-
PDF
- Split View
-
Views
-
Cite
Cite
Marta Kicia, Maria Wesolowska, Zaneta Kopacz, Martin Kváč, Bohumil Sak, Magdalena Sokulska, Kamil Cebulski, Andrzej B Hendrich, Andrzej Pozowski, Disseminated Infection of Encephalitozoon cuniculi Associated With Osteolysis of Hip Periprosthetic Tissue, Clinical Infectious Diseases, Volume 67, Issue 8, 15 October 2018, Pages 1228–1234, https://doi.org/10.1093/cid/ciy256
Close - Share Icon Share
Abstract
Among patients with hip joint endoprosthesis, periprosthetic osteolysis is the most common complication following primary arthroplasty, and subsequent implant loosening is the leading cause of arthroplasty revision. Causes of stability loss, though not always evident, can be mechanical, allergic, or infectious (bacterial and fungal agents) in nature. Microsporidia, widespread opportunistic fungal pathogens that infect most human tissues, are a potential infectious cause of stability loss. Infections caused by Encephalitozoon species—one of the most common microsporidial pathogens in humans—primarily localize to intestinal and respiratory tracts, but can disseminate to tissues throughout the body.
We examined 53 immunocompetent patients, 23 after revision and 30 after primary hip arthroplasty, for infection by Encephalitozoon species. Periprosthetic tissue, urine sediments, and stool samples were tested by microscopic examination and genus-specific nested polymerase chain reaction followed by genotyping.
Ten patients had Encephalitozoon-positive periprosthetic tissues, 9 (39%) after revision and 1 (3.3%) after primary hip arthroplasty. Among the tissue-positive postrevision patients, 7 had a positive urine sample and 1 had a positive stool sample. Encephalitozoon cuniculi genotype II was identified in 88.8% (16/18) of samples. Two urine samples were positive for a novel Encephalitozoon species.
Encephalitozoon cuniculi should be considered as a cause of osteolysis in hip periprosthetic tissue, leading to a loss of implant stability.
(See the Editorial Commentary by Peel on pages 1235–6.)
Periprosthetic osteolysis is the most common complication after primary hip arthroplasty and can lead to implant loosening and arthroplasty revision. With the increasing incidence of total hip replacement, implant destabilization cases present a challenging problem. As bone resorption is multifactorial, there may be many causes of joint stability loss and not all of them have been proven. The most common are aseptic mechanical and biological causes connected with the wear of the materials on the rubbing surfaces and the degradation of bone tissue quality [1]. In these processes, osteolysis results from a local inflammatory response to particulate wear debris and allergic reactions to metal ions from implant materials [1–4]. Infectious agents such as bacteria and fungi are particularly devastating, affecting periprosthetic tissues in up to 8% of patients following hip arthroplasty, leading to osteolysis and implant destabilization. Such infections are a major challenge with orthopedic implants, and the incidence of deep hip implant infections is expected to rise significantly in the near future [5]. The majority of confirmed periprosthetic infections to date have been caused by bacteria, particularly Staphylococcus (S. aureus, S. epidermidis, S. lugdunensis), Streptococcus species, Enterococcus faecalis, and Propionibacterium species [6–10]. Reports on fungal causes of implant loosening are comparatively rare, with Candida and Aspergillus species most frequently identified as the causative fungal agent [11–13]. However, infectious causes of implant loosening are likely to be underestimated due to difficulties in diagnosing deep implant infection.
