-
PDF
- Split View
-
Views
-
Cite
Cite
Fernanda Q Onofrio, Curtis Cooper, Sergio M Borgia, Marie-Louise Vachon, Alnoor Ramji, Leslie B Lilly, Alexander Wong, Joshua Booth, Izza Sattar, Heidy Morales, Samuel Lee, Brian Conway, Jordan J Feld, Salvage Therapy with Sofosbuvir/Velpatasvir/Voxilaprevir in DAA-experienced Patients: Results from a Prospective Canadian Registry, Clinical Infectious Diseases, Volume 72, Issue 11, 1 June 2021, Pages e799–e805, https://doi.org/10.1093/cid/ciaa1510
Close - Share Icon Share
Abstract
Despite the current highly effective therapies with direct-acting antiviral agents (DAAs), some patients with chronic hepatitis C virus (HCV) infection still do not achieve sustained virological response (SVR) and require retreatment. Sofosbuvir/velpatasvir/voxilaprevir (SVV) is recommended as the first-line retreatment option for most patients. The aim of this study was to evaluate the efficacy of SVV as salvage therapy after at least one course of DAA.
Data were collected on all HCV-infected patients who failed DAAs and were prescribed SVV from a prospective Canadian registry (CANUHC) including 17 sites across Canada. Factors associated with failure to achieve SVR with SVV therapy and the utility of RAS testing and ribavirin use were evaluated.
A total of 128 patients received SVV after non-SVR with DAA treatment: 80% male, median age 57.5 (31–86), 44% cirrhotic, and 17 patients post liver transplant. First line regimens included: sofosbuvir/velpatasvir (27.3%), sofosbuvir/ledipasvir (26.5%), grazoprevir/elbasvir (12.5%), other (33.5%). Ribavirin was added to SVV in 26 patients due to past sofosbuvir/velpatasvir use (n = 8), complex resistance associated substitution profiles (n = 16) and/or cirrhosis (n = 9). Overall SVR rate was 96% (123/128). Of 35 patients who previously failed sofosbuvir/velpatasvir, 31 (88.5%) achieved SVR compared to 92 of 93 (99%) among those receiving any other regimen (P = .01).
Similar to reports from phase 3 clinical trials, SVV proved highly effective as salvage therapy for patients who failed a previous DAA therapy. Those who failed SVV had at least 2 of the following factors: genotype 3, presence of cirrhosis, past liver transplantation, past exposure to sofosbuvir/velpatasvir and/or complex resistance profiles.
Hepatitis C treatment has changed dramatically over the past 10 years. In 2015 the advent of all-oral interferon-free direct-acting antiviral agents (DAAs) revolutionized the treatment of HCV with few side effects and high sustained virological response (SVR) rates (>95%), regardless of HCV genotype, disease stage, or treatment history [1, 2]. However, despite the current highly effective therapies with DAAs, 2%–5% of patients still do not achieve SVR and require retreatment [3, 4]. These patients are a minority, but given the size of the HCV-infected population — 71 million people worldwide [5] — the absolute number of such patients is substantial and will increase as more patients are treated for HCV infection [6].
Factors associated with treatment failure include infection with genotypes 1a or 3, presence of cirrhosis, and the presence of viral resistance-associated substitutions (RASs) [7–9], which are nucleic acid substitutions in the HCV viral genome associated with reduced response to DAAs. Indeed, RASs have been shown to confer cross-resistance among some DAAs of the same class [10] and when in the NS5A region, can persist long-term, being detectable many years after completing treatment [11]. In 2017, sofosbuvir/velpatasvir/voxilaprevir (SVV), a combination of an NS5B polymerase inhibitor, an NS5A inhibitor, and an NS3/4 protease inhibitor, which targets 3 major steps in HCV replication, was developed to be used in cirrhotic patients with genotype 3 infection and those of any genotype or disease stage (except decompensated cirrhosis) who failed prior treatment [12].
Two large phase III trials (POLARIS-1 and POLARIS-4) [6] have assessed the efficacy of SVV in patients with HCV of any genotype, with or without cirrhosis, carrying multiple RASs, who had failed a previous DAA-based regimen. In POLARIS-1, patients were included if they had failed a previous NS5A-based regimen, whilst in POLARIS-4 patients treated with any DAA regimen without an NS5A inhibitor were included. The overall cure rate (undetectable HCV RNA 12 weeks after the end of treatment, or SVR12) was 96% and 98% in POLARIS-1 and -4, respectively. Based on these results, SVV was approved and is now recommended as the first-line retreatment option for most patients, irrespective of the presence of RAS [4, 13]. However, POLARIS-1 and -4 included very few patients treated with more recently approved pangenotypic HCV regimens. The aim of this study was to evaluate the efficacy of SVV as retreatment after at least one course of prior DAA treatment in a prospective real-world cohort of patients across Canada.
