-
PDF
- Split View
-
Views
-
Cite
Cite
Hyunah Yoon, Rachel M Wake, Antonio S Nakouzi, Tao Wang, Ilir Agalliu, Caroline T Tiemessen, Nelesh P Govender, Joseph N Jarvis, Thomas S Harrison, Liise-anne Pirofski, Association of Antibody Immunity With Cryptococcal Antigenemia and Mortality in a South African Cohort With Advanced Human Immunodeficiency Virus Disease, Clinical Infectious Diseases, Volume 76, Issue 4, 15 February 2023, Pages 649–657, https://doi.org/10.1093/cid/ciac633
Close - Share Icon Share
Abstract
Asymptomatic cryptococcal antigenemia (positive blood cryptococcal antigen [CrAg]) is associated with increased mortality in individuals with human immunodeficiency virus (HIV) even after adjusting for CD4 count and despite receiving antifungal treatment. The association of antibody immunity with mortality in adults with HIV with cryptococcal antigenemia is unknown.
Cryptococcal capsular glucuronoxylomannan (GXM)- and naturally occurring β-glucans (laminarin, curdlan)-binding antibodies were measured in blood samples of 197 South Africans with HIV who underwent CrAg screening and were followed up to 6 months. Associations between antibody titers, CrAg status, and all-cause mortality were sought using logistic and Cox regression, respectively.
Compared with CrAg-negative individuals (n = 130), CrAg-positive individuals (n = 67) had significantly higher IgG1 (median, 6672; interquartile range [IQR], 4696–10 414 vs 5343, 3808–7722 μg/mL; P = .007), IgG2 (1467, 813–2607 vs 1036, 519–2012 μg/mL; P = .01), and GXM-IgG (1:170, 61–412 vs 1:117, 47–176; P = .0009) and lower curdlan-IgG (1:47, 11–133 vs 1:93, 40–206; P = .01) titers. GXM-IgG was associated directly with cryptococcal antigenemia adjusted for CD4 count and antiretroviral therapy use (odds ratio, 1.64; 95% confidence interval [CI], 1.21 to 2.22). Among CrAg-positive individuals, GXM-IgG was inversely associated with mortality at 6 months adjusted for CD4 count and tuberculosis (hazard ratio, 0.50; 95% CI, .33 to .77).
The inverse association of GXM-IgG with mortality in CrAg-positive individuals suggests that GXM-IgG titer may have prognostic value in those individuals. Prospective longitudinal studies to investigate this hypothesis and identify mechanisms by which antibody may protect against mortality are warranted.
Despite an ongoing international effort to end cryptococcal-associated deaths [1], cryptococcosis remains a major cause of morbidity and mortality among people with human immunodeficiency virus (HIV). Most cases occur in sub-Saharan Africa, where substantial research efforts have advanced treatment and prevention of cryptococcosis [2, 3]. The implementation of cryptococcal antigen (CrAg) screening in individuals with CD4 counts <200 cells/mm3 makes it possible to identify individuals with HIV at risk for developing cryptococcal meningitis (CM) before the onset of symptoms, thereby facilitating earlier diagnostic testing and treatment [2].
While the incidence of CM has declined with CrAg screening and preemptive treatment programs, mortality remains higher in individuals with HIV with asymptomatic cryptococcal antigenemia than in those without cryptococcal antigenemia, even after adjustment for CD4 count and despite preemptive antifungal therapy [3, 4]. Non-CD4 T-cell-associated factors are likely to affect the pathogenesis of cryptococcosis since cryptococcosis occurs in individuals with HIV with CD4 T-cell counts >200 cells/mm3 [5] and in individuals without HIV [6]. Previous studies have shown that Cryptococcus neoformans (CN) capsular glucuronoxylomannan (GXM) and β-glucan (laminarin, a branched β-[1–3]-glucan and curdlan, a linear β-[1–3]-glucan)-binding antibody levels are associated with CrAg or CM status in individuals with and without HIV [6–9]. In this study, we investigated associations between antibody immunity, cryptococcal antigenemia, and mortality in a South African cohort with advanced HIV with and without asymptomatic cryptococcal antigenemia.
METHODS
Study Design
This is a retrospective study of serum samples obtained at enrollment in a prospective cohort study of asymptomatic CrAg-positive and CrAg-negative adults with HIV followed over 6 months after CrAg screening at 2 large urban hospitals in Johannesburg, South Africa [4]. Participants were screened for asymptomatic/subclinical CM (positive cerebrospinal fluid using culture, India ink microscopy, or CrAg test) when logistically possible. All participants were started on antifungal therapy and then antiretroviral therapy (ART) per local management guidelines (National Department of Health, South Africa, 2015). Baseline demographic, clinical, and mortality data for up to 6 months were collected.