Microsporidia are a group of intracellular fungi [14] that infect vertebrate and invertebrate hosts. Fifteen species are known to infect humans, and species from the genus Encephalitozoon have the greatest clinical significance. Encephalitozoon species infect several cell types in mammalian hosts, including epithelial and endothelial cells, fibroblasts, macrophages, and astrocytes [15]. Although the primary sites of infection are the small intestine and respiratory tract, as opportunistic pathogens, they can cause disseminated infection, particularly in hosts with impaired immune function [16]. Symptomatic infections, including diarrhea, pneumonia, hepatitis, cholangitis, peritonitis, and nephritis [15, 17–20], occur most frequently in people with AIDS and recipients of solid organ or bone marrow transplants [19, 21]. There have been also few reports of disease in immunocompetent hosts [22, 23]. Recently, it has been shown that Encephalitozoon persists in immunocompetent hosts and can be reactivated [16]. We hypothesized that Encephalitozoon migrates to periprosthetic tissue during an inflammatory response to hip joint implants, where they could contribute to periprosthetic osteolysis. The aim of this study was to determine the occurrence of disseminated Encephalitozoon species infections among patients following total hip arthroplasty, both primary and revision resulting from periprosthetic osteolysis. Here, we report that Encephalitozoon cuniculi can disseminate to periprosthetic tissue and may contribute to hip implant stability loss.
METHODS
Patients
We examined samples from 2 groups of patients who had been under the care of the Nonresident Department of Traumatic-Orthopedic Surgery, Provincial Specialist Hospital in Wroclaw, Poland, between January 2013 and November 2014. The first group comprised patients who underwent revision total hip arthroplasty due to implant loosening because of periprosthetic osteolytic lesions (RHA group). The second group, which served as a control, comprised patients who underwent primary total hip arthroplasty (THA group). Exclusion criteria for both groups were (1) undergoing immunosuppressive treatment; (2) HIV infection; (3) history of inflammatory processes treated with antibiotics also outside the locomotor organs (eg, peritonitis, adnexitis); and, additionally in case of RHA patients, (4) previous revision arthroplasty and (5) periprosthetic joint infection (PJI) according to criteria of the Infectious Diseases Society of America or the Musculoskeletal Infection Society [24, 25]. In all RHA patients, diagnosis of infection was based on the erythrocyte sedimentation rate, C-reactive protein values, scintigraphy, and examination of the area around the prosthesis for the presence of pathogenic microorganisms. Puncture of periprosthetic tissue for culture was performed before surgery. In the event that the first puncture was aseptic, the puncture was repeated after 6 weeks. In addition, histopathological examination, immunohistochemical analysis, and culture of gram-positive and gram-negative aerobes and anaerobes were performed on periprosthetic tissue taken intraoperatively. Aseptic loosening was diagnosed when the patient had radiological signs of loosening without symptoms of infection. Primary and revision total hip arthroplasties were made with cement or cementless metal-on-polyethylene or polyethylene-on-ceramic articulations. All surgeries were performed by the same team with the same main surgeon and in 1 standard, nonlaminar airflow operating room in accordance with the same procedures. Based on previous data, risk factors of PJI were identified as diabetes mellitus, rheumatoid arthritis, a history of renal insufficiency, or malignancy [10, 11, 26, 27].
Sample Collection
Urine sediments, following centrifugation at 252g for 20 minutes, and stool specimens were collected in the direct postoperative period. Samples of periprosthetic tissue from RHA patients, and fragments of hip joint capsules and synovial membrane from the THA group, were collected intraoperatively. Samples were collected aseptically. All samples were examined immediately by microscopy or stored, without preservative, at –20°C for 1–2 weeks before molecular analyses.
Microscopic Examination
Slides from fresh tissue samples were prepared by mechanical homogenization using sterile mortar and pestle. Standard Calcofluor M2R and trichrome-based staining were used to detect microsporidial spores [28]. Standard histological examination of tissues slides stained by hematoxylin-eosin were also performed. Direct immunofluorescence assay was performed using A700FLR 1X. Microspor-FA kit (Waterborne, New Orleans, Louisiana) with fluorescein–labeled polyclonal antibody made to outer spore wall antigenic sites (epitopes) of Encephalitozoon intestinalis, following the manufacturer’s instructions.
