-
PDF
- Split View
-
Views
-
Cite
Cite
Caroline Charlier, Sylvain Poirée, Christophe Delavaud, Gaby Khoury, Clémence Richaud, Alexandre Leclercq, Olivier Hélénon, Marc Lecuit, MONALISA Study Group, Imaging of Human Neurolisteriosis: A Prospective Study of 71 Cases, Clinical Infectious Diseases, Volume 67, Issue 9, 1 November 2018, Pages 1419–1426, https://doi.org/10.1093/cid/ciy449
Close - Share Icon Share
Abstract
Neurolisteriosis ranks among the most severe neurological infections. Its radiological features have not been thoroughly studied. We describe here the neuroradiological features of neurolisteriosis and assess their prognostic value.
Patients with microbiologically proven neurolisteriosis were enrolled from November 2009 to October 2013 in MONALISA study. Magnetic resonance and computed tomography images were studied by 2 independent neuroradiologists. Predictors of 3-month mortality were determined using logistic regression.
Seventy-one patients were included; 42 were men (59%). Mean age was 64 years. Sixty patients (85%) reported signs of encephalitis, with clinical brainstem involvement in 16 (23%). Images were abnormal in 87% of cases (62/71). Main neuroradiological images were meningeal enhancement (25/71, 35%), abscess(es), or nodular image(s) evocative of abscess (10/71, 14%), hemorrhages (11/71, 15%), contrast-enhancing ventricles, or hydrocephalus (7/71, 10%). White-matter images (42/71, 59%), dilated Virchow-Robin spaces (22/71, 31%), and cerebral atrophy were also reported (34/71, 48%). Brainstem involvement (meningeal enhancement, abscess) was reported in only 7/71 cases (10%). Three-month survival was lower in patients with hydrocephalus or contrast-enhancing ventricles (1/7 [14%] than without [47/64, 73%], P = .005) and in patients with parenchymal images (abscess[es], nodule[s]\, or white matter images; 25/46 [54%] vs 23/25 without [92%], P = .004). Parenchymal images were associated with lower 3-month survival in the multivariable model (odds ratio 5.60, 95% confidence interval [1.42–29.6], P = .02).
Neurolisteriosis presents as a combination of neuroradiological images, none being specific. Radiological signs of rhombencephalitis are uncommon, whereas, unexpectedly, hemorrhagic images are frequent. The negative prognostic value of parenchymal neuroradiological images was evidenced.
NCT01520597
Listeria monocytogenes (Lm) is a foodborne pathogen responsible for listeriosis, a severe systemic infection affecting pregnant women, immunosuppressed individuals, and the elderly. Three main clinical forms are described: maternal-neonatal infections, septicemia, and neurolisteriosis. Neurolisteriosis typically presents as a meningoencephalitis (evidenced in 84% of cases) or less commonly as a meningitis without encephalitis (13% of cases) [1–3]. In some Western countries, Lm is one of the leading etiological agents of meningo-encephalitis in adults, together with Herpes simplex virus, Varicella zoster virus and Mycobacterium tuberculosis [4, 5]; and is associated with the highest morbi-mortality, despite appropriate antimicrobial therapy. Three-months mortality is around 30%, with persisting neurological impairment reported in 44% of surviving patients [3]. Most neurolisteriosis cases are therefore managed in intensive care management (60%), with 33% requiring mechanical ventilation [3]. The radiological presentation of neurolisteriosis remains poorly characterized and only reported as scattered observations focusing on brain abscesses or rhombencephalitis [6–9]. Yet, careful evaluation of central nervous system imaging in patients with neurolisteriosis is critical for a better understanding of the pathophysiology of this deadly infection, and to help refine clinical care.
MONALISA is a national prospective cohort study of invasive listeriosis implemented in France since 2009, compiling all cases of invasive listeriosis reported through national mandatory reporting, whose exhaustiveness was recently estimated around 87% [10]. For all included cases, a large data set of clinical features and clinical isolates were collected [3]. The clinical results of this study were recently published, and new prognostic factors for neurolisteriosis were evidenced: concomitant positive blood cultures, the use of adjunctive dexamethasone or mechanical ventilation requirement were independently associated with higher 3-month mortality; encephalitis signs and the number of neurological symptoms were also associated with a higher-risk of persisting neurological impairment [3]. Radiological data have not been analyzed in this clinical study. No correlation between radiological and clinical data has ever been reported, and the impact of radiological abnormal images has not been studied to our knowledge. We took advantage of this collection of neuroradiological imaging data of patients with neurolisteriosis to characterize the radiological features of neurolisteriosis and determine their prognostic value.
METHODS
Study Population
Neurolisteriosis cases were microbiologically confirmed and defined as follow: isolation of Listeria monocytogenes from the cerebrospinal fluid (CSF) or brain abscess, isolation of Listeria monocytogenes from blood cultures in patients with otherwise unexplained neurological symptoms (altered consciousness, seizures, nuchal rigidity, or focal neurological symptoms), isolation of Listeria monocytogenes from blood cultures and in CSF by quantitative polymerase chain reaction (qPCR). They were included from November 2009 to October 2013 from the MONALISA national prospective observational cohort study, whose design has been reported [3].
All patients or their legal representatives when unable to consent provided written informed consent. In accordance with French legislation, the study received institutional review board approval by the local ethics committee (Comité de Protection des Personnes Ile De France III, November 6, 2009). Clinical and biological data were available at baseline and at 3-month follow-up. Radiological files were collected from clinicians. The study is registered at Clinical Trials (NCT01520597).
Radiological Evaluation
As cases were scattered all over France, neuroimaging was performed according to local hospital practices. Brain computed tomography (CT) and magnetic resonance imaging (MRI) performed from baseline were sent by the clinicians along with clinical data. They analyzed by two neuroradiologists highly experienced in neuroimaging of infections. Images were analyzed according to a preestablished checklist. There was no abnormal image evidenced that was not listed in the checklist. Two independent analyses were performed; in case of discrepancy, a third was performed with both radiologists to reach consensus.
DEFINITIONS
- Meningeal enhancement was defined after injection of gadolinium chelates on T1-weighted images on MRI or after injection of iodine contrast medium on helicoidal CT scan. Meningeal enhancement was classified as leptomeningeal (involvement of pia mater or subarachnoid spaces) or pachymeningeal (involvement of dura mater).
- Brain abscesses were defined as nodules or masses with peripheral enhancement after injection after injection of gadolinium chelates on T1-weighted images on MRI, or of iodine contrast media on helicoidal CT scan. When available, hyper intensity on MRI diffusion weighting images was also taken into account to confirm the diagnosis of brain abscess.
- Nodules evocative of abscess were defined as enhanced nodules after injection of contrast media, but lacking typical peripheral enhancement.
