-
PDF
- Split View
-
Views
-
Cite
Cite
Allassane F Ouattara, Catherine M Bjerum, Méité Aboulaye, Olivier Kouadio, Vanga K Marius, Britt Andersen, Daphne Lew, Charles W Goss, Gary J Weil, Benjamin G Koudou, Christopher L King, Semiannual Treatment of Albendazole Alone is Efficacious for Treatment of Lymphatic Filariasis: A Randomized Open-label Trial in Cote d’Ivoire, Clinical Infectious Diseases, Volume 74, Issue 12, 15 June 2022, Pages 2200–2208, https://doi.org/10.1093/cid/ciab194
Close - Share Icon Share
Abstract
Ivermectin (IVM) plus albendazole (ALB), or IA, is widely used in mass drug administration (MDA) programs that aim to eliminate lymphatic filariasis (LF) in Africa. However, IVM can cause severe adverse events in persons with heavy Loa loa infections that are common in Central Africa. ALB is safe in loiasis, but more information is needed on its efficacy for LF. This study compared the efficacy and safety of 3 years of semiannual treatment with ALB to annual IA in persons with bancroftian filariasis.
Adults with Wuchereria bancrofti microfilaremia (Mf) were randomized to receive either 3 annual doses of IA (N = 52), 6 semiannual doses of ALB 400 mg (N = 45), or 6 semiannual doses of ALB 800 mg (N = 47). The primary outcome is amicrofilaremia at 36 months.
IA was more effective for completely clearing Mf than ALB 400mg or ALB 800mg (79%, 95% confidence interval [CI]: 67–91; vs 48%, 95% CI: 32–66 and 57%, 95% CI: 41–73, respectively). Mean percentage reductions in Mf counts at 36 months relative to baseline tended to be greater after IA (98%, 95% CI: 88–100) than after ALB 400 mg (88%, 95% CI: 78–98) and ALB 800 mg (89%, 95% CI: 79–99) (P = .07 and P = .06, respectively). Adult worm nest numbers (assessed by ultrasound) were reduced in all treatment groups. Treatments were well tolerated.
Repeated semiannual treatment with ALB is macrofilaricidal for W. bancrofti and leads to sustained reductions in Mf counts. This is a safe and effective regimen that could be used as MDA to eliminate LF in areas where ivermectin cannot be used.
NCT02974049
Lymphatic filariasis (LF) is a leading cause of preventable disability in the world, and it has been targeted for elimination as a global health problem by 2030 [1]. An estimated 323 million people are at risk for LF in sub-Saharan Africa [1]. Elimination efforts in sub-Saharan Africa utilize annual mass drug administration (MDA) with a combination of ivermectin (IVM) and albendazole (ALB) (IA) have significantly reduced LF in sub-Saharan countries [2, 3]. However, IVM cannot be used in Central African countries where LF is coendemic with Loa loa because of the risk of serious adverse events (AE) in persons with high level L. loa microfilaremia (Mf) [4, 5]. In contrast to IVM, ALB has no direct effect on Mf, but it has embryotoxic and macrofilaricidal activity against adult filarial worms. Thus, repeated treatment with ALB alone slowly reduces Mf counts in blood [6, 7]. In 2012, the World Health Organization (WHO) proposed a provisional strategy of MDA with ALB together with integrated vector management to eliminate LF in areas coendemic for L. loa [8], where an estimated 1.4 million are at risk for serious AEs with ivermectin [9]. When the current trial began, there were no data on the efficacy of semiannual treatment with ALB alone for clearing W. bancrofti Mf, although semiannual treatment with albendazole paired with ivermectin [10] or diethylcarbamazine (DEC) [11] showed greater efficacy for clearing Mf compared to annual treatment indicating potent benefits of more frequent ALB treatment. Recent community MDA trials with semiannual ALB 400 mg alone conducted in the Republic of Congo and Democratic Republic of the Congo demonstrated significant reductions in circulating filarial antigen levels and in Mf counts [12, 13]. However, these studies did not have a comparator group, and insecticide-treated bed nets could have contributed to the observed results. Moreover, a recent review suggested that ALB alone has little ability to clear MF or kill W. bancrofti adult worms after a few rounds of treatment [14]. Here we report results of a randomized clinical trial that compared the impact of three years of either semiannual ALB to that of annual IA treatment (the current standard MDA regimen for West Africa) in individuals with W. bancrofti infections in Côte d’Ivoire.
METHODS
Study Design and Participants
This was a randomized, open label, controlled, parallel-group study of participants recruited from Agboville District, Côte d’Ivoire. LF is endemic in Côte d’Ivoire, but L. loa is not. The study protocol was approved by institutional review boards (UH Cleveland Medical Center IRB #08-13-13) and (Comité National d’Ethique et de la Recherche, CNER, N: 008/MSLS/CNER/-kp) and registered with ClinicalTrials.gov (NCT02974049). Screening for W. bancrofti infection was performed with finger-stick blood samples from 4910 individuals using a rapid test for CFA (filariasis test strip [FTS], Alere Inc., Waltham, Massachusetts, USA). FTS positive individuals had night blood testing for Mf as described below. All participants provided written informed consent and 189 individuals were enrolled (Figure 1). Inclusion criteria were ≥50 Mf/mL, age 18–70 years, no recent acute illness, and no treatment with ALB or IVM within the last 5 years. Exclusion criteria included a positive pregnancy test; a history of chronic kidney or liver disease, or serum alanine transaminase, aspartate transaminase or creatinine levels >1.5 times the upper limits of normal or severe anemia (blood hemoglobin <7 gm/dL). Persons with onchocerca microfiladermia or positive Ov16 antibody test were excluded. The protocol was amended to reevaluate participants for presence of Mf and filarial antigen in persons treated with ALB alone at 48 months.
![CONSORT diagram shows screening, randomization, and follow-up. Participants enrolled in each treatment arm were retested for LF infection (FTS, Mf, and presence of worm nests by ultrasound in men with nests at baseline) at 6, 12, 24 and 36 months. Note that the treatment arm IVM + DEC + ALB is not included in this report; it was part of a separate study that used the same IA comparator arm [15]. Abbreviations: ALB, albendazole; DEC, diethylcarbamazine; FTS, filariasis test strip; IA, ivermectin plus albendazole, IVM, ivermectin; LF, lymphatic filariasis; Mf, microfilaria.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/cid/74/12/10.1093_cid_ciab194/2/m_ciab194f0001.jpeg?Expires=1750381107&Signature=3mZzxXXNKY0ZBvkRMDDazflrD3PpqD-rZTZmgHilzM7FKW7LfjTcGnk4-cpvN6s7nr3h9c7hux-W6QjGZufLCAefPcViahHIIw8axEaTkz4quVkRdBYdjhj9Gf-K3ImarUEfaLKloSlaWH67xyIZePFjDkTXK1hGWVm25BqF~YzcaF~1oZdel202cYbjwh~j3k4A-0Naj9BurQg6BmHhJs~sPOuF~VhEh6g9E98a3YyhCvTCiVGoaL631QgSF26dU-~5JEbY3bZndox6eMyLTt1-rZ02Y97cnMUv5pdpYKUnVmjTqJnsuOw549JYb2EZhmp6ASDNcopHMmP1Ck4xmg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
CONSORT diagram shows screening, randomization, and follow-up. Participants enrolled in each treatment arm were retested for LF infection (FTS, Mf, and presence of worm nests by ultrasound in men with nests at baseline) at 6, 12, 24 and 36 months. Note that the treatment arm IVM + DEC + ALB is not included in this report; it was part of a separate study that used the same IA comparator arm [15]. Abbreviations: ALB, albendazole; DEC, diethylcarbamazine; FTS, filariasis test strip; IA, ivermectin plus albendazole, IVM, ivermectin; LF, lymphatic filariasis; Mf, microfilaria.
Randomization and Masking
Two studies used the same IA treatment control group, but this report only presents data from one of the studies. Results from the other study has been published [15]. A computer-generated block randomization table assigned participants to 1 of 4 treatment arms, with 3 arms reported here as follows: (A) Three annual doses of co-administered IVM 200 µg/kg (Merck & Co., Kenilworth, New Jersey, USA) plus ALB 400 mg (GlaxoSmithKline, Uxbridge, United Kingdom); (B) semiannual ALB 400 mg alone or (C) semiannual ALB 800 mg alone each provided every 6 months for 3 years for a total 6 doses. The study coordinator who distributed medications had sole access to the randomization table. Investigators who evaluated AEs, performed ultrasounds, or read Mf slides were masked to treatment assignments.
