-
PDF
- Split View
-
Views
-
Cite
Cite
Cinthya Maria Neves Varandas, José Lucas Sena da Silva, Janeusa Rita L Primo, Maria de Fátima S P de Oliveira, Otávio Moreno-Carvalho, Lourdes Farre, Achiléa L Bittencourt, Early Juvenile Human T-cell Lymphotropic Virus Type-1–Associated Myelopathy/Tropical Spastic Paraparesis: Study of 25 Patients, Clinical Infectious Diseases, Volume 67, Issue 9, 1 November 2018, Pages 1427–1433, https://doi.org/10.1093/cid/ciy289
Close - Share Icon Share
Abstract
Human T-cell lymphotropic virus type-1 (HTLV-1) may cause severe diseases such as HTLV-1–associated myelopathy/tropical spastic paraparesis (HAM/TSP) and infective dermatitis associated with HTLV-1 (IDH). The clinical characteristics and progression of 25 early onset HAM/TSP associated or not to IDH were described.
Following-up 37 IDH patients with neurological examinations, 54% developed HAM/TSP. To these cases were added 5 cases of juvenile HAM/TSP. The patients were HTLV-1+ and were submitted to dermatological and neurological examinations. Diagnosis of HAM/TSP was performed according to Osame et al (1990) and Castro-Costa et al (2006) criteria.
Twenty-one patients were classified as definite HAM/TSP by both criteria, 3 as probable HAM/TSP by Osame et al, and another as probable HAM/TSP according to Castro-Costa et al Median age at onset of neurological manifestations was 9 years for the IDH/HAM/TSP group and 16 years for the HAM/TSP group (P = .045). In 12 patients, the onset of neurological manifestations occurred when they were less than 10 years of age. In the group IDH/HAM/TSP, the neurological symptoms always begun during the period of activity of IDH. The progression of HAM/TSP evaluated in 17 cases was heterogeneous, and 3 had rapid progressive course.
The juvenile HAM/TSP may occur very early and also presents marked female predominance. Progression of IDH to HAM/TSP before 19 years of age is frequent (54%). Rapid progressive form may also occur in early HAM/TSP. As juvenile IDH and HAM/TSP are due to vertical transmission through breastfeeding, it is very important to avoid this pathway of infection.
Human T-cell lymphotropic virus type-1 (HTLV-1) infects 5 to 10 million people worldwide [1]. The highest areas of prevalence are located in Southwestern Japan, the Caribbean, sub-Saharan Africa, South America, and foci in the Middle East and Australo-Melanesia. In Salvador, Bahia (Brazil), a city whose population is predominantly of African descent, the mean prevalence of HTLV-1 is 1.76% [2]. This retrovirus has been implicated in several diseases, such as HTLV-1–associated myelopathy/tropical spastic paraparesis (HAM/TSP) and infective dermatitis associated to HTLV-1 (IDH). Most carriers remain asymptomatic; however, ~ 10% develop diseases [3].
HAM/TSP is a myelopathy generally of insidious onset characterized by mild sensory involvement, bladder disturbances, and slow progressive spastic paraparesis that affects mainly the pyramidal tracts [4]. However, cases with rapid progressive disease have been reported [5]. Even though HAM/TSP has been characterized as an adult-onset disease [6, 7], 27 cases with early onset have already described, 10 of them registered in the state of Bahia, Brazil [3]. IDH is a recurrent, infected chronic eczema that affects HTLV-1 vertically infected children [8]. The association of HAM/TSP and IDH (IDH/HAM/TSP) has already been described in childhood and adolescence [8].
The aim of this study was to evaluate the clinical characteristics and progression of early onset HAM/TSP associated or not to IDH. Here we describe 25 of these patients.