- Diffuse cerebral edema was defined as diffuse hyper intense signal of the cerebral white matter with effacement of the sulci on T2-weighted or Flair MRI sequences or as diffuse cerebral white matter hypodensity with effacement of the sulci on helicoidal CT scan.
- Nonspecific white matter images were defined as nonspecific hyper intense signal of the white matter on T2-weighted MRI sequences or as non-specific white matter hypodensities on helicoidal CT scan.
- Cerebral atrophy was defined on both MRI and CT scan as a parenchymal volume loss with enlarged cerebral sulci (cortical atrophy) and/or ventriculomegaly without bulging of the third ventricular recesses (central atrophy).
- Dilated Virchow Robin spaces were defined as linear fluid signal nonenhancing spaces located in the basal ganglia or midbrain.
- Cerebral herniation was defined as a shift of cerebral tissue from its normal location into an adjacent space.
- Contrast-enhancing ventricles were defined as ventricles with enhancement of the ependymal lining after injection of gadolinium chelates on T1-weighted images on MRI or after injection of iodine contrast media on helicoidal CT scan.
- Hydrocephalus was defined as an enlargement of the ventricles without cerebral atrophy.
- Radiological vasculitis was defined as enhanced cortical grey matter after injection of gadolinium chelates on T1-weighted images on MRI or after injection of iodine contrast media on helicoidal CT scan, possibly associated with hemorrhages or ischemic lesions.
- Hemorrhage was defined as a lesion with hyper intense signal on T1-weighted sequences or marked low signal on GRET2* weighted sequences on MRI, or as a spontaneously hyper dense lesion (not calcified) on helicoidal CT scan.
- Ischemic images were defined as focal lesions of the cortical or subcortical matter with increased diffusion weight imaging (DWI) signal and reduced apparent diffusion coefficient (ADC) values on MRI or hypodensity after injection of iodine contrast media on helicoidal CT scan.
- Clinical encephalitis was defined by the presence of at least one of the following symptoms, with no alternative cause than listeriosis identified: altered consciousness (score on Glasgow Coma Scale (GCS) <15), seizures, new onset of neurological symptoms, and abnormality on electroencephalography [3, 11].
Statistical Analysis
The sample size was a convenient sample, determined by the number of available images during the study period. All tests were performed with R software (version 3.4.0). All tests were 2-tailed and P-values < .05 (calculated by χ2 test, Fisher exact test, or Student t test) were considered significant. For the multivariable analysis of mortality, candidate variables were the variables showing association at a significance level of P < .10 in the univariable analysis; the 3 clinical variables previously shown associated most strongly associated with mortality in neurolisteriosis were included in the multivariable model, and a stepwise selection was done [3]. Inter-rater agreement has been quantified by Cohen’s kappa statistics. Disagreement was considered if one radiologist described one lesion as present when the other one described it as absent or not interpretable. The situation where one radiologist answered that a lesion was absent when the other one described it as uninterpretable was not considered as a disagreement. Confidence intervals were computed using the bootstrap method, that is, numerical resampling. Agreement was not computed for following signs owing to their low prevalence (< 3%, ie, <3 patients, for at least 1 of the radiologists): brain herniation, brain edema, radiological vasculitis, and ventricular involvement. Observer agreement was categorized by kappa values as poor (<0.39), moderate (0.40–0.59), good (0.60–0.79), or excellent (>0.80) [12].
RESULTS
Characteristics of the Study Population
The patients’ characteristics are described in Table 1. In sum, 59% were men (42/71). Sixty patients (85%) reported encephalitis signs including clinical brainstem symptoms in 23% (16/71). Patients with neuroimaging available were similar to those of the French neurolisteriosis cohort in terms of age, sex, number and frequency of comorbidities, GCS, rate of encephalitis and focal signs, cerebrospinal fluid features, proportion of isolates with virulent clonal complexes, death, and persisting impairment rates (P >0.1, data not shown) [3].
Characteristics of 71 Patients With Neurolisteriosis and Brain Imaging Available
| Characteristics . | Patients With Neuroimaging, N = 71 . |
|---|---|
| Epidemiological features | |
| Median age, years (no· evaluated) | 64 ± 18 (71) |
| Male sex, no·/ no· evaluated (%) | 42/71 (59) |
| Geographical origin (no· evaluated) | (71) |
| France, no·/ no· evaluated (%) | 57/71 (80) |
| Other European country, no·/ no· evaluated (%) | 4/71(6) |
| North America | 4/71(6) |
| Africa | 3/71 (4) |
| Other areas | 3/71 (4) |
| Past history | |
| Mean number of associated comorbidities per patient (no· evaluated) | 3 ± 2 (71) |
| Immunosuppressive comorbidities | |
| Median number of immunosuppressive comorbiditiesa | 2 ± 2 |
| Administration of any immunosuppressive therapy in the past 5 years | 23/71 (32) |
| Including corticosteroids | (16/71 (23)) |
| Solid organ cancerb | 10/71 (14) |
| Hematological malignancyc | 7/71 (10) |
| Diabetes mellitus | 10/71 (14) |
| Inflammatory bowel disease | 5/71 (7) |