METHODS
Study Design
Patients were recruited from the Canadian Network Undertaking against HCV (CANUHC) cohort, a prospective multi-center real-world study evaluating patients treated with DAAs in various sites across Canada. This study included HCV infected-patients who failed a previous DAA treatment and were retreated with SVV. The primary outcome was achievement of SVR12, with analysis of correlates of failure to achieve this outcome. Variables that were considered included baseline demographics, HCV genotype, presence of cirrhosis and cirrhosis complications, specific type of previous DAA therapy, ribavirin use, and RAS testing/RAS pattern detected by next generation sequencing (Illumina, San Diego, CA) with a ≥15% threshold. The study was approved at each of the participating centers’ Research Ethics Board and by the Research Ethics Board of University Health Network, University of Toronto. The funders had no participation in the study design, analysis, or decision to publish.
Study Population
All adult HCV-infected patients who failed at least one DAA treatment and were prescribed salvage therapy with SVV for 12 weeks from January 2019 to January 2020 from any of the 17 CANUHC sites across Canada were included. Patients who had not completed SVV treatment or in whom SVR12 could not be ascertained were excluded.
Endpoints
The primary outcome was achievement of SVR12 after therapy with SVV. SVR12 was defined as negative HCV RNA after 12 weeks of completing treatment with SVV. Secondary outcomes included the factors associated with failure to achieve SVR, the efficacy of ribavirin use and utility of RAS testing.
Statistical Analysis
Categorical variables were reported as frequencies (percentages) and continuous variables as median (range). Categorical variables were compared using the Chi squared or the Fisher’s exact tests; continuous variables were compared using the Student’s t test, the Mann-Whitney U test or the Kruskal-Wallis test, as appropriate. All tests were 2-sided and used a significance level of 0.05. Data handling and analysis were performed with SPSS (Version 25.0, SPSS INC, NY).
RESULTS
A total of 128 patients were treated with SVV after failing a previous DAA regimen. Eight patients were initially enrolled but not included in the analysis due to loss to follow up or inability to ascertain SVR12 (Figure 1). Baseline characteristics for the cohort appear in Table 1. The overall mean age of the cohort was 57.5 years (31–86) and 80% were male. Coinfection with HIV was present in 7% of patients and coinfection with HBV in 1.6%. Two patients were coinfected HCV-HBV-HIV. Diabetes was reported in 17% and the mean BMI was 28 (SD 5.6). A diagnosis of cirrhosis was documented in 44% of the cohort, with a history of prior decompensation before but not at the time of retreatment in 23% of these (13/56), or 10% of the overall cohort. Seventeen (13%) patients had undergone prior liver transplantation. HCV genotype distribution was 60% genotype 1 (63 patients G1a, 12 patients G1b, and 2 patients G1 with unknown sub-genotype), 30% genotype 3, 5% genotype 4, 3% genotype 2, 0.7% genotype 6. A history of previous hepatocellular carcinoma (HCC) was present in 19 patients. Five patients had active HCC at the time of retreatment, including 3 with recurrent HCC.
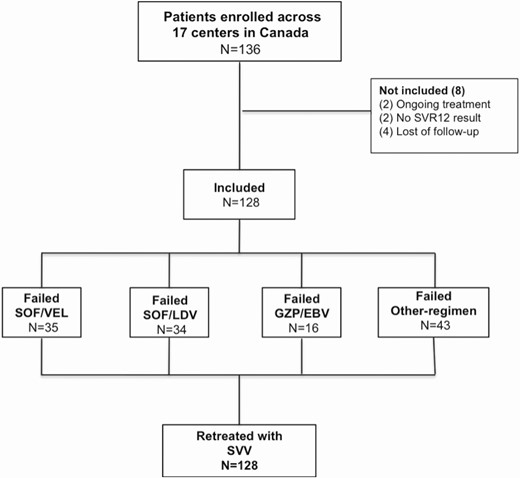
Flowchart of patients included in the study according to previous DAA treatment. Abbreviations: EBV, Elbatasvir; GZP, Grazoprevir; LDV, Ledipasvir; SOF, Sofosbuvir; SVR, Sustained Virological Response; SVV, Sofosbuvir-Velpatasvir-Voxilaprevir.