MATERIAL AND METHODS
Study Population
Between 2002 and 2017, in an outpatient clinic of the Faculty of Medicine of the Federal University of Bahia, Brazil, 25 juvenile patients were diagnosed with HAM/TSP, 20 of them associated with IDH. All patients were from the state of Bahia, Brazil, African descendants and had low socioeconomic status. The patients were serologically positive for HTLV-1 (ELISA with confirmation by Western blot and/or polymerase chain reaction [PCR]—PCR targeting HTLV-1 tax gene in peripheral blood mononuclear cells) and seronegative for HIV1/2, syphilis, and hepatitis B and C. They were submitted to dermatological and neurological examination and the HAM/TSP follow-up was ≥ 1year, median of 8 years (from 1 to 15 years) for the IDH/HAM/TSP, and median of 2 years (from 1 to 3 years) for the HAM/TSP group without IDH (Table 1). Obstetric, breastfeeding, and neuro-psychomotor development (NPMD) data were collected. The mothers and siblings were also submitted for HTLV-1 serology and dermatological and neurological examinations. The 20 IDH/HAM/TSP patients were found in a cohort of 42 IDH patients (it is important to note that neurological examination was only possible in 37 of them). Because of this, they were being followed up since IDH diagnosis. Therefore, the total follow-up period for patients with IDH/HAM/TSP was longer, 10 years in median (from 2 to 20 years). The HAM/TSP patients without IDH were identified among the siblings of the IDH patients. According to the World Health Organization, adolescence was defined as ≤18 years [9].
Clinical Data of 25 Patients With Myelopathy Associated With Human T-cell Lymphotropic Virus Type-1
| . | IDH/HAM/TSP Groupa (20 Cases) . | HAM/TSP Group (5 Cases) . | ||
|---|---|---|---|---|
| Time of breastfeedingb | 30 (0.4–60) | 24 (24–48) | ||
| Male/female ratio | 1/4 | 1/4 | ||
| Age at onset of neurological symptomsc | 9 (3–18) | 16 (11–18) | ||
| Age at HAM/TSP diagnosisc | 12,5 (4–20) | 17 (11–18) | ||
| Time of follow-up of HAM/TSPc | 8 (1–15) | 2 (1–3) | ||
| Duration of HAM/TSPd | 10 (2–20) | 3 (1–16) | ||
| Age at onset of IDH symptomsc | 2 (0.4–9) | … | ||
| Age at IDH diagnosisc | 8.5 (1–16) | … | ||
| Age at IDH remissionc,e | 17.50 (11–26) | … | ||
| Time between both diagnosesc | 4 (0–10) | … | ||
| Initial OMDS (grades)f | 1 (1–8) | 1 (1–2) | ||
| Final OMDS | 3 (1–9) | 3 (2–3) | ||
| Initial EDSS | 2 (1–7.5) | 1.5 (1–2.5) | ||
| Final EDSS | 3 (2–8) | 2.5 (1–3.5) | ||
| Neurological manifestations | Initial | At last visit | Initial | At last visitf |
| Sensory symptoms | 63% | 84% | 60% | 80% |
| Urinary complaints | 42% | 58% | 20% | 60% |
| Constipation | 37% | 74% | 20% | 40% |
| Piramidal signs | 84% | 89% | 80% | 80% |
| Gait disturbances | 79% | 100% | 40% | 100% |
| . | IDH/HAM/TSP Groupa (20 Cases) . | HAM/TSP Group (5 Cases) . | ||
|---|---|---|---|---|
| Time of breastfeedingb | 30 (0.4–60) | 24 (24–48) | ||
| Male/female ratio | 1/4 | 1/4 | ||
| Age at onset of neurological symptomsc | 9 (3–18) | 16 (11–18) | ||
| Age at HAM/TSP diagnosisc | 12,5 (4–20) | 17 (11–18) | ||
| Time of follow-up of HAM/TSPc | 8 (1–15) | 2 (1–3) | ||
| Duration of HAM/TSPd | 10 (2–20) | 3 (1–16) | ||
| Age at onset of IDH symptomsc | 2 (0.4–9) | … | ||
| Age at IDH diagnosisc | 8.5 (1–16) | … | ||
| Age at IDH remissionc,e | 17.50 (11–26) | … | ||
| Time between both diagnosesc | 4 (0–10) | … | ||
| Initial OMDS (grades)f | 1 (1–8) | 1 (1–2) | ||
| Final OMDS | 3 (1–9) | 3 (2–3) | ||
| Initial EDSS | 2 (1–7.5) | 1.5 (1–2.5) | ||
| Final EDSS | 3 (2–8) | 2.5 (1–3.5) | ||
| Neurological manifestations | Initial | At last visit | Initial | At last visitf |
| Sensory symptoms | 63% | 84% | 60% | 80% |
| Urinary complaints | 42% | 58% | 20% | 60% |
| Constipation | 37% | 74% | 20% | 40% |
| Piramidal signs | 84% | 89% | 80% | 80% |
| Gait disturbances | 79% | 100% | 40% | 100% |
Abbreviations: EDSS, expanded disability status score; HAM/TSP, HTLV-1–associated myelopathy/tropical spastic paraparesis; IDH, infective dermatitis associated with HTLV-1; OMDS, Osame motor disability score.