DNA Isolation
A total of 200 mg of stool, periprosthetic tissue, and urine sediment was homogenized by bead disruption using a Precellys 24 Instrument (Bertin Technologies, France). Total DNA was extracted using the PSP Spin Stool DNA Kit (Stratec Molecular, Birkenfeld, Germany) for stool or DNeasy Blood and Tissue Kit (Qiagen, Hilden, Germany) for tissue and urine, following the manufacturers’ instructions. Extracted DNA was stored at –20°C until polymerase chain reaction (PCR) amplification.
Molecular Examination
Genomic DNA was analyzed for microsporidia presence by nested PCR to amplify partial sequence of the 16S ribosomal RNA (rRNA) gene, the entire internal transcribed spacer (ITS) region, and a partial sequence of 5.8S rRNA gene of Encephalitozoon genus with primer pairs ITS580F/ITS580R in primary and MSP3/MSP4A in secondary reactions [29, 30]. The PCR conditions were as previously described [22]. DNA extracted from E. cuniculi genotype III spores was used as a positive control.
Sequencing and Phylogenetic Analyses
PCR products were sequenced in both directions using the Sanger sequencing method. Amplification and sequencing of each sample was repeated twice. Nucleotide sequences were edited using the program ChromasPro 2.1.5 and aligned with each other and with reference sequences from GenBank (www.ncbi.nlm.nih.gov/blast) using MAFFT version 7 software (http://mafft.cbrc.jp/alignment/software/). Phylogenetic analyses were performed using MEGA6 software and trees were inferred by the maximum likelihood method. Bootstrap support for branching was based on 1000 pseudoreplicates.
Nucleotide Sequence Accession Numbers
The partial sequences of 16S rRNA gene, complete sequences of ITS and partial sequences of 5.8S rRNA gene obtained in this study have been deposited in GenBank under accession numbers MF062415–MF062431 and MF078363.
Statistical Analysis
The χ2 or Fisher exact tests were used to compare categorical variables (sex, place of residence, risk factors of PJI) between Encephalitozoon-positive and -negative patients, whereas continuous variables (age, time after primary hip arthroplasty) was compared using Student t test. A P value of <.05 was considered significant.
Ethical Considerations
This study was approved by the Human Research Ethics Committee of Wroclaw Medical University (agreement number KB-549/2012). Written informed consent was obtained from every participant prior to examination.
RESULTS
Patients
A total of 53 individuals, 30 THA and 23 RHA, were screened for microsporidial infection. The mean age of RHA patients was 70 ± 11.7 years, and ranged between 47 and 89 years. THA patients had a mean age of 69 ± 8.7 years, and ranged between 51 and 89 years. Overall, the male-to-female ratio was 6 (26%) to 17 (74%) in the RHA group, and 8 (27%) to 22 (73%) in the THA group.
Complete blood cell count, electrolytes, blood glucose, erythrocyte sedimentation rate and C-reactive protein values, creatinine levels, and urine tests were within the normal range in all patients. Ectopic ossification prophylaxis was applied in all patients and consisted of nonsteroidal anti-inflammatory drugs administered for 10 days to 4 weeks after surgery. Patients were not undergoing immunosuppressive treatment.
Non–rapidly progressive osteolysis was observed in all RHA patients. The process began with stability loss in some endoprosthesis elements. Early stability loss (up to 3 years after primary arthroplasty) was estimated clinically (pain) and radiologically (radiolucent area around the implants). The osteolytic lesions were localized in the hip bone acetabulum, mostly within zone 3, and in the femur, within zone 1 and 7 (Gruen zone). Periprosthetic tissue retrieved at the time of revision operations consisted of a conglomerate of necrotic tissues with excessive congestion originating from a humoral immune response. Moreover, there was a localized inflammatory response to polyethylene debris and abrasion particles of polyethylene. Microbiological tests failed to detect bacterial, viral, or fungal infections in tissues from RHA patients, and therefore implant loosening was clinically classified as aseptic.
