-
PDF
- Split View
-
Views
-
Cite
Cite
Jaime Lora-Tamayo, Éric Senneville, Alba Ribera, Louis Bernard, Michel Dupon, Valérie Zeller, Ho Kwong Li, Cédric Arvieux, Martin Clauss, Ilker Uçkay, Dace Vigante, Tristan Ferry, José Antonio Iribarren, Trisha N. Peel, Parham Sendi, Nina Gorišek Miksić, Dolors Rodríguez-Pardo, María Dolores del Toro, Marta Fernández-Sampedro, Ulrike Dapunt, Kaisa Huotari, Joshua S. Davis, Julián Palomino, Danielle Neut, Benjamin M. Clark, Thomas Gottlieb, Rihard Trebše, Alex Soriano, Alberto Bahamonde, Laura Guío, Alicia Rico, Mauro J. C. Salles, M. José G. Pais, Natividad Benito, Melchor Riera, Lucía Gómez, Craig A. Aboltins, Jaime Esteban, Juan Pablo Horcajada, Karina O’Connell, Matteo Ferrari, Gábor Skaliczki, Rafael San Juan, Javier Cobo, Mar Sánchez-Somolinos, Antonio Ramos, Efthymia Giannitsioti, Alfredo Jover-Sáenz, Josu Mirena Baraia-Etxaburu, José María Barbero, Peter F. M. Choong, Nathalie Asseray, Séverine Ansart, Gwenäel Le Moal, Werner Zimmerli, Javier Ariza, for the Group of Investigators for Streptococcal Prosthetic Joint Infection , The Not-So-Good Prognosis of Streptococcal Periprosthetic Joint Infection Managed by Implant Retention: The Results of a Large Multicenter Study, Clinical Infectious Diseases, Volume 64, Issue 12, 15 June 2017, Pages 1742–1752, https://doi.org/10.1093/cid/cix227
Close - Share Icon Share
Abstract
Streptococci are not an infrequent cause of periprosthetic joint infection (PJI). Management by debridement, antibiotics, and implant retention (DAIR) is thought to produce a good prognosis, but little is known about the real likelihood of success.
A retrospective, observational, multicenter, international study was performed during 2003–2012. Eligible patients had a streptococcal PJI that was managed with DAIR. The primary endpoint was failure, defined as death related to infection, relapse/persistence of infection, or the need for salvage therapy.
Overall, 462 cases were included (median age 72 years, 50% men). The most frequent species was Streptococcus agalactiae (34%), and 52% of all cases were hematogenous. Antibiotic treatment was primarily using β-lactams, and 37% of patients received rifampin. Outcomes were evaluable in 444 patients: failure occurred in 187 (42.1%; 95% confidence interval, 37.5%–46.7%) after a median of 62 days from debridement; patients without failure were followed up for a median of 802 days. Independent predictors (hazard ratios) of failure were rheumatoid arthritis (2.36), late post-surgical infection (2.20), and bacteremia (1.69). Independent predictors of success were exchange of removable components (0.60), early use of rifampin (0.98 per day of treatment within the first 30 days), and long treatments (≥21 days) with β-lactams, either as monotherapy (0.48) or in combination with rifampin (0.34).
This is the largest series to our knowledge of streptococcal PJI managed by DAIR, showing a worse prognosis than previously reported. The beneficial effects of exchanging the removable components and of β-lactams are confirmed and maybe also a potential benefit from adding rifampin.
Periprosthetic joint infection (PJI) is a dreaded complication of joint replacement [1, 2]. Removal of the infected foreign body is the rule for any given device-associated infection. However, debridement, antibiotics, and implant retention (DAIR) may be attempted in some acute cases of PJI [2–4]. When strict selection of patients is followed, the success rate may reach >85% [4–7].
Streptococci are responsible for PJI in 4–12% of cases [8, 9] especially in hematogenous infections [10, 11]. Some studies have suggested that streptococcal PJI may have a more favorable outcome compared with other etiologies [12–14], but this has been contested by others [15]. In fact, the success rate of streptococcal PJI (mostly Streptococcus agalactiae) treated with DAIR varies from 22% to 100%, presumably depending on the selection criteria used [6, 13, 15–18] (Supplementary Table 1). Thus, the real success rate for patients managed by DAIR remains uncertain.
The optimal antimicrobial treatment for streptococcal PJI is also unknown. Current guidelines recommend the use of β-lactams [2, 4], but these antibiotics may have a very high minimal biofilm eradication concentration [19, 20]. The role of alternative compounds with a better antibiofilm profile [21] has not been consistently explored in clinical studies.
Our aim was to analyze the clinical presentations and outcomes of a large cohort of patients with streptococcal PJI managed by DAIR, focusing on the impact of antimicrobial therapy.
METHODS
Setting and Patients
This was a multicenter retrospective study performed in 52 hospitals from 15 nations between 2003 and 2012. Patients were included if they had suffered a PJI that was caused by streptococci and initially managed by DAIR. Eighty-one cases included here have previously been published [6, 15, 22].
PJI was defined according to Infectious Diseases Society of America (IDSA) guidelines as the presence of a sinus tract communicating with the prosthesis, acute inflammation on histologic examination, purulence surrounding the prosthesis, and/or ≥2 evaluable samples yielding the same organism [4]. Polymicrobial cases were also included if streptococci were isolated from the beginning, but we excluded cases of streptococcal superinfection. Microorganisms were identified following standard criteria [23], after samples had been inoculated in liquid and solid media and incubated for ≥7 days. Enterococci, obligate anaerobes (i.e., Peptostreptococcus spp.) or nutritionally variant streptococci (i.e., Abiotrophia spp.) were not included.
PJI was classified as early postoperative, if the symptoms began within the first 3 months after the prosthesis was placed, and late post-surgical, if they started thereafter. The episode was considered acute hematogenous, if it occurred after an uneventful postoperative course and after microbiologically confirmed or clinically suspected streptococcal bacteremia. A contiguous spread was considered, if the PJI occurred in a limb with either infectious cellulitis, or a soft tissue abscess. New radiographical signs of infection were taken as a surrogate marker of chronicity (i.e., periprosthetic radiolucency, bone sclerosis, or osteolytic lesions). Chronic renal failure was defined as a baseline creatinine >150 µmol/L; immunosuppressant therapy was recorded if the patient received, was receiving glucocorticoid, or other immunosuppressant drug therapy.
Data were recorded in a Microsoft-Access database. All cases were critically reviewed by one author (J. L.-T.), and any doubts or inconsistencies were double-checked by the investigator at each hospital.
Clinical and Surgical Management
DAIR has been described elsewhere [2, 3, 24]. Briefly, it comprises thorough surgical debridement of all purulent collections and necrotic tissues surrounding the prosthesis. Mobile parts of the device (i.e., the polyethylene liner) are exchanged if feasible. DAIR is recommended in patients who meet the criteria proposed by the IDSA guidelines [4]. Patients with early postoperative (<1 month) or acute hematogenous PJI with ≤3 weeks of symptoms qualify for DAIR if they have a soundly fixed prosthesis, good periprosthetic soft tissues condition, and antibiotics are available with a reasonable activity against biofilm-embedded bacteria. In the present study, these criteria were not strictly met by many patients, and the decision to undergo DAIR was taken by individual medical group on a case by case basis.
Outcome and Follow-up
Patients were followed until death, treatment failure, removal or replacement of the prosthesis, or until loss to follow-up. Overall Failure was the primary endpoint and was considered in cases of: (12) death related to the infection;64(ii) need for salvage therapy to control the infection, including supplementary surgical debridements >30 days after the first debridement, prosthesis removal (due to any cause during the first year after debridement, or due to streptococcal persistence or relapse, or superinfection by other microorganisms), or the need for supplementary courses of antibiotics beyond the initially scheduled treatment (including chronic suppressive antimicrobial therapy); and/or (iii) persistent signs of infection at the last visit or follow-up appointment.
Given the retrospective nature of this study, and to avoid a survivor bias when analyzing the impact of antimicrobial therapy, several failure dynamics were studied:
Early Failure was considered to have occurred in patients who met the failure criteria within the first 30 days after surgical debridement.
Late Failure was considered to have occurred in patients who met the failure criteria beyond the first 30 days after debridement but who were still under antimicrobial therapy. In this group, only antimicrobials received during the first 30 days were analyzed.
Failure after Therapy was considered to have occurred in patients who met the failure criteria once they had finished the scheduled therapy. In this analysis, the antibiotics received throughout treatment were included.
Statistical Analysis
Categorical parameters were compared with the χ2 test or Fisher exact test, and continuous variables were compared with the Mann–Whitney U test or Kruskal–Wallis test. Parameters associated with Overall Failure, Late Failure, and Failure after Therapy were identified by Kaplan–Meier curves (long-rank test), univariate, and multivariate Cox regression. For the analysis of Early Failure, logistic regression were performed. All analyses were 2-tailed, and a P value < .05 was considered statistically significant.
RESULTS
Description of the Series
Overall, 922 cases of PJI were recorded, of which 92 (10.0%) were excluded for various reasons, leaving a cohort of 830 cases. We initially managed 462 (55.7%) by DAIR, and these cases were used as the focus of this analysis (Supplementary Figure 1).
The median age was 72 years (interquartile range [IQR], 65–78 years), and 50% were men. The most frequent type of PJI was hematogenous (52%), which occurred more frequently in men, in patients with malignancy and in those with knee prostheses. Patients with hematogenous PJI more frequently presented with bacteremia and elevated temperature, along with higher leukocyte counts and C-reactive protein (CRP) levels (Table 1).