aThe dermatological aspects of 17 of these cases were described previously [8].
bValues expressed in median (months) and range.
cValues expressed in median (years) and range.
dDuration of HAM/TSP was calculated since onset of neurological manifestations.
eRemission occurred in only 8 patients.
fFour patients were not included, 3 of them due to the short follow-up and the other one due to the absence of motor disabilities.
Clinical Data of 25 Patients With Myelopathy Associated With Human T-cell Lymphotropic Virus Type-1
| . | IDH/HAM/TSP Groupa (20 Cases) . | HAM/TSP Group (5 Cases) . | ||
|---|---|---|---|---|
| Time of breastfeedingb | 30 (0.4–60) | 24 (24–48) | ||
| Male/female ratio | 1/4 | 1/4 | ||
| Age at onset of neurological symptomsc | 9 (3–18) | 16 (11–18) | ||
| Age at HAM/TSP diagnosisc | 12,5 (4–20) | 17 (11–18) | ||
| Time of follow-up of HAM/TSPc | 8 (1–15) | 2 (1–3) | ||
| Duration of HAM/TSPd | 10 (2–20) | 3 (1–16) | ||
| Age at onset of IDH symptomsc | 2 (0.4–9) | … | ||
| Age at IDH diagnosisc | 8.5 (1–16) | … | ||
| Age at IDH remissionc,e | 17.50 (11–26) | … | ||
| Time between both diagnosesc | 4 (0–10) | … | ||
| Initial OMDS (grades)f | 1 (1–8) | 1 (1–2) | ||
| Final OMDS | 3 (1–9) | 3 (2–3) | ||
| Initial EDSS | 2 (1–7.5) | 1.5 (1–2.5) | ||
| Final EDSS | 3 (2–8) | 2.5 (1–3.5) | ||
| Neurological manifestations | Initial | At last visit | Initial | At last visitf |
| Sensory symptoms | 63% | 84% | 60% | 80% |
| Urinary complaints | 42% | 58% | 20% | 60% |
| Constipation | 37% | 74% | 20% | 40% |
| Piramidal signs | 84% | 89% | 80% | 80% |
| Gait disturbances | 79% | 100% | 40% | 100% |
| . | IDH/HAM/TSP Groupa (20 Cases) . | HAM/TSP Group (5 Cases) . | ||
|---|---|---|---|---|
| Time of breastfeedingb | 30 (0.4–60) | 24 (24–48) | ||
| Male/female ratio | 1/4 | 1/4 | ||
| Age at onset of neurological symptomsc | 9 (3–18) | 16 (11–18) | ||
| Age at HAM/TSP diagnosisc | 12,5 (4–20) | 17 (11–18) | ||
| Time of follow-up of HAM/TSPc | 8 (1–15) | 2 (1–3) | ||
| Duration of HAM/TSPd | 10 (2–20) | 3 (1–16) | ||
| Age at onset of IDH symptomsc | 2 (0.4–9) | … | ||
| Age at IDH diagnosisc | 8.5 (1–16) | … | ||
| Age at IDH remissionc,e | 17.50 (11–26) | … | ||
| Time between both diagnosesc | 4 (0–10) | … | ||
| Initial OMDS (grades)f | 1 (1–8) | 1 (1–2) | ||
| Final OMDS | 3 (1–9) | 3 (2–3) | ||
| Initial EDSS | 2 (1–7.5) | 1.5 (1–2.5) | ||
| Final EDSS | 3 (2–8) | 2.5 (1–3.5) | ||
| Neurological manifestations | Initial | At last visit | Initial | At last visitf |
| Sensory symptoms | 63% | 84% | 60% | 80% |
| Urinary complaints | 42% | 58% | 20% | 60% |
| Constipation | 37% | 74% | 20% | 40% |
| Piramidal signs | 84% | 89% | 80% | 80% |
| Gait disturbances | 79% | 100% | 40% | 100% |
Abbreviations: EDSS, expanded disability status score; HAM/TSP, HTLV-1–associated myelopathy/tropical spastic paraparesis; IDH, infective dermatitis associated with HTLV-1; OMDS, Osame motor disability score.
