-
PDF
- Split View
-
Views
-
Cite
Cite
Edward K. Thomsen, Nelly Sanuku, Manasseh Baea, Samson Satofan, Elit Maki, Bart Lombore, Mark S. Schmidt, Peter M. Siba, Gary J. Weil, James W. Kazura, Lawrence L. Fleckenstein, Christopher L. King, Efficacy, Safety, and Pharmacokinetics of Coadministered Diethylcarbamazine, Albendazole, and Ivermectin for Treatment of Bancroftian Filariasis, Clinical Infectious Diseases, Volume 62, Issue 3, 1 February 2016, Pages 334–341, https://doi.org/10.1093/cid/civ882
Close - Share Icon Share
Abstract
Background. Available treatments for lymphatic filariasis (LF) are limited in their longterm clearance of microfilaria from the blood. The safety and efficacy of a single-dose triple-drug therapy of the antifilarial drugs diethylcarbamazine (DEC), ivermectin (IVM), and albendazole (ALB) for LF are unknown.
Methods. We performed a pilot study to test the efficacy, safety, and pharmacokinetics of single-dose DEC, IVM, and ALB in Wuchereria bancrofti-infected Papua New Guineans. Adults were randomized into 2 treatment arms, DEC 6 mg/kg + ALB 400 mg (N = 12) or DEC 6 mg/kg + ALB 400 mg + IVM 200 μg/kg (N = 12), and monitored for microfilaria, parasite antigenemia, adverse events (AEs), and serum drug levels.
Results. Triple-drug therapy induced >2-log reductions in microfilaria levels at 36 and 168 hours after treatment compared with approximately 1-log reduction with 2 drugs. All 12 individuals who received 3 drugs were microfilaria negative 1 year after treatment, whereas 11 of 12 individuals in the 2-drug regimen were microfilaria positive. In 6 participants followed 2 years after treatment, those who received 3 drugs remained microfilaria negative. AEs, particularly fever, myalgias, pruritus, and proteinuria/hematuria, occurred in 83% vs 50% of those receiving triple-drug compared to 2-drug treatment respectively (P = .021); all resolved within 7 days after treatment. No serious AEs were observed in either group. There was no significant effect of IVM on DEC or ALB drug levels.
Conclusions. Triple-drug therapy is safe and more effective than DEC + ALB for Bancroftian filariasis and has the potential to accelerate elimination of lymphatic filariasis.
Clinical Trials Registration. NCT01975441.
Wuchereria bancrofti is a mosquito-transmitted, chronically disabling nematode infection that causes lymphedema, elephantiasis, and hydroceles. Wuchereria bancrofti is endemic in 73 countries, infecting approximately 100 million people [1]. The World Health Organization has targeted lymphatic filariasis (LF) for global elimination by 2020 [2]. Since there is no drug that reliably kills or sterilizes adult filarial worms, the focus of the Global Programme to Eliminate Lymphatic Filariasis (GPELF) has been on the use of mass drug administration (MDA) to reduce the source of microfilaria in endemic populations and thereby interrupt transmission. The current MDA strategy is to provide repeated, annual doses of albendazole (ALB) with either diethylcarbamazine (DEC) or ivermectin (IVM) for the lifespan of adult worms (typically 5–7 years) [3]. Mathematical models of LF transmission suggest that the most potent drug combination currently recommended (annual DEC + ALB) will require high compliance (>70%) with MDA for 5–7 years in order to achieve elimination targets, particularly in areas with moderate to high endemicity [4]. Clinical trials have shown that a single dose of this combination clears microfilaria in only approximately 25% of participants at 12 months [5–8]. Drug regimens with better activity against microfilaria would prevent new infections that would otherwise have to be treated in later years. This could significantly improve the chances for eliminating LF in resource poor settings.
Addition of the potent microfilaricide IVM to DEC +ALB may improve microfilaria clearance and provide a more long-lasting effect than the widely used 2-drug regimen [7–9]. Prior community studies have shown that MDA with IVM plus DEC was more effective for reducing microfilaria rates than DEC alone [10]. A single dose of IVM completely cleared microfilaria in 35% of participants and reduced the geometric mean microfilaria level by >98% at 1 year; 2 years after a single treatment, 20% of participants remained microfilaria negative, and geometric mean microfilaria levels remained reduced by >90% [11]. Similar but less profound effects were observed for single-dose IVM in a trial conducted in Tanzania [12]. It is possible that simultaneous treatment with IVM + DEC + ALB could reduce the number of rounds of MDA required to reach LF elimination, and this could have a transformative impact on the 60% of LF-exposed populations that reside outside of sub-Saharan Africa.