| Other auto-immune diseased | 4/71 (6) |
| HIV infection | 2/71 (3) |
| Non- immunosuppressive comorbidities | |
| Hypertension | 29/71 (41) |
| Chronic respiratory disease | 9/71 (13) |
| Renal insufficiency | 5/71 (7) |
| Other conditione | 5/71 (7) |
| Age below 40, no identified comorbidity | 6/71 (8) |
| Clinical features | |
| Fever | 70/71 (99) |
| Nuchal rigidity | 48/71 (67) |
| Encephalitis | 60/71 (85) |
| Seizures | 15/71 (21) |
| Mean Glasgow coma scale score, no. <8 (coma) / no· evaluated (%) | 12 ± 3, 9/71 (13) |
| Aphasia | 15/71 (21) |
| Limb motor deficiency | 14/71 (20) |
| Limb sensory loss | 4/71 (6) |
| Any brainstem symptom | 16/71 (23) |
| 3rd nerve | 5/71 (7) |
| 5th nerve | 1/71 (1) |
| 6th nerve | 4/71 (6) |
| 7th nerve | 5/71 (7) |
| 8th nerve | 4/71 (6) |
| Cerebellar syndrome | 3/71 (4) |
| Extrapyramidal syndrome | 3/71 (4) |
| Biological features | |
| Cerebrospinal fluid abnormality, including white-cell count > 10 cells /mm3 | 69/69 (100), 67/69 (97) |
| Cerebrospinal fluid culture positive | 63/69 (91) |
| Cerebrospinal fluid nucleated cells number median [IQ25-IQ75] | 470 [182–1028] |
| Cerebrospinal fluid protein level (g/L) median [IQ25-IQ75] | 2.0 [1.3–2.9] |
| Cerebrospinal fluid glucose level (g/L) median [IQ25-IQ75] | 0.5 g/L [0,3-0,7] |
| Blood culture positive | 38/71 (54) |
| Lymphopenia <1500 /mm3, no· / no· evaluated (%) | 56/71 (79) |
| Monocytopenia <200 /mm3, no· / no· evaluated (%) | 10/71 (14) |
| Clonal complexes | |
| Hypervirulent clones (CC1, 2, 4, or 6) | 43/71 (61) |
| Hypovirulent clones (CC9 or 121) | 2/71 (3) |
| Other clones (others) | 26/71 (37) |
| Outcome | |
| 3-month mortality | 23/71 (32) |
| Persisting impairment | 21/48 (44) |
| Characteristics . | Patients With Neuroimaging, N = 71 . |
|---|---|
| Epidemiological features | |
| Median age, years (no· evaluated) | 64 ± 18 (71) |
| Male sex, no·/ no· evaluated (%) | 42/71 (59) |
| Geographical origin (no· evaluated) | (71) |
| France, no·/ no· evaluated (%) | 57/71 (80) |
| Other European country, no·/ no· evaluated (%) | 4/71(6) |
| North America | 4/71(6) |
| Africa | 3/71 (4) |
| Other areas | 3/71 (4) |
| Past history | |
| Mean number of associated comorbidities per patient (no· evaluated) | 3 ± 2 (71) |
| Immunosuppressive comorbidities | |
| Median number of immunosuppressive comorbiditiesa | 2 ± 2 |
| Administration of any immunosuppressive therapy in the past 5 years | 23/71 (32) |
| Including corticosteroids | (16/71 (23)) |
| Solid organ cancerb | 10/71 (14) |
| Hematological malignancyc | 7/71 (10) |
| Diabetes mellitus | 10/71 (14) |
| Inflammatory bowel disease | 5/71 (7) |
| Other auto-immune diseased | 4/71 (6) |
| HIV infection | 2/71 (3) |
| Non- immunosuppressive comorbidities | |
| Hypertension | 29/71 (41) |
| Chronic respiratory disease | 9/71 (13) |
| Renal insufficiency | 5/71 (7) |
| Other conditione | 5/71 (7) |
| Age below 40, no identified comorbidity | 6/71 (8) |
| Clinical features | |
| Fever | 70/71 (99) |
| Nuchal rigidity | 48/71 (67) |
| Encephalitis | 60/71 (85) |
| Seizures | 15/71 (21) |
| Mean Glasgow coma scale score, no. <8 (coma) / no· evaluated (%) | 12 ± 3, 9/71 (13) |
| Aphasia | 15/71 (21) |
| Limb motor deficiency | 14/71 (20) |
| Limb sensory loss | 4/71 (6) |
| Any brainstem symptom | 16/71 (23) |
| 3rd nerve | 5/71 (7) |
| 5th nerve | 1/71 (1) |
| 6th nerve | 4/71 (6) |
| 7th nerve | 5/71 (7) |
| 8th nerve | 4/71 (6) |
| Cerebellar syndrome | 3/71 (4) |
| Extrapyramidal syndrome | 3/71 (4) |
| Biological features | |
| Cerebrospinal fluid abnormality, including white-cell count > 10 cells /mm3 | 69/69 (100), 67/69 (97) |
| Cerebrospinal fluid culture positive | 63/69 (91) |
| Cerebrospinal fluid nucleated cells number median [IQ25-IQ75] | 470 [182–1028] |
| Cerebrospinal fluid protein level (g/L) median [IQ25-IQ75] | 2.0 [1.3–2.9] |
| Cerebrospinal fluid glucose level (g/L) median [IQ25-IQ75] | 0.5 g/L [0,3-0,7] |
| Blood culture positive | 38/71 (54) |
| Lymphopenia <1500 /mm3, no· / no· evaluated (%) | 56/71 (79) |
| Monocytopenia <200 /mm3, no· / no· evaluated (%) | 10/71 (14) |
| Clonal complexes | |
| Hypervirulent clones (CC1, 2, 4, or 6) | 43/71 (61) |
| Hypovirulent clones (CC9 or 121) | 2/71 (3) |
| Other clones (others) | 26/71 (37) |
| Outcome | |
| 3-month mortality | 23/71 (32) |
| Persisting impairment | 21/48 (44) |
Abbreviations: HIV, human immunodeficiency virus; IQ, interquartile.
aImmunosuppressive comorbidities included: daily alcohol uptake >3 drinks/day, cirrhosis, diabetes mellitus, end-stage renal disease, solid organ cancer, hematological malignancy, hematopoietic stem cell transplantation, solid organ transplantation, asplenia, preexisting neutropenia, preexisting lymphopenia, HIV infection, inflammatory bowel disease, inflammatory rheumatic disorder, other auto-immune disease, congenital immune deficiency, age >70 years, prescription of corticosteroids or other immunosuppressive therapy in the past 5 years.
bThey were hepatocarcinoma, breast or prostate cancers (n = 2 each), skin, ethmoidal, brain, and lung cancers (n = 1 each). Of them, 4/10 (40%) were considered as cured.
cThey were chronic lymphoid leukemia (n = 5), chronic myeloid leukemia and Waldenstrom’s macroglobulinemia (n = 1 each).
dThey were rheumatoid arthritis and giant cell arteritis (n = 2 each).
eThey were seizures and chronic liver diseases (n = 2 each).
