status (SES) variable was generated, giving a score of +1 for each of the following: (a) The participant's mother or father had completed primary school, (b) the household had access to drinking water from a source other than Lake Albert, (c) the household had access to a latrine, or (d) the household had electricity. The SES composite score ranges from 0 (low) to 4 (high).
Anthropometry
Using the World Health Organization (WHO) ANTHRO package, participants’ weight, length, age, and sex were used to determine the weight-for-age (WAZ), length-for-age (LAZ), and weight-for-length (WLZ) z-scores. We applied these z-scores to determine anthropometric undernutrition categories of underweight (WAZ < −2), stunting (LAZ < −2), and wasting (WLZ < −2).
Blood Biomarkers
A venous blood sample was drawn from each participant, and hemoglobin was measured as part of a complete blood count using the Sysmex pocH-100i Automated Hematology Analyzer (McKesson, Irving, Texas, USA). Anemia was defined as hemoglobin <11.0 g/dL. Plasma specimens were used to detect coinfections with human immunodeficiency virus (HIV) (Abbott, Determine HIV-1/2 Ag/Ab Combo, 7D2648) and malaria (Abbott, Bioline Malaria Ag P.f, 05FK50) using point-of-care lateral flow assays.
Stool Examination
Two stool samples were collected on consecutive days from each participant. The Kato-Katz method was used to quantify EPG in duplicate slides of two stool samples for S. mansoni, hookworm, Trichuris trichiura, and Ascaris lumbricoides infections. S. mansoni infection intensity was categorized as light (1–99 EPG), moderate (100–399 EPG), or heavy (≥400 EPG) [1]. Fecal occult blood (FOB) was detected using a point-of-care lateral flow assay (BioPanda, RAPG-FOB-001).
Statistical Analysis
Multivariable linear regression models were used to assess associations between S. mansoni intensity and hemoglobin concentrations. Multivariable log-binomial regression models were used to estimate the risk ratio (RR) between S. mansoni intensity and categorical outcomes (anemia and dichotomized anthropometric undernutrition). Bivariate analysis was used for regression model building and considered factors of age, sex, SES, hookworm, malaria, and HIV. Based on bivariate associations with P values < 0.1, fully adjusted models included covariates of age, SES score, and malaria co-infection. Continuous variables were natural log-transformed when included in regression models, and P values < 0.05 were considered statistically significant. Statistical analyses were conducted using SAS Studio 3.8 (SAS Institute Inc., Cary, North Carolina, USA).
RESULTS
Descriptive Statistics
Descriptive statistics are reported in Supplementary Table 1. There were 354 PSAC with S. mansoni infection included for analysis. The median age was 36 months (interquartile range [IQR] 28–42), and 51.1% were male. The median S. mansoni intensity was 72 EPG (IQR 24–258) with 56.8% light, 24.0% moderate, and 19.2% heavy infection intensity categories. With respect to coinfections, there were 0 cases of Ascaris, 0 cases of Trichuris, 3 cases of hookworm (0.9%), 56 cases of malaria (15.8%), and 4 cases of HIV (1.1%). The median hemoglobin concentration was 10.8 g/dL, and 200 participants (56.5%) were categorized as anemic. FOB was detected in 27.3% of participants. With respect to anthropometric undernutrition categories, 2.9% were underweight, 18.6% were stunted, and 1.1% were wasted.
Hemoglobin and Anemia
The relationship between continuous infection intensity (EPG) and hemoglobin concentration (g/dL) is shown in Supplementary Figure 1. Regression models adjusting for age, SES, and malaria show that higher continuous infection intensity was associated with lower hemoglobin concentration (ß = −0.014, SE = 0.003, P < .0001), and heavy infection intensity category was associated with lower hemoglobin compared to light infection intensity category (ß = −0.067, standard error [SE] = 0.014, P < .0001) shown in Figure 1 and reported in Supplementary Table 2. These associations remained significant after including FOB as an additional covariate into the models. Similarly, higher continuous infection intensity was associated with a greater risk for anemia (RR = 1.05, 95% CI 1.01–1.10, P = .0281), and heavy infection intensity category was associated with a greater risk for anemia compared to light infection (RR = 1.15, 95% CI 1.01–1.30, P = .0286, Figure 1 and Supplementary Table 3).
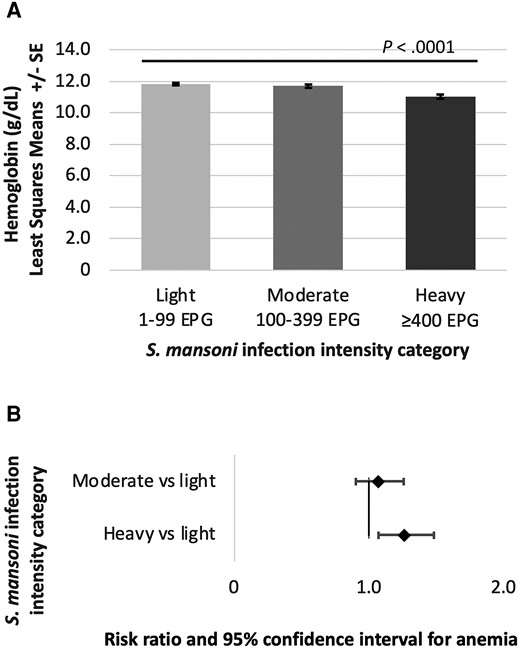
S. mansoni infection intensity category and (A) association with hemoglobin concentration and (B) risk for anemia (Hb < 11.0 g/dL). Hemoglobin least squares means reported in panel A and risk ratios reported in panel B have been adjusted for age, socioeconomic status, and malaria infection. Abbreviations: EPG, eggs per gram of stool; S. mansoni, Schistosoma mansoni.