Baseline Features, Clinical Presentation, Surgical Management, Outcome, and Comparative Analysis of Hematogenous and Non-hematogenous Cases
| . | All Patients (n = 462) . | Non-hematogenous Cases (n = 220) . | Hematogenous Cases (n = 242) . | P . |
|---|---|---|---|---|
| Baseline features | ||||
| Sex (men) | 232 (50%) | 121 (45%) | 111 (54%) | .050 |
| Age (years)a | 72 (65–78) | 72 (64–78) | 72 (65–78) | .986 |
| Diabetes | 111 (24%) | 50 (23%) | 61 (25%) | .533 |
| Renal chronic disease | 45 (10%) | 20 (9%) | 25 (10%) | .654 |
| Rheumatoid arthritis | 37 (8%) | 15 (7%) | 22 (9%) | .369 |
| Immunosuppressive therapy | 49 (11%) | 22 (10%) | 27 (11%) | .687 |
| Malignancy | 29 (6%) | 7 (3%) | 22 (9%) | .009 |
| Liver cirrhosis | 19 (4%) | 9 (4%) | 10 (4%) | .982 |
| Chronic lung disease | 56 (12%) | 27 (12%) | 29 (12%) | .924 |
| Chronic heart disease | 128 (28%) | 54 (25%) | 74 (31%) | .148 |
| Prosthesis location (knee) | 273 (59%) | 117 (53%) | 156 (65%) | .014 |
| Revision prosthesis | 114 (25%) | 48 (22%) | 66 (27%) | .174 |
| Clinical presentation and microbiological data | ||||
| Temperature > 37°C | 300 (66%) | 110 (51%) | 190 (80%) | <.001 |
| Sinus tract | 62 (14%) | 46 (21%) | 16 (7%) | <.001 |
| Leukocyte count (×10E9/L)a | 12.0 (8.5–15.4) | 11.0 (7.3–14.6) | 13.0 (9.6–16.0) | .001 |
| C-reactive protein at diagnosis (mg/L)a | 186 (85–283) | 135 (55–230) | 234 (130–305) | <.001 |
| Rx signs of infection | 85 (18%) | 41 (19%) | 44 (18%) | .900 |
| Bacteremia | 138 (31%) | 35 (17%) | 103 (45%) | <.001 |
| Penicillin MIC > 0.125 mg/Lf | 24/425 (6%) | 15/199 (8%) | 9/226 (4%) | .113 |
| Polymicrobial infection | 63 (14%) | 52 (24%) | 11 (5%) | <.001 |
| Surgical management | ||||
| Time to debridement (days)a,b | 5 (2–13) | 5 (2–16) | 5 (2–12) | .688 |
| Exchange of removable components c | 220/418 (53%) | 100/200 (50%) | 120/218 (55%) | .302 |
| Need for ≥2 debridements | 42 (9%) | 21 (10%) | 21 (9%) | .797 |
| Outcomed | ||||
| Overall failuree | 187/444 (42%) | 92/210 (44%) | 95/234 (41%) | .494 |
| Early failured,e | 55/187 (29%) | 25/92 (27%) | 30/95 (32%) | .509 |
| Late failured,e | 71/187 (38%) | 34/92 (37%) | 37/95 (39%) | .779 |
| Failure after therapye | 61/187 (33%) | 33/92 (36%) | 28/95 (30%) | .351 |
| . | All Patients (n = 462) . | Non-hematogenous Cases (n = 220) . | Hematogenous Cases (n = 242) . | P . |
|---|---|---|---|---|
| Baseline features | ||||
| Sex (men) | 232 (50%) | 121 (45%) | 111 (54%) | .050 |
| Age (years)a | 72 (65–78) | 72 (64–78) | 72 (65–78) | .986 |
| Diabetes | 111 (24%) | 50 (23%) | 61 (25%) | .533 |
| Renal chronic disease | 45 (10%) | 20 (9%) | 25 (10%) | .654 |
| Rheumatoid arthritis | 37 (8%) | 15 (7%) | 22 (9%) | .369 |
| Immunosuppressive therapy | 49 (11%) | 22 (10%) | 27 (11%) | .687 |
| Malignancy | 29 (6%) | 7 (3%) | 22 (9%) | .009 |
| Liver cirrhosis | 19 (4%) | 9 (4%) | 10 (4%) | .982 |
| Chronic lung disease | 56 (12%) | 27 (12%) | 29 (12%) | .924 |
| Chronic heart disease | 128 (28%) | 54 (25%) | 74 (31%) | .148 |
| Prosthesis location (knee) | 273 (59%) | 117 (53%) | 156 (65%) | .014 |
| Revision prosthesis | 114 (25%) | 48 (22%) | 66 (27%) | .174 |
| Clinical presentation and microbiological data | ||||
| Temperature > 37°C | 300 (66%) | 110 (51%) | 190 (80%) | <.001 |
| Sinus tract | 62 (14%) | 46 (21%) | 16 (7%) | <.001 |
| Leukocyte count (×10E9/L)a | 12.0 (8.5–15.4) | 11.0 (7.3–14.6) | 13.0 (9.6–16.0) | .001 |
| C-reactive protein at diagnosis (mg/L)a | 186 (85–283) | 135 (55–230) | 234 (130–305) | <.001 |
| Rx signs of infection | 85 (18%) | 41 (19%) | 44 (18%) | .900 |
| Bacteremia | 138 (31%) | 35 (17%) | 103 (45%) | <.001 |
| Penicillin MIC > 0.125 mg/Lf | 24/425 (6%) | 15/199 (8%) | 9/226 (4%) | .113 |
| Polymicrobial infection | 63 (14%) | 52 (24%) | 11 (5%) | <.001 |
| Surgical management | ||||
| Time to debridement (days)a,b | 5 (2–13) | 5 (2–16) | 5 (2–12) | .688 |
| Exchange of removable components c | 220/418 (53%) | 100/200 (50%) | 120/218 (55%) | .302 |
| Need for ≥2 debridements | 42 (9%) | 21 (10%) | 21 (9%) | .797 |
| Outcomed | ||||
| Overall failuree | 187/444 (42%) | 92/210 (44%) | 95/234 (41%) | .494 |
| Early failured,e | 55/187 (29%) | 25/92 (27%) | 30/95 (32%) | .509 |
| Late failured,e | 71/187 (38%) | 34/92 (37%) | 37/95 (39%) | .779 |
| Failure after therapye | 61/187 (33%) | 33/92 (36%) | 28/95 (30%) | .351 |
Statistical significance (ie, P < .05) are shown in bold.
Abbreviation: MIC, minimal inhibitory concentration.
Data expressed as count and (percentage) except for acontinuous variables (median and interquartile range).
bTime from onset of symptoms to surgical debridement.
cData available in 418 cases.
d444 patients evaluable for outcome.
ePercentages given over the whole of failures.
fData available in 425 patients.
Baseline Features, Clinical Presentation, Surgical Management, Outcome, and Comparative Analysis of Hematogenous and Non-hematogenous Cases
| . | All Patients (n = 462) . | Non-hematogenous Cases (n = 220) . | Hematogenous Cases (n = 242) . | P . |
|---|---|---|---|---|
| Baseline features | ||||
| Sex (men) | 232 (50%) | 121 (45%) | 111 (54%) | .050 |
| Age (years)a | 72 (65–78) | 72 (64–78) | 72 (65–78) | .986 |
| Diabetes | 111 (24%) | 50 (23%) | 61 (25%) | .533 |
| Renal chronic disease | 45 (10%) | 20 (9%) | 25 (10%) | .654 |
| Rheumatoid arthritis | 37 (8%) | 15 (7%) | 22 (9%) | .369 |
| Immunosuppressive therapy | 49 (11%) | 22 (10%) | 27 (11%) | .687 |
| Malignancy | 29 (6%) | 7 (3%) | 22 (9%) | .009 |
| Liver cirrhosis | 19 (4%) | 9 (4%) | 10 (4%) | .982 |
| Chronic lung disease | 56 (12%) | 27 (12%) | 29 (12%) | .924 |
| Chronic heart disease | 128 (28%) | 54 (25%) | 74 (31%) | .148 |
| Prosthesis location (knee) | 273 (59%) | 117 (53%) | 156 (65%) | .014 |
| Revision prosthesis | 114 (25%) | 48 (22%) | 66 (27%) | .174 |
| Clinical presentation and microbiological data | ||||
| Temperature > 37°C | 300 (66%) | 110 (51%) | 190 (80%) | <.001 |
| Sinus tract | 62 (14%) | 46 (21%) | 16 (7%) | <.001 |
| Leukocyte count (×10E9/L)a | 12.0 (8.5–15.4) | 11.0 (7.3–14.6) | 13.0 (9.6–16.0) | .001 |
| C-reactive protein at diagnosis (mg/L)a | 186 (85–283) | 135 (55–230) | 234 (130–305) | <.001 |
| Rx signs of infection | 85 (18%) | 41 (19%) | 44 (18%) | .900 |
| Bacteremia | 138 (31%) | 35 (17%) | 103 (45%) | <.001 |
| Penicillin MIC > 0.125 mg/Lf | 24/425 (6%) | 15/199 (8%) | 9/226 (4%) | .113 |
| Polymicrobial infection | 63 (14%) | 52 (24%) | 11 (5%) | <.001 |
| Surgical management | ||||
| Time to debridement (days)a,b | 5 (2–13) | 5 (2–16) | 5 (2–12) | .688 |
| Exchange of removable components c | 220/418 (53%) | 100/200 (50%) | 120/218 (55%) | .302 |
| Need for ≥2 debridements | 42 (9%) | 21 (10%) | 21 (9%) | .797 |
| Outcomed | ||||