Since the tolerability and potential drug interactions of this 3-drug combination have never been investigated, we undertook a pilot study to compare the safety, tolerability, pharmacokinetics, and efficacy of IVM + DEC + ALB vs DEC + ALB in individuals with Bancroftian filariasis.
METHODS
Study Location
Participants were recruited from the villages of Tau 1 and 2 (3.6718 S,142.7254 E), Dreikikir District, in East Sepik Province, Papua New Guinea, with microfilaria rates of 10%–44%. No prior treatment for LF has ever been given in these 2 communities. All participants where hospitalized at the Dreikikir Health Center for the initial days following treatment.
Selection of Study Participants and Study Design
Study inclusion and exclusion criteria were as follows: aged 18–60 years, >100 microfilaria /mL, no prior antifilarial medications, or free from any acute or chronic illnesses. If inclusion and exclusion criteria were fulfilled, enrolled individuals had a 1-mL venous night blood sample (after 2200 hours) and serum biochemistries measured to ensure that alanine transaminase (ALT), aspartate transaminase (AST), and creatinine were <1.5 times normal; hemoglobin levels were >7 gm/dL; and there was no significant urine proteinuria, hematuria, or glucosuria by dipstick measurement 1 to 2 weeks prior to hospitalization (Figure 1). A total of 903 individuals were prescreened, 53 were enrolled, and 24 met the final inclusion and exclusion criteria and agreed to participate in the study. One individual withdrew 72 hours after treatment and 2 individuals failed to return on day 7 but did have blood drawn 1 year later. Eleven individuals were unavailable at the 2-year follow-up (Figure 1). This was a single-blinded, parallel-group, randomized study with 2 treatment arms. Participants were stratified by sex and randomly assigned to 1 of 2 treatment groups: DEC 6 mg/kg + ALB 400 mg or DEC 6 mg/kg + IVM 200 μg/kg + ALB 400 mg. A blood sample was taken to establish baseline ALT, AST, and creatinine levels immediately prior to treatment. Participants were given a breakfast of peanut butter and biscuits prior to observed administration of drugs. Blood draws were performed at 0, 1, 2, 3, 4, 6, 8, 12, 24, 36, 48, and 72 hours and 7 days following experimental drug treatment (Figure 1).

Study design. Abbreviations: ALB, albendazole; ALT, alanine transaminase; AST, aspartate transaminase; BP, blood pressure; Cr, creatinine; DEC, diethylcarbamazine; F, female; Hgb, hemoglobin; hx, history; IV, intravenous; IVM, ivermectin; LF, lymphatic filariasis; M, male; mf, microfilaria; NL, normal; PE, physical examination; U/A, urinanalysis.
Parasitological, Biochemical, and Drug Testing
Parasitemia levels were assessed by passing 1 mL of anticoagulated, nocturnally collected blood through a 5-µm polycarbonate filter (EMD Millipore Corp., Billerica, Massachusetts), washed with filtered water, placed on glass slides, dried, and stained with Giemsa. Microfilariae were counted by microscopy. Antigen levels were measured using the Og4C3 mAb–based assay [13]. Plasma for drug levels was stored at −80°C. Evaluation of AST, ALT, and creatinine levels was performed using a Vitros DT-60 II biochemistry analyzer (Ortho Clinical Diagnostics, Rochester, New York). Glucose, blood, protein, nitrites, and leukocytes were measured with a urine dipstick (Multistix 10 SG, Bayer/Seimens, Malvern, Pennsylvania), with severity graded according to the manufacturer's protocol. Hemoglobin levels were evaluated on a HemoCue Hb 201+ machine (HemoCue Inc. Cypress, California).
All pharmacokinetic analyses were performed at the University of Iowa's College of Pharmacy. The concentration of DEC was determined using high-performance liquid chromatography with mass spectrometric detection [14]. IVM concentrations were determined using a previously published extraction methodology [15]. The linear range of the calibration curve was 0.20–400 ng/mL from 0.20 mL plasma.
Clinical Evaluation and Adverse Events Recording
The night prior to drug dosing, a thorough clinical assessment was performed. Circumference of all limbs was recorded at each time point. Scrotal abnormalities were classified as edema, hernia, or hydrocele, and any hydroceles graded as smaller or larger than a fist.