aThe dermatological aspects of 17 of these cases were described previously [8].
bValues expressed in median (months) and range.
cValues expressed in median (years) and range.
dDuration of HAM/TSP was calculated since onset of neurological manifestations.
eRemission occurred in only 8 patients.
fFour patients were not included, 3 of them due to the short follow-up and the other one due to the absence of motor disabilities.
IDH diagnosis was made according to established criteria [8]. In Figure 1, the dermatologic lesions of one representative patient are shown. Remission of IDH was considered when a patient off treatment remained free of disease for at least 6 months. HAM/TSP was diagnosed in accordance with Osame et al criteria (1990) [4]. HAM/TSP cases were classified as definite or probable. HAM/TSP was considered as definite when both progressive paraparesis and anti-HTLV-1 antibodies in blood and cerebrospinal fluid (CSF) were present, whereas the probable HAM/TSP represented progressive myelopathy in patients with anti-HTLV-1 antibodies in CSF or blood but not in both [4]. Neurological disability was assessed using Osame motor disability score (OMDS) [10] and using the expanded disability status score (EDSS) [11]. The Castro-Costa et al (1996) [12] classification was also employed. The duration of HAM/TSP was defined as the time of onset of neurological manifestations to the last visit and the duration of HAM/TSP follow-up from diagnosis to the last visit. The interval between the diagnoses of IDH and HAM/TSP was also recorded. Neurological manifestations were judged to be asymmetric when either motor weakness, spasticity, or sensory impairment of lower extremities differed clearly between sides. For CSF specimens, obtained in all patients, except three, cell counts and total protein and immunoglobulin levels were measured. Direct microorganism search (using Ziehl and Gram stains and India ink) and serological analysis for some infections (HTLV-1 infection, toxoplasmosis, cysticercosis, syphilis, and schistosomiasis) was also performed. PCR analysis using DNA amplification of the proviral tax sequences of HTLV-1 were also performed in CSF. Besides the 25 HAM/TSP patients, 3 other patients had neurological manifestations but were excluded from the study, 2 because they had no paraparesis and did not perform CSF examination, and one other with paraparesis because she presented antibodies anti-Schistosoma mansoni in the liquor.
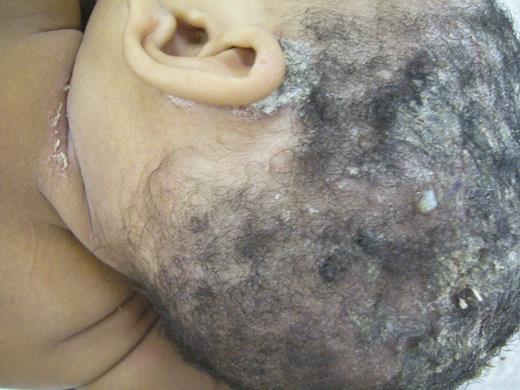
Dermatological lesions of a representative infective dermatitis associated with Human T-cell Lymphotropic Virus Type-1 (IDH) patient. Dermatological aspects of an IDH 2-years-old female with an extensive erythematous-scaly lesion covered with adherent yellowish crusts involving the scalp, the retroauricular region and the neck. Abbreviation: IDH, infective dermatitis associated with Human T-cell Lymphotropic Virus Type-1.