Study Population
Eligible individuals were adults with HIV aged ≥ 18 years with CD4 count <100 cells/mm3. Consecutive CrAg-positive and 1:2 ratio of CrAg-negative individuals attending study sites following CrAg screening were invited to participate. Exclusion criteria were a previous diagnosis of CM or symptoms or signs of CM.
Sample Collection and Processing
Serum samples collected at baseline were frozen and stored at –20oC. Before analysis, they were heat-inactivated at 56oC for 30 minutes to inactivate HIV and/or other heat-sensitive viruses as well as complement and stored at 4oC.
Antibody Measurements
Serum immunoglobulin (Ig) and cryptococcal capsular GXM polysaccharide-, and β-glucan (laminarin, curdlan)-binding antibodies were analyzed. The latter bind conserved microbial determinants that are found on the cryptococcal cell wall and that of other fungi [10]. IgM, IgG1, and IgG2 concentrations were measured using a Luminex platform (Austin, TX) and quantified in units of micrograms per milliliter as previously described [7, 11]. GXM-IgM/IgG/IgA and β-glucan-binding IgM/IgG were detected by antigen-capture enzyme-linked immunosorbent assay (ELISA) using plates coated, respectively, with GXM (from CN strain 24067) and laminarin or curdlan and reported as inverse titers as previously described [7, 11]. These assays have been used extensively for the detection of human GXM- and β-glucan-binding antibodies [11–15].
Ethics
Samples were obtained during a cohort study [4] approved by the ethics committees at the University of the Witwatersrand and the London School of Hygiene and Tropical Medicine. All patients provided written or witnessed verbal informed consent, and deidentified serum samples were studied under an Albert Einstein College of Medicine Institutional Review Board–approved protocol (1989–228). Samples were transported outside of South Africa with the permission of the local research ethics committee.
Statistical Analyses
Bivariate Analysis
Baseline demographics, clinical characteristics, and antibody levels stratified by plasma CrAg status were compared using the Wilcoxon rank sum test for continuous variables and the Fisher exact or χ2 test for categorical variables. All tests were 2-sided (α = 0.05). Correlations between measured antibody variables and between antibody variables and plasma CrAg titer were assessed using the Spearman correlation coefficient.
Model Building
The association between antibody levels and cryptococcal antigenemia was estimated using multivariable logistic regression, and the association between antibody levels and time to mortality was estimated using Cox proportional hazards regression. An a priori decision was made to include CD4 count in the model. Other variables were included in the full model if the P value was <.20 in the univariable model. Variables assessed include ART use prior to enrollment, tuberculosis diagnosed within 6 months prior to or on the day of enrollment, and log-transformed antibody levels. Total IgG1 and IgG2 were not included in the full model as they may biologically be in the same causal pathway as the antigen-specific antibodies. Variables were selected by backward elimination and checked for confounding and interaction with the main independent variable of interest, GXM-IgG. In the survival analysis, GXM-IgG was evaluated as both a continuous and a categorical variable in tertiles to demonstrate dose-response as Kaplan-Meier curves. Hosmer-Lemeshow goodness of fit and Schoenfeld residuals were assessed in the logistic regression and Cox model, respectively.
Mediation Analysis
We fitted a linear regression model for the mediator (log of GXM-IgG) with the exposure status (CrAg positive vs CrAg negative) as the independent variable. The Cox proportional hazards model for the outcome (mortality by 6 months) was built with the exposure status and the mediator as independent variables and potential confounders as covariables. The model also included the exposure-mediator interaction term to assess for potential interactions. Regmedint package in R was used for the analysis [16, 17].
Principal Component Analysis
Baseline antibody markers were analyzed using the principal component analysis (PCA) to reduce complex correlated datasets into a series of linear, noncorrelated principal components (PCs) and avoid multiple comparisons as described [6]. Log-transformed antibody markers were normalized to the mean. Differences in top PC scores by CrAg status (positive vs negative) and mortality were examined using a linear regression model. All statistical analyses were performed using R and Stata/IC 16.1 software (StataCorp LLC, College Station, TX).
RESULTS
Study Cohort
Demographics and clinical characteristics of the 130 CrAg-negative and 67 CrAg-positive cases are shown in Table 1. The median age was 39 years, 51% were male, 97% self-identified as Black race; there was with no difference between the CrAg status groups. Compared with CrAg-negative individuals, CrAg-positive individuals had lower CD4 counts (median 27 vs 40.5 cells/mm3; P = .003) and a greater proportion were on ART prior to enrollment (23.9% vs 10.0%; P = .01). The median plasma CrAg titer was 1:40 (interquartile range [IQR], 5–320). The prevalence of tuberculosis within the past 6 months did not differ between the groups. There were significantly more deaths in the CrAg-positive group than in the CrAg-negative group (25.4% vs 8.5%; hazard ratio [HR], 3.43; 95% confidence interval [CI], 1.61 to 7.33; Table 1, Supplementary Table 1).