Objective adverse events (AEs) were based on serial physical examinations, biochemical evaluations, and urinalysis every 4 hours for the first 12 hours then at 24, 48, and 72 hours and 7 days after treatment (Figure 1). AEs were defined as any one of the following: any increase in ALT, AST, or creatinine measurement >1.5 times the upper limit of the reference range; tympanic temperature >37.8°C; increase in lymph node tenderness, swelling, or pain from baseline; or increase in proteinuria or hematuria from baseline based on urine dipstick measurement. A scoring system was used for proteinuria and hematuria based on severity and duration of the abnormal dipstick measurement. First hematuria (or proteinuria) was scored as negative (0), mild (1+), moderate (2+), and severe (3+) with a negative assigned a score of 0, mild a score of 1, moderate a score of 2, and severe a score of 3. If an abnormal urine dipstick measurement was observed on just 1 testing day post-treatment (eg, at 24, 48, and 72 hours and day 7 post-treatment), a score of 1 was given. If the urine dipstick measurement remained abnormal for 2 days, a score of 2 was given to a maximum of 4 if the urinalysis remained abnormal for the 4 testing time points. A final score for each treatment group was determined by adding the sum of all these values for the12 individuals in each group.
Subjective AEs were assessed by asking participants about symptoms that they may have experienced after taking the medication that either increased in severity or was experienced for the first time following treatment. Participants were asked to categorize any symptoms as mild (does not interfere with daily activity), moderate (interferes with daily activity), or severe (warrants hospital admission).
Informed Consent and Regulatory Monitoring
Institutional review boards at the University Hospitals Case Medical Center, Cleveland, Ohio, the Papua New Guinea Institute of Medical Research, and the Medical Research Advisory Committee of Papua New Guinea approved study protocols and documents. The trial was registered at ClinicalTrials.gov (NCT01975441).
Data Treatment and Analyses
The study was powered to look at drug interactions and AEs, with changes in microfilaria levels as a secondary outcome. Objective and subjective AEs were compared separately using Mann–Whitney–Wilcoxon rank sum test. Differences in microfilaremia levels were assessed using student t test of log-transformed data. A general linear model was used to examine the independent effects of treatment and microfilaremia levels on the frequency of AEs.
For each participant, pharmacokinetic parameters were estimated by plotting the plasma concentrations (of DEC, ALB, albendazole sulfoxide [ALBSO], albendazole sulfone [ALBSO2], or IVM) vs time using noncompartmental analysis (WinNonlin v5.0, Pharsight Corporation, Cary, North Carolina). The maximum plasma concentration (Cmax) and time of maximum concentration (Tmax) were observed directly from the concentration–time curve. The half-life, area under the curve, and drug–drug interactions were calculated as previously described [14–17] and are included in the Supplementary Materials.
RESULTS
Population Characteristics and Impact of Treatment on Infection Levels
Before treatment study participants in the 2 treatment groups had similar infection intensities, age, weight, and hemoglobin levels (Table 1). A single dose of DEC + ALB + IVM resulted in almost total elimination of microfilaria at 36 hours and 7 days after treatment, and no participant was microfilaremic 12 months after treatment (Figure 2). By contrast, a single dose of DEC + ALB resulted in less dramatic reductions in microfilaria levels at 36 hours and 7 days, and 10 of 11 participants remained microfilaremic at the 12-month time point. Twelve participants who had not moved away and agreed to have an additional night blood sample drawn (by chance, 6 in each treatment group) were examined for microfilaria levels 2 years following treatment (Table 2). All 6 individuals who received the single 3-drug treatment remained amicrofilaremic at 2 years (P = .047, compared with those receiving 2 drugs; Table 2). DEC + ALB + IVM also resulted in greater decreases in filarial antigen levels compared with DEC + ALB at 12 months (Figure 3). All participants in both treatment groups remained antigen positive at 12 months and at 2 years.