Follow-up
Most of the patients (IDH/HAM/TSP and HAM/TSP) were examined at least once a year; however, in some cases this period was longer due to the socioeconomic conditions of this population. At each visit, neurological and dermatological examinations were performed including measurement of OMDS and EDSS. The differentiation between slow and rapid progression was made according to Olindo et al (1995) [5]. Seven of IDH/HAM/TSP patients have been previously published [13–15].
Statistical Analysis
GraphPad Prism 5 was used to plot graphs and perform statistical analyses. The Mann-Whitney U-test was used to compare data between groups. P-values < .05 were considered statistically significant.
Ethical Considerations
The study was approved by the Institutional Review Board of the Professor Edgard Santos Teaching Hospital of the Federal University of Bahia, and the parents or legal guardians of the participants gave written informed consent.
RESULTS
Twenty-one of the 25 patients were classified as definite HAM/TSP by Osame et al (1990) [4] and Castro-Costa et al (2006) criteria [12], as they were submitted to liquor examination and had motor disabilities. Another 3 patients were regarded as probable HAM/TSP by Osame et al (1990) [4] because they presented paraparesis with OMDS ≥ 2, but they did not accept to perform lumbar puncture. Another patient was classified as probable HAM/TSP according to Castro-Costa el al. [12] because during the 7 years of follow-up the only neurological manifestations were a positive Babinski sign and hyperreflexia besides the presence of antibodies anti-HTLV-1 in the liquor as well as PCR positive for tax. The median age at HAM/TSP diagnosis was 12.5 years for the IDH/HAM/TSP group and 17 years for the HAM/TSP group (Table 1). Diagnosis was performed after 18 years of age in 4 patients at 19, 19, 20, and 22 years of age, but neurological manifestations started earlier, when they were between 11 and 18 years of age.
The neurologic manifestations began earlier in the patients with IDH association, as well as the HAM/TSP diagnosis (Table 1). The median age at the onset of neurological manifestations was 9 years (ranging from 3 to 18 years) for the IDH/HAM/TSP group and 16 years (ranging from 11 to 18 years) for HAM/TSP group (P = .045). In 12 patients, 11 associated with IDH, the onset of neurological manifestations occurred when they were less than 10 years of age. The onset of these manifestations occurred in the other 13 patients when they had 10 to 18 years of age. From a total of 25 patients, 20 were females (ratio of females to males 4/1). Excluding 3 patients from whom no familial information was obtained, all were born from deliveries without intercurrences, and they had no history of NPMD impairment or familial neurodegenerative diseases. The information about duration of breastfeeding was obtained in 21 patients, in 20 their mothers were HTLV-1 seropositive, and in another, whose mother was seronegative, the child was breastfed by another woman with unknown serology. The duration of breastfeeding varied from 1 week to 60 months (median 30 months) in the group IDH/HAM/TSP and from 24 to 48 months (median 24 months) in the other group. No patient received blood transfusion. Nine patients had 1 to 2 normal pregnancies and were advised not to breastfeed their children.
From the CSF obtained in 22 patients, 21 had antibodies to HTLV-1 and were positive for HTLV-1 infection by PCR. The other one had no antibodies to HTLV-1, but HTLV-1 was detected in the CSF by PCR. Pleocytosis (>4 cells/mm3) was found in 11 of 25 patients, varying from 7.3 to 46 cells/mm3. Only one patient with initial disease (OMDS 1) had hyperproteinorrachia (95 mg/dL), associated with a cell count of 46 cells/mm3. This patient had initially rapid progressive disease.
The clinical aspects of HAM/TSP in both groups are shown in Table 1. The pyramidal signs were the most prevalent initial neurologic manifestations in both groups of patients, followed by gait and sensory disturbances. Asymmetry of neurologic manifestations was observed in 20% of the patients.