Baseline and Clinical Characteristics of Participants by Cryptococcal Antigen Status
| Covariable . | Total (n = 197) . | CrAg-Negative (n = 130) . | CrAg-Positive (n = 67) . | P Value . |
|---|---|---|---|---|
| Age, years | 39.0 (32.4–47.1) | 39.1 (33.0–47.8) | 38.7 (31.7–47.1) | .67 |
| Sex | ||||
| ȃMale | 100 (50.8) | 70 (53.9) | 30 (44.8) | .23 |
| ȃFemale | 97 (49.2) | 60 (46.2) | 37 (55.2) | |
| Race | ||||
| ȃBlack | 191 (97.0) | 125 (96.2) | 66 (98.5) | .67 |
| ȃOther | 6 (3.1) | 5 (3.9) | 1 (1.5) | |
| CD4 count, cells/mm3 | 33 (12–61) | 40.5 (16–64) | 27 (7–40) | .003 |
| Plasma CrAg titers | 1:40 (5–320) | Not applicable | 1:40 (5–320) | |
| Baseline blood fungal growth | 5 (2.5) | 0 | 5 (7.5) | .002 |
| Tuberculosis in the past 6 monthsa | 51 (25.9) | 33 (25.4) | 18 (26.9) | .82 |
| Tuberculosis treatment | 29 (14.7) | 18 (13.9) | 11 (16.4) | .63 |
| Antiretroviral therapy prior to enrollment | 29 (14.7) | 13 (10.0) | 16 (23.9) | .01 |
| Vital status at 6 months | ||||
| ȃAlive | 160 (81.2) | 113 (86.9) | 47 (70.2) | .01 |
| ȃDead | 28 (14.2) | 11 (8.5) | 17 (25.4) | |
| ȃUnknown | 9 (4.6) | 6 (4.6) | 3 (4.5) |
| Covariable . | Total (n = 197) . | CrAg-Negative (n = 130) . | CrAg-Positive (n = 67) . | P Value . |
|---|---|---|---|---|
| Age, years | 39.0 (32.4–47.1) | 39.1 (33.0–47.8) | 38.7 (31.7–47.1) | .67 |
| Sex | ||||
| ȃMale | 100 (50.8) | 70 (53.9) | 30 (44.8) | .23 |
| ȃFemale | 97 (49.2) | 60 (46.2) | 37 (55.2) | |
| Race | ||||
| ȃBlack | 191 (97.0) | 125 (96.2) | 66 (98.5) | .67 |
| ȃOther | 6 (3.1) | 5 (3.9) | 1 (1.5) | |
| CD4 count, cells/mm3 | 33 (12–61) | 40.5 (16–64) | 27 (7–40) | .003 |
| Plasma CrAg titers | 1:40 (5–320) | Not applicable | 1:40 (5–320) | |
| Baseline blood fungal growth | 5 (2.5) | 0 | 5 (7.5) | .002 |
| Tuberculosis in the past 6 monthsa | 51 (25.9) | 33 (25.4) | 18 (26.9) | .82 |
| Tuberculosis treatment | 29 (14.7) | 18 (13.9) | 11 (16.4) | .63 |
| Antiretroviral therapy prior to enrollment | 29 (14.7) | 13 (10.0) | 16 (23.9) | .01 |
| Vital status at 6 months | ||||
| ȃAlive | 160 (81.2) | 113 (86.9) | 47 (70.2) | .01 |
| ȃDead | 28 (14.2) | 11 (8.5) | 17 (25.4) | |
| ȃUnknown | 9 (4.6) | 6 (4.6) | 3 (4.5) |
Categorical variables are summarized with counts; percentages and P values are based on the Fisher exact or χ2 test. Continuous variables are summarized with medians, interquartile ranges, and P values based on Wilcoxon rank sum test. P values < .05 are bolded.
Abbreviations: CrAg, cryptococcal antigen.
Includes pulmonary, disseminated, or extrapulmonary Mycobacterium tuberculosis infection diagnosed within 6 months prior to or on the day of enrollment.