| Overall failuree | 187/444 (42%) | 92/210 (44%) | 95/234 (41%) | .494 |
| Early failured,e | 55/187 (29%) | 25/92 (27%) | 30/95 (32%) | .509 |
| Late failured,e | 71/187 (38%) | 34/92 (37%) | 37/95 (39%) | .779 |
| Failure after therapye | 61/187 (33%) | 33/92 (36%) | 28/95 (30%) | .351 |
| . | All Patients (n = 462) . | Non-hematogenous Cases (n = 220) . | Hematogenous Cases (n = 242) . | P . |
|---|---|---|---|---|
| Baseline features | ||||
| Sex (men) | 232 (50%) | 121 (45%) | 111 (54%) | .050 |
| Age (years)a | 72 (65–78) | 72 (64–78) | 72 (65–78) | .986 |
| Diabetes | 111 (24%) | 50 (23%) | 61 (25%) | .533 |
| Renal chronic disease | 45 (10%) | 20 (9%) | 25 (10%) | .654 |
| Rheumatoid arthritis | 37 (8%) | 15 (7%) | 22 (9%) | .369 |
| Immunosuppressive therapy | 49 (11%) | 22 (10%) | 27 (11%) | .687 |
| Malignancy | 29 (6%) | 7 (3%) | 22 (9%) | .009 |
| Liver cirrhosis | 19 (4%) | 9 (4%) | 10 (4%) | .982 |
| Chronic lung disease | 56 (12%) | 27 (12%) | 29 (12%) | .924 |
| Chronic heart disease | 128 (28%) | 54 (25%) | 74 (31%) | .148 |
| Prosthesis location (knee) | 273 (59%) | 117 (53%) | 156 (65%) | .014 |
| Revision prosthesis | 114 (25%) | 48 (22%) | 66 (27%) | .174 |
| Clinical presentation and microbiological data | ||||
| Temperature > 37°C | 300 (66%) | 110 (51%) | 190 (80%) | <.001 |
| Sinus tract | 62 (14%) | 46 (21%) | 16 (7%) | <.001 |
| Leukocyte count (×10E9/L)a | 12.0 (8.5–15.4) | 11.0 (7.3–14.6) | 13.0 (9.6–16.0) | .001 |
| C-reactive protein at diagnosis (mg/L)a | 186 (85–283) | 135 (55–230) | 234 (130–305) | <.001 |
| Rx signs of infection | 85 (18%) | 41 (19%) | 44 (18%) | .900 |
| Bacteremia | 138 (31%) | 35 (17%) | 103 (45%) | <.001 |
| Penicillin MIC > 0.125 mg/Lf | 24/425 (6%) | 15/199 (8%) | 9/226 (4%) | .113 |
| Polymicrobial infection | 63 (14%) | 52 (24%) | 11 (5%) | <.001 |
| Surgical management | ||||
| Time to debridement (days)a,b | 5 (2–13) | 5 (2–16) | 5 (2–12) | .688 |
| Exchange of removable components c | 220/418 (53%) | 100/200 (50%) | 120/218 (55%) | .302 |
| Need for ≥2 debridements | 42 (9%) | 21 (10%) | 21 (9%) | .797 |
| Outcomed | ||||
| Overall failuree | 187/444 (42%) | 92/210 (44%) | 95/234 (41%) | .494 |
| Early failured,e | 55/187 (29%) | 25/92 (27%) | 30/95 (32%) | .509 |
| Late failured,e | 71/187 (38%) | 34/92 (37%) | 37/95 (39%) | .779 |
| Failure after therapye | 61/187 (33%) | 33/92 (36%) | 28/95 (30%) | .351 |
Statistical significance (ie, P < .05) are shown in bold.
Abbreviation: MIC, minimal inhibitory concentration.
Data expressed as count and (percentage) except for acontinuous variables (median and interquartile range).
bTime from onset of symptoms to surgical debridement.
cData available in 418 cases.
d444 patients evaluable for outcome.
ePercentages given over the whole of failures.
fData available in 425 patients.
The most frequent species was S. agalactiae (159 cases [34.4%]) (Table 2). There were 63 (14%) polymicrobial infections that were typically postoperative (83%), presented less frequently with fever (51% vs. 68%, P = .007) and more frequently with a sinus tract (34% vs. 10%, P < .001), and had lower CRP levels (80 mg/L [IQR 41–150] vs. 202 mg/L [IQR 110–291], P < .001).
| Streptococcus . | ||
|---|---|---|
| S. agalactiae | 159 (34.4%) | |
| S. pyogenes | 36 (7.8%) | |
| S. pneumoniae | 21 (4.5%) | |
| Other large-colony β-haemolytic streptococci | 121 (26.2%) | |
| S. dysagalactiae | 49 (10.6%) | |
| Group G streptococci | 40 (8.7%) | |
| Other β-haemolytic streptococci | 28 (6.1%) | |
| S. equisimilis | 4 (0.9%) | |
| S. anginosus group | 32 (6.9%) | |
| S. anginosus | 17 (3.7%) | |
| S. constellatus | 8 (1.7%) | |
| S. milleri | 4 (0.9%) | |
| S. intermedius | 3 (0.6%) | |
| Viridans group | 86 (18.6%) | |
| Unspecified viridans streptococci | 25 (5.4%) | |
| S. mitis | 25 (5.4%) | |
| S. oralis | 17 (3.7%) | |
| S. sanguis | 10 (2.2%) | |
| S. salivarius | 4 (0.9%) | |
| S. gordonii | 2 (0.4%) | |
| S. mutans | 2 (0.4%) | |
| S. parasanguis | 1 (0.2%) | |
| Other streptococci | 7 (1.5%) | |
| S. bovis | 6 (1.3%) | |
| S. canis | 1 (0.2%) | |
| Other microorganisms (polymicrobial episodes) | ||
| Gram positive microorganisms | 59 | |
| Staphylococcus aureus | 29 | |
| Coagulase-negative staphylococci | 15 | |
| Enterococcus faecalis | 7 | |
| Corynebacterium striatum | 2 | |
| Other Gram-positive microorganismsa | 6 | |
| Gram negative microorganisms | 19 | |
| Enterobacteriaceaec | 15 | |
| Nonfermentative Gram-negative bacillib | 2 | |
| Anaerobe Gram-negative microorganismsd | 2 | |
| Streptococcus . | ||
|---|---|---|
| S. agalactiae | 159 (34.4%) | |
| S. pyogenes | 36 (7.8%) | |
| S. pneumoniae | 21 (4.5%) | |
| Other large-colony β-haemolytic streptococci | 121 (26.2%) | |
| S. dysagalactiae | 49 (10.6%) | |
| Group G streptococci | 40 (8.7%) | |
| Other β-haemolytic streptococci | 28 (6.1%) | |
| S. equisimilis | 4 (0.9%) | |
| S. anginosus group | 32 (6.9%) | |
| S. anginosus | 17 (3.7%) | |
| S. constellatus | 8 (1.7%) | |
| S. milleri | 4 (0.9%) | |
| S. intermedius | 3 (0.6%) | |
| Viridans group | 86 (18.6%) | |
| Unspecified viridans streptococci | 25 (5.4%) | |
| S. mitis | 25 (5.4%) | |
| S. oralis | 17 (3.7%) | |
| S. sanguis | 10 (2.2%) | |
| S. salivarius | 4 (0.9%) | |
| S. gordonii | 2 (0.4%) | |
| S. mutans | 2 (0.4%) | |
| S. parasanguis | 1 (0.2%) | |
| Other streptococci | 7 (1.5%) | |
| S. bovis | 6 (1.3%) | |
| S. canis | 1 (0.2%) | |
| Other microorganisms (polymicrobial episodes) | ||
| Gram positive microorganisms | 59 | |
| Staphylococcus aureus | 29 | |
| Coagulase-negative staphylococci | 15 | |
| Enterococcus faecalis | 7 | |
| Corynebacterium striatum | 2 | |
| Other Gram-positive microorganismsa | 6 | |
| Gram negative microorganisms | 19 | |
| Enterobacteriaceaec | 15 | |
| Nonfermentative Gram-negative bacillib | 2 | |
| Anaerobe Gram-negative microorganismsd | 2 | |
The values in parentheses represent the relative number of each specific streptococcal species or stretpcoccal group in rapport to the total number of episodes of streptococcal periprosthetic joint infection (thus, it only applies to streptococci). Bold indicates the number and percentages of the streptococcal groups observed in the study (it distinguishes it from the specific number and percentages of each streptococcal species included in the study).
aIncludes Aerococcus viridans (n = 1), Arcanobacterium haemolyticus (n = 1), Bacillus spp (n = 2), Lactobacillus acidophilus (n = 1) and Peptostreptococcus spp (n = 1).
bIncludes Pseudomonas aeruginosa (n = 1), Acinetobacter baumannii (n = 1).
cIncludes Escherichia coli (n = 5), Klebsiella pneumoniae (n = 1), Enterobacter cloacae (n = 4), Proteus mirabilis (n = 3), Serratia sp. (n = 1), and Citrobacter sp. (n = 1).
dIncludes Veillonella spp. and Prevotella spp.