In the IDH/HAM/TSP group, the median age of IDH onset was 2 years, and the median period between the diagnoses of both diseases was 4 years (Table 1). Moreover, neurological manifestations occurred before IDH remission, except in one case in which HAM/TSP onset and IDH remission occurred in the same year.
Four pairs of siblings were observed; in 2 pairs all patients had IDH/HAM/TSP, and in the other 2 pairs, each one had one patient with IDH/HAM/TSP, and the other with only HAM/TSP. In respect to the patients’ mothers, only 18 of the 21 could be examined. Ten had HAM/TSP (one of them with associated adulthood IDH), 7 were asymptomatic carriers, and one was seronegative for HTLV-1.
OMDS and EDSS Parameters and Neurological Evolution
The initial and final grades of OMDS and EDSS are included in Table 1. No differences were observed between these two parameters (either initial or final) between the groups IDH/HAM/TSP and HAM/TSP, although follow-up time was much longer in the IDH/HAM/TSP group in relation to the other group. The evolution of the OMDS since the HAM/TSP diagnosis was exemplified in Figure 2. Seven of the 25 patients (3 from the IDH/HAM/TSP group and 4 from the HAM/TSP group) were not included in this evaluation because they had only ≤ 2 years of follow-up. An eighth patient was not included because she did not present motor disabilities (OMDS = 0). However, one patient with 2 years of follow-up was included because he had 10 years of disease and his motor disability increased until OMDS 9.
![Evolution of the OMDS parameter during follow-up since HAM/TSP diagnosis. A, IDH/HAM/TSP patients with rapidly progressive myelopathy (n = 3). B, IDH/HAM/TSP patients with initial rapidly progressive myelopathy, increasing two grades of OMDS in only 1 or 2 years but after that the progression decreased in intensity (n = 3). C, IDH/HAM/TSP patients with myelopathy presenting slow progression (three representative cases). All patients represented here had HAM/TSP with IDH. The evaluation of OMDS parameter was based in established criteria [10] and the evaluation of its progression was based on the Olindo et al’s criteria [5]. Abbreviations: HAM/TSP, HTLV-1–associated myelopathy/tropical spastic paraparesis; IDH, infective dermatitis associated with HTLV-1; OMDS, Osame motor disability score.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/cid/67/9/10.1093_cid_ciy289/1/m_ciy28902.jpeg?Expires=1750295999&Signature=1bBDNA2QqYYQ3vWl9NbXhpC7FaItvmwtL4kkiFNtpHNbLF33bFGeBSfx78CLGyYR1pDAItcWEL52uFgsBv74wdg2VgfenZWcRolXzBGWIVJVMi4HcdQMsaYu4a8xxoB~dry-kXGSfmtoVJz0qTlLG5kdZyqJmGjqx6eywTYbvw7jMRQmTCcbcyIi~THrosmoe5TLaHoITfp5MST7GzuiQX6D0Na7AO75S6BzXpcPrvzCs1jeI6Ilnln9TZHWev0EN8cZtpcX-VYjhg15cNJ-8t2daF-bLxxq7KqGfn7H6Zb4HTgebXbAmGBTXxakuINllwdY~-FOjPzgdr9EPFnyMg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Evolution of the OMDS parameter during follow-up since HAM/TSP diagnosis. A, IDH/HAM/TSP patients with rapidly progressive myelopathy (n = 3). B, IDH/HAM/TSP patients with initial rapidly progressive myelopathy, increasing two grades of OMDS in only 1 or 2 years but after that the progression decreased in intensity (n = 3). C, IDH/HAM/TSP patients with myelopathy presenting slow progression (three representative cases). All patients represented here had HAM/TSP with IDH. The evaluation of OMDS parameter was based in established criteria [10] and the evaluation of its progression was based on the Olindo et al’s criteria [5]. Abbreviations: HAM/TSP, HTLV-1–associated myelopathy/tropical spastic paraparesis; IDH, infective dermatitis associated with HTLV-1; OMDS, Osame motor disability score.