Procedures
Enrollment procedures evaluated symptoms, measured vital signs, and performed a physical examination before treatment. This was repeated 24 hours after treatment, and trained village health workers contacted participants daily through day 7 to record and manage AEs. These were scored using a modified version of the National Cancer Institute Common Terminology Criteria for Adverse Events, v4.0. Treatment was directly observed to assure all tablets were swallowed.
Detection of Infections
Filarial antigenemia was detected with filariasis test strips according to the manufacturer’s instructions. Positive tests were scored semi-quantitatively from negative (0) and 1, 2, and 3 as previously described [16]. Individuals with positive FTS were checked for Mf by membrane filtration of 2 1-mL aliquots of venous night blood as previously described [15]. Two microscopists independently read Giemsa-stained filters. A third microscopist read filters with discordant results. Prior to treatment, scrotal ultrasound examinations were performed on men in the supine position using a SonoScape S8 portable ultrasound with a 5–10 MHz linear array transducer (International Diagnostic Devices, Las Vegas, Nevada, USA). Adult worm nests were identified by worm movement [17, 18] and digitally recorded. Participants with worm nests at baseline were rescanned at 6, 12, 24, and 36 months. The presence or absence of worm nests, the number and location, the maximum diameter, and the degree of lymphatic vessel dilation were recorded. Worm nests not identified on previous examinations were considered to be new, incident infections.
Outcomes
Primary endpoints were complete clearance of Mf and CFA from blood 36 months post-treatment. The same parameters at 12 and 24 months were secondary endpoints. Other secondary endpoints included mean percent reduction in Mf, reductions in CFA test scores, and percent inactivation of adult filarial worm nests (compared to baseline) at all time points post-treatment.
Statistical Analysis
Sample size calculation was based on the following assumptions for the hypothesis that semiannual ALB monotherapy is non-inferior for clearing W. bancrofti Mf compared to annual IA administration at 36 months. The margin of equivalence was set at 15%, α = 0.05, and we assumed that ≥70% of persons enrolled would be followed through 36 months. We assumed that 3 annual doses of IA would achieve complete Mf clearance in 95% of participants at 36 months. A sample of 70 participants in each treatment arm would provide the trial with a power of 80% to test the hypothesis that at least 80% of participants would have complete Mf clearance after 6 ALB doses. An intention to treat (ITT) analysis was performed for all individuals who were tested at 36 months for the primary outcome assessment.
Baseline sample characteristics between treatment arms, and subsequent Mf and FTS clearance rates were compared using the χ 2 test and relative risk analysis. A negative binomial generalized estimating equation model (PROC GENMOD in SAS Institute, Cary, North Carolina, USA) compared changes in Mf and worm nest counts across time and between treatment arms. The model included the follow-up time points of 6, 12, 24, and 36 months and interaction between treatment and time as fixed effects. The model adjusted for baseline Mf levels. An unstructured correlation was used to account for correlation among repeated measurements.
RESULTS
Enrollment, Treatment Assignment, and Baseline Filariasis Test Results
Screening of 4910 adults yielded 189 eligible individuals (all Mf and CFA positive), 144 of whom were randomized to treatment with annual IA, or semiannual ALB 400 mg or ALB 800 mg. Participants were enrolled between 5 February and 13 September 2015. Thirty individuals withdrew from the study after enrollment (10 from IA, 11 from ALB 400-mg, and 9 from ALB 800-mg arms), and 2 died of unrelated causes. Baseline demographics, Mf counts, and CFA levels were similar in the 3 arms (Table 1). Participants were mostly men (82%, n = 118) because of higher infection prevalence in males and reluctance of women to participate in the treatment trial. Pre-treatment Mf counts ranged from 51 to 2551 Mf/mL with an overall geometric mean of 193 Mf/mL.
| Characteristics . | IA . | ALB 400 mg . | ALB 800 mg . |
|---|---|---|---|
| . | (N = 52) . | (N = 45) . | (N = 47) . |
| Age in years, median (IQR) | 35 (19) | 40 (20) | 39 (21) |
| Male sex (%) | 46 (88%) | 33 (73%) | 39 (83%) |
| Geometric mean Mf count, Mf/mL | 190 | 177 | 204 |
| (range) | (51–1065) | (56–1050) | (52–2251) |
| Men with worm nests/number examined (%) | 34/42 (80%) | 11/27 (41%) | 17/31 (54%) |
| Mean worm nestsa | 2.5 | 2.3 | 3.2 |
| (range) | (1–6) | (1–4) | (1–8) |
| FTS scoreb | 2.8 ± 0.5 | 2.8 ± 0.5 | 2.7 ± 0.4 |
| Characteristics . | IA . | ALB 400 mg . | ALB 800 mg . |
|---|---|---|---|
| . | (N = 52) . | (N = 45) . | (N = 47) . |
| Age in years, median (IQR) | 35 (19) | 40 (20) | 39 (21) |
| Male sex (%) | 46 (88%) | 33 (73%) | 39 (83%) |
| Geometric mean Mf count, Mf/mL | 190 | 177 | 204 |
| (range) | (51–1065) | (56–1050) | (52–2251) |
| Men with worm nests/number examined (%) | 34/42 (80%) | 11/27 (41%) | 17/31 (54%) |
| Mean worm nestsa | 2.5 | 2.3 | 3.2 |
| (range) | (1–6) | (1–4) | (1–8) |
| FTS scoreb | 2.8 ± 0.5 | 2.8 ± 0.5 | 2.7 ± 0.4 |
Abbreviations: ALB, albendazole; FTS, filariasis test strip; IA, ivermectin plus albendazole; IQR, interquartile range.
aIn men with 1one or more worm nests detected by ultrasound.
bAverage FTS score ± SD of 1, 2, or 3.
| Characteristics . | IA . | ALB 400 mg . | ALB 800 mg . |
|---|---|---|---|
| . | (N = 52) . | (N = 45) . | (N = 47) . |
| Age in years, median (IQR) | 35 (19) | 40 (20) | 39 (21) |
| Male sex (%) | 46 (88%) | 33 (73%) | 39 (83%) |
| Geometric mean Mf count, Mf/mL | 190 | 177 | 204 |
| (range) | (51–1065) | (56–1050) | (52–2251) |
| Men with worm nests/number examined (%) | 34/42 (80%) | 11/27 (41%) | 17/31 (54%) |
| Mean worm nestsa | 2.5 | 2.3 | 3.2 |
| (range) | (1–6) | (1–4) | (1–8) |
| FTS scoreb | 2.8 ± 0.5 | 2.8 ± 0.5 | 2.7 ± 0.4 |
| Characteristics . | IA . | ALB 400 mg . | ALB 800 mg . |
|---|---|---|---|
| . | (N = 52) . | (N = 45) . | (N = 47) . |
| Age in years, median (IQR) | 35 (19) | 40 (20) | 39 (21) |
| Male sex (%) | 46 (88%) | 33 (73%) | 39 (83%) |
| Geometric mean Mf count, Mf/mL | 190 | 177 | 204 |
| (range) | (51–1065) | (56–1050) | (52–2251) |
| Men with worm nests/number examined (%) | 34/42 (80%) | 11/27 (41%) | 17/31 (54%) |
| Mean worm nestsa | 2.5 | 2.3 | 3.2 |
| (range) | (1–6) | (1–4) | (1–8) |
| FTS scoreb | 2.8 ± 0.5 | 2.8 ± 0.5 | 2.7 ± 0.4 |
Abbreviations: ALB, albendazole; FTS, filariasis test strip; IA, ivermectin plus albendazole; IQR, interquartile range.
aIn men with 1one or more worm nests detected by ultrasound.
bAverage FTS score ± SD of 1, 2, or 3.