| Streptococcus . | ||
|---|---|---|
| S. agalactiae | 159 (34.4%) | |
| S. pyogenes | 36 (7.8%) | |
| S. pneumoniae | 21 (4.5%) | |
| Other large-colony β-haemolytic streptococci | 121 (26.2%) | |
| S. dysagalactiae | 49 (10.6%) | |
| Group G streptococci | 40 (8.7%) | |
| Other β-haemolytic streptococci | 28 (6.1%) | |
| S. equisimilis | 4 (0.9%) | |
| S. anginosus group | 32 (6.9%) | |
| S. anginosus | 17 (3.7%) | |
| S. constellatus | 8 (1.7%) | |
| S. milleri | 4 (0.9%) | |
| S. intermedius | 3 (0.6%) | |
| Viridans group | 86 (18.6%) | |
| Unspecified viridans streptococci | 25 (5.4%) | |
| S. mitis | 25 (5.4%) | |
| S. oralis | 17 (3.7%) | |
| S. sanguis | 10 (2.2%) | |
| S. salivarius | 4 (0.9%) | |
| S. gordonii | 2 (0.4%) | |
| S. mutans | 2 (0.4%) | |
| S. parasanguis | 1 (0.2%) | |
| Other streptococci | 7 (1.5%) | |
| S. bovis | 6 (1.3%) | |
| S. canis | 1 (0.2%) | |
| Other microorganisms (polymicrobial episodes) | ||
| Gram positive microorganisms | 59 | |
| Staphylococcus aureus | 29 | |
| Coagulase-negative staphylococci | 15 | |
| Enterococcus faecalis | 7 | |
| Corynebacterium striatum | 2 | |
| Other Gram-positive microorganismsa | 6 | |
| Gram negative microorganisms | 19 | |
| Enterobacteriaceaec | 15 | |
| Nonfermentative Gram-negative bacillib | 2 | |
| Anaerobe Gram-negative microorganismsd | 2 | |
| Streptococcus . | ||
|---|---|---|
| S. agalactiae | 159 (34.4%) | |
| S. pyogenes | 36 (7.8%) | |
| S. pneumoniae | 21 (4.5%) | |
| Other large-colony β-haemolytic streptococci | 121 (26.2%) | |
| S. dysagalactiae | 49 (10.6%) | |
| Group G streptococci | 40 (8.7%) | |
| Other β-haemolytic streptococci | 28 (6.1%) | |
| S. equisimilis | 4 (0.9%) | |
| S. anginosus group | 32 (6.9%) | |
| S. anginosus | 17 (3.7%) | |
| S. constellatus | 8 (1.7%) | |
| S. milleri | 4 (0.9%) | |
| S. intermedius | 3 (0.6%) | |
| Viridans group | 86 (18.6%) | |
| Unspecified viridans streptococci | 25 (5.4%) | |
| S. mitis | 25 (5.4%) | |
| S. oralis | 17 (3.7%) | |
| S. sanguis | 10 (2.2%) | |
| S. salivarius | 4 (0.9%) | |
| S. gordonii | 2 (0.4%) | |
| S. mutans | 2 (0.4%) | |
| S. parasanguis | 1 (0.2%) | |
| Other streptococci | 7 (1.5%) | |
| S. bovis | 6 (1.3%) | |
| S. canis | 1 (0.2%) | |
| Other microorganisms (polymicrobial episodes) | ||
| Gram positive microorganisms | 59 | |
| Staphylococcus aureus | 29 | |
| Coagulase-negative staphylococci | 15 | |
| Enterococcus faecalis | 7 | |
| Corynebacterium striatum | 2 | |
| Other Gram-positive microorganismsa | 6 | |
| Gram negative microorganisms | 19 | |
| Enterobacteriaceaec | 15 | |
| Nonfermentative Gram-negative bacillib | 2 | |
| Anaerobe Gram-negative microorganismsd | 2 | |
The values in parentheses represent the relative number of each specific streptococcal species or stretpcoccal group in rapport to the total number of episodes of streptococcal periprosthetic joint infection (thus, it only applies to streptococci). Bold indicates the number and percentages of the streptococcal groups observed in the study (it distinguishes it from the specific number and percentages of each streptococcal species included in the study).
aIncludes Aerococcus viridans (n = 1), Arcanobacterium haemolyticus (n = 1), Bacillus spp (n = 2), Lactobacillus acidophilus (n = 1) and Peptostreptococcus spp (n = 1).
bIncludes Pseudomonas aeruginosa (n = 1), Acinetobacter baumannii (n = 1).
cIncludes Escherichia coli (n = 5), Klebsiella pneumoniae (n = 1), Enterobacter cloacae (n = 4), Proteus mirabilis (n = 3), Serratia sp. (n = 1), and Citrobacter sp. (n = 1).
dIncludes Veillonella spp. and Prevotella spp.
Baseline features, clinical presentation, and management were similar among the streptococcal species (Supplementary Table 2). Exceptions to this were the higher rate of patients with rheumatoid arthritis among episodes caused by S. pyogenes, and the higher rate of chronic lung disease and malignancy in PJI due to S. pneumoniae. Pneumococcal PJI was also more frequently hematogenous, occurred more frequently with knee prostheses, and presented with a higher leukocyte count. Penicillin minimum inhibitory concentration (MIC) was >0.125 mg/L in 24/425 cases (6%).
DAIR Management
Patients underwent debridement after a median of 5 days (IQR 2–13) from the onset of symptoms. Removable components were exchanged in 53% of cases, this being highly variable across participating centers (Supplementary Figure 2). The median number of different antimicrobial classes prescribed per patient was 2 (range 1–6). Patients were usually treated with β-lactams, which were given intravenously for a mean time of 21 days ± 20 days. Rifampin-based combinations were significantly used (i.e., during >21 days) in 37% of patients, but this fraction was also highly variable across the participating hospitals (in those recruiting >10 patients, it ranged from 18% to 88%) (Supplementary Figure 2). Alternative antimicrobials such as fluoroquinolones, clindamycin, or linezolid were used less often (Supplementary Table 3). In patients not failing while on treatment, antimicrobial therapy was continued for a median of 91 days (IQR, 58–171 days).
Outcome
The primary endpoint was evaluable in 444 patients (96.1%). Overall Failure occurred in 187 patients (42.1%; 95% confidence interval [CI], 37.5%–46.7%) after a median of 62 days from debridement (IQR, 25–160 days); by contrast, 257 patients (57.1%) did not fail and were followed up for a median of 802 days (IQR, 507–1339 days) (Figure 1A). Success rates were highly variable among the participating centers (Supplementary Figure 2), with it ranging from 44% to 91% among hospitals recruiting >10 patients.
![Kaplan-Meier curves of patients with streptococcal periprosthetic joint infection according to the criteria for indicating debridement and implant retention. A, Kaplan-Meier curve of all evaluable patients (n = 444, 187 failures). Causes of failure were due to the streptococcal infection in 147 cases (79%), the other reasons being prosthesis removal due to orthopedic causes (15 patients [8%]), and superinfection by other microorganisms (25 cases [13%]). Death related to periprosthetic joint infection was observed in 11 cases (2%). B, Black continuous line: patients meeting IDSA criteria for DAIR (see text): 81 failures in 221 episodes of infection; grey dotted line: patients not meeting IDSA criteria for DAIR: 106 failures in 223 episodes of infection; long-rank test, P = .017. Reasons for not fulfilling the IDSA criteria were (more than 1 motive per patient is possible): in 67 patients (30%) symptoms duration was longer than 21 days; 90 patients (40%) had a post-surgical infection with symptoms beginning beyond the first month after the placement of the prosthesis; 61 patients (27%) presented with a sinus tract; and in 80 cases (36%) there were radiographic signs of prosthesis loosening and/or chronic infection. C, Post-surgical cases (i.e., non-hematogenous cases) (n = 189, 82 failures): black continuous line: cases with symptoms beginning within the first 30 days after the placement of the prosthesis (n = 78, 25 failures); grey continuous line: cases with symptoms beginning within 31 and 90 days after the placement of the prosthesis (n = 41, 13 failures); black dotted line: cases with symptoms beginning beyond 90 days after the placement of the prosthesis (n = 70, 44 failures). Long-rank test, P < .001. Abbreviations: DAIR, debridement, antibiotics, and implant retention; IDSA, Infectious Diseases Society of America.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/cid/64/12/10.1093_cid_cix227/2/m_cix22701.jpeg?Expires=1750196820&Signature=DCDfbGoP6vi0CSRnKPeuQUsJ11Mti0-b1CKdvUVIT~-N1IfJZ3v2gdSU-gscSpss5B4Y3vaYI2bshs53xTE1jCbOHEJkwkJMBOU-10VXOqAcMjA9e9vOnx4z~VX6hCh9iDYf8UmR17g9hvY5IHoyfmUTexkDsIZyI~D59T6CxEeMi6-kOTj2LJyMzBHg-gJ5~uIoe8uQQub09cX3SIRroRFRXRjkZLYQbjNkVja6nnQM72sF3mnHPsDS60BopOx7tOciHF5iDN88JlsE7a5QeJvP6-KhYbY9eusnJi1L4ki01fK53PCOrZqf5aMIyWlnNGnk5Nq8weDEA4XFn7wOzw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Kaplan-Meier curves of patients with streptococcal periprosthetic joint infection according to the criteria for indicating debridement and implant retention. A, Kaplan-Meier curve of all evaluable patients (n = 444, 187 failures). Causes of failure were due to the streptococcal infection in 147 cases (79%), the other reasons being prosthesis removal due to orthopedic causes (15 patients [8%]), and superinfection by other microorganisms (25 cases [13%]). Death related to periprosthetic joint infection was observed in 11 cases (2%). B, Black continuous line: patients meeting IDSA criteria for DAIR (see text): 81 failures in 221 episodes of infection; grey dotted line: patients not meeting IDSA criteria for DAIR: 106 failures in 223 episodes of infection; long-rank test, P = .017. Reasons for not fulfilling the IDSA criteria were (more than 1 motive per patient is possible): in 67 patients (30%) symptoms duration was longer than 21 days; 90 patients (40%) had a post-surgical infection with symptoms beginning beyond the first month after the placement of the prosthesis; 61 patients (27%) presented with a sinus tract; and in 80 cases (36%) there were radiographic signs of prosthesis loosening and/or chronic infection. C, Post-surgical cases (i.e., non-hematogenous cases) (n = 189, 82 failures): black continuous line: cases with symptoms beginning within the first 30 days after the placement of the prosthesis (n = 78, 25 failures); grey continuous line: cases with symptoms beginning within 31 and 90 days after the placement of the prosthesis (n = 41, 13 failures); black dotted line: cases with symptoms beginning beyond 90 days after the placement of the prosthesis (n = 70, 44 failures). Long-rank test, P < .001. Abbreviations: DAIR, debridement, antibiotics, and implant retention; IDSA, Infectious Diseases Society of America.