Baseline and Clinical Characteristics of Participants by Cryptococcal Antigen Status
| Covariable . | Total (n = 197) . | CrAg-Negative (n = 130) . | CrAg-Positive (n = 67) . | P Value . |
|---|---|---|---|---|
| Age, years | 39.0 (32.4–47.1) | 39.1 (33.0–47.8) | 38.7 (31.7–47.1) | .67 |
| Sex | ||||
| ȃMale | 100 (50.8) | 70 (53.9) | 30 (44.8) | .23 |
| ȃFemale | 97 (49.2) | 60 (46.2) | 37 (55.2) | |
| Race | ||||
| ȃBlack | 191 (97.0) | 125 (96.2) | 66 (98.5) | .67 |
| ȃOther | 6 (3.1) | 5 (3.9) | 1 (1.5) | |
| CD4 count, cells/mm3 | 33 (12–61) | 40.5 (16–64) | 27 (7–40) | .003 |
| Plasma CrAg titers | 1:40 (5–320) | Not applicable | 1:40 (5–320) | |
| Baseline blood fungal growth | 5 (2.5) | 0 | 5 (7.5) | .002 |
| Tuberculosis in the past 6 monthsa | 51 (25.9) | 33 (25.4) | 18 (26.9) | .82 |
| Tuberculosis treatment | 29 (14.7) | 18 (13.9) | 11 (16.4) | .63 |
| Antiretroviral therapy prior to enrollment | 29 (14.7) | 13 (10.0) | 16 (23.9) | .01 |
| Vital status at 6 months | ||||
| ȃAlive | 160 (81.2) | 113 (86.9) | 47 (70.2) | .01 |
| ȃDead | 28 (14.2) | 11 (8.5) | 17 (25.4) | |
| ȃUnknown | 9 (4.6) | 6 (4.6) | 3 (4.5) |
| Covariable . | Total (n = 197) . | CrAg-Negative (n = 130) . | CrAg-Positive (n = 67) . | P Value . |
|---|---|---|---|---|
| Age, years | 39.0 (32.4–47.1) | 39.1 (33.0–47.8) | 38.7 (31.7–47.1) | .67 |
| Sex | ||||
| ȃMale | 100 (50.8) | 70 (53.9) | 30 (44.8) | .23 |
| ȃFemale | 97 (49.2) | 60 (46.2) | 37 (55.2) | |
| Race | ||||
| ȃBlack | 191 (97.0) | 125 (96.2) | 66 (98.5) | .67 |
| ȃOther | 6 (3.1) | 5 (3.9) | 1 (1.5) | |
| CD4 count, cells/mm3 | 33 (12–61) | 40.5 (16–64) | 27 (7–40) | .003 |
| Plasma CrAg titers | 1:40 (5–320) | Not applicable | 1:40 (5–320) | |
| Baseline blood fungal growth | 5 (2.5) | 0 | 5 (7.5) | .002 |
| Tuberculosis in the past 6 monthsa | 51 (25.9) | 33 (25.4) | 18 (26.9) | .82 |
| Tuberculosis treatment | 29 (14.7) | 18 (13.9) | 11 (16.4) | .63 |
| Antiretroviral therapy prior to enrollment | 29 (14.7) | 13 (10.0) | 16 (23.9) | .01 |
| Vital status at 6 months | ||||
| ȃAlive | 160 (81.2) | 113 (86.9) | 47 (70.2) | .01 |
| ȃDead | 28 (14.2) | 11 (8.5) | 17 (25.4) | |
| ȃUnknown | 9 (4.6) | 6 (4.6) | 3 (4.5) |
Categorical variables are summarized with counts; percentages and P values are based on the Fisher exact or χ2 test. Continuous variables are summarized with medians, interquartile ranges, and P values based on Wilcoxon rank sum test. P values < .05 are bolded.
Abbreviations: CrAg, cryptococcal antigen.
Includes pulmonary, disseminated, or extrapulmonary Mycobacterium tuberculosis infection diagnosed within 6 months prior to or on the day of enrollment.
Antibody Marker Levels
In the bivariate analysis, CrAg-positive individuals had higher inverse titers of GXM-IgG (median, 169.5; IQR, 61.1–411.9 vs 117.3, 47.0–176.3; P = .0009), higher concentrations of IgG1 (6672.2, 4695.5–10 414 vs 5343.4, 3807.7–7721.9 μg/mL; P = .007) and IgG2 (1467.4, 812.8–2607 vs 1036.0, 519.2–2012.4 μg/mL; P = .01), and lower curdlan-IgG (46.6, 11.1–133.0 vs 93.2, 39.6–205.9; P = .01) than CrAg-negative individuals (Figure 1). There were no between-group differences in laminarin-binding antibodies. There was collinearity among GXM-IgG, GXM-IgM, and GXM-IgA (Supplementary Figure 1).
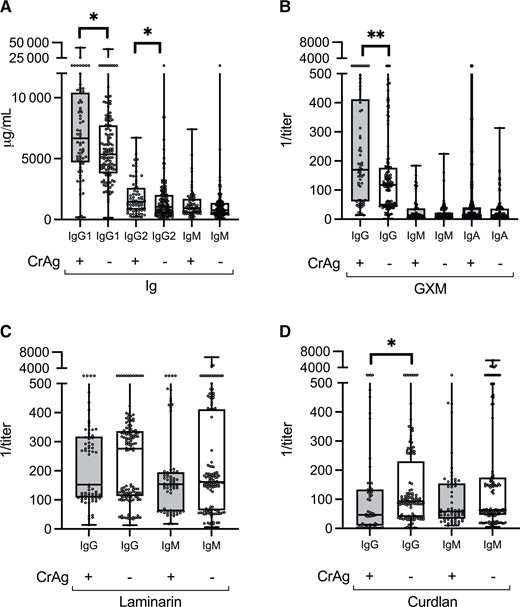
Total and antigen-specific antibody levels in 130 CrAg-negative and 67 CrAg-positive individuals at baseline. Total Ig levels were determined using Luminex; GXM, laminarin, and curdlan antibody titers were determined using enzyme-linked immunosorbent assay. Ig concentrations in units of micrograms per milliliter (A) and the inverse of GXM (B), laminarin (C), and curdlan (D) titers, depicted as medians and interquartile ranges, are shown on the y-axis for each group shown on the x-axis. CrAg status is represented by (+) or (–) to indicate positive or negative CrAg status, respectively. *P < .05; **P ≤ .001. Wilcoxon rank sum test. Abbreviations: CrAg, cryptococcal antigen; GXM, glucuronoxylomannan; Ig, immunoglobulin.