According to Olindo et al (2005) criteria [5], within the IDH/HAM/TSP group, 3 patients demonstrated rapidly progressive myelopathy (Figure 2A). One of them with 6 years of disease progressed to OMDS 7 and died due to rheumatic cardiomyopathy at 14 years of age without remission of IDH. The second patient followed-up during 15 years progressed from OMDS 4 to OMDS 9 and is now 29 years old ; her IDH remission only occurred when she was 26 years old. The third patient, 19 years old at admission, with OMDS 8, progressed in 2 years to OMDS 9. During 10 years of disease, this patient progressed until OMDS 9 and died at 21 years of age due to kidney failure. These cases with rapidly progressive myelopathy had the onset of disease before 12 years of age, and the IDH onset occurred when they were ≤ 2 years old. Three other patients of the IDH/HAM/TSP group initially progressed faster, increasing 2 grades of OMDS in only 1 or 2 years; however, shortly thereafter the progression decreased in intensity, increasing later only in one of them to OMDS 6 (Figure 2B). In these patients the onset of HAM/TSP also occurred before 12 years of age, and in 2 of them the IDH had not yet disappeared at 19 and 27 years of age, respectively. These patients cannot be considered as having rapid progression based on the Olindo et al’s criteria [5]. From the remaining 11 patients, 10 had a slow progression (Figure 2C and Supplementary Figure 1A–G), and one other was stable in grade 2 during 5 years (Supplementary Figure 1H).
Patients’ Treatment
For all patients, vitamin C was prescribed, and in 5 patients additional courses of pulse therapy with methylprednisolone have been employed without modification of the motor disabilities. One patient, who was 12 years old at admission, had the diagnosis of IDH and previously used high-dose corticosteroids for the eczematous disease; during follow-up it was not possible to withdraw the corticosteroids because of the continuous relapses of IDH. By the last follow-up visit, the patient still used corticosteroids and presented active disease, without remission 11 years after admission. Her HAM/TSP had only one degree of elevation during five years. As previously described, only 2 HAM/TSP patients died both with associated IDH.
DISCUSSION
Here we report 25 pediatric HTLV-1 carriers who developed HAM/TSP, 20 of them associated with IDH, all from the state of Bahia, Brazil. The patients were classified in 3 ways, 21 as definite HAM/TSP according to both criteria [4, 12], 3 as probable HAM/TSP according to Osame et al [4], and one associated with IDH, as probable HAM/TSP in agreement with Castro-Costa et al [12]. As previously observed [16], there was concordance between both classifications in reference to definite HAM/TSP. The Castro Costa et al classification [12] enables the diagnosis of initial forms of myelopathy when the patients are mono- or oligosymptomatic as occurred in one patient evaluated here. The myelopathy may begin very early as observed in one case presented here, at 3 years of age. There is a report on a 3-years-old patient diagnosed as probable HAM/TSP whose neurological manifestations began even earlier [17]. In half of the patients of the current study, the disease started when they were 10 years old or less.
The mode of HTLV-1 transmission was vertical probably through breastfeeding. Curiously, in one case, the transmission was probably horizontal by breastfeeding because the patient’s mother was seronegative and the child was breastfed by another woman of unknown serology. A marked female predominance was observed in the cases studied here, as occurs in the adult form of HAM/TSP [6]. The increased incidence rate of HAM/TSP in females cannot be explained in juvenile HAM/TSP by the male-to-female predominant transmission during sexual intercourse as it was suggested in adults [18], because the transmission was vertical.
The major initial manifestations of early onset HAM/TSP were pyramidal signs followed by gait disturbances and sensory symptoms. Asymmetry of symptomatology was observed in 20% of the patients. These aspects do not differ from those observed in adulthood HAM/TSP [16, 19, 20].
The positivity of CSF was assessed by 2 strategies, enzyme-linked immunosorbent assay (ELISA) test to detect HTLV-1 antibodies and PCR to amplify HTLV-1 provirus. The use of these 2 strategies enabled the demonstration of positivity of the CSF in a patient with negative ELISA test. Moreover, it was assessed that 22 HAM/TSP patients had infected cells in the CSF. The cellularity was increased in the CSF in 8 patients and the protein level in only one. These findings were consistent with previous reports, which had already shown that CSF of adult patients with HAM/TSP may present normal results [20]. One study even showed that, over time, these findings tend to become slight or even absent after the second year of disease [21]. So far, this is the largest series of cases describing early progression of IDH to HAM/TSP. Among us, in 2005, 6 patients with IDH and HAM/TSP have been described, they are included in the present series, now with a longer follow-up [14]. In a systematic search and review of the literature about early onset HTLV-1 diseases, among 29 cases of early onset HAM/TSP, 55.6% were associated with IDH [3].