| Clinical Features . | n = 128 . |
|---|---|
| Age [years], median (range) | 59 [31–86] |
| Male, n (%) | 102 (79.6) |
| Coinfected HIV, n (%) | 9/127 (7.0) |
| Coinfected HBV, n (%) | 2/127 (1.6) |
| Coinfected HIV-HBV, n (%) | 2/127 (1.6) |
| Diabetes, n (%) | 19/110 (17.2) |
| Body Mass Index [kg/m2], mean (SD) | 28.5 (5.6)a |
| eGFR [mL/min], mean (SD) | 89 (21.5)b |
| Genotype, n (%) | |
| 1 | 77 (60) |
| 2 | 4 (3) |
| 3 | 39 (30) |
| 4 | 6 (5) |
| 6 | 1 (0.7) |
| 1a/4 | 1 (0.7) |
| Cirrhosis, n (%) | 56 (44) |
| Prior (but not current) decompensated cirrhosis, n (%) | 13 (10) |
| Past HCC, n (%) | 19 (15) |
| Current HCC, n (%) | 5 (4) |
| Past DAA therapy, n (%) | |
| sofosbuvir/velpatasvir | 35 (27.3) |
| sofosbuvir/ledipasvir | 34 (26.5) |
| grazoprevir/elbasvir | 16 (12.5) |
| other regimen | 43 (33.5) |
| Clinical Features . | n = 128 . |
|---|---|
| Age [years], median (range) | 59 [31–86] |
| Male, n (%) | 102 (79.6) |
| Coinfected HIV, n (%) | 9/127 (7.0) |
| Coinfected HBV, n (%) | 2/127 (1.6) |
| Coinfected HIV-HBV, n (%) | 2/127 (1.6) |
| Diabetes, n (%) | 19/110 (17.2) |
| Body Mass Index [kg/m2], mean (SD) | 28.5 (5.6)a |
| eGFR [mL/min], mean (SD) | 89 (21.5)b |
| Genotype, n (%) | |
| 1 | 77 (60) |
| 2 | 4 (3) |
| 3 | 39 (30) |
| 4 | 6 (5) |
| 6 | 1 (0.7) |
| 1a/4 | 1 (0.7) |
| Cirrhosis, n (%) | 56 (44) |
| Prior (but not current) decompensated cirrhosis, n (%) | 13 (10) |
| Past HCC, n (%) | 19 (15) |
| Current HCC, n (%) | 5 (4) |
| Past DAA therapy, n (%) | |
| sofosbuvir/velpatasvir | 35 (27.3) |
| sofosbuvir/ledipasvir | 34 (26.5) |
| grazoprevir/elbasvir | 16 (12.5) |
| other regimen | 43 (33.5) |
Data are presented as median [IQR] or no. (%).
Abbreviations: DAA, direct-acting antiviral; HCC hepatocellular carcinoma; SVV, /sofosbuvir/velpatasvir/voxilaprevir, an = 69, bn = 97.
| Clinical Features . | n = 128 . |
|---|---|
| Age [years], median (range) | 59 [31–86] |
| Male, n (%) | 102 (79.6) |
| Coinfected HIV, n (%) | 9/127 (7.0) |
| Coinfected HBV, n (%) | 2/127 (1.6) |
| Coinfected HIV-HBV, n (%) | 2/127 (1.6) |
| Diabetes, n (%) | 19/110 (17.2) |
| Body Mass Index [kg/m2], mean (SD) | 28.5 (5.6)a |
| eGFR [mL/min], mean (SD) | 89 (21.5)b |
| Genotype, n (%) | |
| 1 | 77 (60) |
| 2 | 4 (3) |
| 3 | 39 (30) |
| 4 | 6 (5) |
| 6 | 1 (0.7) |
| 1a/4 | 1 (0.7) |
| Cirrhosis, n (%) | 56 (44) |
| Prior (but not current) decompensated cirrhosis, n (%) | 13 (10) |
| Past HCC, n (%) | 19 (15) |
| Current HCC, n (%) | 5 (4) |
| Past DAA therapy, n (%) | |
| sofosbuvir/velpatasvir | 35 (27.3) |
| sofosbuvir/ledipasvir | 34 (26.5) |
| grazoprevir/elbasvir | 16 (12.5) |
| other regimen | 43 (33.5) |
| Clinical Features . | n = 128 . |
|---|---|
| Age [years], median (range) | 59 [31–86] |
| Male, n (%) | 102 (79.6) |
| Coinfected HIV, n (%) | 9/127 (7.0) |
| Coinfected HBV, n (%) | 2/127 (1.6) |
| Coinfected HIV-HBV, n (%) | 2/127 (1.6) |
| Diabetes, n (%) | 19/110 (17.2) |
| Body Mass Index [kg/m2], mean (SD) | 28.5 (5.6)a |
| eGFR [mL/min], mean (SD) | 89 (21.5)b |
| Genotype, n (%) | |
| 1 | 77 (60) |
| 2 | 4 (3) |
| 3 | 39 (30) |
| 4 | 6 (5) |
| 6 | 1 (0.7) |
| 1a/4 | 1 (0.7) |
| Cirrhosis, n (%) | 56 (44) |
| Prior (but not current) decompensated cirrhosis, n (%) | 13 (10) |
| Past HCC, n (%) | 19 (15) |
| Current HCC, n (%) | 5 (4) |
| Past DAA therapy, n (%) | |
| sofosbuvir/velpatasvir | 35 (27.3) |
| sofosbuvir/ledipasvir | 34 (26.5) |
| grazoprevir/elbasvir | 16 (12.5) |
| other regimen | 43 (33.5) |
Data are presented as median [IQR] or no. (%).