Periprosthetic tissues obtained during primary arthroplasty from THA patients consisted of fragments of joint capsules and synovial membrane. These tissues exhibited localized inflammation, large overgrowth of synovial membrane, reduced tissue elasticity values, and scar tissue formation, but T cells were not detected.
Encephalitozoon Analysis
Encephalitozoon DNA was detected in 10 of the 53 (18.9%) patients in the study. Among the positive patients, 9 were from the RHA group (90%), representing 39% (9/23) of the patients in that group (Table 1), and 1 was from the THA group, representing 3.3% (1/30) of the patients in that group.
| Characteristic . | Encephalitozoon Species . | P Value . | |
|---|---|---|---|
| . | Positive (n = 9) . | Negative (n = 14) . | . |
| Age, y, median (range) | 71 (61–89) | 70 (47–82) | .396 |
| Sex, No. (%) | |||
| Male | 0 (0) | 6 (42.9) | .048 |
| Female | 9 (100) | 8 (57.1) | .048 |
| Setting of residence, No. (%) | |||
| Industrial | 8 (88.9) | 8 (57.1) | .176 |
| Rural | 1 (11.1) | 6 (42.9) | .176 |
| Risk factors for periprosthetic joint infectiona, No. (%) | |||
| Diabetes mellitus | 1 (11.1) | 1 (7.1) | 1.000 |
| Rheumatoid arthritis | 1 (11.1) | 0 | .391 |
| Time after primary hip arthroplasty, y, median (range) | 14 (7–21) | 13 (5–19) | .277 |
| Characteristic . | Encephalitozoon Species . | P Value . | |
|---|---|---|---|
| . | Positive (n = 9) . | Negative (n = 14) . | . |
| Age, y, median (range) | 71 (61–89) | 70 (47–82) | .396 |
| Sex, No. (%) | |||
| Male | 0 (0) | 6 (42.9) | .048 |
| Female | 9 (100) | 8 (57.1) | .048 |
| Setting of residence, No. (%) | |||
| Industrial | 8 (88.9) | 8 (57.1) | .176 |
| Rural | 1 (11.1) | 6 (42.9) | .176 |
| Risk factors for periprosthetic joint infectiona, No. (%) | |||
| Diabetes mellitus | 1 (11.1) | 1 (7.1) | 1.000 |
| Rheumatoid arthritis | 1 (11.1) | 0 | .391 |
| Time after primary hip arthroplasty, y, median (range) | 14 (7–21) | 13 (5–19) | .277 |
aBecause no history of renal insufficiency or malignancy was identified in Encephalitozoon-positive patients after revision total hip arthroplasty, these risk factors are not specified.
| Characteristic . | Encephalitozoon Species . | P Value . | |
|---|---|---|---|
| . | Positive (n = 9) . | Negative (n = 14) . | . |
| Age, y, median (range) | 71 (61–89) | 70 (47–82) | .396 |
| Sex, No. (%) | |||
| Male | 0 (0) | 6 (42.9) | .048 |
| Female | 9 (100) | 8 (57.1) | .048 |
| Setting of residence, No. (%) | |||
| Industrial | 8 (88.9) | 8 (57.1) | .176 |
| Rural | 1 (11.1) | 6 (42.9) | .176 |
| Risk factors for periprosthetic joint infectiona, No. (%) | |||
| Diabetes mellitus | 1 (11.1) | 1 (7.1) | 1.000 |
| Rheumatoid arthritis | 1 (11.1) | 0 | .391 |
| Time after primary hip arthroplasty, y, median (range) | 14 (7–21) | 13 (5–19) | .277 |
| Characteristic . | Encephalitozoon Species . | P Value . | |
|---|---|---|---|
| . | Positive (n = 9) . | Negative (n = 14) . | . |
| Age, y, median (range) | 71 (61–89) | 70 (47–82) | .396 |
| Sex, No. (%) | |||
| Male | 0 (0) | 6 (42.9) | .048 |
| Female | 9 (100) | 8 (57.1) | .048 |
| Setting of residence, No. (%) | |||
| Industrial | 8 (88.9) | 8 (57.1) | .176 |
| Rural | 1 (11.1) | 6 (42.9) | .176 |
| Risk factors for periprosthetic joint infectiona, No. (%) | |||
| Diabetes mellitus | 1 (11.1) | 1 (7.1) | 1.000 |
| Rheumatoid arthritis | 1 (11.1) | 0 | .391 |
| Time after primary hip arthroplasty, y, median (range) | 14 (7–21) | 13 (5–19) | .277 |
aBecause no history of renal insufficiency or malignancy was identified in Encephalitozoon-positive patients after revision total hip arthroplasty, these risk factors are not specified.