Effects of Treatment on Microfilaremia
Treatment with IA administered once per year for 3 years cleared Mf in 34%, 26%, 54%, and 79% of participants at 6, 12, 24, and 36 months, respectively (Table 2); semiannual ALB cleared Mf more slowly and less completely. ALB400mg cleared Mf in 3%, 14%, 24%, and 48% of participants, and ALB 800 mg cleared Mf in 5%, 17%, 30%, and 57% of participants at the same time points. At 36 months, ALB at both 400 mg and 800 mg was inferior to IA by −31% and −22%, respectively, which was outside our predefined 15% noninferiority margin (P = .389 and P = .12).
| . | . | Months Post-Initiation of Treatment . | . | . | . |
|---|---|---|---|---|---|
| Treatment Group . | Characteristic . | 6 . | 12 . | 24 . | 36 . |
| IVM/ALB (IA) × 3 | No. of participants | 44 | 42 | 39 | 42 |
| Participants with complete Mf clearance | 15 | 11 | 21 | 33 | |
| % with complete Mf clearance (95% CI)a | 34% (20, 48) | 26% (13, 39) | 54% (38, 70) | 79% (67, 91) | |
| ALB 400 mg × 6 | No. of participants | 39 | 36 | 34 | 33 |
| Participants with complete Mf clearance | 1 | 5 | 8 | 16 | |
| % with complete Mf clearance (95% CI) | 3% (0, 8) | 14% (3, 25) | 24% (10, 38) | 48% (32, 66) | |
| P-value (χ 2 compared to IA × 3) | <.001 | .262 | .010 | .008 | |
| Risk ratio vs IA × 3 (95% CI) | 1.5 (1.2, 1.8) | 1.2 (0.9, 1.5) | 1.7 (1.1, 2.4) | 2.4 (1.2, 4.7) | |
| P-value | .0005 | .175 | .011 | .005 | |
| ALB 800 mg × 6 | No. of participants | 40 | 41 | 37 | 37 |
| Participants with complete Mf clearance | 2 | 7 | 11 | 21 | |
| % with complete Mf clearance (95% CI) | 5% (0, 12) | 17% (6, 29) | 30% (10, 38) | 57% (41, 73) | |
| P-value (χ 2 compared to IA × 3) | <.001 | .426 | .011 | .053 | |
| Risk ratio vs IA × 3 (95% CI) | 1.4 (1.2, 1.8) | 1.1 (.9, 1.4) | 1.5 (1.0, 2.3) | 2.0 (1.0, 4.0) | |
| P-value | .0014 | .316 | .039 | .045 |
| . | . | Months Post-Initiation of Treatment . | . | . | . |
|---|---|---|---|---|---|
| Treatment Group . | Characteristic . | 6 . | 12 . | 24 . | 36 . |
| IVM/ALB (IA) × 3 | No. of participants | 44 | 42 | 39 | 42 |
| Participants with complete Mf clearance | 15 | 11 | 21 | 33 | |
| % with complete Mf clearance (95% CI)a | 34% (20, 48) | 26% (13, 39) | 54% (38, 70) | 79% (67, 91) | |
| ALB 400 mg × 6 | No. of participants | 39 | 36 | 34 | 33 |
| Participants with complete Mf clearance | 1 | 5 | 8 | 16 | |
| % with complete Mf clearance (95% CI) | 3% (0, 8) | 14% (3, 25) | 24% (10, 38) | 48% (32, 66) | |
| P-value (χ 2 compared to IA × 3) | <.001 | .262 | .010 | .008 | |
| Risk ratio vs IA × 3 (95% CI) | 1.5 (1.2, 1.8) | 1.2 (0.9, 1.5) | 1.7 (1.1, 2.4) | 2.4 (1.2, 4.7) | |
| P-value | .0005 | .175 | .011 | .005 | |
| ALB 800 mg × 6 | No. of participants | 40 | 41 | 37 | 37 |
| Participants with complete Mf clearance | 2 | 7 | 11 | 21 | |
| % with complete Mf clearance (95% CI) | 5% (0, 12) | 17% (6, 29) | 30% (10, 38) | 57% (41, 73) | |
| P-value (χ 2 compared to IA × 3) | <.001 | .426 | .011 | .053 | |
| Risk ratio vs IA × 3 (95% CI) | 1.4 (1.2, 1.8) | 1.1 (.9, 1.4) | 1.5 (1.0, 2.3) | 2.0 (1.0, 4.0) | |
| P-value | .0014 | .316 | .039 | .045 |
Abbreviations: ALB, albendazole; CI, confidence interval; IA, ivermectin plus albendazole; IVM, ivermectin; Mf, microfilaria.
aCI denotes using binomial “exact” calculation.
| . | . | Months Post-Initiation of Treatment . | . | . | . |
|---|---|---|---|---|---|
| Treatment Group . | Characteristic . | 6 . | 12 . | 24 . | 36 . |
| IVM/ALB (IA) × 3 | No. of participants | 44 | 42 | 39 | 42 |
| Participants with complete Mf clearance | 15 | 11 | 21 | 33 | |
| % with complete Mf clearance (95% CI)a | 34% (20, 48) | 26% (13, 39) | 54% (38, 70) | 79% (67, 91) | |
| ALB 400 mg × 6 | No. of participants | 39 | 36 | 34 | 33 |
| Participants with complete Mf clearance | 1 | 5 | 8 | 16 | |
| % with complete Mf clearance (95% CI) | 3% (0, 8) | 14% (3, 25) | 24% (10, 38) | 48% (32, 66) | |
| P-value (χ 2 compared to IA × 3) | <.001 | .262 | .010 | .008 | |
| Risk ratio vs IA × 3 (95% CI) | 1.5 (1.2, 1.8) | 1.2 (0.9, 1.5) | 1.7 (1.1, 2.4) | 2.4 (1.2, 4.7) | |
| P-value | .0005 | .175 | .011 | .005 | |
| ALB 800 mg × 6 | No. of participants | 40 | 41 | 37 | 37 |
| Participants with complete Mf clearance | 2 | 7 | 11 | 21 | |
| % with complete Mf clearance (95% CI) | 5% (0, 12) | 17% (6, 29) | 30% (10, 38) | 57% (41, 73) | |
| P-value (χ 2 compared to IA × 3) | <.001 | .426 | .011 | .053 | |
| Risk ratio vs IA × 3 (95% CI) | 1.4 (1.2, 1.8) | 1.1 (.9, 1.4) | 1.5 (1.0, 2.3) | 2.0 (1.0, 4.0) | |
| P-value | .0014 | .316 | .039 | .045 |
| . | . | Months Post-Initiation of Treatment . | . | . | . |
|---|---|---|---|---|---|
| Treatment Group . | Characteristic . | 6 . | 12 . | 24 . | 36 . |
| IVM/ALB (IA) × 3 | No. of participants | 44 | 42 | 39 | 42 |
| Participants with complete Mf clearance | 15 | 11 | 21 | 33 | |
| % with complete Mf clearance (95% CI)a | 34% (20, 48) | 26% (13, 39) | 54% (38, 70) | 79% (67, 91) | |
| ALB 400 mg × 6 | No. of participants | 39 | 36 | 34 | 33 |
| Participants with complete Mf clearance | 1 | 5 | 8 | 16 | |
| % with complete Mf clearance (95% CI) | 3% (0, 8) | 14% (3, 25) | 24% (10, 38) | 48% (32, 66) | |
| P-value (χ 2 compared to IA × 3) | <.001 | .262 | .010 | .008 | |
| Risk ratio vs IA × 3 (95% CI) | 1.5 (1.2, 1.8) | 1.2 (0.9, 1.5) | 1.7 (1.1, 2.4) | 2.4 (1.2, 4.7) | |
| P-value | .0005 | .175 | .011 | .005 | |
| ALB 800 mg × 6 | No. of participants | 40 | 41 | 37 | 37 |
| Participants with complete Mf clearance | 2 | 7 | 11 | 21 | |
| % with complete Mf clearance (95% CI) | 5% (0, 12) | 17% (6, 29) | 30% (10, 38) | 57% (41, 73) | |
| P-value (χ 2 compared to IA × 3) | <.001 | .426 | .011 | .053 | |
| Risk ratio vs IA × 3 (95% CI) | 1.4 (1.2, 1.8) | 1.1 (.9, 1.4) | 1.5 (1.0, 2.3) | 2.0 (1.0, 4.0) | |
| P-value | .0014 | .316 | .039 | .045 |
Abbreviations: ALB, albendazole; CI, confidence interval; IA, ivermectin plus albendazole; IVM, ivermectin; Mf, microfilaria.
aCI denotes using binomial “exact” calculation.
Changes in Mf counts after treatment were assessed by 2 methods: percent reduction compared to baseline and absolute decreases in Mf count adjusting for baseline Mf levels using a generalized estimating equation. With respect to the first analysis, twice-yearly treatment with ALB 400 mg or ALB 800 mg progressively reduced blood Mf levels to achieve 88% (95% confidence interval [CI]: 78, 98) and 89% (95% CI: 79, 99) mean % reductions in Mf counts at 36 months, respectively. These reductions in Mf counts were equivalent between ALB doses (P = .98, .75, .64, and .93 at 6, 12, 24, and 36 months, respectively). By contrast, annual IA treatment reduced Mf levels to a much greater extent at interim points, although percent reductions converged at 36 months for all 3 treatment arms and were not statistically different. The percent reduction in Mf counts from baseline to 36 months for the IA treatment group was 98% (95% CI: 88, 100). Figure 2 shows absolute reductions in Mf counts using model adjusted Mf values. Microfilaria counts decreased rapidly after the first dose of IA, whereas semiannual ALB alone resulted in slower reductions in Mf counts. Reductions in Mf were similar in all 3 treatment arms at 36 months.