Abbreviations: DAA, direct-acting antiviral; HCC hepatocellular carcinoma; SVV, /sofosbuvir/velpatasvir/voxilaprevir, an = 69, bn = 97.
First line regimens included: sofosbuvir/velpatasvir (SOF/VEL) (27.3%), sofosbuvir/ledipasvir (SOF/LDV) (26.5%), grazoprevir/elbasvir (GZR/EBR) (12.5%), and other combinations (33.5%), including sofosbuvir and RBV, sofosbuvir plus daclatasvir plus RBV, pegylated interferon/sofosbuvir plus RBV, paritaprevir/ritonavir/ombitasvir plus dasabuvir. SOF/LDV was the most common past DAA regimen used in patients with genotype 1 (43.5%). The most commonly used prior regimen in patients with genotype 3 infection was SOF/VEL (50%). Only 9 patients out of 128 failed more than one DAA regimen prior to receiving SVV, of whom 5 had genotype 1 infection (3 G1a, 1 G1b, 1 G1 unspecified).
The overall SVR12 rate with SVV was 96% (123/128). All patients previously treated with SOF/LDV and GZR/EBR achieved SVR after retreatment with SVV (Table 2). Of the 5 (4%) patients who did not achieve SVR with SVV, 3 were cirrhotic, 3 were status post-transplant, 4 were previously treated with SOF/VEL, and all 5 had at least dual class (NS3 and NS5A) resistance (Table 3). Notably 3 out of 5 patients who failed SVV had genotype 3 infection. One patient who failed SVV had active HCC. Fourteen out of 17 (82%) post-transplant patients achieved SVR12 compared to 109 out of 111 (98%) without a transplant (P = .01). The SVR12 rate was lower in patients previously treated with SOF/VEL (31 of 35, 88.5%) when compared to other DAA combinations (92 of 93, 99%) (P = .01) (Figure 2A). No serious adverse events related to SVV were reported and no patients discontinued therapy prematurely due to side effects or hepatic decompensation, including the 13 patients with a previous history of decompensation.
| . | SVR (n = 123) . | Non-SVR (n = 5) . | P value . |
|---|---|---|---|
| Mean Age (years) | 58 | 55 | .69 |
| Male % | 97 (79) | 5 (100) | .58 |
| HCV Genotype | .38 | ||
| 1 | 76 (62) | 1 (20) | |
| 2 | 3 (2) | 1 (20) | |
| 3 | 36 (29) | 3 (60) | |
| 4 | 6 (5) | 0 (0) | |
| 6 | 1 (0.8) | 0 (0) | |
| 1b/4 | 1 (0.8) | 0 (0) | |
| Cirrhosis | 53 (43) | 3 (60) | .65 |
| Decompensated cirrhosis | 12 (10) | 1 (20) | .55 |
| Past HCC | 18 (15) | 1 (20) | .55 |
| Current HCC | 4 (3) | 1 (20) | .18 |
| Past liver transplantation | 14 (11) | 3 (60) | .01 |
| Prior DAA therapy | |||
| sofosbuvir/velpatasvir | 31 (25.2) | 4 (80) | .01 |
| sofosbuvir/ledipasvir | 34 (27.6) | 0 (0) | |
| grazoprevir/elbasvir | 16 (13) | 0 (0) | |
| other regimen | 42 (34.1) | 1 (20) |
| . | SVR (n = 123) . | Non-SVR (n = 5) . | P value . |
|---|---|---|---|
| Mean Age (years) | 58 | 55 | .69 |
| Male % | 97 (79) | 5 (100) | .58 |
| HCV Genotype | .38 | ||
| 1 | 76 (62) | 1 (20) | |
| 2 | 3 (2) | 1 (20) | |
| 3 | 36 (29) | 3 (60) | |
| 4 | 6 (5) | 0 (0) | |
| 6 | 1 (0.8) | 0 (0) | |
| 1b/4 | 1 (0.8) | 0 (0) | |
| Cirrhosis | 53 (43) | 3 (60) | .65 |
| Decompensated cirrhosis | 12 (10) | 1 (20) | .55 |
| Past HCC | 18 (15) | 1 (20) | .55 |
| Current HCC | 4 (3) | 1 (20) | .18 |
| Past liver transplantation | 14 (11) | 3 (60) | .01 |
| Prior DAA therapy | |||
| sofosbuvir/velpatasvir | 31 (25.2) | 4 (80) | .01 |
| sofosbuvir/ledipasvir | 34 (27.6) | 0 (0) | |
| grazoprevir/elbasvir | 16 (13) | 0 (0) | |
| other regimen | 42 (34.1) | 1 (20) |
Data are presented as no. (%).
