-
PDF
- Split View
-
Views
-
Cite
Cite
Cristina Díez, Juan Berenguer, Luis Ibañez-Samaniego, Elba Llop, Leire Pérez-Latorre, María V Catalina, Víctor Hontañón, María A Jiménez-Sousa, Teresa Aldámiz-Echevarría, Javier Martínez, José Luis Calleja, Agustín Albillos, José M Bellón, Salvador Resino, Juan González-García, Rafael Bañares, Persistence of Clinically Significant Portal Hypertension After Eradication of Hepatitis C Virus in Patients With Advanced Cirrhosis, Clinical Infectious Diseases, Volume 71, Issue 10, 15 November 2020, Pages 2726–2729, https://doi.org/10.1093/cid/ciaa502
Close - Share Icon Share
Abstract
This prospective study of 34 patients with HCV cirrhosis (17 HIV positive) with baseline clinically significant portal hypertension (CSPH; HVPG ≥10 mmHg) and SVR after DAA therapy showed that disappearance of CSPH (primary endpoint) is a rare event (6/18 patients; 18%), indicating a persistent risk of clinical progression or death.
(See the Editorial Commentary by Seth and Sherman on pages 2730–1.)
Hepatic venous pressure gradient (HVPG) is the most accurate predictor of liver-related outcomes in patients with compensated cirrhosis [1] and has been independently associated with survival in patients with decompensated cirrhosis [2]. The impact of direct-acting antiviral agent (DAA)–based therapy on HVPG in patients with hepatitis C virus (HCV)–related cirrhosis has been assessed in the short term in 2 studies, neither of which included patients with both human immunodeficiency virus (HIV) and HCV [3, 4]. We assessed changes in HVPG following sustained virologic response (SVR) after all-oral DAA therapy in patients with HCV-related cirrhosis with or without HIV. We also assessed the relationship between changes in HVPG and changes in plasma biomarkers that are characteristic of advanced liver disease.
METHODS
We performed a multicenter prospective study of patients with advanced HCV-related cirrhosis initiating anti-HCV therapy with all-oral DAAs between January and December 2015 in 4 centers in Madrid, Spain. The eligibility criteria for this study were as follows:
Presence of HCV-RNA in plasma
Advanced cirrhosis, defined as any of the following criteria: (1) history of liver decompensation, (2) Child-Pugh-Turcotte score >6, and (3) liver stiffness ≥25 kPa
Noninclusion on the liver transplant waiting list
Prospect of initiation of all-oral DAA therapy
Clinically significant portal hypertension, defined as an HVPG ≥10 mmHg.
The study was approved by the Ethics Committee of Hospital General Universitario Gregorio Marañón.
Clinical and laboratory variables were collected at baseline, every 4 weeks during anti-HCV treatment, and every 12 weeks after discontinuation of therapy until 48 weeks after the completion of treatment. Liver fibrosis, HVPG, and biomarkers were assessed at baseline and 48 weeks after discontinuation of antiviral treatment. Liver fibrosis was assessed using transient elastography (FibroScan; Echosens, Paris, France). HVPG determinations were performed following well-established recommendations [5]. We analyzed various plasma biomarkers related to the following: (1) bacterial translocation, including lipopolysaccharide-binding protein (LBP) and soluble CD14 (sCD14); (2) inflammatory cytokines, including interleukin (IL) 6 and IFN-γ–inducible protein 10 (IP-10); (3) biomarkers of endothelial dysfunction, including soluble intercellular cell adhesion molecule 1 (sICAM1) and soluble vascular cell adhesion molecule 1 (sVCAM1); and (4) coagulation biomarkers, including plasminogen activator inhibitor-1 (PAI-1) and D-dimer [6].
The primary endpoint was a decrease in HVPG to <10 mmHg, the parameter defining clinically significant portal hypertension. The secondary endpoint was the achievement of a clinically significant decrease in HVPG, defined as one of the following goals: (1) an HVPG <10 mmHg in patients with compensated cirrhosis without esophageal varices, (2) a decrease in HVPG ≥10% in patients with compensated cirrhosis and esophageal varices, or (3) an HVPG <12 mmHg or a decrease ≥20% in HVPG in patients with decompensated cirrhosis [7].