The 20 patients of the IDH/HAM/TSP group were identified among a cohort of 37 IDH patients neurologically evaluated, corresponding to 54% of them. These findings indicate a very high frequency of IDH progression to HAM/TSP, suggesting that IDH may predispose to HAM/TSP development. The factors involved in this frequent association are not known. The appearance of neurological symptoms during the activity of IDH suggests a possible relationship between these 2 inflammatory processes. It is known that both diseases present an over-production of proinflammatory cytokines in relation to asymptomatic carriers [22].
An interesting finding was the high frequency (55%) of HAM/TSP in the patients’ mothers. In the 27 cases of early onset HAM/TSP revised in the literature [3], this percentage was 22%. Clustering of HAM/TSP and IDH has been previously demonstrated in Bahia, Brazil, including some of the cases described here [23]. For better understanding of the familial characteristics of HAM/TSP and IDH, genetic studies must be performed because it is known that some HLA alleles are related to the development of these diseases [24, 25].
The disease progression evaluated in 17 cases, was divided into 4 groups: (1) Three cases with rapid progressive course; (2) 3 with initial rapid onset; (3) 10 with slow progressive course; (4) one without progression (in plateau). As shown, the progression of HAM/TSP was heterogeneous as described in adult patients [5, 19, 26]. We demonstrated the relation between fast progression of HAM/TP and vertical transmission of the virus, differently from adulthood myelopathy, where fast progression has been related to transmission via blood transfusion and to more advanced age [20, 27]. Also, Nakagawa et al (1995) [28] showed that a group of adult patients whose initial neurological symptoms had been described as occurring below 15 years had a slower progression of disease in relation to other group with initial symptoms after 15 years of age. However, Kuroda et al (1991) [26] verified that only young age at onset was significantly associated with rapid deterioration of disease. Here, rapid progressive course was also associated with a long-lasting IDH despite treatment. Three patients, also with associated IDH, had an initially rapid progression increasing grades in one or 2 years with a later slowing. A Brazilian study about adulthood HAM/TSP suggests that the progress of the motor disabilities occurs mainly during the first year and then becomes stable [29]. However, one of them showed a progressive motor deterioration, finally exhibiting a 2-degree increase in OMDS in just 1 year. The onset of neurological manifestations also occurred before 12 years of age and in 2 of them the IDH had not yet disappeared at 19 and 27 years of age, respectively, also indicating the severity of IDH.
Some patients used steroid therapy without obvious improvement. However, there was one case that used corticosteroids during all the follow-up on account of the marked and recurrent IDH that improved exclusively with this therapy. Coincidentally, this patient had a very slow progression.
It is important for pediatricians in endemic areas to consider HAM/TSP in patients with myelopathy symptomatology. This disease inflicts great impairment to life, and this is much worse when the disease occurs very early in life.
As early HAM/TSP is due to vertical transmission through breastfeeding, it is very important to avoid this pathway of infection through the detection of HTLV-1 positive mothers in prenatal care so as to advise them to refrain from breastfeeding.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank Dr Vitoria Regina Almeida Rego, who provided us with facilities at the Dermatologic Outpatient Clinic of the Federal University of Bahia.
Funding. This work was supported by the Fundação de Amparo à Pesquisa do Estado da Bahia (FAPESB) [grant number RED0028/2012 to L. F.] and Conselho Nacional de Desenvolvimento Científico e Tecnológico – CNPq [grant number 409985/2016-3 to A. L. B.].
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
REEFERENCES
Author notes
L. F. and A. L. B. contributed equally to this article as corresponding and senior authors.