| Treatment Group . | N . | Male/Female . | Geometric Mean Microfilaremia (microfilaria /mL) ± 95% CI (range) . | Filarial Antigen (unit/mL) Geometric Mean ± 95% CI . | Median Age, y (range) . | Mean Weight, kg ± SD . | Mean Hemoglobin, g/dL ± SD . |
|---|---|---|---|---|---|---|---|
| Diethylcarbamazine + albendazole + ivermectin | 12 | 6/6 | 1558 ± 2322 (209–13 776) | 3881 ± 1227 | 30 (19–59) | 53 ± 9 | 11.2 ± 1.3 |
| Diethylcarbamazine + albendazole | 12 | 6/6 | 1857 ± 2191 (133–13 333) | 3347 ± 1018 | 28 (19–50) | 49 ± 8 | 11.0 ± 1.2 |
| Treatment Group . | N . | Male/Female . | Geometric Mean Microfilaremia (microfilaria /mL) ± 95% CI (range) . | Filarial Antigen (unit/mL) Geometric Mean ± 95% CI . | Median Age, y (range) . | Mean Weight, kg ± SD . | Mean Hemoglobin, g/dL ± SD . |
|---|---|---|---|---|---|---|---|
| Diethylcarbamazine + albendazole + ivermectin | 12 | 6/6 | 1558 ± 2322 (209–13 776) | 3881 ± 1227 | 30 (19–59) | 53 ± 9 | 11.2 ± 1.3 |
| Diethylcarbamazine + albendazole | 12 | 6/6 | 1857 ± 2191 (133–13 333) | 3347 ± 1018 | 28 (19–50) | 49 ± 8 | 11.0 ± 1.2 |
Abbreviations: CI, confidence interval; SD, standard deviation.
| Treatment Group . | N . | Male/Female . | Geometric Mean Microfilaremia (microfilaria /mL) ± 95% CI (range) . | Filarial Antigen (unit/mL) Geometric Mean ± 95% CI . | Median Age, y (range) . | Mean Weight, kg ± SD . | Mean Hemoglobin, g/dL ± SD . |
|---|---|---|---|---|---|---|---|
| Diethylcarbamazine + albendazole + ivermectin | 12 | 6/6 | 1558 ± 2322 (209–13 776) | 3881 ± 1227 | 30 (19–59) | 53 ± 9 | 11.2 ± 1.3 |
| Diethylcarbamazine + albendazole | 12 | 6/6 | 1857 ± 2191 (133–13 333) | 3347 ± 1018 | 28 (19–50) | 49 ± 8 | 11.0 ± 1.2 |
| Treatment Group . | N . | Male/Female . | Geometric Mean Microfilaremia (microfilaria /mL) ± 95% CI (range) . | Filarial Antigen (unit/mL) Geometric Mean ± 95% CI . | Median Age, y (range) . | Mean Weight, kg ± SD . | Mean Hemoglobin, g/dL ± SD . |
|---|---|---|---|---|---|---|---|
| Diethylcarbamazine + albendazole + ivermectin | 12 | 6/6 | 1558 ± 2322 (209–13 776) | 3881 ± 1227 | 30 (19–59) | 53 ± 9 | 11.2 ± 1.3 |
| Diethylcarbamazine + albendazole | 12 | 6/6 | 1857 ± 2191 (133–13 333) | 3347 ± 1018 | 28 (19–50) | 49 ± 8 | 11.0 ± 1.2 |
Abbreviations: CI, confidence interval; SD, standard deviation.
| Treatment . | Time Post-Treatment . | ||||
|---|---|---|---|---|---|
| Pre-Treatment . | 36 h . | 7 d . | 1 y . | 2 y . | |
| Diethylcarbamazine + albendazole | 3235a | 4 | 9 | 10 | 0 |
| 1599 | 10 | 8 | 11 | 44 | |
| 1562 | 1 | 627 | 22 | 0 | |
| 1095 | 27 | 49 | 7 | 4 | |
| 1107 | 13 | 1 | 27 | 13 | |
| 1747 | 87 | 947 | 29 | 37 | |
| Diethylcarbamazine + albendazole + ivermectin | 689 | 0 | 0 | 0 | 0 |
| 677 | 1 | 1 | 0 | 0 | |
| 1034 | 6 | 8 | 0 | 0 | |
| 1476 | 1 | 4 | 0 | 0 | |
| 1857 | 0 | 0 | 0 | 0 | |
| 2509 | 0 | 0 | 0 | 0 | |
| Treatment . | Time Post-Treatment . | ||||
|---|---|---|---|---|---|
| Pre-Treatment . | 36 h . | 7 d . | 1 y . | 2 y . | |
| Diethylcarbamazine + albendazole | 3235a | 4 | 9 | 10 | 0 |
| 1599 | 10 | 8 | 11 | 44 | |
| 1562 | 1 | 627 | 22 | 0 | |
| 1095 | 27 | 49 | 7 | 4 | |
| 1107 | 13 | 1 | 27 | 13 | |
| 1747 | 87 | 947 | 29 | 37 | |
| Diethylcarbamazine + albendazole + ivermectin | 689 | 0 | 0 | 0 | 0 |
| 677 | 1 | 1 | 0 | 0 | |
| 1034 | 6 | 8 | 0 | 0 | |
| 1476 | 1 | 4 | 0 | 0 | |
| 1857 | 0 | 0 | 0 | 0 | |
| 2509 | 0 | 0 | 0 | 0 | |
a Microfilaria per milliliter of filtered whole blood.