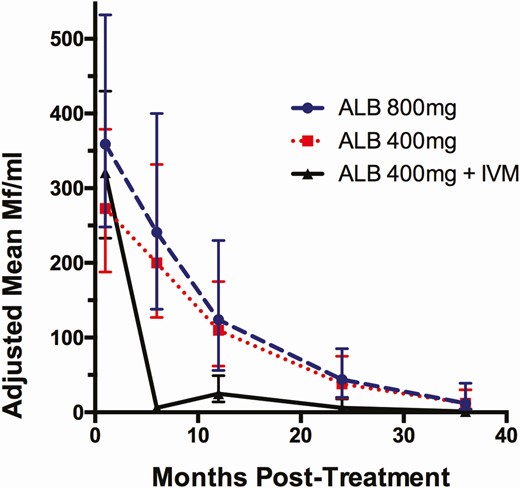
Comparison of adjusted mean Mf counts (95% CI) in the 3 treatment groups using a negative binomial generalized estimating equation. Treatment with IA was superior for reducing Mf counts compared to ALB 400 mg at 6, 12, and 24 but not at 36 months (P < .001, .027, .03, .067, respectively). For ALB 800 mg compared with IA, P-values at the same time points were P < .001, .029, .08, and .064. There were no significant differences at any time point for reductions in Mf counts after ALB 400 mg or ALB 800 mg (see text for P values). Numbers of participants for each time point are shown in Table 2. Abbreviations: ALB, albendazole; CI, confidence interval; IA, ivermectin plus albendazole; Mf, microfilaria.
Effects of Treatment on Circulating Filarial Antigenemia
FTS scores significantly decreased by 44%, 50%, and 42% in IA, ALB 400-mg and ALB 800-mg arms, respectively, by 12 months (Figure 3, P < .001). FTS scores did not decrease further at 24 and 36 months even though participants continued to receive treatment. Few participants completely cleared CFA by 36 months (4/42 [10%] cleared CFA after IA, 2/33 [6%] after ALB 400 mg, and 1/37 [3%] after ALB 800 mg).
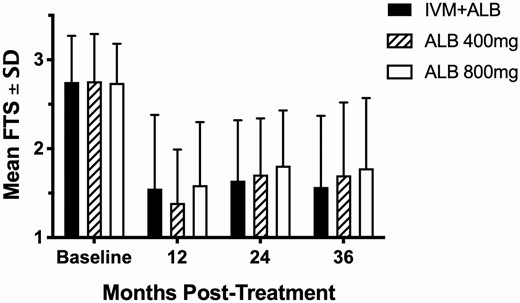
Mean (±SD) FTS score at each time point following treatment. At 12, 24, and 36 months, FTS scores were significantly lower than baseline for all 3 treatment groups (P < .001 by the Mann-Whitney U test) and equivalent across individual time points for each group (P > .2, all time points). Abbreviations: ALB, albendazole; FTS, filariasis test strip; IVM, ivermectin.
Effects of Treatment on Filarial Adult Worm Nests
Prior to treatment, 62 of 118 (53%) male study participants had motile worm nests in scrotal lymphatics as determined by ultrasound, with 34, 11, and 17 men having detectable worms in the IA, ALB 400-mg, and ALB 800-mg groups, respectively (Table 1). Table 3 shows a minority of individuals had complete inactivation of all adult worm nests at all time points that was similar between treatment arms. Of note, after the first 1 or 2 doses of ALB, with or without IVM, no further reduction in complete nest inactivation was observed.
Participants With Inactivation of All Detectable Worm Nests After Treatment
| Treatment Group and Outcomes . | Months After Initial Treatment . | . | . | . |
|---|---|---|---|---|
| . | 6 . | 12 . | 24 . | 36 . |
| IVM/ALB × 3 | ||||
| Participants with worm nests (no.)a | 28 | 28 | 27 | 25 |
| Participants with complete inactivation of all worm nests | 5 | 9 | 11 | 8 |
| % complete inactivation (95% CI) | 18% (4, 30) | 32% (15, 47) | 41% (25, 63) | 32% (14, 50) |
| ALB 400 mg × 6 | ||||
| Participants with worm nests (no.)a | 10 | 9 | 8 | 9 |
| Participants with complete inactivation of all worm nests | 2 | 2 | 1 | 2 |
| % complete inactivation (95% CI) | 20% (0, 45) | 22% (0, 49) | 13% (0, 36) | 22% (0, 49) |
| P-value | 1.0 | .69 | .21 | .69 |
| ALB 800 mg × 6 | ||||
| Participants with worm nests (no.)a | 15 | 14 | 12 | 9 |
| Participants with complete inactivation of all worm nests | 2 | 3 | 2 | 3 |
| % complete inactivation (95% CI) | 13% (0, 30) | 21% (0, 42) | 17% (0, 39) | 33% (5, 65) |
| P-value | 1.00 | .69 | .15 | 1.00 |
| Treatment Group and Outcomes . | Months After Initial Treatment . | . | . | . |
|---|---|---|---|---|
| . | 6 . | 12 . | 24 . | 36 . |
| IVM/ALB × 3 | ||||
| Participants with worm nests (no.)a | 28 | 28 | 27 | 25 |
| Participants with complete inactivation of all worm nests | 5 | 9 | 11 | 8 |
| % complete inactivation (95% CI) | 18% (4, 30) | 32% (15, 47) | 41% (25, 63) | 32% (14, 50) |
| ALB 400 mg × 6 | ||||
| Participants with worm nests (no.)a | 10 | 9 | 8 | 9 |
| Participants with complete inactivation of all worm nests | 2 | 2 | 1 | 2 |
| % complete inactivation (95% CI) | 20% (0, 45) | 22% (0, 49) | 13% (0, 36) | 22% (0, 49) |
| P-value | 1.0 | .69 | .21 | .69 |
| ALB 800 mg × 6 | ||||
| Participants with worm nests (no.)a | 15 | 14 | 12 | 9 |
| Participants with complete inactivation of all worm nests | 2 | 3 | 2 | 3 |
| % complete inactivation (95% CI) | 13% (0, 30) | 21% (0, 42) | 17% (0, 39) | 33% (5, 65) |
| P-value | 1.00 | .69 | .15 | 1.00 |
Abbreviations: ALB, albendazole; CI, confidence interval; IA, ivermectin plus albendazole;
aOnly data from participants with worm nests detected by ultrasound at baseline are included in this table.
Participants With Inactivation of All Detectable Worm Nests After Treatment
| Treatment Group and Outcomes . | Months After Initial Treatment . | . | . | . |
|---|---|---|---|---|
| . | 6 . | 12 . | 24 . | 36 . |
| IVM/ALB × 3 | ||||
| Participants with worm nests (no.)a | 28 | 28 | 27 | 25 |
| Participants with complete inactivation of all worm nests | 5 | 9 | 11 | 8 |
| % complete inactivation (95% CI) | 18% (4, 30) | 32% (15, 47) | 41% (25, 63) | 32% (14, 50) |
| ALB 400 mg × 6 | ||||
| Participants with worm nests (no.)a | 10 | 9 | 8 | 9 |
| Participants with complete inactivation of all worm nests | 2 | 2 | 1 | 2 |
| % complete inactivation (95% CI) | 20% (0, 45) | 22% (0, 49) | 13% (0, 36) | 22% (0, 49) |
| P-value | 1.0 | .69 | .21 | .69 |
| ALB 800 mg × 6 | ||||
| Participants with worm nests (no.)a | 15 | 14 | 12 | 9 |
| Participants with complete inactivation of all worm nests | 2 | 3 | 2 | 3 |
| % complete inactivation (95% CI) | 13% (0, 30) | 21% (0, 42) | 17% (0, 39) | 33% (5, 65) |
| P-value | 1.00 | .69 | .15 | 1.00 |
| Treatment Group and Outcomes . | Months After Initial Treatment . | . | . | . |
|---|---|---|---|---|
| . | 6 . | 12 . | 24 . | 36 . |
| IVM/ALB × 3 | ||||
| Participants with worm nests (no.)a | 28 | 28 | 27 | 25 |
| Participants with complete inactivation of all worm nests | 5 | 9 | 11 | 8 |
| % complete inactivation (95% CI) | 18% (4, 30) | 32% (15, 47) | 41% (25, 63) | 32% (14, 50) |
| ALB 400 mg × 6 | ||||
| Participants with worm nests (no.)a | 10 | 9 | 8 | 9 |
| Participants with complete inactivation of all worm nests | 2 | 2 | 1 | 2 |
| % complete inactivation (95% CI) | 20% (0, 45) | 22% (0, 49) | 13% (0, 36) | 22% (0, 49) |
| P-value | 1.0 | .69 | .21 | .69 |
| ALB 800 mg × 6 | ||||
| Participants with worm nests (no.)a | 15 | 14 | 12 | 9 |
| Participants with complete inactivation of all worm nests | 2 | 3 | 2 | 3 |
| % complete inactivation (95% CI) | 13% (0, 30) | 21% (0, 42) | 17% (0, 39) | 33% (5, 65) |
| P-value | 1.00 | .69 | .15 | 1.00 |
Abbreviations: ALB, albendazole; CI, confidence interval; IA, ivermectin plus albendazole;
aOnly data from participants with worm nests detected by ultrasound at baseline are included in this table.