Pearson’s correlation coefficient and Spearman’s ρ were used to assess the correlations between HPVG and changes in liver stiffness and plasma biomarkers. Univariable and multivariable logistic regression analyses were used to identify baseline variables associated with the primary and secondary outcomes. Linear mixed models for longitudinal data were used to account for repeated measures of plasma biomarkers, with primary and secondary endpoints and time and the interaction between them taken as fixed effects and the patient as a random effect.
RESULTS
A total of 57 patients signed the informed-consent document and underwent a portal hemodynamic study. Six patients were not included in the analysis because of a baseline HVPG <10 mmHg. Of the 51 remaining patients, 17 were excluded from the analysis for different reasons: 7 because they did not achieve SVR and did not undergo a second hemodynamic study (discontinuation of treatment, n = 4; relapse of HCV, n = 2; and death, n = 1), 5 because they refused to undergo a second hemodynamic study at 48 weeks despite having achieved an SVR, and 5 because they initiated nonselective ß-blockers during the study period. In comparison with the 34 patients included in the final analysis, the 17 patients not included were less likely to have HIV and had a lower serum albumin concentration. However, no differences were found between the groups in age, sex, body mass index, liver decompensation, high alcohol intake, methadone use, statin use, prior anti-HCV therapy, HCV genotype, HCV-RNA, platelet count, Child-Pugh-Turcotte score, model for end-stage liver disease score, liver stiffness, or HVPG (Supplementary Table 1).
Of the 34 patients included in the final analysis (all with SVR), 17 had decompensated liver cirrhosis and 17 had HIV. Baseline characteristics are shown in Supplementary Table 2. Anti-HCV regimens included ledipasvir/sofosbuvir (n = 13), ombitasvir/paritaprevir/ritonavir plus dasabuvir (n = 9), daclatasvir/sofosbuvir (n = 7), simeprevir/sofosbuvir (n = 4), and sofosbuvir/ribavirin (n = 1).
From baseline to week 48 after completion of therapy, the platelet count increased by 9 × 109/L (P = .038) and the serum albumin concentration by 0.50 g/dL (P < .001) (Supplementary Table 3).
HVPG decreased significantly from baseline to week 48 after completing HCV therapy, independently of previous decompensation or having HIV (Figure 1A). The median (interquartile range [IQR]) decrease in HVPG was 3.1 (2.0–4.9) mmHg overall, 3.5 (2.3–5.5) mmHg for compensated patients, 2.5 (1.3–4.4) mmHg for decompensated patients, 2.5 (0.5–3.5) mmHg for HCV-monoinfected patients, and 4.0 (2.0–5.5) mmHg for patients with HIV/HCV (Supplementary Figure 1). Of note, HVPG increased from baseline to week 48 after completion of therapy in 4 patients (11.8%), 3 of whom were HCV monoinfected and 2 of whom had liver decompensation.