| Treatment . | Time Post-Treatment . | ||||
|---|---|---|---|---|---|
| Pre-Treatment . | 36 h . | 7 d . | 1 y . | 2 y . | |
| Diethylcarbamazine + albendazole | 3235a | 4 | 9 | 10 | 0 |
| 1599 | 10 | 8 | 11 | 44 | |
| 1562 | 1 | 627 | 22 | 0 | |
| 1095 | 27 | 49 | 7 | 4 | |
| 1107 | 13 | 1 | 27 | 13 | |
| 1747 | 87 | 947 | 29 | 37 | |
| Diethylcarbamazine + albendazole + ivermectin | 689 | 0 | 0 | 0 | 0 |
| 677 | 1 | 1 | 0 | 0 | |
| 1034 | 6 | 8 | 0 | 0 | |
| 1476 | 1 | 4 | 0 | 0 | |
| 1857 | 0 | 0 | 0 | 0 | |
| 2509 | 0 | 0 | 0 | 0 | |
| Treatment . | Time Post-Treatment . | ||||
|---|---|---|---|---|---|
| Pre-Treatment . | 36 h . | 7 d . | 1 y . | 2 y . | |
| Diethylcarbamazine + albendazole | 3235a | 4 | 9 | 10 | 0 |
| 1599 | 10 | 8 | 11 | 44 | |
| 1562 | 1 | 627 | 22 | 0 | |
| 1095 | 27 | 49 | 7 | 4 | |
| 1107 | 13 | 1 | 27 | 13 | |
| 1747 | 87 | 947 | 29 | 37 | |
| Diethylcarbamazine + albendazole + ivermectin | 689 | 0 | 0 | 0 | 0 |
| 677 | 1 | 1 | 0 | 0 | |
| 1034 | 6 | 8 | 0 | 0 | |
| 1476 | 1 | 4 | 0 | 0 | |
| 1857 | 0 | 0 | 0 | 0 | |
| 2509 | 0 | 0 | 0 | 0 | |
a Microfilaria per milliliter of filtered whole blood.
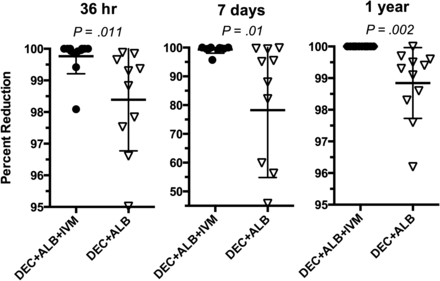
Percent reduction in microfilaria compared to pretreatment levels at 36 hours, 7 days, and 1 year following treatment with diethylcarbamazine (DEC) + albendazole (ALB) + ivermectin (IVM) or DEC + ALB. Microfilaria levels were determined by filtering 1 mL of anticoagulated, nocturnally collected peripheral venous blood through a nucleopore filter. Significance determined by Student t test.
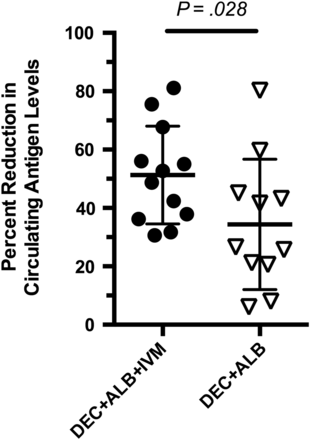
Percent reduction in serum filarial antigen levels 1 year following treatment compared to levels before treatment in participants receiving a single dose of diethylcarbamazine (DEC) + albendazole (ALB) + ivermectin (IVM) or DEC + ALB. Antigen levels were measure using the Og4C3 assay. Significance determined by Student t test.