Characteristics of 71 Patients With Neurolisteriosis and Brain Imaging Available
| Characteristics . | Patients With Neuroimaging, N = 71 . |
|---|---|
| Epidemiological features | |
| Median age, years (no· evaluated) | 64 ± 18 (71) |
| Male sex, no·/ no· evaluated (%) | 42/71 (59) |
| Geographical origin (no· evaluated) | (71) |
| France, no·/ no· evaluated (%) | 57/71 (80) |
| Other European country, no·/ no· evaluated (%) | 4/71(6) |
| North America | 4/71(6) |
| Africa | 3/71 (4) |
| Other areas | 3/71 (4) |
| Past history | |
| Mean number of associated comorbidities per patient (no· evaluated) | 3 ± 2 (71) |
| Immunosuppressive comorbidities | |
| Median number of immunosuppressive comorbiditiesa | 2 ± 2 |
| Administration of any immunosuppressive therapy in the past 5 years | 23/71 (32) |
| Including corticosteroids | (16/71 (23)) |
| Solid organ cancerb | 10/71 (14) |
| Hematological malignancyc | 7/71 (10) |
| Diabetes mellitus | 10/71 (14) |
| Inflammatory bowel disease | 5/71 (7) |
| Other auto-immune diseased | 4/71 (6) |
| HIV infection | 2/71 (3) |
| Non- immunosuppressive comorbidities | |
| Hypertension | 29/71 (41) |
| Chronic respiratory disease | 9/71 (13) |
| Renal insufficiency | 5/71 (7) |
| Other conditione | 5/71 (7) |
| Age below 40, no identified comorbidity | 6/71 (8) |
| Clinical features | |
| Fever | 70/71 (99) |
| Nuchal rigidity | 48/71 (67) |
| Encephalitis | 60/71 (85) |
| Seizures | 15/71 (21) |
| Mean Glasgow coma scale score, no. <8 (coma) / no· evaluated (%) | 12 ± 3, 9/71 (13) |
| Aphasia | 15/71 (21) |
| Limb motor deficiency | 14/71 (20) |
| Limb sensory loss | 4/71 (6) |
| Any brainstem symptom | 16/71 (23) |
| 3rd nerve | 5/71 (7) |
| 5th nerve | 1/71 (1) |
| 6th nerve | 4/71 (6) |
| 7th nerve | 5/71 (7) |
| 8th nerve | 4/71 (6) |
| Cerebellar syndrome | 3/71 (4) |
| Extrapyramidal syndrome | 3/71 (4) |
| Biological features | |
| Cerebrospinal fluid abnormality, including white-cell count > 10 cells /mm3 | 69/69 (100), 67/69 (97) |
| Cerebrospinal fluid culture positive | 63/69 (91) |
| Cerebrospinal fluid nucleated cells number median [IQ25-IQ75] | 470 [182–1028] |
| Cerebrospinal fluid protein level (g/L) median [IQ25-IQ75] | 2.0 [1.3–2.9] |
| Cerebrospinal fluid glucose level (g/L) median [IQ25-IQ75] | 0.5 g/L [0,3-0,7] |
| Blood culture positive | 38/71 (54) |
| Lymphopenia <1500 /mm3, no· / no· evaluated (%) | 56/71 (79) |
| Monocytopenia <200 /mm3, no· / no· evaluated (%) | 10/71 (14) |
| Clonal complexes | |
| Hypervirulent clones (CC1, 2, 4, or 6) | 43/71 (61) |
| Hypovirulent clones (CC9 or 121) | 2/71 (3) |
| Other clones (others) | 26/71 (37) |
| Outcome | |
| 3-month mortality | 23/71 (32) |
| Persisting impairment | 21/48 (44) |
| Characteristics . | Patients With Neuroimaging, N = 71 . |
|---|---|
| Epidemiological features | |
| Median age, years (no· evaluated) | 64 ± 18 (71) |
| Male sex, no·/ no· evaluated (%) | 42/71 (59) |
| Geographical origin (no· evaluated) | (71) |
| France, no·/ no· evaluated (%) | 57/71 (80) |
| Other European country, no·/ no· evaluated (%) | 4/71(6) |
| North America | 4/71(6) |
| Africa | 3/71 (4) |
| Other areas | 3/71 (4) |
| Past history | |
| Mean number of associated comorbidities per patient (no· evaluated) | 3 ± 2 (71) |
| Immunosuppressive comorbidities | |
| Median number of immunosuppressive comorbiditiesa | 2 ± 2 |
| Administration of any immunosuppressive therapy in the past 5 years | 23/71 (32) |
| Including corticosteroids | (16/71 (23)) |
| Solid organ cancerb | 10/71 (14) |
| Hematological malignancyc | 7/71 (10) |
| Diabetes mellitus | 10/71 (14) |
| Inflammatory bowel disease | 5/71 (7) |
| Other auto-immune diseased | 4/71 (6) |
| HIV infection | 2/71 (3) |
| Non- immunosuppressive comorbidities | |
| Hypertension | 29/71 (41) |
| Chronic respiratory disease | 9/71 (13) |
| Renal insufficiency | 5/71 (7) |
| Other conditione | 5/71 (7) |
| Age below 40, no identified comorbidity | 6/71 (8) |
| Clinical features | |
| Fever | 70/71 (99) |
| Nuchal rigidity | 48/71 (67) |
| Encephalitis | 60/71 (85) |
| Seizures | 15/71 (21) |
| Mean Glasgow coma scale score, no. <8 (coma) / no· evaluated (%) | 12 ± 3, 9/71 (13) |
| Aphasia | 15/71 (21) |
| Limb motor deficiency | 14/71 (20) |
| Limb sensory loss | 4/71 (6) |
| Any brainstem symptom | 16/71 (23) |
| 3rd nerve | 5/71 (7) |
| 5th nerve | 1/71 (1) |
| 6th nerve | 4/71 (6) |
| 7th nerve | 5/71 (7) |
| 8th nerve | 4/71 (6) |
| Cerebellar syndrome | 3/71 (4) |
| Extrapyramidal syndrome | 3/71 (4) |
| Biological features | |
| Cerebrospinal fluid abnormality, including white-cell count > 10 cells /mm3 | 69/69 (100), 67/69 (97) |
| Cerebrospinal fluid culture positive | 63/69 (91) |
| Cerebrospinal fluid nucleated cells number median [IQ25-IQ75] | 470 [182–1028] |
| Cerebrospinal fluid protein level (g/L) median [IQ25-IQ75] | 2.0 [1.3–2.9] |
| Cerebrospinal fluid glucose level (g/L) median [IQ25-IQ75] | 0.5 g/L [0,3-0,7] |
| Blood culture positive | 38/71 (54) |
| Lymphopenia <1500 /mm3, no· / no· evaluated (%) | 56/71 (79) |
| Monocytopenia <200 /mm3, no· / no· evaluated (%) | 10/71 (14) |
| Clonal complexes | |
| Hypervirulent clones (CC1, 2, 4, or 6) | 43/71 (61) |
| Hypovirulent clones (CC9 or 121) | 2/71 (3) |
| Other clones (others) | 26/71 (37) |
| Outcome | |
| 3-month mortality | 23/71 (32) |
| Persisting impairment | 21/48 (44) |
Abbreviations: HIV, human immunodeficiency virus; IQ, interquartile.
aImmunosuppressive comorbidities included: daily alcohol uptake >3 drinks/day, cirrhosis, diabetes mellitus, end-stage renal disease, solid organ cancer, hematological malignancy, hematopoietic stem cell transplantation, solid organ transplantation, asplenia, preexisting neutropenia, preexisting lymphopenia, HIV infection, inflammatory bowel disease, inflammatory rheumatic disorder, other auto-immune disease, congenital immune deficiency, age >70 years, prescription of corticosteroids or other immunosuppressive therapy in the past 5 years.
bThey were hepatocarcinoma, breast or prostate cancers (n = 2 each), skin, ethmoidal, brain, and lung cancers (n = 1 each). Of them, 4/10 (40%) were considered as cured.
cThey were chronic lymphoid leukemia (n = 5), chronic myeloid leukemia and Waldenstrom’s macroglobulinemia (n = 1 each).
dThey were rheumatoid arthritis and giant cell arteritis (n = 2 each).
eThey were seizures and chronic liver diseases (n = 2 each).