Treatment with IA and ALB 800 mg significantly reduced the number of detectable worm nests at 12 and 24 months without further reduction by 36 months (Table 4). ALB 400 mg failed to significantly reduce worm nest numbers at any time point. However, ALB 400-mg treatment group had insufficient power to detect reductions in worm nests because relatively few men in that group had worm nests visible at baseline. There was no difference in the reduction in worm nest between treatment arms. The mean diameter of persistently active worm nests and the degree of lymphatic vessel dilatation were not significantly changed at any time point in any arm.
Adjusted Mean Numbers of Worm Nests (95% CI) at Baseline and Following Treatment
| Months Post Initiation of Treatment . | ALB + IVM . | ALB 400 mg alone . | ALB 800 mg alone . |
|---|---|---|---|
| 0 | 2.31 (1.76, 3.03) | 2.14 (1.31, 3.5) | 3.01 (2.1, 4.31) |
| 6 | 2.39 (1.79, 3.19) | 1.84 (1.07, 3.16) | 3.06 (2.11, 4.45) |
| 12 | 1.22 (0.85, 1.77) | 1.69 (0.94, 3.03) | 1.48 (0.92, 2.39) |
| 24 | 1.00 (0.67, 1.51) | 1.64 (0.89, 3.04) | 1.23 (0.71, 2.15) |
| 36 | 1.12 (0.75, 1.67) | 1.9 (1.09, 3.32) | 1.22 (0.65, 2.28) |
| Months Post Initiation of Treatment . | ALB + IVM . | ALB 400 mg alone . | ALB 800 mg alone . |
|---|---|---|---|
| 0 | 2.31 (1.76, 3.03) | 2.14 (1.31, 3.5) | 3.01 (2.1, 4.31) |
| 6 | 2.39 (1.79, 3.19) | 1.84 (1.07, 3.16) | 3.06 (2.11, 4.45) |
| 12 | 1.22 (0.85, 1.77) | 1.69 (0.94, 3.03) | 1.48 (0.92, 2.39) |
| 24 | 1.00 (0.67, 1.51) | 1.64 (0.89, 3.04) | 1.23 (0.71, 2.15) |
| 36 | 1.12 (0.75, 1.67) | 1.9 (1.09, 3.32) | 1.22 (0.65, 2.28) |
Table 4 only includes data from men with detectable worm nests at baseline. There were no significant differences across treatment arms (P = .3 or greater at each time point). There was a significant decrease in worm nests in the IA and ALB 800-mg treatment arms between baseline and 12, 24, and 36 months post initiation of treatment (P = .002, P < .001 and P < .001 for IA, respectively, and P = .005, P = .002, and P = .006 for ALB 800 mg) but not ALB 400 mg (P = .5, P = .4, and P = .7). A negative binomial generalized estimating equation model that included random effects for subjects was used to calculate means, confidence intervals, and P-value.
Abbreviations: ALB, albendazole; CI, confidence interval; IVM, ivermectin.
Adjusted Mean Numbers of Worm Nests (95% CI) at Baseline and Following Treatment
| Months Post Initiation of Treatment . | ALB + IVM . | ALB 400 mg alone . | ALB 800 mg alone . |
|---|---|---|---|
| 0 | 2.31 (1.76, 3.03) | 2.14 (1.31, 3.5) | 3.01 (2.1, 4.31) |
| 6 | 2.39 (1.79, 3.19) | 1.84 (1.07, 3.16) | 3.06 (2.11, 4.45) |
| 12 | 1.22 (0.85, 1.77) | 1.69 (0.94, 3.03) | 1.48 (0.92, 2.39) |
| 24 | 1.00 (0.67, 1.51) | 1.64 (0.89, 3.04) | 1.23 (0.71, 2.15) |
| 36 | 1.12 (0.75, 1.67) | 1.9 (1.09, 3.32) | 1.22 (0.65, 2.28) |
| Months Post Initiation of Treatment . | ALB + IVM . | ALB 400 mg alone . | ALB 800 mg alone . |
|---|---|---|---|
| 0 | 2.31 (1.76, 3.03) | 2.14 (1.31, 3.5) | 3.01 (2.1, 4.31) |
| 6 | 2.39 (1.79, 3.19) | 1.84 (1.07, 3.16) | 3.06 (2.11, 4.45) |
| 12 | 1.22 (0.85, 1.77) | 1.69 (0.94, 3.03) | 1.48 (0.92, 2.39) |
| 24 | 1.00 (0.67, 1.51) | 1.64 (0.89, 3.04) | 1.23 (0.71, 2.15) |
| 36 | 1.12 (0.75, 1.67) | 1.9 (1.09, 3.32) | 1.22 (0.65, 2.28) |
Table 4 only includes data from men with detectable worm nests at baseline. There were no significant differences across treatment arms (P = .3 or greater at each time point). There was a significant decrease in worm nests in the IA and ALB 800-mg treatment arms between baseline and 12, 24, and 36 months post initiation of treatment (P = .002, P < .001 and P < .001 for IA, respectively, and P = .005, P = .002, and P = .006 for ALB 800 mg) but not ALB 400 mg (P = .5, P = .4, and P = .7). A negative binomial generalized estimating equation model that included random effects for subjects was used to calculate means, confidence intervals, and P-value.
Abbreviations: ALB, albendazole; CI, confidence interval; IVM, ivermectin.
Presence of Microfilaria and Circulating Filarial antigen 48 Months After Treatment
To assess potential sustained effects of six rounds of ALB, we examined Mf levels and CFA positivity at 48 months (ie, 18 months after the last ALB treatment, Table 6) among individuals willing to participate in the ALB alone treatment arms. The participants in the ALB + IVM arm were not examined. Among the individuals examined at 48 months, Mf clearance increased from 46% at 36 months to 79% at 48 months who had received ALB 400 mg and from 48% to 78%, respectively, in those treated with ALB 800 mg. All but one participant in both arms who was Mf-negative at 36 months remained negative at 48 months. With respect to CFA, 23/24 (96%) individuals in the ALB 400-mg group were positive at 36 months and 15/24 (63%) at 48 months. In ALB 800-mg arm 26/27 (96%) were CFA positive at 36 months and 21/27 (78%) at 48 months.