Adverse Events Following Treatment
Objective and subjective AEs were mild to moderate in severity and were experienced by participants in both treatment groups (Table 3). The first AEs started 4 hours after treatment, typically they were subjective findings such as nausea and headaches. These were usually followed by arthralgias and pruritus that often presented by 8 hours after treatment. Two participants also developed mild inguinal tenderness at that time. One individual developed a fever at 8 hours post-treatment. In other individuals that developed fevers following treatment, elevated temperatures were present by 12 hours post-treatment. Temperatures often exceeded 39°C and were successfully treated with acetaminophen. Seven individuals developed hematuria and/or proteinuria, 3 at 24 hours post-treatment and the remaining by 48 hours. Hematuria and/or proteinuria resolved by 72 hours in 3 of 7 individuals, and the remainder resolved by 7 days. Abnormal transaminases were confined to AST and followed a similar kinetics of hematuria and/or proteinuria, with all resolving by 7 days after treatment.
Number and Frequency of Adverse Events Experienced by Study Participants After Treatment
| Adverse Event . | Regimen . | |
|---|---|---|
| Diethylcarbamazine + Albendazole (N = 12) . | Diethylcarbamazine + Albendazole + Ivermectin (N = 12) . | |
| Objective findings | ||
| Fever | 2 (17) | 6 (50) |
| Lymphadenitis | 5 (42) | 3 (25) |
| Hepatic (alanine transaminase\aspartate transaminase) abnormalitiesa | 2 (17) | 1 (8 |
| Proteinuria | 1 (8) | 3 (25) |
| Hematuria | 0 (0) | 4 (33) |
| Transient low blood pressure | 1 (8) | 1 (8) |
| Subjective findings | ||
| Headache | 3 (25) | 6 (50) |
| Joint pain | 5 (42) | 7 (58) |
| Nausea | 1 (8) | 3 (25) |
| Cough | 0 (0) | 5 (42) |
| Abdominal pain | 1 (8) | 4 (33) |
| Itch | 3 (25) | 5 (42) |
| Weakness | 0 (0) | 2 (17) |
| Dizziness | 2 (17) | 1 (8) |
| Adverse Event . | Regimen . | |
|---|---|---|
| Diethylcarbamazine + Albendazole (N = 12) . | Diethylcarbamazine + Albendazole + Ivermectin (N = 12) . | |
| Objective findings | ||
| Fever | 2 (17) | 6 (50) |
| Lymphadenitis | 5 (42) | 3 (25) |
| Hepatic (alanine transaminase\aspartate transaminase) abnormalitiesa | 2 (17) | 1 (8 |
| Proteinuria | 1 (8) | 3 (25) |
| Hematuria | 0 (0) | 4 (33) |
| Transient low blood pressure | 1 (8) | 1 (8) |
| Subjective findings | ||
| Headache | 3 (25) | 6 (50) |
| Joint pain | 5 (42) | 7 (58) |
| Nausea | 1 (8) | 3 (25) |
| Cough | 0 (0) | 5 (42) |
| Abdominal pain | 1 (8) | 4 (33) |
| Itch | 3 (25) | 5 (42) |
| Weakness | 0 (0) | 2 (17) |
| Dizziness | 2 (17) | 1 (8) |
a >1.5 times the upper limit of normal.
Number and Frequency of Adverse Events Experienced by Study Participants After Treatment
| Adverse Event . | Regimen . | |
|---|---|---|
| Diethylcarbamazine + Albendazole (N = 12) . | Diethylcarbamazine + Albendazole + Ivermectin (N = 12) . | |
| Objective findings | ||
| Fever | 2 (17) | 6 (50) |
| Lymphadenitis | 5 (42) | 3 (25) |
| Hepatic (alanine transaminase\aspartate transaminase) abnormalitiesa | 2 (17) | 1 (8 |
| Proteinuria | 1 (8) | 3 (25) |
| Hematuria | 0 (0) | 4 (33) |
| Transient low blood pressure | 1 (8) | 1 (8) |
| Subjective findings | ||
| Headache | 3 (25) | 6 (50) |
| Joint pain | 5 (42) | 7 (58) |
| Nausea | 1 (8) | 3 (25) |
| Cough | 0 (0) | 5 (42) |
| Abdominal pain | 1 (8) | 4 (33) |
| Itch | 3 (25) | 5 (42) |
| Weakness | 0 (0) | 2 (17) |
| Dizziness | 2 (17) | 1 (8) |
| Adverse Event . | Regimen . | |
|---|---|---|
| Diethylcarbamazine + Albendazole (N = 12) . | Diethylcarbamazine + Albendazole + Ivermectin (N = 12) . | |
| Objective findings | ||
| Fever | 2 (17) | 6 (50) |
| Lymphadenitis | 5 (42) | 3 (25) |
| Hepatic (alanine transaminase\aspartate transaminase) abnormalitiesa | 2 (17) | 1 (8 |
| Proteinuria | 1 (8) | 3 (25) |
| Hematuria | 0 (0) | 4 (33) |
| Transient low blood pressure | 1 (8) | 1 (8) |
| Subjective findings | ||
| Headache | 3 (25) | 6 (50) |
| Joint pain | 5 (42) | 7 (58) |
| Nausea | 1 (8) | 3 (25) |
| Cough | 0 (0) | 5 (42) |
| Abdominal pain | 1 (8) | 4 (33) |
| Itch | 3 (25) | 5 (42) |
| Weakness | 0 (0) | 2 (17) |
| Dizziness | 2 (17) | 1 (8) |
a >1.5 times the upper limit of normal.