Neuroradiological Presentation at Baseline
The first radiological procedure was performed after a median time interval of 2 days from the time of the diagnosis procedure (blood or cerebrospinal fluid sample; interquartile range, 0–11 days). The neuroradiological features are detailed in Table 2 and summarized in Figure 1. Altogether, abnormal neuroradiological findings were found in 62 of the 71 patients (83%). Meningeal enhancement was reported in 25/71 (35%). It consisted in pachy- or lepto-meningeal enhancement, focal (n = 2) or diffuse (n = 23). Twelve patients had meningeal enhancement evidenced before lumbar puncture was performed, excluding the possibility of nonspecific dural and/or arachnoid enhancement, which can be observed after lumbar puncture [13].
Neuroradiological Features of 71 Patients With Neurolisteriosis and Neuroimaging
| Neuroradiological Finding . | Lesions . | . | ||
|---|---|---|---|---|
| No. of Patients With Radiological Lesionsa, N = 71 . | No. of Patients With Lesions on MRIa, N = 46 . | No. of Patients With Lesions on CTa, N = 30 . | P Value CT vs MRI . | |
| Meninges | ||||
| Lepto and/or pachy-meningeal enhancement | 25/71 (35) | 20/46 (43) | 6/30 (20) | .048 |
| Parenchyma | ||||
| Brain abscessb | 4/71 (6) | 3/46 (7) | 1/30 (3) | NS |
| Nodule evocative of abscessb | 7//71 (10) | 7/46 (15) | 1/30 (3) | NS |
| Nonspecific white matter lesion | 42/71 (59) | 30/46 (65) | 14/30 (47) | NS |
| Atrophyc | 34/71 (48) | 24/46 (52) | 12/30 (40) | NS |
| Dilated Virchow-Robin spaces | 22/71 (31) | 20/46 (43) | 6/30 (20) | .048 |
| Cerebral herniationd | 2/71 (2) | 2/46 (4) | 1/30 (3) | NS |
| Diffuse cerebral edema | 1/71 (1) | 1/46 (2) | - | NS |
| Ventricles | ||||
| Contrast-enhancing ventricles | 2/71 (3) | 2/46 (4) | 1/30 (3) | NS |
| Hydrocephalus | 6/71 (9) | 1/46 (2) | 6/30 (20) | .01 |
| Brain vessels | ||||
| Radiological vasculitise | 3/71 (5) | 3/46 (7) | 1/30 (3) | NS |
| Hemorrhage | 11/71 (15) | 10/46 (22) | 1/30 (3) | .04 |
| Ischemiaf | 8/71 (11) | 8/46 (17) | 1/30 (3) | NS |
| Concomitant tumor imageg | 5/71 (7) | 4/46 (8) | 2/30 (6) | NS |
| Normal | 9/71 (13) | 2/46 (4) | 7/30 (23) | .02 |
| Neuroradiological Finding . | Lesions . | . | ||
|---|---|---|---|---|
| No. of Patients With Radiological Lesionsa, N = 71 . | No. of Patients With Lesions on MRIa, N = 46 . | No. of Patients With Lesions on CTa, N = 30 . | P Value CT vs MRI . | |
| Meninges | ||||
| Lepto and/or pachy-meningeal enhancement | 25/71 (35) | 20/46 (43) | 6/30 (20) | .048 |
| Parenchyma | ||||
| Brain abscessb | 4/71 (6) | 3/46 (7) | 1/30 (3) | NS |
| Nodule evocative of abscessb | 7//71 (10) | 7/46 (15) | 1/30 (3) | NS |
| Nonspecific white matter lesion | 42/71 (59) | 30/46 (65) | 14/30 (47) | NS |
| Atrophyc | 34/71 (48) | 24/46 (52) | 12/30 (40) | NS |
| Dilated Virchow-Robin spaces | 22/71 (31) | 20/46 (43) | 6/30 (20) | .048 |
| Cerebral herniationd | 2/71 (2) | 2/46 (4) | 1/30 (3) | NS |
| Diffuse cerebral edema | 1/71 (1) | 1/46 (2) | - | NS |
| Ventricles | ||||
| Contrast-enhancing ventricles | 2/71 (3) | 2/46 (4) | 1/30 (3) | NS |
| Hydrocephalus | 6/71 (9) | 1/46 (2) | 6/30 (20) | .01 |
| Brain vessels | ||||
| Radiological vasculitise | 3/71 (5) | 3/46 (7) | 1/30 (3) | NS |
| Hemorrhage | 11/71 (15) | 10/46 (22) | 1/30 (3) | .04 |
| Ischemiaf | 8/71 (11) | 8/46 (17) | 1/30 (3) | NS |
| Concomitant tumor imageg | 5/71 (7) | 4/46 (8) | 2/30 (6) | NS |
| Normal | 9/71 (13) | 2/46 (4) | 7/30 (23) | .02 |
Abbreviations: CT, computed tomography; MRI, magnetic resonance imaging; NS, not significant.
aData presented are those from exams 1 and 2. When 1 patient had benefited from 2 exams with the same procedure (computed tomography or magnetic resonance), data from the first exam were retained for analysis. Patients could have images evidenced in both procedures; therefore, total numbers are not the sum of MRI plus CT scan numbers.
bBrain abscesses and nodules were localized in the frontal lobe, temporal lobe (n = 1, each), thalami (n = 2), cerebellum (n = 1), brainstem (n = 4), or brainstem and occipital lobe (n = 1).
cAtrophy was classified as diffuse in 27/33 (82%; cortical in 6, subcortical in 7, and both cortical and subcortical in 14) and as focal in 6/33 (18%).
dCerebral herniation was classified as uncal or subfalcine (n = 1 each).
eRadiological vasculitis was defined as enhanced cortical grey matter after injection of gadolinium chelates on T1-weighted images on MRI or after infection of iodine contrast media on helicoidal CT scan, possibly associated with hemorrhages or ischemic lesions.
fIschemic images were considered as sequellar lesions in 3/8 cases, and recent in 5/8. In the 5 images reflecting recent lesions, they could be related to the radiological vasculitis in 2/5 cases; altogether, they were subtentorial in 3/5 and supratentorial in 2/5 cases.
gTumor images included meningioma (n = 2) and histologically confirmed malignant brain tumor (n = 3).