Associations of Antibody Markers With CrAg Status
Higher GXM-IgG and lower curdlan-IgG were each associated with CrAg-positive status adjusted for CD4 count and ART use prior to enrollment in the multivariable logistic regression model (adjusted odds ratio [aOR], 1.64; 64% increase in odds per log increase in GXM-IgG titer; 95% CI, 1.21 to 2.22 and aOR, 0.71; 29% reduction in odds per log increase in curdlan-IgG titer; 95% CI, .58 to .87, respectively; Figure 2).
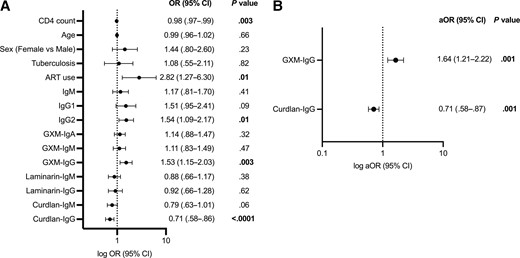
Forest plots of the univariable (A) and multivariable (B) logistic regression analysis estimating the association between antibody markers and plasma cryptococcal antigen (CrAg) status in a cohort of 130 CrAg-negative and 67 CrAg-positive individuals. Antibody variables were log-transformed. Covariables with P < .20 in the univariable analysis were included in the multivariable analysis (CD4 count and ART use). Tuberculosis includes pulmonary, disseminated, or extrapulmonary infection diagnosed within 6 months prior to enrollment. ART use includes treatment history prior to enrollment. P values < .05 are bolded. Abbreviations: aOR, adjusted odds ratio; ART, antiretroviral therapy; CI, confidence interval; CrAg, cryptococcal antigen; GXM, glucuronoxylomannan; Ig, immunoglobulin; OR, odds ratio.
Correlation Between Plasma CrAg Titer and Antibody Levels
In the CrAg-positive group (n = 67), there were inverse correlations between CrAg titer and IgG1 (ρ=−0.33; P = .007), IgG2 (ρ=−0.29; P = .02), laminarin-IgG (ρ=−0.24; P = .05), and, while not significant, GXM-IgG (ρ=−0.19; P = .13; Supplementary Table 2).
Associations of Antibody Markers With Mortality
Variables significantly associated with death within 6 months in the univariable analysis included positive plasma CrAg (HR, 3.43; 95% CI, 1.61 to 7.33), CrAg titer (HR, 1.19; 19% increase in hazard per log increase in titer; 95% CI, 1.05 to 1.36), tuberculosis diagnosis within the prior 6 months (HR, 2.66; 95% CI, 1.26 to 5.59), and GXM-IgM (HR, 0.67; 33% decrease in hazard per log increase in titer; 95% CI, .45 to 1.00; Supplementary Table 1). In the multivariable Cox proportional hazards analysis, there was an interaction between CrAg status and GXM-IgG (interaction term, P = .04), so the model was stratified by CrAg status. In CrAg-positive individuals, GXM-IgG was inversely associated with mortality in both unadjusted (HR, 0.55; a 1.8-fold reduction per log increase in titer; 95% CI, .36 to .84) and adjusted (HR, 0.50; a 2-fold reduction per log increase in titer; 95% CI, .33 to .77) model for CD4 count and tuberculosis (Table 2, Figure 3). GXM-IgG was not associated with mortality in the CrAg-negative cohort. GXM-IgM was not associated with mortality in the multivariable model.

Kaplan-Meier curves for survival by baseline GXM-IgG categories. The range of inverse titers of GXM-IgG was categorized into tertiles: low (1.9–62.2), medium (62.8–172.0), and high (176.3–3453.3) GXM-IgG. Curves are shown for the 67 CrAg-positive individuals (A) and 130 CrAg-negative individuals (B). GXM-IgG was log-transformed in the survival analysis. Abbreviations: CrAg, cryptococcal antigen; GXM, glucuronoxylomannan; Ig, immunoglobulin.