Overall, 10 of 12 (83%) individuals in the 3-drug treatment group developed 1 or more objective AEs compared with 6 of 12 (50%, P = .19, Fischer exact test) in the 2-drug group. Median number of AEs per person in the 3-drug group was 2 compared with 0.5 in the 1-drug group (P = .094). Hematuria and/or proteinuria also predominantly occurred in the 3-drug treatment group. A scoring system was used based on severity and duration of the hematuria and/or proteinuria (see “Methods” section), with individuals in the 3-drug group having a score of 16 vs 1 in the 2-drug group.
With respect to subjective AEs, the proportion of individuals who developed 1 or more complaints, including headache, nausea, pruritus, abdominal pain, weakness, and arthralgia (Table 2), was similar between the 2 treatment groups (9 of 12 [75%] in the 3-drug group and 7 of 12 [58%] in the 2-drug group). Individuals in the 3-drug group tended to have more subjective AEs (median = 2.5) compared with those in the 2-drug group (median = 1, P = .091).
When both subjective and objective AEs were combined, individuals on 3-drug therapy had significantly more AEs (median = 4.5) compared with those receiving 2-drug treatment (median = 2.5, P = .021). Microfilaremia levels prior to treatment (or closely related reduction in microfilaria levels at 36 hours) were related to the number of AEs independent of treatment (P = .09).
Drug Interactions and Pharmacokinetics
Drug concentration–time curves are shown in Figure 4 and Supplementary Figure 1. The pharmacokinetic parameter estimates for DEC, ALB, ALBSO, and ALBO2 with and without IVM administration are presented in Supplementary Table 1, along with parameter estimates for IVM. No significant drug interactions were observed (P > .05 for all treatment group comparisons).
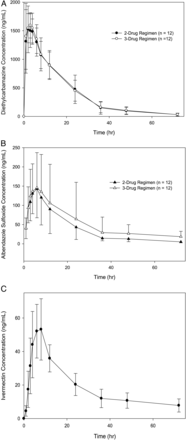
Mean serum plasma levels (± standard deviation) of (A) diethylcarbamazine (DEC), (B) albendazole (ALB) sulfoxide and (C) ivermectin (IVM) at different times following treatment with DEC + ALB + IVM or DEC + ALB.
Geometric mean parameter ratios [(with IVM)/(without IVM)] of Cmax, AUC0–t, and AUC0–∞ for each analyte (DEC, ALBSO, and ALBSO2) are presented with 90% confidence intervals (CIs) in Supplementary Table 2. DEC Cmax levels in the 2 treatment groups almost met the commonly used limits for no clinically relevant effect (80%–125%) [17–19]. DEC AUC0–t and AUC0–∞ 90% CIs obtained in this study were only slightly outside of these limits but were completely within the slightly less stringent limits of 70%–143%. Ninety percent CIs for the parameter estimates of ALBSO and ALBSO2 were quite broad, because of the small samples sizes in this study.