Independent predictors of a poor outcome were rheumatoid arthritis (hazard ratio [HR], 2.36), late post-surgical infection (HR, 2.20), and bacteremia (HR, 1.69). The exchange of removable components was independently associated with a favorable outcome (HR, 0.60) (Table 3). No one streptococcal species was associated with a higher likelihood of Overall Failure, although a nonsignificant better prognosis was observed for S. pneumoniae (24% failure). A high penicillin MIC (>0.125 mg/L) was also not associated with failure. Also, polymicrobial cases were not associated with a higher likelihood of failure, even when S. aureus was involved (data not shown).
| . | All Evaluable Cases—Overall Failure (n = 444, 187 Failures) . | Evaluable Cases Not Failing within the First 30 days (n = 389, 132 Failures) . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable . | Categories . | Failures/n . | HR (95%CI) . | P . | aHR (95%CI) . | P . | Failures/n . | HR (95%CI) . | P . | aHR (95%CI) . | P . |
| Sex | Female | 90/225 | 0.86 (0.65–1.14) | .30 | 60/195 | 0.75 (0.53–1.06) | .10 | – | – | ||
| Malea | 97/219 | 72/194 | |||||||||
| Age (per year) | … | … | 1.00 (0.99–1.01) | .93 | … | 0.99 (0.98–1.01) | .32 | ||||
| Diabetes | Yes | 50/108 | 1.16 (0.84–1.60) | .38 | 36/94 | 1.20 (0.82–1.76) | .36 | ||||
| Noa | 137/336 | 96/295 | |||||||||
| Renal Chronic Disease | Yes | 24/44 | 1.58 (1.03–2.43) | .05 | 1.55 (0.97–2.48) | .07 | 16/36 | 1.57 (0.93–2.65) | .09 | … | … |
| Noa | 163/400 | 116/353 | |||||||||
| Rheumatoid arthritis | Yes | 24/37 | 2.23 (1.45–3.43) | <.01 | 2.36 (1.50–3.72) | <.01 | 14/27 | 2.04 (1.17–3.54) | .02 | … | … |
| Noa | 163/407 | 118/362 | |||||||||
| Immunosuppressive therapy | Yes | 29/48 | 1.86 (1.25–2.76) | <.01 | – | – | 21/40 | 2.08 (1.31–3.32) | <.01 | 1.66 (0.99–2.18) | .055 |
| Noa | 158/396 | 111/349 | |||||||||
| Malignancy | Yes | 11/28 | 0.90 (0.49–1.66) | .73 | 10/27 | 1.20 (0.63–2.29) | .59 | ||||
| Noa | 176/416 | 122/362 | |||||||||
| Prosthesis location | Knee | 116/263 | 1.05 (0.95–1.16) | .31 | 82/229 | 1.09 (0.91–1.29) | .36 | ||||
| Othera | 71/181 | 50/160 | |||||||||
| Revision prosthesis | Yes | 60/112 | 1.60 (1.18–2.17) | <.01 | 1.37 (0.98–1.90) | .06 | 42/94 | 1.66 (1.15–2.40) | <.01 | 1.47 (0.99–2.18) | .06 |
| Noa | 127/332 | 90/295 | |||||||||
| Hematogenous infection | Yes | 95/234 | 0.90 (0.68–1.20) | .48 | 65/204 | 0.84 (0.60–1.18) | .32 | ||||
| Noa | 92/210 | 67/185 | |||||||||
| Late post-surgical infectiond | Yes | 44/70 | 1.41 (1.19–1.67) | <.01 | 2.20 (1.51–3.20) | <.01 | 31/57 | 1.28 (1.12–1.46) | <.01 | 1.69 (1.10–2.60) | .02 |
| Noa | 143/374 | 101/332 | |||||||||
| Temperature >37°C | Yes | 122/288 | 1.08 (0.79–1.46) | .65 | 85/251 | 1.05 (0.73–1.52) | .78 | ||||
| Noa | 60/149 | 42/132 | |||||||||
| Sinus tract | Yes | 27/61 | 1.12 (0.75–1.69) | .58 | 21/55 | 1.29 (0.81–2.06) | .30 | ||||
| Noa | 155/378 | 107/330 | |||||||||
| Rx signs of infection | Yes | 39/80 | 1.08 (0.99–1.19) | .11 | 25/66 | 1.21 (0.77–1.91) | .42 | ||||
| Noa | 98/251 | 72/225 | |||||||||
| Leukocytes (per unit/µL) | … | 1.00 (1.00–1.00) | .21 | … | 1.00 (1.00–1.00) | .11 | |||||
| C-reactive protein | Per mg/L | … | 1.00 (1.00–1.00) | .91 | … | 1.00 (1.00–1.00) | .76 | ||||
| Penicillin MIC | >0.125 mg/L | 8/23 | 0.80 (0.40–1.63) | .53 | 4/19 | 0.58 (0.21–1.56) | .24 | ||||
| ≤0.125 mg/La | 161/384 | 111/334 | |||||||||
| Bacteriemia | Yes | 63/132 | 1.44 (1.06–1.96) | .02 | 1.69 (1.19–2.40) | <.01 | 39/108 | 1.23 (0.84–1.79) | .30 | ||
| Noa | 110/290 | 83/263 | |||||||||
| Polymicrobial infection | Yes | 28/59 | 1.17 (0.78–1.74) | .46 | 21/52 | 1.27 (0.80–2.03) | .32 | ||||
| Noa | 159/385 | 111/337 | |||||||||
| Time to debridementb | Per day | … | 1.00 (1.00–1.00) | .06 | – | – | … | 1.00 (1.00–1.00) | .01 | 1.00 (1.00–1.00) | .05 |
| >7 days | 82/173 | 1.28 (0.96–1.71) | .09 | 61/152 | 1.45 (1.03–2.05) | .03 | |||||
| ≤7 daysa | 105/271 | 71/237 | |||||||||
| >21 days | 35/67 | 1.33 (0.92–1.92) | .14 | 27/59 | 1.51 (0.99–2.31) | .07 | |||||
| ≤21 daysa | 152/377 | 105/330 | |||||||||
| Polyethylene exchange | Yes | 73/211 | 0.59 (0.44–0.80) | <.01 | 0.60 (0.44–0.81) | <.01 | 53/191 | 0.60 (0.42–0.86) | <.01 | 0.65 (0.50–0.93) | .02 |
| Noa | 98/190 | 68/160 | |||||||||
| Need for ≥2 debridements | Yes | 41/80 | 1.41 (1.00–2.00) | .05 | 1.38 (0.96–1.99) | .08 | 30/69 | 1.53 (1.02–2.30) | .05 | 1.68 (1.10–2.57) | .02 |
| Noa | 146/364 | 102/320 | |||||||||
| Treatment with rifampinc | Per day | … | … | … | 0.99 (0.97–1.00) | .05 | 0.98 (0.96–0.998) | .03 | |||
| >14 days | … | … | 33/116 | 0.72 (0.48–1.06) | .09 | ||||||
| ≤14adays | … | … | 99/273 | ||||||||
| Treatment with β-lactamsc | Per day | … | … | … | 0.99 (0.98–1.01) | .99 | |||||
| >14 days | … | … | 87/270 | 0.85 (0.59–1.22) | .39 | ||||||
| ≤14a days | … | … | 45/119 | ||||||||
| Treatment with glycopeptidesc | Days | … | … | … | 1.04 (1.02–1.06) | <.01 | 1.04 (1.02–1.06) | <.01 | |||
| >14 days | … | … | 16/29 | 2.37 (1.40–4.00) | <.01 | ||||||
| ≤14a days | … | … | 116/360 | ||||||||
| Treatment with co-trimoxazolec | Days | … | … | … | 1.03 (1.00–1.06) | .04 | 1.04 (1.002–1.08) | .04 | |||
| >14 days | … | … | 6/9 | 2.33 (1.03–5.30) | .04 | ||||||
| ≤14a days | … | … | 126/380 | ||||||||
| . | All Evaluable Cases—Overall Failure (n = 444, 187 Failures) . | Evaluable Cases Not Failing within the First 30 days (n = 389, 132 Failures) . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable . | Categories . | Failures/n . | HR (95%CI) . | P . | aHR (95%CI) . | P . | Failures/n . | HR (95%CI) . | P . | aHR (95%CI) . | P . |
| Sex | Female | 90/225 | 0.86 (0.65–1.14) | .30 | 60/195 | 0.75 (0.53–1.06) | .10 | – | – | ||
| Malea | 97/219 | 72/194 | |||||||||
| Age (per year) | … | … | 1.00 (0.99–1.01) | .93 | … | 0.99 (0.98–1.01) | .32 | ||||
| Diabetes | Yes | 50/108 | 1.16 (0.84–1.60) | .38 | 36/94 | 1.20 (0.82–1.76) | .36 | ||||
| Noa | 137/336 | 96/295 | |||||||||
| Renal Chronic Disease | Yes | 24/44 | 1.58 (1.03–2.43) | .05 | 1.55 (0.97–2.48) | .07 | 16/36 | 1.57 (0.93–2.65) | .09 | … | … |
| Noa | 163/400 | 116/353 | |||||||||
| Rheumatoid arthritis | Yes | 24/37 | 2.23 (1.45–3.43) | <.01 | 2.36 (1.50–3.72) | <.01 | 14/27 | 2.04 (1.17–3.54) | .02 | … | … |
| Noa | 163/407 | 118/362 | |||||||||
| Immunosuppressive therapy | Yes | 29/48 | 1.86 (1.25–2.76) | <.01 | – | – | 21/40 | 2.08 (1.31–3.32) | <.01 | 1.66 (0.99–2.18) | .055 |
| Noa | 158/396 | 111/349 | |||||||||
| Malignancy | Yes | 11/28 | 0.90 (0.49–1.66) | .73 | 10/27 | 1.20 (0.63–2.29) | .59 | ||||
| Noa | 176/416 | 122/362 | |||||||||
| Prosthesis location | Knee | 116/263 | 1.05 (0.95–1.16) | .31 | 82/229 | 1.09 (0.91–1.29) | .36 | ||||