Neuroradiological Features of 71 Patients With Neurolisteriosis and Neuroimaging
| Neuroradiological Finding . | Lesions . | . | ||
|---|---|---|---|---|
| No. of Patients With Radiological Lesionsa, N = 71 . | No. of Patients With Lesions on MRIa, N = 46 . | No. of Patients With Lesions on CTa, N = 30 . | P Value CT vs MRI . | |
| Meninges | ||||
| Lepto and/or pachy-meningeal enhancement | 25/71 (35) | 20/46 (43) | 6/30 (20) | .048 |
| Parenchyma | ||||
| Brain abscessb | 4/71 (6) | 3/46 (7) | 1/30 (3) | NS |
| Nodule evocative of abscessb | 7//71 (10) | 7/46 (15) | 1/30 (3) | NS |
| Nonspecific white matter lesion | 42/71 (59) | 30/46 (65) | 14/30 (47) | NS |
| Atrophyc | 34/71 (48) | 24/46 (52) | 12/30 (40) | NS |
| Dilated Virchow-Robin spaces | 22/71 (31) | 20/46 (43) | 6/30 (20) | .048 |
| Cerebral herniationd | 2/71 (2) | 2/46 (4) | 1/30 (3) | NS |
| Diffuse cerebral edema | 1/71 (1) | 1/46 (2) | - | NS |
| Ventricles | ||||
| Contrast-enhancing ventricles | 2/71 (3) | 2/46 (4) | 1/30 (3) | NS |
| Hydrocephalus | 6/71 (9) | 1/46 (2) | 6/30 (20) | .01 |
| Brain vessels | ||||
| Radiological vasculitise | 3/71 (5) | 3/46 (7) | 1/30 (3) | NS |
| Hemorrhage | 11/71 (15) | 10/46 (22) | 1/30 (3) | .04 |
| Ischemiaf | 8/71 (11) | 8/46 (17) | 1/30 (3) | NS |
| Concomitant tumor imageg | 5/71 (7) | 4/46 (8) | 2/30 (6) | NS |
| Normal | 9/71 (13) | 2/46 (4) | 7/30 (23) | .02 |
| Neuroradiological Finding . | Lesions . | . | ||
|---|---|---|---|---|
| No. of Patients With Radiological Lesionsa, N = 71 . | No. of Patients With Lesions on MRIa, N = 46 . | No. of Patients With Lesions on CTa, N = 30 . | P Value CT vs MRI . | |
| Meninges | ||||
| Lepto and/or pachy-meningeal enhancement | 25/71 (35) | 20/46 (43) | 6/30 (20) | .048 |
| Parenchyma | ||||
| Brain abscessb | 4/71 (6) | 3/46 (7) | 1/30 (3) | NS |
| Nodule evocative of abscessb | 7//71 (10) | 7/46 (15) | 1/30 (3) | NS |
| Nonspecific white matter lesion | 42/71 (59) | 30/46 (65) | 14/30 (47) | NS |
| Atrophyc | 34/71 (48) | 24/46 (52) | 12/30 (40) | NS |
| Dilated Virchow-Robin spaces | 22/71 (31) | 20/46 (43) | 6/30 (20) | .048 |
| Cerebral herniationd | 2/71 (2) | 2/46 (4) | 1/30 (3) | NS |
| Diffuse cerebral edema | 1/71 (1) | 1/46 (2) | - | NS |
| Ventricles | ||||
| Contrast-enhancing ventricles | 2/71 (3) | 2/46 (4) | 1/30 (3) | NS |
| Hydrocephalus | 6/71 (9) | 1/46 (2) | 6/30 (20) | .01 |
| Brain vessels | ||||
| Radiological vasculitise | 3/71 (5) | 3/46 (7) | 1/30 (3) | NS |
| Hemorrhage | 11/71 (15) | 10/46 (22) | 1/30 (3) | .04 |
| Ischemiaf | 8/71 (11) | 8/46 (17) | 1/30 (3) | NS |
| Concomitant tumor imageg | 5/71 (7) | 4/46 (8) | 2/30 (6) | NS |
| Normal | 9/71 (13) | 2/46 (4) | 7/30 (23) | .02 |
Abbreviations: CT, computed tomography; MRI, magnetic resonance imaging; NS, not significant.
aData presented are those from exams 1 and 2. When 1 patient had benefited from 2 exams with the same procedure (computed tomography or magnetic resonance), data from the first exam were retained for analysis. Patients could have images evidenced in both procedures; therefore, total numbers are not the sum of MRI plus CT scan numbers.
bBrain abscesses and nodules were localized in the frontal lobe, temporal lobe (n = 1, each), thalami (n = 2), cerebellum (n = 1), brainstem (n = 4), or brainstem and occipital lobe (n = 1).
cAtrophy was classified as diffuse in 27/33 (82%; cortical in 6, subcortical in 7, and both cortical and subcortical in 14) and as focal in 6/33 (18%).
dCerebral herniation was classified as uncal or subfalcine (n = 1 each).
eRadiological vasculitis was defined as enhanced cortical grey matter after injection of gadolinium chelates on T1-weighted images on MRI or after infection of iodine contrast media on helicoidal CT scan, possibly associated with hemorrhages or ischemic lesions.
fIschemic images were considered as sequellar lesions in 3/8 cases, and recent in 5/8. In the 5 images reflecting recent lesions, they could be related to the radiological vasculitis in 2/5 cases; altogether, they were subtentorial in 3/5 and supratentorial in 2/5 cases.
gTumor images included meningioma (n = 2) and histologically confirmed malignant brain tumor (n = 3).
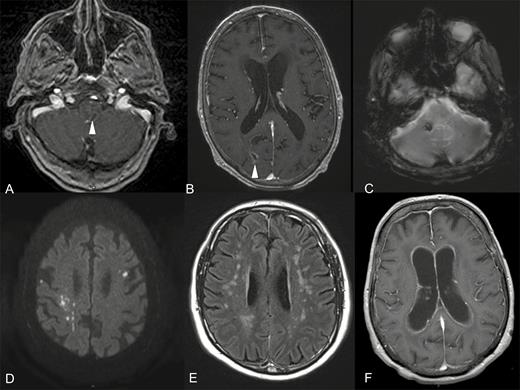
Main patterns of radiological neurolisteriosis in magnetic resonance imaging (MRI) procedures. A, Axial contrast-enhancing T1-weighted MRI with a ring-like enhancing pons image corresponding to an abscess (arrow head). B, Axial contrast-enhancing T1-weighted MRI with a ring-enhancing image of the right parietal lobe corresponding to an abscess (arrow head) with diffuse meningeal enhancement. C, Axial T2 gradient-echo MRI with markedly hypo signal in right cerebellar peduncle corresponding to focal bleeding. D, Axial diffusion weighted MRI with bilateral supra-tentorial cerebral dot-like hyper signals corresponding to ischemic images highly evocating of radiological vasculitis. E, Axial T2 spin-echo MRI with symmetric hyper signals of supra-tentorial white matter. F, Axial contrast-enhancing T1-weighted MRI with hydrocephalus and ventricular ependymal lining enhancement.