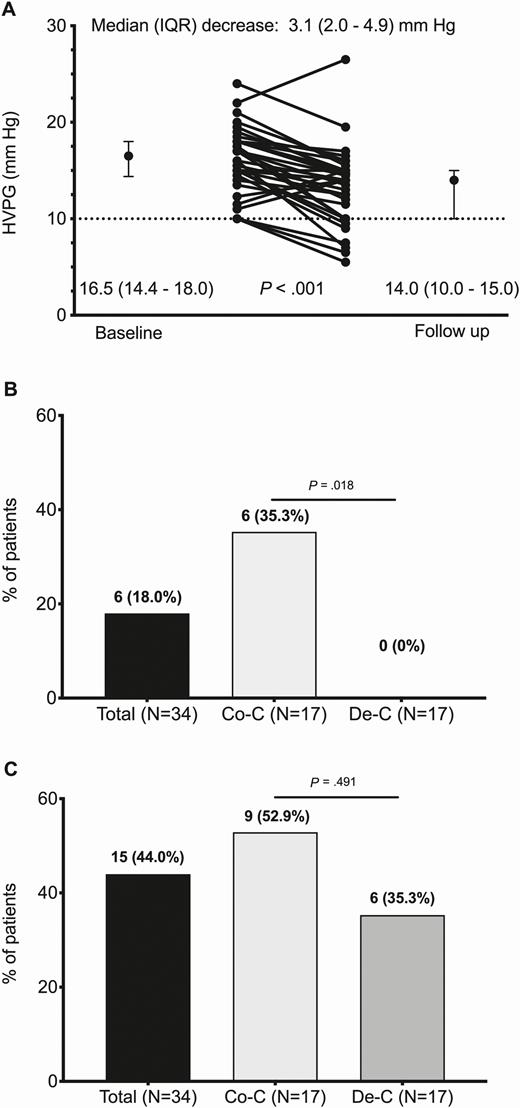
A, Changes in HVPG from baseline to week 48 after completion of HCV therapy. B, Main endpoint defined as a decrease in HVPG to <10 mmHg, the parameter defining clinically significant portal hypertension. C, Secondary endpoint defined as one of the following goals: (1) an HVPG <10 mmHg in patients with compensated cirrhosis without esophageal varices, (2) a decrease ≥10% in HVPG in patients with compensated cirrhosis with esophageal varices, or (3) an HVPG <12 mmHg or a decrease ≥20% in HVPG in patients with decompensated cirrhosis. Abbreviations: Co-C, compensated cirrhosis; De-C, decompensated cirrhosis; HCV, hepatitis C virus; HVPG, hepatic venous pressure gradient; IQR, interquartile range.
Liver stiffness also decreased significantly in all patients, independently of previous decompensation or coinfection with HIV (Supplementary Figure 2). Overall, the median (IQR) decrease in liver stiffness from baseline to week 48 was 7.85 (0.35–15.50) kPa.
A weak nonsignificant correlation was found between changes in liver stiffness and changes in HVPG from baseline to week 48 (Spearman’s ρ, 0.235; P = .196) (Supplementary Figure 3).
The plasma concentration of all plasma biomarkers, except for PAI-1, decreased from baseline to week 48 after completion of therapy, although this decrease was statistically significant only for LBP, IP-10, sVCAM1, and sICAM1 (Supplementary Table 4).
The primary endpoint was achieved by 6 patients overall (18%), all of whom had compensated cirrhosis (35.3%); no patients with decompensated cirrhosis achieved the primary endpoint (P = .018) (Figure 1B). The secondary endpoint was achieved by 15 patients overall (44.0%), by 9 patients with compensated cirrhosis (52.9%) and by 6 patients with decompensated cirrhosis (35.3%) (P = .491) (Figure 1C). In the multivariable analysis, only HVPG was independently associated with the primary endpoint (adjusted odds ratio [95% confidence interval], .53 [.32–.78]; P = .002) (Supplementary Table 5). None of the variables analyzed were associated with the secondary endpoint in the univariable analysis.
None of the patients experienced worsening of liver function or hepatocellular carcinoma during the study period.
DISCUSSION
In this cohort of 34 patients with HCV-related cirrhosis and clinically significant portal hypertension (CSPH), portal pressure decreased significantly after clearance of HCV, irrespective of whether the patients had previous decompensation or whether they had HIV. However, in fewer than 20% of patients, the follow-up hemodynamic study revealed an HVPG <10 mmHg, which is indicative of the disappearance of CSPH. None of the patients with decompensated cirrhosis achieved this primary endpoint, although it was achieved in approximately one-third of those with compensated liver disease. A clinically significant decrease in HVPG (the secondary endpoint) was achieved in more than half of the patients with compensated cirrhosis and slightly more than one-third of patients with decompensated cirrhosis, which is remarkable as this endpoint has been associated with a reduction in the risk of death in patients with portal hypertension [7].