| Othera | 71/181 | 50/160 | |||||||||
| Revision prosthesis | Yes | 60/112 | 1.60 (1.18–2.17) | <.01 | 1.37 (0.98–1.90) | .06 | 42/94 | 1.66 (1.15–2.40) | <.01 | 1.47 (0.99–2.18) | .06 |
| Noa | 127/332 | 90/295 | |||||||||
| Hematogenous infection | Yes | 95/234 | 0.90 (0.68–1.20) | .48 | 65/204 | 0.84 (0.60–1.18) | .32 | ||||
| Noa | 92/210 | 67/185 | |||||||||
| Late post-surgical infectiond | Yes | 44/70 | 1.41 (1.19–1.67) | <.01 | 2.20 (1.51–3.20) | <.01 | 31/57 | 1.28 (1.12–1.46) | <.01 | 1.69 (1.10–2.60) | .02 |
| Noa | 143/374 | 101/332 | |||||||||
| Temperature >37°C | Yes | 122/288 | 1.08 (0.79–1.46) | .65 | 85/251 | 1.05 (0.73–1.52) | .78 | ||||
| Noa | 60/149 | 42/132 | |||||||||
| Sinus tract | Yes | 27/61 | 1.12 (0.75–1.69) | .58 | 21/55 | 1.29 (0.81–2.06) | .30 | ||||
| Noa | 155/378 | 107/330 | |||||||||
| Rx signs of infection | Yes | 39/80 | 1.08 (0.99–1.19) | .11 | 25/66 | 1.21 (0.77–1.91) | .42 | ||||
| Noa | 98/251 | 72/225 | |||||||||
| Leukocytes (per unit/µL) | … | 1.00 (1.00–1.00) | .21 | … | 1.00 (1.00–1.00) | .11 | |||||
| C-reactive protein | Per mg/L | … | 1.00 (1.00–1.00) | .91 | … | 1.00 (1.00–1.00) | .76 | ||||
| Penicillin MIC | >0.125 mg/L | 8/23 | 0.80 (0.40–1.63) | .53 | 4/19 | 0.58 (0.21–1.56) | .24 | ||||
| ≤0.125 mg/La | 161/384 | 111/334 | |||||||||
| Bacteriemia | Yes | 63/132 | 1.44 (1.06–1.96) | .02 | 1.69 (1.19–2.40) | <.01 | 39/108 | 1.23 (0.84–1.79) | .30 | ||
| Noa | 110/290 | 83/263 | |||||||||
| Polymicrobial infection | Yes | 28/59 | 1.17 (0.78–1.74) | .46 | 21/52 | 1.27 (0.80–2.03) | .32 | ||||
| Noa | 159/385 | 111/337 | |||||||||
| Time to debridementb | Per day | … | 1.00 (1.00–1.00) | .06 | – | – | … | 1.00 (1.00–1.00) | .01 | 1.00 (1.00–1.00) | .05 |
| >7 days | 82/173 | 1.28 (0.96–1.71) | .09 | 61/152 | 1.45 (1.03–2.05) | .03 | |||||
| ≤7 daysa | 105/271 | 71/237 | |||||||||
| >21 days | 35/67 | 1.33 (0.92–1.92) | .14 | 27/59 | 1.51 (0.99–2.31) | .07 | |||||
| ≤21 daysa | 152/377 | 105/330 | |||||||||
| Polyethylene exchange | Yes | 73/211 | 0.59 (0.44–0.80) | <.01 | 0.60 (0.44–0.81) | <.01 | 53/191 | 0.60 (0.42–0.86) | <.01 | 0.65 (0.50–0.93) | .02 |
| Noa | 98/190 | 68/160 | |||||||||
| Need for ≥2 debridements | Yes | 41/80 | 1.41 (1.00–2.00) | .05 | 1.38 (0.96–1.99) | .08 | 30/69 | 1.53 (1.02–2.30) | .05 | 1.68 (1.10–2.57) | .02 |
| Noa | 146/364 | 102/320 | |||||||||
| Treatment with rifampinc | Per day | … | … | … | 0.99 (0.97–1.00) | .05 | 0.98 (0.96–0.998) | .03 | |||
| >14 days | … | … | 33/116 | 0.72 (0.48–1.06) | .09 | ||||||
| ≤14adays | … | … | 99/273 | ||||||||
| Treatment with β-lactamsc | Per day | … | … | … | 0.99 (0.98–1.01) | .99 | |||||
| >14 days | … | … | 87/270 | 0.85 (0.59–1.22) | .39 | ||||||
| ≤14a days | … | … | 45/119 | ||||||||
| Treatment with glycopeptidesc | Days | … | … | … | 1.04 (1.02–1.06) | <.01 | 1.04 (1.02–1.06) | <.01 | |||
| >14 days | … | … | 16/29 | 2.37 (1.40–4.00) | <.01 | ||||||
| ≤14a days | … | … | 116/360 | ||||||||
| Treatment with co-trimoxazolec | Days | … | … | … | 1.03 (1.00–1.06) | .04 | 1.04 (1.002–1.08) | .04 | |||
| >14 days | … | … | 6/9 | 2.33 (1.03–5.30) | .04 | ||||||
| ≤14a days | … | … | 126/380 | ||||||||
P value <.10 (which is the cutoff for including a given parameter in the initial model of the multivariate analysis) are shown in bold.
Abbreviations: aHR, adjusted hazard ratio. CI, confidence interval; HR, hazard ratio; MIC, minimal inhibitory concentration.
aReference category.
bTime from onset of symptoms to surgical debridement.
cTreatments considered are those received within the first 30 days after surgical debridement. Overall analysis does not include the influence of antibiotics in order to avoid survivors bias. The initial model of the multivariate analyses was built with variables with a P value ≤ .10 in the univariate analysis, and then selected with a stepwise backward process (variables excluded during this process are marked as “–”).
dNon-hematogenous infection with symptoms beginning beyond 90 days after the prosthesis placement.
| . | All Evaluable Cases—Overall Failure (n = 444, 187 Failures) . | Evaluable Cases Not Failing within the First 30 days (n = 389, 132 Failures) . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable . | Categories . | Failures/n . | HR (95%CI) . | P . | aHR (95%CI) . | P . | Failures/n . | HR (95%CI) . | P . | aHR (95%CI) . | P . |
| Sex | Female | 90/225 | 0.86 (0.65–1.14) | .30 | 60/195 | 0.75 (0.53–1.06) | .10 | – | – | ||
| Malea | 97/219 | 72/194 | |||||||||
| Age (per year) | … | … | 1.00 (0.99–1.01) | .93 | … | 0.99 (0.98–1.01) | .32 | ||||
| Diabetes | Yes | 50/108 | 1.16 (0.84–1.60) | .38 | 36/94 | 1.20 (0.82–1.76) | .36 | ||||
| Noa | 137/336 | 96/295 | |||||||||
| Renal Chronic Disease | Yes | 24/44 | 1.58 (1.03–2.43) | .05 | 1.55 (0.97–2.48) | .07 | 16/36 | 1.57 (0.93–2.65) | .09 | … | … |
| Noa | 163/400 | 116/353 | |||||||||
| Rheumatoid arthritis | Yes | 24/37 | 2.23 (1.45–3.43) | <.01 | 2.36 (1.50–3.72) | <.01 | 14/27 | 2.04 (1.17–3.54) | .02 | … | … |
| Noa | 163/407 | 118/362 | |||||||||
| Immunosuppressive therapy | Yes | 29/48 | 1.86 (1.25–2.76) | <.01 | – | – | 21/40 | 2.08 (1.31–3.32) | <.01 | 1.66 (0.99–2.18) | .055 |
| Noa | 158/396 | 111/349 | |||||||||
| Malignancy | Yes | 11/28 | 0.90 (0.49–1.66) | .73 | 10/27 | 1.20 (0.63–2.29) | .59 | ||||
| Noa | 176/416 | 122/362 | |||||||||
| Prosthesis location | Knee | 116/263 | 1.05 (0.95–1.16) | .31 | 82/229 | 1.09 (0.91–1.29) | .36 | ||||
| Othera | 71/181 | 50/160 | |||||||||
| Revision prosthesis | Yes | 60/112 | 1.60 (1.18–2.17) | <.01 | 1.37 (0.98–1.90) | .06 | 42/94 | 1.66 (1.15–2.40) | <.01 | 1.47 (0.99–2.18) | .06 |
| Noa | 127/332 | 90/295 | |||||||||
| Hematogenous infection | Yes | 95/234 | 0.90 (0.68–1.20) | .48 | 65/204 | 0.84 (0.60–1.18) | .32 | ||||
| Noa | 92/210 | 67/185 | |||||||||
| Late post-surgical infectiond | Yes | 44/70 | 1.41 (1.19–1.67) | <.01 | 2.20 (1.51–3.20) | <.01 | 31/57 | 1.28 (1.12–1.46) | <.01 | 1.69 (1.10–2.60) | .02 |
| Noa | 143/374 | 101/332 | |||||||||
| Temperature >37°C | Yes | 122/288 | 1.08 (0.79–1.46) | .65 | 85/251 | 1.05 (0.73–1.52) | .78 | ||||
| Noa | 60/149 | 42/132 | |||||||||
| Sinus tract | Yes | 27/61 | 1.12 (0.75–1.69) | .58 | 21/55 | 1.29 (0.81–2.06) | .30 | ||||
| Noa | 155/378 | 107/330 | |||||||||
| Rx signs of infection | Yes | 39/80 | 1.08 (0.99–1.19) | .11 | 25/66 | 1.21 (0.77–1.91) | .42 | ||||
| Noa | 98/251 | 72/225 | |||||||||
| Leukocytes (per unit/µL) | … | 1.00 (1.00–1.00) | .21 | … | 1.00 (1.00–1.00) | .11 | |||||
| C-reactive protein | Per mg/L | … | 1.00 (1.00–1.00) | .91 | … | 1.00 (1.00–1.00) | .76 | ||||
| Penicillin MIC | >0.125 mg/L | 8/23 | 0.80 (0.40–1.63) | .53 | 4/19 | 0.58 (0.21–1.56) | .24 | ||||