Among the 9 RHA patients who tested positive for Encephalitozoon DNA, 9 (100%) had positive tissue samples, 7 (78%) had positive urine samples, and 1 (11%) had a positive stool sample. Only a tissue sample was positive in the Encephalitozoon-positive THA patient (Figure 1A). Phylogenetic analysis revealed the presence of E. cuniculi genotype II in all positive tissue samples, a positive stool sample, and in 5 of 7 urine sediments. Previously undescribed Encephalitozoon species were found in the remaining 2 urine sediments (Figure 1A). These novel Encephalitozoon species shared 100% identity with each other, differed from other Encephalitozoon species at the 16S, 5.8S, and ITS loci, and formed a sister group with E. cuniculi (Figure 1A and 1B). No microsporidia lesions were found in tissues by histology, but microscopic analysis of Calcofluor M2R–stained and direct immunofluorescence-labeled smears confirmed the presence of spores (2–3 spores per slide) in tissue samples that tested positive for Encephalitozoon DNA (Figure 2).
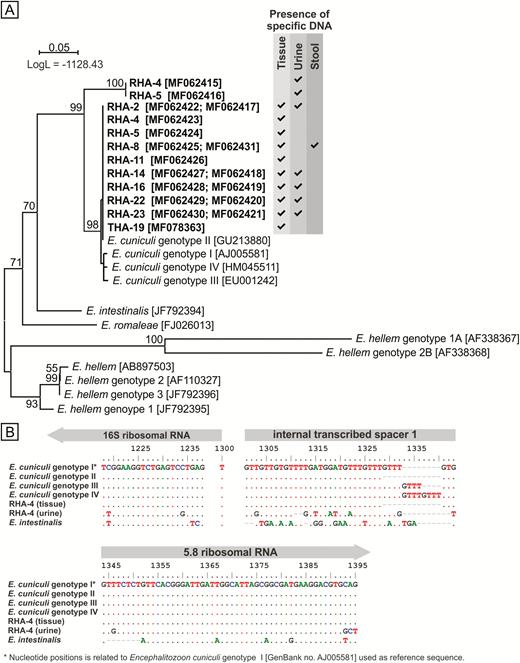
Phylogenetic relationships between novel Encephalitozoon isolates and Encephalitozoon cuniculi identified in this study and the rest of the Encephalitozoon genus. A, Maximum likelihood tree of novel Encephalitozoon internal transcribed spacer (ITS) sequences together with all members of the Encephalitozoon genus. Alignment of partial sequences of the 16S ribosomal RNA (rRNA) gene, complete sequences of ITS, and partial sequences of the 5.8S rRNA gene of one of the novel Encephalitozoon isolates, as well as E. cuniculi identified in the same patient, different E. cuniculi genotypes, and E. intestinalis in (B), reveals single-nucleotide polymorphisms differentiate identified isolates; the scale bar corresponds to a genetic distance of .05 substitutions per site. Abbreviations: LogL, log-likelihood value; RHA, revision total hip arthroplasty.