Stratified Cox Proportional Hazards Regression Model Estimating Glucuronoxylomannan-Immunoglobulin G as a Predictor of Mortality by 6 Months Following Enrollment
| Outcome . | CrAg-Negative (n = 130) . | CrAg-Positive (n = 67) . | ||
|---|---|---|---|---|
| HR (95% CI) . | P Value . | HR (95% CI) . | P Value . | |
| Death, no. (%) | 11 (8.5) | 17 (25.4) | … | |
| Model 1, unadjusted | 1.03 (.58–1.80) | .93 | .55 (.36–.84) | .006 |
| Model 2, adjusted CD4 count | 1.04 (.59–1.82) | .90 | .56 (.36–.86) | .009 |
| Model 3, adjusted CD4 count and tuberculosis | 1.03 (.60–1.76) | .92 | .50 (.33–.77) | .002 |
| Outcome . | CrAg-Negative (n = 130) . | CrAg-Positive (n = 67) . | ||
|---|---|---|---|---|
| HR (95% CI) . | P Value . | HR (95% CI) . | P Value . | |
| Death, no. (%) | 11 (8.5) | 17 (25.4) | … | |
| Model 1, unadjusted | 1.03 (.58–1.80) | .93 | .55 (.36–.84) | .006 |
| Model 2, adjusted CD4 count | 1.04 (.59–1.82) | .90 | .56 (.36–.86) | .009 |
| Model 3, adjusted CD4 count and tuberculosis | 1.03 (.60–1.76) | .92 | .50 (.33–.77) | .002 |
Glucuronoxylomannan-immunoglobulin G was log-transformed and used as a continuous variable. P values <.05 are bolded.
Abbreviations: CI, confidence interval; CrAg, cryptococcal antigen; HR, hazard ratio.
Stratified Cox Proportional Hazards Regression Model Estimating Glucuronoxylomannan-Immunoglobulin G as a Predictor of Mortality by 6 Months Following Enrollment
| Outcome . | CrAg-Negative (n = 130) . | CrAg-Positive (n = 67) . | ||
|---|---|---|---|---|
| HR (95% CI) . | P Value . | HR (95% CI) . | P Value . | |
| Death, no. (%) | 11 (8.5) | 17 (25.4) | … | |
| Model 1, unadjusted | 1.03 (.58–1.80) | .93 | .55 (.36–.84) | .006 |
| Model 2, adjusted CD4 count | 1.04 (.59–1.82) | .90 | .56 (.36–.86) | .009 |
| Model 3, adjusted CD4 count and tuberculosis | 1.03 (.60–1.76) | .92 | .50 (.33–.77) | .002 |
| Outcome . | CrAg-Negative (n = 130) . | CrAg-Positive (n = 67) . | ||
|---|---|---|---|---|
| HR (95% CI) . | P Value . | HR (95% CI) . | P Value . | |
| Death, no. (%) | 11 (8.5) | 17 (25.4) | … | |
| Model 1, unadjusted | 1.03 (.58–1.80) | .93 | .55 (.36–.84) | .006 |
| Model 2, adjusted CD4 count | 1.04 (.59–1.82) | .90 | .56 (.36–.86) | .009 |
| Model 3, adjusted CD4 count and tuberculosis | 1.03 (.60–1.76) | .92 | .50 (.33–.77) | .002 |
Glucuronoxylomannan-immunoglobulin G was log-transformed and used as a continuous variable. P values <.05 are bolded.
Abbreviations: CI, confidence interval; CrAg, cryptococcal antigen; HR, hazard ratio.
Mediation Analysis
Mediation analysis [16, 18] was performed to determine whether the influence of the exposure (CrAg status) on the potential mediator (GXM-IgG) explains some or all of the significance of the association between an exposure and an outcome (mortality) statistically. A significant interaction between the exposure and mediator variable was detected, and CrAg status was significantly associated with GXM-IgG and mortality. The direct effect of CrAg status on mortality was 1.71 (95% CI, .88 to 2.54), the indirect effect mediated by GXM-IgG was –0.35 (95% CI, –.67 to –.03), and –57% of the total effect on mortality was mediated by GXM-IgG (95% CI, –1.21 to .08; Supplementary Figure 2).
Principal Component Analysis
Most of the variance in the dataset was explained by PC1 (35%) and PC2 (19%), which were used to explore associations between antibody markers and CrAg status and mortality. PC1 was composed primarily of GXM-IgA, GXM-IgG, and GXM-IgM. PC2 was composed of curdlan-IgG, laminarin-IgG, and a negative loading of laminarin-IgM (Supplementary Figure 3). Among CrAg-positive individuals, the PC1 score had a significant inverse association with 6-month mortality. Mean PC1 score was 0.32 among the CrAg-positive individuals who survived and –0.66 among the CrAg-positive individuals who died, a difference of 0.98 (95% CI, .04 to 1.92; P = .04), indicating that higher levels of GXM-IgA, GXM-IgG, and GXM-IgM were associated with higher survival rates in this population (Supplementary Table 3, Supplementary Figure 4). There was no association between PC score and mortality in the CrAg-negative group. No association was found between PC score and CrAg status in the overall cohort.