| ≤0.125 mg/La | 161/384 | 111/334 | |||||||||
| Bacteriemia | Yes | 63/132 | 1.44 (1.06–1.96) | .02 | 1.69 (1.19–2.40) | <.01 | 39/108 | 1.23 (0.84–1.79) | .30 | ||
| Noa | 110/290 | 83/263 | |||||||||
| Polymicrobial infection | Yes | 28/59 | 1.17 (0.78–1.74) | .46 | 21/52 | 1.27 (0.80–2.03) | .32 | ||||
| Noa | 159/385 | 111/337 | |||||||||
| Time to debridementb | Per day | … | 1.00 (1.00–1.00) | .06 | – | – | … | 1.00 (1.00–1.00) | .01 | 1.00 (1.00–1.00) | .05 |
| >7 days | 82/173 | 1.28 (0.96–1.71) | .09 | 61/152 | 1.45 (1.03–2.05) | .03 | |||||
| ≤7 daysa | 105/271 | 71/237 | |||||||||
| >21 days | 35/67 | 1.33 (0.92–1.92) | .14 | 27/59 | 1.51 (0.99–2.31) | .07 | |||||
| ≤21 daysa | 152/377 | 105/330 | |||||||||
| Polyethylene exchange | Yes | 73/211 | 0.59 (0.44–0.80) | <.01 | 0.60 (0.44–0.81) | <.01 | 53/191 | 0.60 (0.42–0.86) | <.01 | 0.65 (0.50–0.93) | .02 |
| Noa | 98/190 | 68/160 | |||||||||
| Need for ≥2 debridements | Yes | 41/80 | 1.41 (1.00–2.00) | .05 | 1.38 (0.96–1.99) | .08 | 30/69 | 1.53 (1.02–2.30) | .05 | 1.68 (1.10–2.57) | .02 |
| Noa | 146/364 | 102/320 | |||||||||
| Treatment with rifampinc | Per day | … | … | … | 0.99 (0.97–1.00) | .05 | 0.98 (0.96–0.998) | .03 | |||
| >14 days | … | … | 33/116 | 0.72 (0.48–1.06) | .09 | ||||||
| ≤14adays | … | … | 99/273 | ||||||||
| Treatment with β-lactamsc | Per day | … | … | … | 0.99 (0.98–1.01) | .99 | |||||
| >14 days | … | … | 87/270 | 0.85 (0.59–1.22) | .39 | ||||||
| ≤14a days | … | … | 45/119 | ||||||||
| Treatment with glycopeptidesc | Days | … | … | … | 1.04 (1.02–1.06) | <.01 | 1.04 (1.02–1.06) | <.01 | |||
| >14 days | … | … | 16/29 | 2.37 (1.40–4.00) | <.01 | ||||||
| ≤14a days | … | … | 116/360 | ||||||||
| Treatment with co-trimoxazolec | Days | … | … | … | 1.03 (1.00–1.06) | .04 | 1.04 (1.002–1.08) | .04 | |||
| >14 days | … | … | 6/9 | 2.33 (1.03–5.30) | .04 | ||||||
| ≤14a days | … | … | 126/380 | ||||||||
| . | All Evaluable Cases—Overall Failure (n = 444, 187 Failures) . | Evaluable Cases Not Failing within the First 30 days (n = 389, 132 Failures) . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable . | Categories . | Failures/n . | HR (95%CI) . | P . | aHR (95%CI) . | P . | Failures/n . | HR (95%CI) . | P . | aHR (95%CI) . | P . |
| Sex | Female | 90/225 | 0.86 (0.65–1.14) | .30 | 60/195 | 0.75 (0.53–1.06) | .10 | – | – | ||
| Malea | 97/219 | 72/194 | |||||||||
| Age (per year) | … | … | 1.00 (0.99–1.01) | .93 | … | 0.99 (0.98–1.01) | .32 | ||||
| Diabetes | Yes | 50/108 | 1.16 (0.84–1.60) | .38 | 36/94 | 1.20 (0.82–1.76) | .36 | ||||
| Noa | 137/336 | 96/295 | |||||||||
| Renal Chronic Disease | Yes | 24/44 | 1.58 (1.03–2.43) | .05 | 1.55 (0.97–2.48) | .07 | 16/36 | 1.57 (0.93–2.65) | .09 | … | … |
| Noa | 163/400 | 116/353 | |||||||||
| Rheumatoid arthritis | Yes | 24/37 | 2.23 (1.45–3.43) | <.01 | 2.36 (1.50–3.72) | <.01 | 14/27 | 2.04 (1.17–3.54) | .02 | … | … |
| Noa | 163/407 | 118/362 | |||||||||
| Immunosuppressive therapy | Yes | 29/48 | 1.86 (1.25–2.76) | <.01 | – | – | 21/40 | 2.08 (1.31–3.32) | <.01 | 1.66 (0.99–2.18) | .055 |
| Noa | 158/396 | 111/349 | |||||||||
| Malignancy | Yes | 11/28 | 0.90 (0.49–1.66) | .73 | 10/27 | 1.20 (0.63–2.29) | .59 | ||||
| Noa | 176/416 | 122/362 | |||||||||
| Prosthesis location | Knee | 116/263 | 1.05 (0.95–1.16) | .31 | 82/229 | 1.09 (0.91–1.29) | .36 | ||||
| Othera | 71/181 | 50/160 | |||||||||
| Revision prosthesis | Yes | 60/112 | 1.60 (1.18–2.17) | <.01 | 1.37 (0.98–1.90) | .06 | 42/94 | 1.66 (1.15–2.40) | <.01 | 1.47 (0.99–2.18) | .06 |
| Noa | 127/332 | 90/295 | |||||||||
| Hematogenous infection | Yes | 95/234 | 0.90 (0.68–1.20) | .48 | 65/204 | 0.84 (0.60–1.18) | .32 | ||||
| Noa | 92/210 | 67/185 | |||||||||
| Late post-surgical infectiond | Yes | 44/70 | 1.41 (1.19–1.67) | <.01 | 2.20 (1.51–3.20) | <.01 | 31/57 | 1.28 (1.12–1.46) | <.01 | 1.69 (1.10–2.60) | .02 |
| Noa | 143/374 | 101/332 | |||||||||
| Temperature >37°C | Yes | 122/288 | 1.08 (0.79–1.46) | .65 | 85/251 | 1.05 (0.73–1.52) | .78 | ||||
| Noa | 60/149 | 42/132 | |||||||||
| Sinus tract | Yes | 27/61 | 1.12 (0.75–1.69) | .58 | 21/55 | 1.29 (0.81–2.06) | .30 | ||||
| Noa | 155/378 | 107/330 | |||||||||
| Rx signs of infection | Yes | 39/80 | 1.08 (0.99–1.19) | .11 | 25/66 | 1.21 (0.77–1.91) | .42 | ||||
| Noa | 98/251 | 72/225 | |||||||||
| Leukocytes (per unit/µL) | … | 1.00 (1.00–1.00) | .21 | … | 1.00 (1.00–1.00) | .11 | |||||
| C-reactive protein | Per mg/L | … | 1.00 (1.00–1.00) | .91 | … | 1.00 (1.00–1.00) | .76 | ||||
| Penicillin MIC | >0.125 mg/L | 8/23 | 0.80 (0.40–1.63) | .53 | 4/19 | 0.58 (0.21–1.56) | .24 | ||||
| ≤0.125 mg/La | 161/384 | 111/334 | |||||||||
| Bacteriemia | Yes | 63/132 | 1.44 (1.06–1.96) | .02 | 1.69 (1.19–2.40) | <.01 | 39/108 | 1.23 (0.84–1.79) | .30 | ||
| Noa | 110/290 | 83/263 | |||||||||
| Polymicrobial infection | Yes | 28/59 | 1.17 (0.78–1.74) | .46 | 21/52 | 1.27 (0.80–2.03) | .32 | ||||
| Noa | 159/385 | 111/337 | |||||||||
| Time to debridementb | Per day | … | 1.00 (1.00–1.00) | .06 | – | – | … | 1.00 (1.00–1.00) | .01 | 1.00 (1.00–1.00) | .05 |
| >7 days | 82/173 | 1.28 (0.96–1.71) | .09 | 61/152 | 1.45 (1.03–2.05) | .03 | |||||
| ≤7 daysa | 105/271 | 71/237 | |||||||||
| >21 days | 35/67 | 1.33 (0.92–1.92) | .14 | 27/59 | 1.51 (0.99–2.31) | .07 | |||||
| ≤21 daysa | 152/377 | 105/330 | |||||||||
| Polyethylene exchange | Yes | 73/211 | 0.59 (0.44–0.80) | <.01 | 0.60 (0.44–0.81) | <.01 | 53/191 | 0.60 (0.42–0.86) | <.01 | 0.65 (0.50–0.93) | .02 |
| Noa | 98/190 | 68/160 | |||||||||
| Need for ≥2 debridements | Yes | 41/80 | 1.41 (1.00–2.00) | .05 | 1.38 (0.96–1.99) | .08 | 30/69 | 1.53 (1.02–2.30) | .05 | 1.68 (1.10–2.57) | .02 |
| Noa | 146/364 | 102/320 | |||||||||
| Treatment with rifampinc | Per day | … | … | … | 0.99 (0.97–1.00) | .05 | 0.98 (0.96–0.998) | .03 | |||
| >14 days | … | … | 33/116 | 0.72 (0.48–1.06) | .09 | ||||||
| ≤14adays | … | … | 99/273 | ||||||||
| Treatment with β-lactamsc | Per day | … | … | … | 0.99 (0.98–1.01) | .99 | |||||
| >14 days | … | … | 87/270 | 0.85 (0.59–1.22) | .39 | ||||||
| ≤14a days | … | … | 45/119 | ||||||||
| Treatment with glycopeptidesc | Days | … | … | … | 1.04 (1.02–1.06) | <.01 | 1.04 (1.02–1.06) | <.01 | |||
| >14 days | … | … | 16/29 | 2.37 (1.40–4.00) | <.01 | ||||||
| ≤14a days | … | … | 116/360 | ||||||||
| Treatment with co-trimoxazolec | Days | … | … | … | 1.03 (1.00–1.06) | .04 | 1.04 (1.002–1.08) | .04 | |||
| >14 days | … | … | 6/9 | 2.33 (1.03–5.30) | .04 | ||||||
| ≤14a days | … | … | 126/380 | ||||||||
P value <.10 (which is the cutoff for including a given parameter in the initial model of the multivariate analysis) are shown in bold.