Abscesses and/or nodular images evocative of abscesses (see Methods) were reported in 10/71 cases (14%). They were unique or multiple (1 to 6, median number of 2), with diameter ranging from 7 to 18 mm. They were infratentorial (brainstem) in 5/10 cases (50%), supratentorial in 4/10 cases (40%), and both supra- and infratentorial in 1 case (1/10, 10%).
Hemorrhages were reported in an unexpected high proportion of cases (11/71, 15%). They involved parenchyma in all cases and were classified as supratentorial in 7 cases, subtentorial in 1, and both supratentorial and subtentorial in 3 cases. They consisted in petechial images (7/11) or hematoma (4/11). Parenchymal hemorrhages could also be associated with subarachnoid hemorrhage (n = 2) or ventricular hemorrhage (n = 1). In 7/11 cases concomitant predisposing conditions were evidenced: hypertension (n = 3), preexisting tumoral (n = 2) or vascular (n = 1) lesions, anticoagulation and thrombocytopenia (< 20000/mm3; n = 1 each).
Importantly, major ventricular involvement (contrast-enhancing ventricles or hydrocephalus) were evidenced in 7 cases (7/71, 10%). Nonspecific deep white-matter images were also frequently reported (42/71, 59%). In all but 2 cases, they could be attributed to age (> 50 years, n = 39), and/or concomitant hypertension (n = 20), or human immunodeficiency virus infection (n = 1).
All combinations of radiological signs were observed (data not shown). Altogether, brainstem involvement with meningeal enhancement or abscess(es) was reported in only 7/71 cases (10%). No involvement of choroid plexuses was found. MRI detected more meningeal enhancement, dilated Virchow Robin spaces and hydrocephalus than CT scan (P < .05, Table 2).
Correlation with Clinical and Microbiological Features
Clinical and radiological data were compared (Supplementary Figure S1). No correlation between radiological presentation and the number of immunosuppressive comorbidities was found, except for vascular images (ischemia or hemorrhage) that were more frequently reported in patients reporting above 2 immunosuppressing comorbidities (12/19) that in others (8/46, P = .0002). All patients radiologically diagnosed with hemorrhages, ventricles involvement (contrast-enhancement or hydrocephalus), cerebral herniation, cerebral edema, or vasculitis also had clinical signs of encephalitis involvement as defined above. However, there was no correlation between clinical signs and neuroradiological findings. Indeed, only 4/7 (57%) patients with radiological evidence of brainstem involvement exhibited brainstem symptoms (namely, cranial nerve involvement) at the time of evaluation. Furthermore, 12/16 (75%) of patients exhibiting clinical brainstem signs did not have brainstem neuroradiological lesions on MRI (n = 10) or CT scan (n = 2; Supplementary Figure S1A). Similarly, 6/25 (24%) patients with meningeal enhancement did not report nuchal rigidity (Supplementary Figure S1B), although all of them (25/25) had alterations of the cerebrospinal fluid (defined as cerebrospinal fluid nucleated cells count >4/mm3 and/or CSF protein >0.5g/L and/or evidence of viable L. monocytogenes in the cerebrospinal fluid). A patient with brain abscess/nodule did not report clinical signs of encephalitis (1/10). Finally, no correlation was evidenced between isolate genotype (either hyper- or hypovirulent, as reported in Maury et al, [14]) and radiological presentation (data not shown).
Relationship Between Baseline Brain Images and Outcome
Patients with neurolisteriosis and ventricle involvement (hydrocephalus or contrast-enhancement) and those with parenchymal images (abscess(es), nodule(s) and/ or nonspecific white matter images) evidenced significantly lower 3-month survival in the univariate analysis (respectively, 1/7 [14%] in patients with ventricle involvement vs 47/64 [73%, P = .005] in those without and 25/46 [54%] in patients with parenchymal images vs 23/25 [92%] in those without, P = .004; Supplementary Table S1). Parenchymal lesions (abscess[es], nodule[s] and/ or nonspecific white matter lesions) remained associated with poor 3-month survival for patients in the multivariable model (Table 3). No link between radiological pattern and long-term impairment was evidenced (data not shown).
Independent Predictors for Mortality in 71 Adults With Neurolisteriosis in Multivariate Analysis
| Parameter . | Odds Ratio (95% CI) . | P Value . |
|---|---|---|
| Parenchymal involvement with abscess(es), nodule(s), and/or nonspecific white matter images | 5.60 (1.42–29.6) | .02 |
| Hydrocephalus or contrast-enhancing ventricles | 5.96 (0.73–130.17) | .14 |
| Positive blood cultures | 3.59 (1.06–13.78) | .04 |
| Ongoing organ neoplasia | 5.03 (0.67–51.5) | .12 |
| Adjunctive dexamethasone for meningitis | 1.64 (0.24–9.38) | .58 |
| Parameter . | Odds Ratio (95% CI) . | P Value . |
|---|---|---|
| Parenchymal involvement with abscess(es), nodule(s), and/or nonspecific white matter images | 5.60 (1.42–29.6) | .02 |
| Hydrocephalus or contrast-enhancing ventricles | 5.96 (0.73–130.17) | .14 |
| Positive blood cultures | 3.59 (1.06–13.78) | .04 |
| Ongoing organ neoplasia | 5.03 (0.67–51.5) | .12 |
| Adjunctive dexamethasone for meningitis | 1.64 (0.24–9.38) | .58 |
Odds ratio calculated from the multivariate model.
Abbreviation: CI, confidence interval.
Independent Predictors for Mortality in 71 Adults With Neurolisteriosis in Multivariate Analysis
| Parameter . | Odds Ratio (95% CI) . | P Value . |
|---|---|---|
| Parenchymal involvement with abscess(es), nodule(s), and/or nonspecific white matter images | 5.60 (1.42–29.6) | .02 |
| Hydrocephalus or contrast-enhancing ventricles | 5.96 (0.73–130.17) | .14 |
| Positive blood cultures | 3.59 (1.06–13.78) | .04 |
| Ongoing organ neoplasia | 5.03 (0.67–51.5) | .12 |
| Adjunctive dexamethasone for meningitis | 1.64 (0.24–9.38) | .58 |
| Parameter . | Odds Ratio (95% CI) . | P Value . |
|---|---|---|
| Parenchymal involvement with abscess(es), nodule(s), and/or nonspecific white matter images | 5.60 (1.42–29.6) | .02 |
| Hydrocephalus or contrast-enhancing ventricles | 5.96 (0.73–130.17) | .14 |
| Positive blood cultures | 3.59 (1.06–13.78) | .04 |
| Ongoing organ neoplasia | 5.03 (0.67–51.5) | .12 |
| Adjunctive dexamethasone for meningitis | 1.64 (0.24–9.38) | .58 |
Odds ratio calculated from the multivariate model.
Abbreviation: CI, confidence interval.