Adverse Events Following Treatment
One hundred thirty-three individuals (92%) were evaluated for treatment-associated AEs 24 hours after treatment (Table 5). Most AEs were mild (grade 1) and were more common in the IA arm compared to ALB 400-mg or ALB 800-mg arms. Moderate (grade 2) AEs occurred infrequently, and rates did not differ by treatment group. Headache, fatigue, and muscle aches were the most common AEs reported. There were no severe or serious AEs. Post-treatment fevers and changes in vital signs were uncommon and did not differ between groups. There were no moderate AEs, and few mild AEs were reported between days 2 and 7 post-treatment.
| . | IA . | ALB 400 mg . | ALB 800 mg . |
|---|---|---|---|
| . | (N = 48) . | (N = 43) . | (N = 42) . |
| At least 1 AE (%) | 19 (39.6) | 7 (16.3)** | 11 (26.2) |
| Individuals with 2 or more AEs (%) | 7 (14.6) | 2 (4.7) | 1 (2.4) |
| Severe or serious AEs | 0 | 0 | 0 |
| Fever ≥ 38°C (%) | 3 (6.3) | 1 (2.3) | 0 |
| Hemodynamic changes | 1 | 2 | 1 |
| Overall grade 1 AEs (total) | 32 | 7* | 12* |
| Overall grade 1 AEs (subjective only) | 20 | 5 | 9 |
| Overall grade 2 AEs (total)a | 5 | 2 | 0 |
| Grade 2 fever ≥ 38.5°C | 2 (4.2) | 1 (2.3) | 0 |
| Grade 2 fatigue | 0 | 1 (2.3) | 0 |
| Grade 2 nausea/vomiting | 1 (2.1) | 0 | 0 |
| Grade 2 muscle ache | 1 2.1) | 0 | 0 |
| Grade 2 lightheaded | 1 (2.1) | 0 | 0 |
| . | IA . | ALB 400 mg . | ALB 800 mg . |
|---|---|---|---|
| . | (N = 48) . | (N = 43) . | (N = 42) . |
| At least 1 AE (%) | 19 (39.6) | 7 (16.3)** | 11 (26.2) |
| Individuals with 2 or more AEs (%) | 7 (14.6) | 2 (4.7) | 1 (2.4) |
| Severe or serious AEs | 0 | 0 | 0 |
| Fever ≥ 38°C (%) | 3 (6.3) | 1 (2.3) | 0 |
| Hemodynamic changes | 1 | 2 | 1 |
| Overall grade 1 AEs (total) | 32 | 7* | 12* |
| Overall grade 1 AEs (subjective only) | 20 | 5 | 9 |
| Overall grade 2 AEs (total)a | 5 | 2 | 0 |
| Grade 2 fever ≥ 38.5°C | 2 (4.2) | 1 (2.3) | 0 |
| Grade 2 fatigue | 0 | 1 (2.3) | 0 |
| Grade 2 nausea/vomiting | 1 (2.1) | 0 | 0 |
| Grade 2 muscle ache | 1 2.1) | 0 | 0 |
| Grade 2 lightheaded | 1 (2.1) | 0 | 0 |
Abbreviations: ALB, albendazole; CI, confidence interval; IA, ivermectin plus albendazole; IVM, ivermectin; Mf, microfilaria.
aCompared to pretreatment clinical assessments, any new or worsening symptoms, changes in vital signs or new abnormal physical examination findings were considered to be drug-related AEs.
*P < .05 and **P < .01 compared to IA arm.
¶4 individuals had 1 grade 2 event, 1 individual had 3 grade 2 events.
| . | IA . | ALB 400 mg . | ALB 800 mg . |
|---|---|---|---|
| . | (N = 48) . | (N = 43) . | (N = 42) . |
| At least 1 AE (%) | 19 (39.6) | 7 (16.3)** | 11 (26.2) |
| Individuals with 2 or more AEs (%) | 7 (14.6) | 2 (4.7) | 1 (2.4) |
| Severe or serious AEs | 0 | 0 | 0 |
| Fever ≥ 38°C (%) | 3 (6.3) | 1 (2.3) | 0 |
| Hemodynamic changes | 1 | 2 | 1 |
| Overall grade 1 AEs (total) | 32 | 7* | 12* |
| Overall grade 1 AEs (subjective only) | 20 | 5 | 9 |
| Overall grade 2 AEs (total)a | 5 | 2 | 0 |
| Grade 2 fever ≥ 38.5°C | 2 (4.2) | 1 (2.3) | 0 |
| Grade 2 fatigue | 0 | 1 (2.3) | 0 |
| Grade 2 nausea/vomiting | 1 (2.1) | 0 | 0 |
| Grade 2 muscle ache | 1 2.1) | 0 | 0 |
| Grade 2 lightheaded | 1 (2.1) | 0 | 0 |
| . | IA . | ALB 400 mg . | ALB 800 mg . |
|---|---|---|---|
| . | (N = 48) . | (N = 43) . | (N = 42) . |
| At least 1 AE (%) | 19 (39.6) | 7 (16.3)** | 11 (26.2) |
| Individuals with 2 or more AEs (%) | 7 (14.6) | 2 (4.7) | 1 (2.4) |
| Severe or serious AEs | 0 | 0 | 0 |
| Fever ≥ 38°C (%) | 3 (6.3) | 1 (2.3) | 0 |
| Hemodynamic changes | 1 | 2 | 1 |
| Overall grade 1 AEs (total) | 32 | 7* | 12* |
| Overall grade 1 AEs (subjective only) | 20 | 5 | 9 |
| Overall grade 2 AEs (total)a | 5 | 2 | 0 |
| Grade 2 fever ≥ 38.5°C | 2 (4.2) | 1 (2.3) | 0 |
| Grade 2 fatigue | 0 | 1 (2.3) | 0 |
| Grade 2 nausea/vomiting | 1 (2.1) | 0 | 0 |
| Grade 2 muscle ache | 1 2.1) | 0 | 0 |
| Grade 2 lightheaded | 1 (2.1) | 0 | 0 |
Abbreviations: ALB, albendazole; CI, confidence interval; IA, ivermectin plus albendazole; IVM, ivermectin; Mf, microfilaria.
aCompared to pretreatment clinical assessments, any new or worsening symptoms, changes in vital signs or new abnormal physical examination findings were considered to be drug-related AEs.
*P < .05 and **P < .01 compared to IA arm.
¶4 individuals had 1 grade 2 event, 1 individual had 3 grade 2 events.
Microfilaria (Mf) Levels and Circulating Filarial Antigen (CFA) Positivity Changes from 36 to 48 Months After Initiation of Treatment
| Albendazole 400-mg arm . | . | . | . | . | . |
|---|---|---|---|---|---|
| . | Baseline Mf/mL . | 36 months Mf/mL . | 48 months Mf/mL . | CFAa (36 months) . | CFAa (48 months) . |
| 332 | 465 | 55 | 2 | 2 | |
| 529 | 282 | 113 | 3 | 3 | |
| 215 | 250 | 0 | 2 | 3 | |
| 968 | 193 | 221 | 3 | 2 | |
| 95 | 119 | 0 | 2 | 1 | |
| 391 | 32 | 0 | 2 | 0 | |
| 106 | 16 | 5 | 3 | 3 | |
| 944 | 14 | 0 | 2 | 1 | |
| 274 | 11 | 10 | 2 | 2 | |
| 83 | 1 | 0 | 2 | 1 | |
| 337 | 1 | 0 | 2 | 1 | |
| 274 | 1 | 0 | 2 | 1 | |
| 171 | 1 | 0 | 2 | 2 | |
| 222 | 0 | 0 | 2 | 0 | |
| 79 | 0 | 0 | 1 | 1 | |
| 109 | 0 | 0 | 0 | 0 | |
| 614 | 0 | 0 | 3 | 2 | |
| 209 | 0 | 0 | 3 | 0 | |
| 55 | 0 | 0 | 1 | 0 | |
| 644 | 0 | 0 | 1 | 0 | |
| 210 | 0 | 0 | 2 | 1 | |
| 76 | 0 | 0 | 1 | 0 | |
| 203 | 0 | 0 | 1 | 0 | |
| 132 | 0 | 0 | 2 | 0 | |
| N negative (% cleared) | 11/24 (46%) | 19/24 (79%) | 1/24 (4%) | 9/24 (38%) | |
| Albendazole 800-mg arm | |||||
| Baseline Mf/mL | 36 months Mf/mL | 48 months Mf/mL | CFAa (36 months) | CFAa (48 months) | |
| 2050 | 611 | 13 | 3 | 3 | |
| 976 | 268 | 33 | 3 | 3 | |
| 177 | 86 | 44 | 3 | 2 | |
| 518 | 63 | 0 | 3 | 2 | |
| 860 | 62 | 97 | 2 | 3 | |
| 519 | 33 | 5 | 1 | 2 | |
| 86 | 29 | 0 | 1 | 2 | |
| 168 | 28 | 0 | 1 | 1 | |
| 221 | 22 | 0 | 3 | 3 | |
| 70 | 7 | 0 | 2 | 1 | |
| 445 | 7 | 0 | 3 | 3 | |
| 97 | 6 | 0 | 1 | 0 | |
| 91 | 2 | 0 | 1 | 0 | |
| 61 | 1 | 0 | 2 | 1 | |
| 1050 | 0 | 0 | 2 | 1 | |
| 470 | 0 | 0 | 1 | 1 | |
| 70 | 0 | 0 | 1 | 0 | |
| 307 | 0 | 0 | 3 | 0 | |
| 116 | 0 | 4 | 0 | 1 | |
| 70 | 0 | 0 | 2 | 2 | |
| 110 | 0 | 0 | 2 | 1 | |
| 59 | 0 | 0 | 1 | 1 | |
| 575 | 0 | 0 | 1 | 0 | |
| 89 | 0 | 0 | 1 | 0 | |