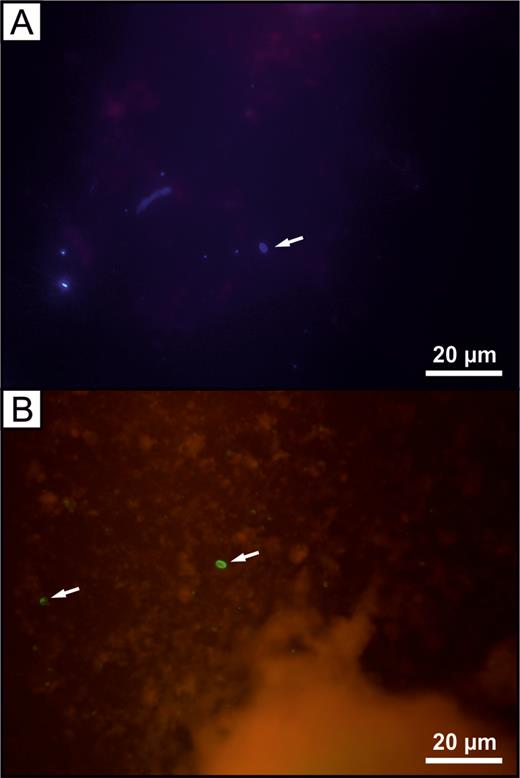
Microscopic examination of homogenized tissue from a patient with revision total hip arthroplasty. A, Single microsporidial spore (arrow) stained with Calcofluor M2R. B, Encephalitozoon cuniculi spores (arrows) labeled with anti–outer spore wall epitopes fluorescein–conjugated polyclonal antibodies.
In examining Encephalitozoon-positive and -negative subgroups in RHA patients (Table 1), there was a significant association between the Encephalitozoon infection and sex (P = .048). Encephalitozoon infection was not correlated with average age, place of residence, diabetes mellitus, or rheumatoid arthritis. None of the Encephalitozoon-positive patients had a history of renal insufficiency or malignancy. The average elapsed time between primary hip arthroplasty and revision surgery was similar in Encephalitozoon-positive and -negative RHA subgroups and ranged from 7 to 21 years and 5 to 19 years, respectively (Table 1). Thus, late stability loss (>3 years after primary arthroplasty) was observed in all of the patients tested. Articulation materials did not differ between Encephalitozoon-positive and -negative patients, suggesting that type of debris generated does not significantly affect the infection outcome. Also, width and location of the radiolucent zones around the mandrel of the prosthesis, according to Gruen system, and around prosthesis acetabulum, according to the DeLee-Charnley system, did not differ significantly between Encephalitozoon-positive and -negative patients and depended mostly on the duration of implant loosening. No patients had clinical signs of microsporidiosis, such as diarrhea or fever.
DISCUSSION
Periprosthetic joint infections are a devastating complication after arthroplasty and are associated with high morbidity [31]. The diagnosis of orthopedic implant infection remains challenging, so the pathogenesis of implant failure remains poorly understood. The chronic periprosthetic infectious process is oligosymptomatic, which makes it difficult to differentiate from aseptic implant loosening, despite the existence of infection biomarkers and criteria [7, 25, 32]. Consequently, more than one-tenth of patients with suspected aseptic loosening have misdiagnosed PJIs and will not receive appropriate treatment [6, 7]. Moreover, mechanical and infective agents are not mutually exclusive and may coexist as a cause of osteolysis, with infection following aseptic loosening [6]. In a proposed mechanism of aseptic implant loosening, implant-derived wear particles are opsonized by danger-associated molecular patterns from damaged host tissue, and are recognized by Toll-like receptors, leading to chronic inflammation and foreign body reaction [2, 4, 33]. In an infection, an inflammatory response is initiated when pathogen-associated molecular patterns are recognized by Toll-like receptors also [4].