DISCUSSION
In this retrospective, serological study of CrAg-positive and CrAg-negative participants with HIV with CD4<100 cells/mm3, higher enrollment GXM-IgG and lower curdlan-IgG titers were associated with CrAg-positive status but not CrAg titer. In addition, for CrAg-positive participants, enrollment GXM-IgG, prior to antifungal therapy, was inversely associated with mortality and a partial mediator on the causal pathway between CrAg and mortality by 6 months. This suggests that lower levels of GXM-IgG may contribute, in part, to excess mortality in CrAg-positive individuals with HIV [3, 4] and echoes a pre-AIDS era study in which an absence of cryptococcal antibodies was a poor prognostic factor in patients with CM [19].
Our data show that CrAg-positive participants with HIV with CD4 counts <100 cells/mm3 had higher IgG1 and IgG2 levels than CrAg-negative participants. However, IgG2 levels in both CrAg-positive and CrAg-negative participants were lower than normal values for adults without HIV. HIV is associated with hypergammaglobulinemia, generally manifested as increased IgG1, but IgG2 is decreased [20]. IgG2 is the predominant IgG subclass of human antibodies to capsular polysaccharides, including GXM [13, 21]. Thus, as in a prior study [7], higher IgG2 levels in CrAg-positive than CrAg-negative participants may reflect a cryptococcal response. We did not determine GXM-IgG subclasses in this study.
Higher GXM-IgG in CrAg-positive participants parallels multiple previous studies that found higher GXM-IgG titers in individuals with and without HIV with cryptococcosis than without cryptococcosis [7–9, 15]. Notably, in participants with cryptococcal antigenemia, lower GXM-IgG was associated with mortality, while in an unadjusted analysis of the entire cohort, lower GXM-IgM was associated with mortality. These associations were supported by the PCA, in which the top principal component, primarily GXM-binding antibodies, was inversely associated with CrAg titer and mortality in CrAg-positive individuals. However, the GXM-IgG-specific signal in predicting CrAg-positive status was reduced by adding noise from GXM-IgM and GXM-IgA. While association is not causation, these data suggest that GXM-binding antibodies may have a protective role in those with cryptococcal antigenemia. This is consistent with a large body of data that show that GXM monoclonal antibodies mediate beneficial effects, including anticryptococcal activity in vitro [22]; GXM clearance, including in a clinical trial in patients with CM [23]; and protection against lethal cryptococcal infection in experimental models [24].
As previously reported [7], we found that CrAg-negative participants had detectable GXM-binding antibodies. Most likely, this reflects cryptococcal infection early in life or latency [25] and/or ongoing environmental exposure, though we cannot rule out cross-reactivity with an unknown determinant [21]. GXM-binding antibodies have been detected in individuals with and without HIV with and without a history of cryptococcosis as well as young children [8, 14]. Thus, higher GXM-IgG titers in CrAg-positive compared with CrAg-negative individuals most likely reflect an immune response to fungal growth. HIV infection impairs responses to new but not previously encountered antigens [20]. Although fungal growth is not always detected (or quantified if present) in CrAg-positive persons, it is logical to posit that serum cryptococcal antigenemia emerges when the host defense is unable to contain latent or newly acquired cryptococcal infection. Thus, an increase in GXM-IgG might signal cryptococcosis risk in CrAg-negative individuals. Consistent with this idea, anti-mannan antibodies, which can protect mice against experimental candidiasis [26], were detected in patients with candidiasis prior to the development of positive blood cultures [27].
β-glucan-binding antibodies are part of the natural serum antibody repertoire that binds conserved microbial determinants found on the cryptococcal cell wall and other fungi and is the first line of defense against pathogens [10, 28]. Although we did not find associations between laminarin- or curdlan-binding antibodies and mortality, lower curdlan-IgG was associated with CrAg status, and laminarin-IgG inversely correlated with CrAg titer, a correlate of risk for meningitis [29] and death [30]. Previous reports show associations between lower laminarin-antibody titers and CrAg-positive status [7] as well as HIV-associated cryptococcus-associated immune reconstitution inflammatory syndrome [11]. Evidence that naturally occurring β-glucan-binding antibodies could contribute to host defense against cryptococcosis includes that laminarin-binding antibodies have direct anti-cryptococcal effects in vitro [31], indirect effects on the inflammatory response [11], and protect mice in vivo [32, 33]. Similar to our findings in CrAg-positive participants, candidemic patients had higher β-glucan-associated protein MP65 titers than noncandidemic patients, and survivors had higher titers than nonsurvivors [34].
We found increased mortality in CrAg-positive participants with tuberculosis and lower curdlan-IgM in participants with tuberculosis than without tuberculosis (Supplementary Table 4). Notably, mycobacterial cell walls contain multiple glucans [35], and decreased levels of serum mycobacterial cell wall-binding IgG were associated with disseminated tuberculosis [36]. Given that tuberculosis is an independent risk factor for cryptococcosis [37, 38], more work is needed to identify possible shared susceptibility factors.