| 74 | 0 | 0 | 2 | 1 | |
| 73 | 0 | 0 | 1 | 1 | |
| 210 | 0 | 0 | 1 | 1 | |
| N negative (% cleared) | 13/27 (48%) | 21/27 (78%) | 1/27 (4%) | 6/27 (22%) |
| Albendazole 400-mg arm . | . | . | . | . | . |
|---|---|---|---|---|---|
| . | Baseline Mf/mL . | 36 months Mf/mL . | 48 months Mf/mL . | CFAa (36 months) . | CFAa (48 months) . |
| 332 | 465 | 55 | 2 | 2 | |
| 529 | 282 | 113 | 3 | 3 | |
| 215 | 250 | 0 | 2 | 3 | |
| 968 | 193 | 221 | 3 | 2 | |
| 95 | 119 | 0 | 2 | 1 | |
| 391 | 32 | 0 | 2 | 0 | |
| 106 | 16 | 5 | 3 | 3 | |
| 944 | 14 | 0 | 2 | 1 | |
| 274 | 11 | 10 | 2 | 2 | |
| 83 | 1 | 0 | 2 | 1 | |
| 337 | 1 | 0 | 2 | 1 | |
| 274 | 1 | 0 | 2 | 1 | |
| 171 | 1 | 0 | 2 | 2 | |
| 222 | 0 | 0 | 2 | 0 | |
| 79 | 0 | 0 | 1 | 1 | |
| 109 | 0 | 0 | 0 | 0 | |
| 614 | 0 | 0 | 3 | 2 | |
| 209 | 0 | 0 | 3 | 0 | |
| 55 | 0 | 0 | 1 | 0 | |
| 644 | 0 | 0 | 1 | 0 | |
| 210 | 0 | 0 | 2 | 1 | |
| 76 | 0 | 0 | 1 | 0 | |
| 203 | 0 | 0 | 1 | 0 | |
| 132 | 0 | 0 | 2 | 0 | |
| N negative (% cleared) | 11/24 (46%) | 19/24 (79%) | 1/24 (4%) | 9/24 (38%) | |
| Albendazole 800-mg arm | |||||
| Baseline Mf/mL | 36 months Mf/mL | 48 months Mf/mL | CFAa (36 months) | CFAa (48 months) | |
| 2050 | 611 | 13 | 3 | 3 | |
| 976 | 268 | 33 | 3 | 3 | |
| 177 | 86 | 44 | 3 | 2 | |
| 518 | 63 | 0 | 3 | 2 | |
| 860 | 62 | 97 | 2 | 3 | |
| 519 | 33 | 5 | 1 | 2 | |
| 86 | 29 | 0 | 1 | 2 | |
| 168 | 28 | 0 | 1 | 1 | |
| 221 | 22 | 0 | 3 | 3 | |
| 70 | 7 | 0 | 2 | 1 | |
| 445 | 7 | 0 | 3 | 3 | |
| 97 | 6 | 0 | 1 | 0 | |
| 91 | 2 | 0 | 1 | 0 | |
| 61 | 1 | 0 | 2 | 1 | |
| 1050 | 0 | 0 | 2 | 1 | |
| 470 | 0 | 0 | 1 | 1 | |
| 70 | 0 | 0 | 1 | 0 | |
| 307 | 0 | 0 | 3 | 0 | |
| 116 | 0 | 4 | 0 | 1 | |
| 70 | 0 | 0 | 2 | 2 | |
| 110 | 0 | 0 | 2 | 1 | |
| 59 | 0 | 0 | 1 | 1 | |
| 575 | 0 | 0 | 1 | 0 | |
| 89 | 0 | 0 | 1 | 0 | |
| 74 | 0 | 0 | 2 | 1 | |
| 73 | 0 | 0 | 1 | 1 | |
| 210 | 0 | 0 | 1 | 1 | |
| N negative (% cleared) | 13/27 (48%) | 21/27 (78%) | 1/27 (4%) | 6/27 (22%) |
aCFA is measured using the semi-quantitative filarial test strip (FTS) described in the materials and methods section.
Microfilaria (Mf) Levels and Circulating Filarial Antigen (CFA) Positivity Changes from 36 to 48 Months After Initiation of Treatment
| Albendazole 400-mg arm . | . | . | . | . | . |
|---|---|---|---|---|---|
| . | Baseline Mf/mL . | 36 months Mf/mL . | 48 months Mf/mL . | CFAa (36 months) . | CFAa (48 months) . |
| 332 | 465 | 55 | 2 | 2 | |
| 529 | 282 | 113 | 3 | 3 | |
| 215 | 250 | 0 | 2 | 3 | |
| 968 | 193 | 221 | 3 | 2 | |
| 95 | 119 | 0 | 2 | 1 | |
| 391 | 32 | 0 | 2 | 0 | |
| 106 | 16 | 5 | 3 | 3 | |
| 944 | 14 | 0 | 2 | 1 | |
| 274 | 11 | 10 | 2 | 2 | |
| 83 | 1 | 0 | 2 | 1 | |
| 337 | 1 | 0 | 2 | 1 | |
| 274 | 1 | 0 | 2 | 1 | |
| 171 | 1 | 0 | 2 | 2 | |
| 222 | 0 | 0 | 2 | 0 | |
| 79 | 0 | 0 | 1 | 1 | |
| 109 | 0 | 0 | 0 | 0 | |
| 614 | 0 | 0 | 3 | 2 | |
| 209 | 0 | 0 | 3 | 0 | |
| 55 | 0 | 0 | 1 | 0 | |
| 644 | 0 | 0 | 1 | 0 | |
| 210 | 0 | 0 | 2 | 1 | |
| 76 | 0 | 0 | 1 | 0 | |
| 203 | 0 | 0 | 1 | 0 | |
| 132 | 0 | 0 | 2 | 0 | |
| N negative (% cleared) | 11/24 (46%) | 19/24 (79%) | 1/24 (4%) | 9/24 (38%) | |
| Albendazole 800-mg arm | |||||
| Baseline Mf/mL | 36 months Mf/mL | 48 months Mf/mL | CFAa (36 months) | CFAa (48 months) | |
| 2050 | 611 | 13 | 3 | 3 | |
| 976 | 268 | 33 | 3 | 3 | |
| 177 | 86 | 44 | 3 | 2 | |
| 518 | 63 | 0 | 3 | 2 | |
| 860 | 62 | 97 | 2 | 3 | |
| 519 | 33 | 5 | 1 | 2 | |
| 86 | 29 | 0 | 1 | 2 | |
| 168 | 28 | 0 | 1 | 1 | |
| 221 | 22 | 0 | 3 | 3 | |
| 70 | 7 | 0 | 2 | 1 | |
| 445 | 7 | 0 | 3 | 3 | |
| 97 | 6 | 0 | 1 | 0 | |
| 91 | 2 | 0 | 1 | 0 | |
| 61 | 1 | 0 | 2 | 1 | |
| 1050 | 0 | 0 | 2 | 1 | |
| 470 | 0 | 0 | 1 | 1 | |
| 70 | 0 | 0 | 1 | 0 | |
| 307 | 0 | 0 | 3 | 0 | |
| 116 | 0 | 4 | 0 | 1 | |
| 70 | 0 | 0 | 2 | 2 | |
| 110 | 0 | 0 | 2 | 1 | |
| 59 | 0 | 0 | 1 | 1 | |
| 575 | 0 | 0 | 1 | 0 | |
| 89 | 0 | 0 | 1 | 0 | |
| 74 | 0 | 0 | 2 | 1 | |
| 73 | 0 | 0 | 1 | 1 | |
| 210 | 0 | 0 | 1 | 1 | |
| N negative (% cleared) | 13/27 (48%) | 21/27 (78%) | 1/27 (4%) | 6/27 (22%) |
| Albendazole 400-mg arm . | . | . | . | . | . |
|---|---|---|---|---|---|
| . | Baseline Mf/mL . | 36 months Mf/mL . | 48 months Mf/mL . | CFAa (36 months) . | CFAa (48 months) . |
| 332 | 465 | 55 | 2 | 2 | |
| 529 | 282 | 113 | 3 | 3 | |
| 215 | 250 | 0 | 2 | 3 | |
| 968 | 193 | 221 | 3 | 2 | |
| 95 | 119 | 0 | 2 | 1 | |
| 391 | 32 | 0 | 2 | 0 | |
| 106 | 16 | 5 | 3 | 3 | |
| 944 | 14 | 0 | 2 | 1 | |
| 274 | 11 | 10 | 2 | 2 | |
| 83 | 1 | 0 | 2 | 1 | |
| 337 | 1 | 0 | 2 | 1 | |
| 274 | 1 | 0 | 2 | 1 | |
| 171 | 1 | 0 | 2 | 2 | |
| 222 | 0 | 0 | 2 | 0 | |
| 79 | 0 | 0 | 1 | 1 | |
| 109 | 0 | 0 | 0 | 0 | |
| 614 | 0 | 0 | 3 | 2 | |
| 209 | 0 | 0 | 3 | 0 | |
| 55 | 0 | 0 | 1 | 0 | |
| 644 | 0 | 0 | 1 | 0 | |
| 210 | 0 | 0 | 2 | 1 | |
| 76 | 0 | 0 | 1 | 0 | |
| 203 | 0 | 0 | 1 | 0 | |
| 132 | 0 | 0 | 2 | 0 | |
| N negative (% cleared) | 11/24 (46%) | 19/24 (79%) | 1/24 (4%) | 9/24 (38%) | |
| Albendazole 800-mg arm | |||||
| Baseline Mf/mL | 36 months Mf/mL | 48 months Mf/mL | CFAa (36 months) | CFAa (48 months) | |
| 2050 | 611 | 13 | 3 | 3 | |
| 976 | 268 | 33 | 3 | 3 | |
| 177 | 86 | 44 | 3 | 2 | |
| 518 | 63 | 0 | 3 | 2 | |
| 860 | 62 | 97 | 2 | 3 | |
| 519 | 33 | 5 | 1 | 2 | |
| 86 | 29 | 0 | 1 | 2 | |
| 168 | 28 | 0 | 1 | 1 | |
| 221 | 22 | 0 | 3 | 3 | |
| 70 | 7 | 0 | 2 | 1 | |
| 445 | 7 | 0 | 3 | 3 | |
| 97 | 6 | 0 | 1 | 0 | |
| 91 | 2 | 0 | 1 | 0 | |
| 61 | 1 | 0 | 2 | 1 | |
| 1050 | 0 | 0 | 2 | 1 | |
| 470 | 0 | 0 | 1 | 1 | |
| 70 | 0 | 0 | 1 | 0 | |
| 307 | 0 | 0 | 3 | 0 | |
| 116 | 0 | 4 | 0 | 1 | |
| 70 | 0 | 0 | 2 | 2 | |
| 110 | 0 | 0 | 2 | 1 | |
| 59 | 0 | 0 | 1 | 1 | |
| 575 | 0 | 0 | 1 | 0 | |
| 89 | 0 | 0 | 1 | 0 | |
| 74 | 0 | 0 | 2 | 1 | |
| 73 | 0 | 0 | 1 | 1 | |
| 210 | 0 | 0 | 1 | 1 | |
| N negative (% cleared) | 13/27 (48%) | 21/27 (78%) | 1/27 (4%) | 6/27 (22%) |
aCFA is measured using the semi-quantitative filarial test strip (FTS) described in the materials and methods section.
Discussion