Here, we detected periprosthetic infection caused by E. cuniculi in 39% of patients suffering from hip implant loosening that was previously classified as aseptic. A key question arising from this finding is whether the infection initiated osteolysis or followed as a result of the immune response to the aseptic implant destabilization process, subsequently accelerating ossification. Encephalitozoon cuniculi could persist and replicate inside resting macrophages, where it can evade the immune response and be transported throughout the host [34]. Because aseptic implant loosening is associated with a response to implant biomaterials [3] or metal ions [35] by local immune activation, for example, macrophages, it is more likely that dissemination of E. cuniculi to periprosthetic tissue followed an inflammatory response to debris. During latent infection in an immunocompetent host, persistent microsporidia could provide continuous immune stimulation, leading to chronic inflammation, progressive tissue destruction, and necrotic changes [36]. As opportunistic pathogens without organ specificity, E. cuniculi can readily spread throughout the body. This can occur by direct extension of spores into surrounding cells and by introduction into the vascular system [37]. In experimentally infected animals, the E. cuniculi course of infection is progressive, with dissemination to, and subsequent clearance from, most organs [16, 38]. In immunocompetent hosts, E. cuniculi is largely confined to the spleen, lung, kidney, and liver during the early stages of infection, while chronic infection persists in the lung, kidney, and cecum [15, 38]. Bacterial and fungal species that form part of the normal skin microflora can be accidentally introduced to sterile body sites during replacement surgery, and this is postulated to be a cause of early periprosthetic deep infections that occur 3–12 months after surgery. However, considering that 7–21 years elapsed between primary and revision arthroplasty in the present study, it is more likely that Encephalitozoon arrived at the periprosthetic tissue by hematogenous spread from another site of infection in the body, similar to other fungal or bacterial agents causing late infections [7, 8, 10].
Presence of E. cuniculi in RHA patients’ tissue may results from the chronic inflammation process and continuous recruitment of microsporidia-infected macrophages from other parts of the body. Such lasting immune activity may lead to persistent activation of latent E. cuniculi infection. This mechanism may explain why RHA patients shed spores in urine and stool significantly more frequently compared with the THA group. The single tissue infection in a THA patient may have resulted from a localized inflammatory response which develops even in the absence of a hip implant, resulting in damage of the hip joint. Compared to microsporidial keratitis, which is characterized by the presence of numerous spores in tissues [39], only a few spores of E. cuniculi were found in the periprosthetic tissue of RHA patients. The question of whether a periprosthetic infection can induce osteolysis and cause implant loosening requires further investigation.
The occurrence of Encephalitozoon species infection was significantly associated with the sex of patients, with only women being infected. According to Caicedo et al [40], women have an increased risk of adverse local tissue reactions, which may result from a more aggressive immune response to implant debris, particularly metal articulations, and lead to metal sensitization and aseptic implant loosening. While this is interesting, differences between the sexes was not a major focus of the present study and a larger study should be designed to specifically address this question.
Additionally, 2 RHA patients were infected with 2 different Encephalitozoon taxa: E. cuniculi in tissues and the same novel Encephalitozoon species in urine sediments. Finding the novel genotype exclusively in urine from both patients suggests that it may localize to the urinary tract. Further study is warranted to characterize this genotype, including detailed descriptions of its biology, morphology, and evolutionary relationships.
In conclusion, finding E. cuniculi in periprosthetic tissue from 39% of revision hip arthroplasty patients shows for the first time that this fungus can occupy this unusual extraintestinal niche, and must therefore be considered as a contributing cause of periprosthetic osteolysis and implant destabilization after hip replacement. Increased presence of E. cuniculi spores in urine, not only of patients after hip joint replacement, might be considered as an indicator of chronic inflammatory process in the patient’s body. This is particularly important given the increasing evidence of widespread microsporidial infection in immunocompetent people.
Notes
Disclaimer. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Financial support. This work was supported by the National Science Centre, Poland (grant number DEC-2012/05/D/NZ6/00615); Wroclaw Medical University, Grant for Young Scientists (grant number Pbmn191); and Grant Agency of the Czech Republic (grant number 17-12871S).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.