We used well-established antigen-capture ELISA to detect GXM-, laminarin-, and curdlan-binding antibodies [9, 12, 15]. While previous studies examined the relationship between antibody markers and cryptococcal disease status, we sought associations between antibody markers and all-cause mortality. We used mediation analysis to establish that GXM-IgG partially mediated a causal association between CrAg and mortality and PCA to eliminate multicollinearity between antibody variables. Use of these platforms led to a novel result that implicates GXM-binding antibody levels in survival of CrAg-positive individuals with HIV.
Our study also has limitations. Serum CrAg may have affected GXM-binding antibody measurements; we did not assay for immune complexes. Since our assays were performed on a single sample from one point in time, our data does not address longitudinal or temporal variations in antibody levels. We did not perform functional assays and did not investigate the relationship between B cells and cryptococcal disease status [39] or antibody levels as we did not have access to peripheral blood mononuclear cells. Misclassification bias is possible. Though rare, CM can occur in CrAg-negative individuals [40], and this may have affected the difference in antibody levels by CrAg status. ART use prior to enrollment was associated with cryptococcal antigenemia, most likely reflecting prolonged immune suppression in ART-experienced, nonadherent individuals. While we adjusted for ART use, our understanding of possible interactions between ART and antibody levels is limited. There may have been other unmeasured or latent confounders that could shed more light on the associations between antibody markers, ART, and mortality.
CONCLUSIONS
Levels of IgG1-, IgG2-, GXM-IgG-, and β-glucan-binding antibodies differed in CrAg-positive and CrAg-negative individuals. GXM-IgG was associated with CrAg status as was lower curdlan-IgG, and lower GXM-IgG was associated with an increased risk of all-cause mortality in CrAg-positive individuals. These findings support the hypothesis that GXM- and β-glucan-binding antibodies may enhance cryptococcal host defense, highlighting the need for mechanistic and larger-scale prospective longitudinal clinical studies. Such studies may provide a better understanding of the antifungal activities of GXM- and β-glucan-binding antibodies, identify much needed biomarkers of cryptococcal pathogenesis and mortality, and inform opportunities for earlier risk stratification and therapeutic intervention.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. Conception or design of the work (H. Y., R. W., T. H., N. G., J. J., L. P.), data analysis (H. Y., R. W., T. W., A. I., J. J., T. H., L. P.), data interpretation (all authors), drafting the article (H. Y., R. W., T. H., L. P.), critical revision of the article (all authors), and final approval of the version to be published (all authors).
Acknowledgments. Samples and data collection performed by Neo Legare, Matshediso Mkhwanazi, Siphiwe Kutta, and Tracey Shabangu.
Disclaimer. The views expressed in this publication are those of the author(s) and not necessarily those of the National Institutes of Health (NIH), Centers for Disease Control and Prevention (CDC), National Institute for Health Research (NIHR), NHS, or the UK Department of Health and Social Care.
Financial support. This work was supported by the Einstein-Rockefeller-CUNY Center for AIDS Research, Albert Einstein College of Medicine (grant P30-AI124414); the NIH/National Center for Advancing Translational Service Einstein-Montefiore Clinical and Translational Science Awards (grant UL1TH001073) to H. Y.; NIH (grant R01-AI143453) to L. P.; Meningitis Research Foundation (grant 1604.0), Sir Ratanji Dallal Trust (research, grant), St Georges NHS Trust Charity (research grant), NIHR (grant CL-2019–16-001) to R. W.; South African Research Chairs Initiative of the Department of Science and National Research Foundation (grant 84177) to C. T. T.; Meningitis Research Foundation (grant 1604.0; paid to institution with R. W.), NIH (grant R01AI118511), and a cooperative agreement between the National Health Laboratory Service and US CDC (CDC-RFA-GH15-1575) to N. P. G.; and EDCTP (paid to institution) and NIHR (grant RP-2017-08-ST2-012) using UK aid from the UK government to support global health research to J. N. J.
References
Author notes
H. Y. and R. M. W. contributed equally to this work.
Potential conflicts of interest. N. P. G. reports grants or contracts from NIH, UK MRC, Gates Foundation, CDC, and NHLS Research Trust (paid to institution); payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from the South African HIV Clinicians Society (paid to author); a role as president and council member of FIDSSA; and receipt of equipment, materials, drugs, medical writing, gifts, or other services from Immy– CrAg LFA strips (paid to institution). T. S. H. reports payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Gilead Sciences and Pfizer (paid to author). J. N. J. reports grants from the CDC; speaker fees from Gilead; participation on a data and safety monitoring board as the TSC chair for ASTRO, HARVEST, ARTIST, ACACIA, and CASTLE; and received a drug donation for a clinical trial from Gilead Sciences, Inc. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.