Abbreviations: DAA, direct-acting antiviral; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; LT, liver transplant; SVR, sustained virological response.
| . | SVR (n = 123) . | Non-SVR (n = 5) . | P value . |
|---|---|---|---|
| Mean Age (years) | 58 | 55 | .69 |
| Male % | 97 (79) | 5 (100) | .58 |
| HCV Genotype | .38 | ||
| 1 | 76 (62) | 1 (20) | |
| 2 | 3 (2) | 1 (20) | |
| 3 | 36 (29) | 3 (60) | |
| 4 | 6 (5) | 0 (0) | |
| 6 | 1 (0.8) | 0 (0) | |
| 1b/4 | 1 (0.8) | 0 (0) | |
| Cirrhosis | 53 (43) | 3 (60) | .65 |
| Decompensated cirrhosis | 12 (10) | 1 (20) | .55 |
| Past HCC | 18 (15) | 1 (20) | .55 |
| Current HCC | 4 (3) | 1 (20) | .18 |
| Past liver transplantation | 14 (11) | 3 (60) | .01 |
| Prior DAA therapy | |||
| sofosbuvir/velpatasvir | 31 (25.2) | 4 (80) | .01 |
| sofosbuvir/ledipasvir | 34 (27.6) | 0 (0) | |
| grazoprevir/elbasvir | 16 (13) | 0 (0) | |
| other regimen | 42 (34.1) | 1 (20) |
| . | SVR (n = 123) . | Non-SVR (n = 5) . | P value . |
|---|---|---|---|
| Mean Age (years) | 58 | 55 | .69 |
| Male % | 97 (79) | 5 (100) | .58 |
| HCV Genotype | .38 | ||
| 1 | 76 (62) | 1 (20) | |
| 2 | 3 (2) | 1 (20) | |
| 3 | 36 (29) | 3 (60) | |
| 4 | 6 (5) | 0 (0) | |
| 6 | 1 (0.8) | 0 (0) | |
| 1b/4 | 1 (0.8) | 0 (0) | |
| Cirrhosis | 53 (43) | 3 (60) | .65 |
| Decompensated cirrhosis | 12 (10) | 1 (20) | .55 |
| Past HCC | 18 (15) | 1 (20) | .55 |
| Current HCC | 4 (3) | 1 (20) | .18 |
| Past liver transplantation | 14 (11) | 3 (60) | .01 |
| Prior DAA therapy | |||
| sofosbuvir/velpatasvir | 31 (25.2) | 4 (80) | .01 |
| sofosbuvir/ledipasvir | 34 (27.6) | 0 (0) | |
| grazoprevir/elbasvir | 16 (13) | 0 (0) | |
| other regimen | 42 (34.1) | 1 (20) |
Data are presented as no. (%).
Abbreviations: DAA, direct-acting antiviral; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; LT, liver transplant; SVR, sustained virological response.
| . | Patient #1 . | Patient #2 . | Patient #3 . | Patient #4 . | Patient #5 . |
|---|---|---|---|---|---|
| Sex | male | male | male | male | male |
| Age (years) | 67 | 54 | 55 | 64 | 35 |
| HCV genotype | 2a/2c | 3 | 1b | 3 | 3 |
| Cirrhosis | yes | no | no | yes | yes |
| HCC (past/current) | no/no | no/no | no/no | yes/yes | no/no |
| First DAA regimen | SOF + RBV | - | - | SOF + RBV | - |
| Last DAA regimen | SOF/VEL +RBV | SOF/DAC +RBV | SOF/VEL | SOF/VEL + RBV | SOF/VEL |
| Past liver transplantation | no | yes | yes | yes | no |
| RAS identified prior to SVV treatment | NS3 (I132L) NS5A (L31M) | NS3 (Q168Q) NS5A (nS62L, Y93H) | NS3 (N174S) NS5A (M28V) NS5B (F415Y, N444E) | NS3 (A166S) NS5A (A30K, S62T, Y93H, R404K) | NS3 (A1665) NS5A (562L, Y93H, 5385Q, R404K) |
| . | Patient #1 . | Patient #2 . | Patient #3 . | Patient #4 . | Patient #5 . |
|---|---|---|---|---|---|
| Sex | male | male | male | male | male |
| Age (years) | 67 | 54 | 55 | 64 | 35 |
| HCV genotype | 2a/2c | 3 | 1b | 3 | 3 |
| Cirrhosis | yes | no | no | yes | yes |
| HCC (past/current) | no/no | no/no | no/no | yes/yes | no/no |
| First DAA regimen | SOF + RBV | - | - | SOF + RBV | - |
| Last DAA regimen | SOF/VEL +RBV | SOF/DAC +RBV | SOF/VEL | SOF/VEL + RBV | SOF/VEL |
| Past liver transplantation | no | yes | yes | yes | no |
| RAS identified prior to SVV treatment | NS3 (I132L) NS5A (L31M) | NS3 (Q168Q) NS5A (nS62L, Y93H) | NS3 (N174S) NS5A (M28V) NS5B (F415Y, N444E) | NS3 (A166S) NS5A (A30K, S62T, Y93H, R404K) | NS3 (A1665) NS5A (562L, Y93H, 5385Q, R404K) |
Abbreviations: DAA, direct-acting antiviral; HCC, hepatocellular carcinoma; LT, liver transplantation; RAS, resistance-associated substitution; SOF + RBV, sofosbuvir + ribavirin; SOF/VEL, sofosbuvir/velpatasvir; SOF/DAC, sofosbuvir/daclatasvir; SVV, sofosbuvir-velpatasvir-voxilaprevir.