In a retrospective study of 41 patients with CSPH and SVR after DAA therapy, follow-up HVPG was <10 mmHg in only 10 patients (24%) [3]. A prospective study of 225 patients monoinfected with HCV with cirrhosis and CSPH showed that CSPH persisted in 78% of patients despite SVR after all-oral DAA therapy. This study was limited because the second portal pressure study was performed only 24 weeks after completion of therapy [4].
We also found that viral eradication was associated with a significant decrease in the plasmatic biomarkers of bacterial translocation, inflammation, and endothelial dysfunction, suggestive of improvement in the cirrhosis-associated immune dysfunction [8]. We found that baseline HVPG was the only variable independently associated with the primary endpoint. Liver stiffness decreased in all patients; however, we found a weak and nonsignificant correlation between changes in liver stiffness and changes in HVPG.
Our study is limited by its small sample size. However, it has several strengths, including its prospective design, the relatively long interval between discontinuation of treatment and determination of the follow-up portal hemodynamic study, and the inclusion of a large proportion of patients with HIV/HCV.
In conclusion, our findings suggest that successful DAA therapy in patients with cirrhosis and CSPH is associated with a decrease in HVPG that may reduce the risk of liver complications and death, particularly in compensated patients. However, the frequent persistence of CSPH despite successful antiviral therapy indicates a persistent risk of clinical progression or death. Transient elastography may not be an accurate method for estimating changes in HVPG following SVR.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
The Efectos de la Erradicación del VHC en Pacientes con Cirrosis Avanzada por VHC. Una Aproximación TraslacionalStudy Group. Cristina Díez, Luis Ibáñez, Leire Pérez-Latorre, Diego Rincón, Teresa Aldámiz-Echevarría, Vega Catalina, Pilar Miralles, Teresa Aldámiz-Echevarría, Francisco Tejerina, María C. Gómez-Rico, Esther Alonso, José M. Bellón, Rafael Bañares, and Juan Berenguer(Hospital General Universitario Gregorio Marañón, Madrid, Spain);José Arribas, José I. Bernardino, Carmen Busca, Javier García-Samaniego, Víctor Hontañón, Luz Martín-Carbonero, Rafael Micán, María L. Montes-Ramírez, Victoria Moreno, Antonio Olveira, Ignacio Pérez-Valero, Eulalia Valencia, and Juan González-García(Hospital Universitario La Paz/IdiPAZ, Madrid, Spain);Elba Llop and José Luis Calleja(Hospital Universitario Puerta de Hierro, Madrid, Spain);Javier Martínez and Agustín Albillos(Hospital Universitario Ramón y Cajal, Madrid, Spain);Marta de Miguel, María Yllescas, and Herminia Esteban(Fundación SEIMC/GeSIDA, Madrid, Spain).
Acknowledgments. The authors thank Thomas O’Boyle for writing assistance during the preparation of the manuscript.
Financial support. This work was supported by the Instituto de Salud Carlos III (ISCII) (grant numbers PI14/01094, PI14-01581, PI14CIII/00011); the Spanish AIDS Research Network (grant numbers RD16/0025/0017, RD16/0025/0018), which is included in the Spanish I+D+I Plan and is cofunded by ISCIII-Subdirección General de Evaluacion and European Funding for Regional Development (FEDER); the CIBER Theme-Based Research Area for Liver and Digestive Diseases (CIBEREHD), which is funded by ISCIII.
Potential conflicts of interest. J. B. reports grants from Gilead, Merck Sharpe and Dohme (MSD), and Viiv, and personal fees from Gilead, Janssen, MSD, and Viiv Healthcare, outside the submitted work. R. B. reports educational activity fees from Gilead, Abbvie, and MSD, outside the submitted work. J. G.-G. reports grants and personal fees from Gilead, Merck Sharpe and Dohme, Janssen and Cilag, and Viiv Healthcare, and nonfinancial support from Gilead, outside the submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.