Radiological Evolution
Of the 71 patients, 9 had another neuroimaging procedure performed after at least a 1-week interval, including 6 with the same type of imaging (MRI or CT scan). Images were found stable in 4 cases (including 1 patient with 18-mm abscesses reevaluated after 10 days, 1 patient with parenchymal hematoma reevaluated after 5 weeks, and 1 patient with persisting meningeal enhancement reevaluated after 9 months), or improved in 1 case with brain hematoma (reevaluation after 4 and 6 weeks). One single case developed focal parietal meningitis after 7 days in the context of altered consciousness despite appropriate therapy; he died 1 week later as a consequence of severe neurolisteriosis.
Radiological Lectures Concordance Tests
Inter-rater agreement was strong for all lesions, with Cohen’s kappa index all ranging from good (hydrocephalus, dilated Virchow Robin spaces, atrophy, nonspecific white matter lesion, ischemic images) to excellent (meningeal enhancement, nodular lesion evocative of abscess, abscess[es] and hemorrhages; Supplementary Table S2).
DISCUSSION
The French mandatory reporting made it possible for the first time to collect a large set of radiological files from patients with neurolisteriosis and to analyze them together with a large data set of clinical data [3]. Only culture-proven cases were included. To our knowledge, previous studies have only been focused on specific images like abscesses or rhombencephalitis, while other signs were only reported as scattered observations [6, 9]. This systematic evaluation of neuroradiological features allowed an unbiased description of the neuroradiological features of neurolisteriosis. We could thereby determine that abnormal images were observed in 83% of cases, corresponding to meningeal enhancement, abscess(es) and nodular images (s) evocative of abscess, hemorrhages, ventricular involvement (contrast-enhancing ventricles or hydrocephalus), nonspecific white matter images, and dilated Virchow–Robin spaces and rarely to diffuse cerebral edema and cerebral herniation.
Abscesses, meningeal enhancement, hydrocephalus, ischemia, and brain edema have been reported before as scattered observations [15–19]; however, a systematic analysis of radiological files allowed us to determine the relative frequency of each radiological sign and to point out the rarity of brain abscesses and of brainstem involvement; although they were the cardinal features reported in the neurolisteriosis literature so far [20–22]. Typical abscesses have indeed been reported in numerous case reports, as highlighted by the retrospective work by Cone and colleagues, who compiled 40 cases of Listeria-related brain abscesses published from 1966 to 2003 [6]; they were, however, evidenced in only 6% of our case series after systematic evaluation, suggesting a bias in previous publications. Similarly, the brainstem radiological involvement called “rhombencephalitis,” which is usually considered very suggestive of neurolisteriosis [9, 23] was only reported in 10% of cases; this low sensitivity mirrors clinical data, as brainstem involvement is only reported in 17% of cases [3]. For the first time, we evidenced the unexpected frequency of hemorrhages and ventricular involvement, that, to our knowledge, had been reported only in scattered observations so far [16, 24]. Hemorrhages could occur as a consequence of associated conditions in 64% of cases; they could also reflect a Listeria endothelial tropism, as Listeria monocytogenes might invade a large array of host cells, including endothelial cells, either directly or via infected monocytes [25, 26]. The pathophysiological mechanisms underlying ventricular involvement (contrast-enhancement and/or hydrocephalus) remain to be determined, especially whether this reflects an invasion site from infected CSF or an extension from parenchymal infection [27].
Altogether, these results highlight the nonspecific neuroradiological presentation of neurolisteriosis, which is not associated with any pathognomonic radiological sign or any specific combination of signs, and can affect any brain compartment (parenchyma, blood vessels, meninges, and ventricles). Clinicians should be aware that the absence of radiological rhombencephalitis, brain abscess, or meningeal-enhancement does not rule out neurolisteriosis and should not be taken into account for empiric anti-Listeria therapy when neurolisteriosis is considered. Diagnosis is indeed often delayed until the results of blood and cerebrospinal fluid cultures or qPCR, as clinical signs are nonspecific and direct examination of the cerebrospinal fluid evidences gram-positive rods in only 32% of cases [3].
Some neuroradiological features were associated with poorer 3-month survival in neurolisteriosis in univariate analysis: ventricular involvement with hydrocephalus or contrast-enhancing ventricles and parenchymal imaging combining abscess(es), nodule(s), and/or white matter images, and multivariable analysis (parenchymal images defined above). Although we cannot exclude overfitting of the logistic model, because of the limited sample size, these images should be added to the other parameters shown associated with poor outcome, namely, ongoing cancer, female sex, recent major weight loss, multi-organ failure, aggravation of any preexisting organ dysfunction, mechanical ventilation, monocytopenia, positive blood culture, and adjunctive dexamethasone therapy [3, 28]. Neuroimaging is recommended in encephalitis as a part of the diagnosis procedure [29]. It should be performed for all patients with confirmed neurolisteriosis, as part of the global assessment of patient’s severity. Furthermore, some lesions might require specific management, like ischemic and hemorrhagic signs, and hydrocephalus or cerebral herniation requiring neurosurgical management. Because all patients with such abnormal images do not all exhibit matching clinical symptoms, neuroimaging should also be performed the 14% of patients with neurolisteriosis who do not exhibit clinical signs of encephalitis. In line with other neurological infections, MRI should be preferred to CT scan, as it displays a greater sensitivity (Table 2) [23, 30, 31].
The high inter-rater agreement underlines the reliability of radiological evaluation and strengthen this recommendation. However, our study has limitations. First, we did not set any harmonized neuroimaging protocol in the setting of this observational study, and the data set reflected these heterogeneous local practices. Also, the sample size hampered further statistical analyses. We could not collect radiological files for all patients included in the MONALISA cohort, and selection bias could not be ruled out; however, the study cohort appeared representative of the global picture of neurolisteriosis in France, without evidence for any distinctive clinical feature or increased severity.
CONCLUSIONS
In conclusion, this study shows that neurolisteriosis presents as a combination of neuroradiological images, none of them being specific. Signs of rhombencephalitis are uncommon, whereas, unexpectedly, hemorrhagic signs are frequent. This study identified parenchymal neuroradiological images associated with a negative prognostic value that will help improve the management of this severe infection.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank Philippe Ravaud, methodologist of the MONALISA study, and Raphaël Porcher for inter-rater agreement analyses.
Funding. Institut Pasteur, Inserm, Santé Publique France, Programme Hospitalier de Recherche Clinique, Swiss National Science Foundation.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
Author notes
C. C. and S. P. contributed equally to the work.
- nuclear magnetic resonance
- magnetic resonance imaging
- encephalitis
- computed tomography
- hemorrhage
- hydrocephalus
- listeria monocytogenes
- abscess
- infections
- brain
- brain stem
- diagnostic imaging
- mortality
- patient prognosis
- rhombencephalitis
- cerebral atrophy
- white matter
- virchow-robin space
- neuroradiologists