| . | Patient #1 . | Patient #2 . | Patient #3 . | Patient #4 . | Patient #5 . |
|---|---|---|---|---|---|
| Sex | male | male | male | male | male |
| Age (years) | 67 | 54 | 55 | 64 | 35 |
| HCV genotype | 2a/2c | 3 | 1b | 3 | 3 |
| Cirrhosis | yes | no | no | yes | yes |
| HCC (past/current) | no/no | no/no | no/no | yes/yes | no/no |
| First DAA regimen | SOF + RBV | - | - | SOF + RBV | - |
| Last DAA regimen | SOF/VEL +RBV | SOF/DAC +RBV | SOF/VEL | SOF/VEL + RBV | SOF/VEL |
| Past liver transplantation | no | yes | yes | yes | no |
| RAS identified prior to SVV treatment | NS3 (I132L) NS5A (L31M) | NS3 (Q168Q) NS5A (nS62L, Y93H) | NS3 (N174S) NS5A (M28V) NS5B (F415Y, N444E) | NS3 (A166S) NS5A (A30K, S62T, Y93H, R404K) | NS3 (A1665) NS5A (562L, Y93H, 5385Q, R404K) |
| . | Patient #1 . | Patient #2 . | Patient #3 . | Patient #4 . | Patient #5 . |
|---|---|---|---|---|---|
| Sex | male | male | male | male | male |
| Age (years) | 67 | 54 | 55 | 64 | 35 |
| HCV genotype | 2a/2c | 3 | 1b | 3 | 3 |
| Cirrhosis | yes | no | no | yes | yes |
| HCC (past/current) | no/no | no/no | no/no | yes/yes | no/no |
| First DAA regimen | SOF + RBV | - | - | SOF + RBV | - |
| Last DAA regimen | SOF/VEL +RBV | SOF/DAC +RBV | SOF/VEL | SOF/VEL + RBV | SOF/VEL |
| Past liver transplantation | no | yes | yes | yes | no |
| RAS identified prior to SVV treatment | NS3 (I132L) NS5A (L31M) | NS3 (Q168Q) NS5A (nS62L, Y93H) | NS3 (N174S) NS5A (M28V) NS5B (F415Y, N444E) | NS3 (A166S) NS5A (A30K, S62T, Y93H, R404K) | NS3 (A1665) NS5A (562L, Y93H, 5385Q, R404K) |
Abbreviations: DAA, direct-acting antiviral; HCC, hepatocellular carcinoma; LT, liver transplantation; RAS, resistance-associated substitution; SOF + RBV, sofosbuvir + ribavirin; SOF/VEL, sofosbuvir/velpatasvir; SOF/DAC, sofosbuvir/daclatasvir; SVV, sofosbuvir-velpatasvir-voxilaprevir.
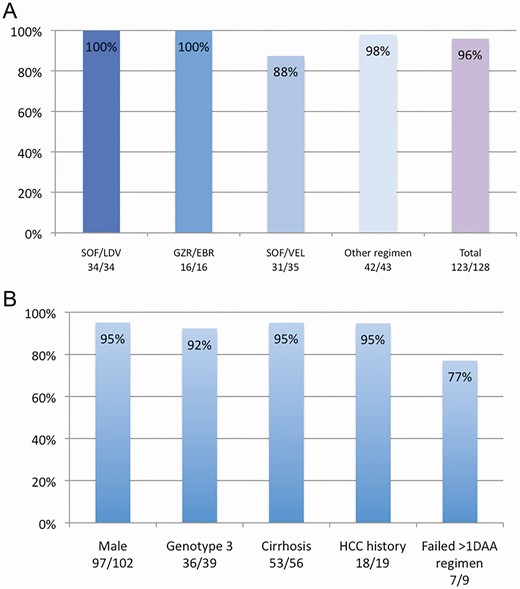
A, SVR12 rate after salvage therapy with SVV according to last DAA therapy. B, SVR12 rate according to relevant clinical features. Abbreviations: EBR, Elbasvir; GZR, Grazoprevir; LDV, Ledipasvir; SOF, Sofosbuvir; VEL, Velpatasvir.
Of 56 patients with cirrhosis, 53 (95%) achieved SVR12 compared to non-cirrhotics (P = .65) (Figure 2B). Of the 13 with a past history of decompensation before SVV use, none experienced decompensation during SVV treatment, and 12 (92%) achieved SVR12.