This randomized clinical trial demonstrated that 6 semiannual rounds of ALB alone is safe and effective for treatment bancroftian filariasis. At 36 months after initiation of treatment, Mf clearance was similar to the standard treatment with 3 annual rounds of ivermectin plus ALB. Our data support the WHO recommendation for using ALB alone to treat LF in areas coendemic for L. loa. Albendazole alone demonstrated a macrofilaricidal effect based on reductions in FTS scores and adult worm nests visible by ultrasound. The delayed decline in Mf counts after ALB treatment is consistent with a pure macrofilaricidal (or adult worm sterilizing) effect without a direct effect of ALB on Mf. Albendazole effects on microfilaremia were dose and time-dependent and clearly inferior to IA until the 36 months timepoint. In spite of ALBs inferior performance compared to IA for complete Mf clearance at 36 months, ALB reduced Mf counts by 88–89% relative to baseline values, and those reductions were comparable to that achieved after annual administration of IA [5, 19]. ALB 800 mg provided no additional efficacy compared to ALB 400 mg for reducing blood Mf levels.
Treatment with ALB alone decreased CFA and inactivated adult worm nests, consistent with partial killing of adult worms [20, 21]. This macrofilaricidal effect was obvious at 12 months (6 months after the second dose of ALB). Additional doses of ALB produced no further decrease in CFA and decline in adult worm nests by 36 months. This suggests that a subset of adult worms may be relatively resistant to ALB. Progressive reductions in Mf counts over time suggest that albendazole’s embryotoxic effect may be more potent than its macrofilaricidal effect. The embryotoxic effect may be related to inhibition of beta-tubulin polymerization required for cell division in embryos. It is interesting that the effects of ALB were not fully reflected by Mf and FTS results at 36 months. Reexamination of many participants in the ALB alone arms at 48 months (ie, 18 months after the last ALB dose) showed further reductions in Mf counts with complete Mf and CFA clearance in many participants. Thus, the embryotoxic and macrofilaricidal effects of ALB are greatly delayed compared to the microfilaricidal effect of ivermectin.
Prior studies have shown ALB alone to be macrofilaricidal for W. bancrofti when it was given at higher doses and with a longer treatment course. Twice daily dosing of 400 mg for 3 weeks killed W. bancrofti adult worms, and this reduced Mf counts later. However, the high frequency of AEs observed and the prolonged treatment would be unacceptable for MDA [22, 23]. Another study showed that daily ALB 400 mg for 7 days was well tolerated and produced an 89% reduction in Mf levels relative to baseline 1 year later [24], but this would be difficult to implement for MDA. In contrast, single dose of ALB 400-mg treatment produced only slight reductions in Mf and CFA levels at 4 months [25, 26] and at later time points after treatment [27–29]. These results suggest ALB provides a comparatively small contribution to early Mf clearance in annual MDA programs when it is combined with IVM or DEC [14]. However, the effects of ALB on adult worms (and secondarily on Mf) are more potent when the drug is provided as part of repeated rounds of MDA. A community-based MDA program in the Republic of the Congo, where ALB 400 mg was administered twice yearly over a course of 3 years, showed that 94% of individuals cleared Mf, and there was a 73% reduction in CFA prevalence [13]. A reanalysis of these data along with results from a similar study in the Democratic Republic of the Congo showed that individuals who took more doses of ALB showed greater Mf clearance [12, 30]. Results from the clinical trial reported here, which also used semiannual ALB, support results from the community-based studies with similar reductions in Mf counts and FTS scores over a similar time period.
A study limitation was that it was open-labeled, which could have biased AE reporting. However, staff evaluating AEs were masked regarding treatment allocation, and AE rates were low in all treatment groups. Another study limitation was that most participants were men. This was an advantage for detection of worm nests, but drug efficacy could differ in females. However, prior pharmacodynamic studies of ALB in combination with DEC and IVM indicated that sexual differences in ALB absorption and efficacy are not significant [31]. A third limitation is that the primary endpoint for this study was complete Mf clearance at 36 months. Follow-up data from 48 months suggest that efficacy observed at 36 months in ALB treatment arms was sustained and improved in many participants by 48 months without additional treatment.
These results show semiannual ALB is a safe and effective treatment for LF. It clears Mf more slowly than IA, but its efficacy approached that of annual IA at 36 months. Our results support the WHO’s recommendation to use semiannual ALB to eliminate LF in areas with significant L. loa coendemicity [8], where LF elimination efforts have lagged because of loiasis.
Notes
Author contributions. All authors contributed substantially to this article. C. K., G. W., and B. K. were responsible for study design, and C. K., C. B., and B. A. performed literature searches. Data were collected by C. B., A. O., O. K., V. M., and B. A.; data were analyzed and interpreted by C. B., A. O., M. A., G. W., B. K., C. G., and C. K. The manuscript was written by A. O., C. B., M. A., B. A., G. W., B. K., and C. K. All authors approved of the final draft.
Acknowledgments. We gratefully acknowledge participation and cooperation of the study participants and the project staff in Cote d’Ivoire who provided essential help with community engagement and the conduct of the study.
Financial support. This work was supported by the Bill and Melinda Gates Foundation (grant number OPPGH 5342). The funders had no role in the study design, data collection, data analysis, or interpretation of data; in the writing of the manuscript; or in the decision to submit for publication. The corresponding author had full access to all study data and had final responsibility for the decision to publish.
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
Author notes
A. F. O. and C. M. B. are joint first authors.