DISCUSSION
In this study, we compared the effects of a new triple-drug regimen (IVM + DEC + ALB) with standard DEC + ALB as a single-dose treatment for Bancroftian filariasis in treatment-naive participants with high-intensity W. bancrofti infections. Triple therapy rapidly eliminated almost all microfilaria from peripheral blood, and, importantly, all participants treated with this regimen were amicrofilaremic 1 and 2 years following treatment. Microfilaria counts also rapidly declined in participants treated with DEC + ALB but less dramatically than in those treated with triple therapy. Also, the 2-drug therapy failed to clear microfilaria in most participants 12 and 24 months after treatment, which is consistent with results from other treatment trials [7–9, 11]. A greater reduction in circulating antigen levels with the triple-drug regimen suggests a higher percentage of adult female worms were killed compared with use of DEC + ALB. Since all treatment participants in both groups had persistently positive filarial antigen tests 1 and 2 years after treatment, neither treatment killed all adults worms [20]. The absence of microfilaria at 1 and 2 years after treatment suggests the triple-drug therapy had embryostatic and/or embryocidal effects on adult female worms.
Participants treated with the triple-drug regimen experienced more AEs than those who received DEC + ALB when both objective and subjective AEs were combined. All AEs were mild to moderate in severity, started 8 hours following treatment, peaked at between 12 and 48 hours, and resolved 7 days later, except in 1 participant who had right inguinal tenderness at day 7. AEs observed were consistent with the well-documented transient AEs that have been reported following treatments that kill microfilaria [7–9]. These AEs include fever, headache, pruritus, arthralgia, tender lymph nodes, and development of proteinuria and/or hemoglobinuria. The spectrum of AEs was similar in the 2 treatment arms, except for urinary abnormalities, which were present almost exclusively in the 3-drug treatment group. Development of proteinuria and/or hemoglobinuria in urine ranged from mild to major, as measured by dipstick, and often persisted for several days. All urine abnormalities resolved by 7 days following treatment. No participant had a significant increase in serum creatinine after treatment. Transient urinary abnormalities were similar to those observed in a previous study following treatment with DEC alone [21]. The proteinuria and/or hemoglobinuria may arise from inflammation due to dead microfilaria in the kidney. Alternatively, participants may develop a transient immune complex associated glomerulonephritis, as based on prior studies in animal models of filariasis [22], renal histology [23–26], and presence of filariasis-specific immune complexes in blood and urine [27] of participants with LF.
Pharmacokinetic studies were performed to determine whether IVM affects drug levels or clearance of DEC and ALB. The absorption and disposition profiles of each drug (and/or ALB metabolites) were not significantly different in participants who received IVM. The pharmacokinetics of DEC and IVM were similar to those seen in previous studies [19, 28, 29]. The pharmacokinetics of ALB in this study was also consistent with that seen in prior studies that reported that ALB is poorly absorbed and rapidly eliminated, primarily through metabolism to ALBSO and ALBSO2 [19, 28, 29]. The wide variation in ALBSO levels after ALB is well known and likely due to differences in absorption of the drug between individuals and differences in ALB metabolism between men and women [30]. There was a clear lack of significant interaction between DEC and IVM. Because of high interpatient variability in ALBSO exposure parameters, definitive conclusions cannot be drawn regarding the lack of an effect of IVM on ALBSO exposure. However, the point estimates for the ratios of geometric means are not suggestive of a major influence of IVM on ALBSO exposure, and in a previous analysis, Awadzi et al [28] failed to detect any substantial interaction between these drugs. Thus, it is unlikely that coadministration of IVM has a clinically significant drug interaction with ALB/ALBSO. It has historically been assumed that the minor metabolite ALBSO2 is inactive against filarial parasites because of its relatively lower abundance in human serum compared with ALBSO and because of the known activity of ALB and ALBSO as antagonists of microtubule formation in nematodes [31]. However, a recent study reported that ALBSO2 prevents binary fission in Wolbachia, an obligate endosymbiont of W. bancrofti [32]. The present analysis is the first to quantify ALBSO2 pharmacokinetics in patients with W. bancrofti infection.
This study has shown that single-dose treatment with IVM +DEC + ALB is safe and more effective for clearing W. bancrofti microfilaria and reducing filarial antigen levels than standard treatment with DEC + ALB. Additional studies are needed to determine the duration of microfilaria clearance after IVM +DEC + ALB and to further establish the safety of this regimen. A “one and done” regimen could have a transformative impact on the global program to eliminate LF by reducing the number of rounds of MDA required to reach elimination targets. This would be especially useful for countries such as Papua New Guinea where it is extremely difficult to provide repeated rounds of MDA to LF-endemic populations, and it could improve chances for global elimination of LF by the target year of 2020.
Notes
Financial support. The Bill and Melinda Gates Foundation and Veterans Affairs Research Service supported this work.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
Author notes
E. K. T. is currently at the Liverpool School of Tropical Medicine, Liverpool, United Kingdom.