RAS testing was performed in 55 (43%) patients, including all those who failed to achieve SVR, revealing lone NS5A in 5.4%, lone NS3 in 13%, and lone NS5B in 2%. Both NS3 and NS5A RAS were seen in 51%, and RAS in all 3 genes were present in 18% (Figure 3A). One patient was tested and had no known RAS identified. Only 1 patient had the S282S/T NS5B RAS associated with sofosbuvir resistance and this patient achieved SVR12. Y93H NS5A RAS were found in 19 patients, of whom 16 (84%) achieved SVR. For those without RAS in both NS3 and NS5A, all achieved SVR12 whereas 24 of 28 (86%) with both NS3 and NS5A RAS achieved SVR12 and 9 of the 10 (90%) with triple NS3 NS5A NS5B achieved SVR12 (P > .05). Of all patients who previously failed SOF/VEL (n = 35), only 51% (18/35) had RAS testing and most of the tested patients presented dual RAS (NS5A NS3) (Figure 3B).

A, RAS distribution among tested patients (n = 55). B, RAS distribution among patients with prior SOF/VEL treatment (n = 18). Abbreviation: RAS, resistance-associated substitution.
Weight-based ribavirin was added to SVV in 26 patients due to past SOF/VEL use (n = 8), complex RAS profiles (n = 16), and/or cirrhosis (n = 9). No clear benefit of adding RBV was observed, with similar rates of SVR with and without its use (ribavirin: 28/31 (90.3%) vs no ribavirin: 95/97 (98%), P = .09). Only 2 of 32 patients with prior exposure to SOF/VEL received RBV in combination with SVV for retreatment and neither achieved SVR.
DISCUSSION
Our study comprises a large North-American cohort of patients who did not respond to DAA therapy and were retreated with SVV. This real-life prospective study included 128 patients from 17 centers across Canada and showed an overall SVR12 rate of 96%. Our overall SVR12 rate was similar to the POLARIS-1 trial [6] and a recent European study [14] that included 179 DAA failure patients retreated with SVV. However, we found that patients previously treated with SOF/VEL had a lower SVR12 rate (88.5%) with SVV than patients treated with other DAA regimens (99%) (P < .01).
Notably few patients with prior SOF/VEL experience were included in the POLARIS-1 trial. Of the four genotype 3 patients in POLARIS‐1 who did not achieve SVR12, 2 had previously received SOF/VEL. Similar to our results, a study of 573 DAA-experienced patients retreated with SVV in the Veterans Affairs (VA) Health system, showed a lower SVR12 rate among patients previously treated with SOF/VEL (84%) than with other regimens (91%) [15]. However, not all studies have replicated this finding. In a small cohort of 31 patients with a mix of HCV genotypes 1 through 5 who had experienced virologic relapse after prior SOF/VEL, retreatment with 12 weeks of SVV resulted in SVR12 for all [16].
The reason for DAA failure is often due to the presence of pre-existing RAS, in both the treatment-naïve, and particularly in the treatment-experienced setting. Large studies have not identified signature RAS patterns that are associated with treatment failure with SVV, which has led many clinicians to forego RAS testing prior to retreatment. Indeed in our cohort, only 55 of 128 (43%) patients had RAS testing performed despite the fact that RAS testing is available and free-of-charge to patient or provider throughout Canada. Although all patients who failed had baseline RAS, and 4 of 5 had dual-class (NS3 and NS5A) resistance detected, no signature RAS was associated with treatment failure in our cohort. While some might argue that this fact suggests RAS testing prior to retreatment may not be necessary, it is only with collection of well-collated RAS data on large numbers of patients that we will have the potential to identify specific substitutions associated with treatment failure. Indeed, after initial reports that RAS did not affect responses to SOF/LDV, subsequent careful analysis showed that for patients with G1a infection and prior interferon/ribavirin exposure, SVR12 was achieved in only 76% with baseline NS5A RAS compared to 97% without [17], highlighting the importance of ongoing data collection. In this study, the SVR12 rate was only 84% (16 of 19) among those with the Y93H RAS in NS5A, which is associated with high-level velpatasvir resistance, particularly in genotype 3 HCV. Although the presence of Y93H was not associated with lower SVR12 in this cohort (16 of 19 (84%) vs 33 of 35 (94%), P = .33), the relatively low rate of RAS testing may have limited the power to see this difference.
RBV was used at the discretion of the treating provider, most commonly in patients with complex RAS profiles and/or cirrhosis. There was no demonstrable benefit of RBV use. In fact, its use was associated with an insignificantly lower SVR12 rate (90% vs 98%). This finding should be interpreted with caution, as RBV was specifically used in patients with a perceived higher risk of nonresponse. However these data would suggest that RBV use is not likely the sole solution for complex RAS profiles. Whether alternative regimens, longer therapy with or without RBV would be of benefit, remains unknown.