Anthropometric Undernutrition
In multivariable models reported in Supplementary Tables 4–6, continuous S. mansoni infection intensity was not associated with the risk for underweight (RR 0.71, 95% CI .45–1.09, P = .1162), stunting (RR 0.89, 95% CI .77–1.03, P = .1229), or wasting (RR 1.33, 95% CI .68–2.63, P = .4027). Similarly, infection intensity categories were not associated with risk for underweight, stunting, or wasting.
DISCUSSION
Baseline findings from the PIP trial demonstrate a high burden of S. mansoni infection intensities in PSAC (24% moderate and 19% heavy) as well as morbidities of anemia (57%) and stunting (19%). These results further reinforce the urgent need for preventive chemotherapy with PZQ among PSAC living in schistosomiasis endemic regions. The 2022 WHO Guidelines on control of schistosomiasis updated recommendations to include children above the age of 2 years in control programs [1], but these are not yet fully implemented in Uganda. Barriers to implementation include the re-education of preventive MDA programs that include PSAC, lack of an approved pediatric formulation, and lack of pharmacokinetic data to guide optimal dosing in this age group. It is imperative to overcome these barriers and ensure that PSAC benefit from control programs as this age group, including children as young as 1–2 years, is most vulnerable to nutritional morbidities of undernutrition, anemia, and impaired linear growth [9].
This is one of the few studies to examine schistosomiasis-related morbidities among PSAC. In this sample, all of whom were infected with S. mansoni, higher infection intensity was associated with an increased risk for anemia. These findings contradict two studies published in 2011 from Uganda [10] and Kenya [11] that report that infection intensity was not found to be associated with risk for anemia. However, a 2017 study from Uganda [7] reported that PSAC with S. mansoni were more likely to be anemic compared to uninfected children. We note the limitation that the present analysis does not include an uninfected control group because the PIP trial only recruited S. mansoni-infected PSAC. Additionally, although we considered potential confounding factors typically associated with these outcomes, residual confounding may remain due to unmeasured factors. Given that FOB did not impact the relationship between infection intensity and anemia, anemia of inflammation may be mechanistically contributing more than iron deficiency anemia, as has been demonstrated among Kenyan school-age children infected with S. mansoni [2] and Filipino school-age children infected with S. japonicum [4].
In this sample, S. mansoni infection intensity was not associated with indicators of anthropometric undernutrition. Without an uninfected control group, it is unclear if the observed burden of stunting is more prominent among PSAC with schistosomiasis. Proportions of underweight and wasting were also very low in this sample, which limits our ability to detect statistical differences by infection intensity in this population. However, separate studies report that S. mansoni infection in PSAC was not found to be associated with underweight, stunting, or wasting compared to uninfected children [7, 10]. The longitudinal results from this trial may discern whether treatment positively impacts anthropometric measures in this age group. While these children experienced relatively high infection intensity, it is also possible that impairments in linear growth due to schistosomiasis are more likely to manifest after a longer duration of persistent infection. Future research should examine the longitudinal impact of PZQ on child growth outcomes for PSAC infected with schistosomiasis, and control programs should be supported in the programmatic inclusion of PSAC age 1–5 years living in endemic regions.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank the Praziquantel in Preschoolers (PIP) trial participants, as well as their families and community leaders. The authors also thank the PIP trial staff for their efforts in facilitating study activities including recruiting and transport of participants, performing pediatric phlebotomy, quantifying schistosome egg burden using the Kato-Katz method, taking anthropometric measures, and conducting laboratory analyses. Additionally, the authors thank the support staff of the Kabatereine Schistosomiasis Research Camp of Lake Albert located in Bugoigo, Uganda.
Financial support. This work was supported by the National Institute of Child Health and Human Development at the National Institutes of Health (grant number R01 HD095562 to J. F. F. and A. L. B.)
References
Author notes
S. C. and C. D. M. contributed equally to this work.
Potential conflicts of interest. A. L. B. reports grants from UKRI Future Leaders Fellowship in Global Health (grant number MR/T041900/1) and Wellcome Trust Biomedical Facilities Grant (Schistosomiasis Snail Resource Facilities—SSR). S. C. reports receipt of the Thrasher Early Career Award. J. F. F. reports a Midcareer Investigator Award in Patient Oriented Research from US National Institutes of Health/NIAID (award number K24 AI112964-06). E. L. W. reports grants from UK Medical Research Council (MRC), UK National Institute for Health and Care Research (NIHR), and European and Developing Countries Clinical Trial Partnership—European Union (EDCTP). All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.