Although it is not clear why patients treated with SOF/VEL fare slightly worse with SVV than those who had failed a different regimen, there are a number of possible explanations. First, SOF/VEL includes 2 of the 3 agents in SVV. As such, any factors associated with SOF/VEL failure, including but not limited to the presence of RAS, may similarly influence the effect of at least 2 of the 3 agents in SVV. We did not identify factors such as drug–drug interactions or adverse events to SOF/VEL or SVV to explain treatment failure. We did find, however, that factors associated with a lower SVR12 rate such as cirrhosis, genotype 3, and multiple RAS were more prevalent among the patients who failed SVV [14–16, 18]. The small number of overall SVV failures precluded multivariable analysis to identify independent factors associated with treatment failure.
All non-SVR patients had at least dual NS3-NS5A RAS, including Y93H, which was present in 3 of 5. All 5 relapsers had previously received a combination including sofosbuvir plus an NS5A inhibitor (1 daclatasvir and 4 velpatasvir) with or without RBV. Degasperi et al [16], similarly reported that all who failed SVV had received an NS5A-containing regimen. However, in contrast to our findings, they did not find a clear association between SVV failure and any specific past regimen, including SOF/VEL. This may partially reflect a smaller proportion of their cohort having received SOF/VEL than in our study (28% in ours vs 20% in their study), however the small numbers preclude strong conclusions. Notably, unlike Degasperi et al., we did not find that cirrhosis was associated with SVV failure.
Our cohort included 44% (56/128) cirrhotic patients of whom 10% (13/128) had a history of prior (but not current) decompensated liver disease (ascites, hepatic encephalopathy, gastrointestinal bleeding) at time of retreatment with SVV. These patients were all treated prior to the FDA warning contraindicating the use of protease inhibitors in patients with decompensated cirrhosis. Fortunately we did not observe further decompensation or other adverse events in the patients with decompensated cirrhosis who received SVV and overall their response rate was 92% (12/13). Our findings notwithstanding, protease inhibitors should not be prescribed in the setting of decompensation and should be used with great caution in patients with a history of such events.
Our study has important limitations. Although it is a relatively large cohort of patients treated after DAA failure, the numbers of patients are still somewhat limited, owing to the very high initial response rates with DAAs. The small number of SVV failures in our cohort limits strong conclusions about the predictors of SVV response. Indeed, although we found that patients treated with SOF/VEL prior to SVV had a lower SVR12 rate, there were potentially other relevant confounders such as a history of liver transplant, cirrhosis and RAS pattern that are difficult to disentangle with small numbers of patients. RAS testing was incomplete in the cohort, which likely reflects clinical practice in many parts of the world, which unfortunately may make it difficult to identify signature RAS patterns that predict SVV failure. Other limitations include incomplete data on the prevalence of diabetes and alcohol use, as well as other correlates of treatment outcome.
SVV was highly effective as a salvage regimen after nonresponse to a course of HCV DAAs. However, response to SVV was lower in patients with at least 2 of the following factors: cirrhosis, genotype 3, past liver transplantation, previous treatment with SOF/VEL, and/or complex resistance profiles. With continued use of SVV as a retreatment strategy, correlates of nonresponse may emerge to better select patients for alternate or intensified retreatment strategies.
Notes
Acknowledgments. The CANUHC network is supported by unrestricted grants from Gilead Science, AbbVie, and Merck.
Disclosures. F. Q. O. has no conflicts of interest to disclose. C. C. has participated on the advisory board of, has served as speaker for, and has received research funding from Gilead, Merck, and AbbVie. S. M. B. has no conflicts of interest to disclose. M. L. V. has been consultant for, and has received lecture honoraria from AbbVie, Gilead, Merck; and has participated in HCV clinical trials of AbbVie and Merck. A. R. has received consulting fees from AbbVie, Allergen, Assembly, Celgene, Gilead, Janssen, Intercept, Galmed, Merck, Springbanks, Novartis, grants from AbbVie, Gilead, Intercept, and Merck, and reports clinical trials with AbbVie, Allergan, Assembly, Gilead, Janssen, Intercept, Novartis, Springbank, and Merck. L. L. has no conflicts of interest to disclose. A. W. has received grants and research support from, has served as speaker of, has received consulting fees, and has participated in clinical trial of Gilead, AbbVie, and Merck. J. B. has no conflicts of interest to disclose. I. S. has no conflicts of interest to disclose. H. M. has no conflicts of interest to disclose. S. L. has received research funds from, and has been served as consultant/ad boards of AbbVie, Gilead, Merck, Novartis, Pendopharm, Oncoustics, London Drugs; and has been speaker of AbbVie, Gilead, Merck, Pendopharm. B. C. has received honoraria, research grants, and has served on advisory boards for AbbVie, Gilead, and Merck. J. F. has received research grants from AbbVie, Alexion, Eiger, Enanta, Gilead, Janssen, Wako/Fujifilm, and has acted as consultant for AbbVie, Enanta, Gilead, Glaxo-Smith Kline, Roche, and Arbutus.
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.