Abbreviations: aHR, adjusted hazard ratio. CI, confidence interval; HR, hazard ratio; MIC, minimal inhibitory concentration.
aReference category.
bTime from onset of symptoms to surgical debridement.
cTreatments considered are those received within the first 30 days after surgical debridement. Overall analysis does not include the influence of antibiotics in order to avoid survivors bias. The initial model of the multivariate analyses was built with variables with a P value ≤ .10 in the univariate analysis, and then selected with a stepwise backward process (variables excluded during this process are marked as “–”).
dNon-hematogenous infection with symptoms beginning beyond 90 days after the prosthesis placement.
Late post-surgical infection was indeed a predictor of bad prognosis, when defined as onset of symptoms beginning >3 months after the prosthesis placement (Figure 1C). Cases with symptoms beginning within the first and third month had a similar prognosis to that of cases with symptoms beginning within the first month after prosthesis placement. No relevant differences were observed in these 2 groups of patients (data not shown).
The failure rate was higher in patients not fulfilling the IDSA criteria for DAIR, namely, 106/223 (48%) versus 81/221 (37%) (long-rank test, P = .017) (Figure 1B). Again, indication of DAIR according to the IDSA criteria was highly variable among participating centers (Supplementary Figure 2), ranging from 33% to 83% in those recruiting >10 patients. Independent predictors of failure among patients meeting the IDSA criteria were rheumatoid arthritis (HR, 2.46 [95% CI, 1.34–4.53]), bacteremia (HR, 1.92 [95% CI, 1.22–3.02]), and male sex (HR, 1.85 [95% CI, 1.18–2.91]). Interestingly, the exchange of removable components during debridement was especially beneficial in patients not meeting the IDSA criteria (37% failures vs. 62%, P < .001), in comparison with patients fulfilling them (failures 33% vs. 39%, P = .286).
Failure Dynamics and Antimicrobial Therapy
Among the 187 patients who failed, 55 (29%) developed Early Failure, 71 (38%) developed Late Failure, and 61 developed Failure after Therapy (33%). Variables independently associated with Early Failure were age, rheumatoid arthritis, late post-surgical infection, bacteremia, and infection by S. pyogenes (Table 4).
Univariate and Multivariate Analysis of Parameters Predicting Early Failure, Late Failure and Failure After Therapy
| . | Early Failure (n = 444, 55 Failures)b . | Late Failure (n = 389, 71 Failures)c . | Failure After Therapy (N = 318, 61 Failures)d . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) . | P . | aOR (95%CI) . | P . | HR (95%CI) . | P . | aHR (95% CI) . | P . | HR (95%CI) . | P . | aHR (95% CI) . | P . | |
| Sex (female) | 1.19 (0.68–2.10) | .540 | 0.50 (0.31–0.81) | .004 | 0.51 (0.30–0.85) | .009 | 1.16 (0.69–1.92) | .572 | ||||
| Age (per year) | 1.03 (0.99–1.01) | .076 | 1.04 (1.00–1.07) | .027 | 1.00 (0.98–1.02) | .995 | 0.99 (0.97–1.01) | .348 | ||||
| Rheumatoid arthritis | 2.98 (1.35–6.56) | .007 | 3.33 (1.40–7.93) | .007 | 2.95 (1.55–5.62) | .004 | - | - | 1.19 (0.37–3.81) | .772 | ||
| Immunosuppressive therapy | 1.49 (0.66–3.66) | .343 | 2.76 (1.56–4.89) | .002 | 2.64 (1.46–4.79) | .001 | 1.51 (0.65–3.51) | .363 | ||||
| Renal chronic disease | 1.67 (0.73–3.81) | .223 | 1.99 (1.05–3.79) | .053 | - | - | 1.17 (0.47–2.91) | .746 | ||||
| Prosthesis location (knee) | 1.04 (0.86–1.26) | .677 | 0.98 (0.83–1.14) | .753 | 1.18 (0.98–1.41) | .073 | - | - | ||||
| Revision prosthesis | 1.53 (0.83–2.81) | .173 | 1.78 (1.09–2.91) | .027 | 1.77 (1.07–2.93) | .027 | 1.56 (0.90–2.70) | .129 | ||||
| Chronic post-surgical inf.e | 1.212 (0.97–1.23) | .091 | 1.41 (1.10–1.81) | .007 | 1.12 (0.92–1.37) | .256 | 1.47 (1.22–1.77) | <.001 | 2.24 (1.24–4.05) | .008 | ||
| Sinus tract | 0.75 (0.31–1.84) | .529 | 1.05 (0.54–2.06) | .881 | 1.61 (0.84–3.11) | .175 | ||||||
| Bacteremia | 2.17 (1.20–3.92) | .011 | 2.23 (1.80–4.20) | .014 | 1.24 (0.74–2.06) | .420 | 1.23 (0.70–2.19) | .478 | ||||
| Rx signs of infection | 1.16 (0.98–1.39) | .091 | - | - | 0.77 (0.40–1.48) | .421 | 2.21 (1.14–4.30) | .025 | - | - | ||
| Infection by S. pyogenes | 3.10 (1.41–6.85) | .005 | 3.31 (1.41–7.77) | .006 | 0.60 (0.19–1.92) | .357 | 1.11 (0.45–2.78) | .821 | ||||
| Infection by virdidans streptococci | 0.71 (0.32–1.57) | .401 | 1.60 (0.94–2.70) | .094 | … | … | 1.01 (0.51–1.98) | .987 | ||||
| Polymicrobial infection | 0.95 (0.41–2.20) | .896 | 1.33 (0.71–2.47) | .385 | 1.23 (0.61–2.49) | .579 | ||||||
| Time to debridement (>7 days)a | 0.96 (0.54–1.72) | .899 | 1.60 (1.00–2.54)a | .050 | 1.70 (1.05–2.75) | .033 | 1.33 (0.80–2.20) | 0.281 | ||||
| Exchange of polyethylene | 0.56 (0.31–1.02) | .059 | … | … | 0.75 (0.46–1.21) | .234 | 0.45 (0.26–0.77) | .033 | 0.44 (0.26–0.76) | .003 | ||
| Need for ≥2 debridements | 1.16 (0.57–2.36) | .683 | 2.26 (1.63–4.36) | <.001 | 2.45 (1.45–4.15) | .001 | 0.60 (0.26–1.40) | .206 | ||||
| Antimicrobial therapy‡ | ||||||||||||
| Β-lactams (without rifampin) | … | … | 1.41 (0.88–2.27) | .155 | 0.62 (0.37–1.03) | .061 | 0.48 (0.28–0.84) | .010 | ||||
| β-lactams + rifampin | … | … | 0.89 (0.47–1.70) | .724 | 0.42 (0.18–0.98) | .025 | 0.34 (0.12–0.96) | .041 | ||||
| Quinolones + rifampin | … | … | 0.19 (0.03–1.36) | .082 | 0.21 (0.03–1.54) | .125 | 1.03 (0.45–2.40) | .940 | ||||
| Glycopeptides without rifampin | … | … | 3.97 (2.08–7.58) | <.001 | 2.82 (1.43–5.53) | .003 | 4.25 (1.32–13.7) | .015 | … | … | ||
| Duration of therapy >120 days | … | … | … | … | 0.54 (0.29–0.90) | .046 | … | … | ||||
| . | Early Failure (n = 444, 55 Failures)b . | Late Failure (n = 389, 71 Failures)c . | Failure After Therapy (N = 318, 61 Failures)d . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) . | P . | aOR (95%CI) . | P . | HR (95%CI) . | P . | aHR (95% CI) . | P . | HR (95%CI) . | P . | aHR (95% CI) . | P . | |
| Sex (female) | 1.19 (0.68–2.10) | .540 | 0.50 (0.31–0.81) | .004 | 0.51 (0.30–0.85) | .009 | 1.16 (0.69–1.92) | .572 | ||||
| Age (per year) | 1.03 (0.99–1.01) | .076 | 1.04 (1.00–1.07) | .027 | 1.00 (0.98–1.02) | .995 | 0.99 (0.97–1.01) | .348 | ||||
| Rheumatoid arthritis | 2.98 (1.35–6.56) | .007 | 3.33 (1.40–7.93) | .007 | 2.95 (1.55–5.62) | .004 | - | - | 1.19 (0.37–3.81) | .772 | ||
| Immunosuppressive therapy | 1.49 (0.66–3.66) | .343 | 2.76 (1.56–4.89) | .002 | 2.64 (1.46–4.79) | .001 | 1.51 (0.65–3.51) | .363 | ||||
| Renal chronic disease | 1.67 (0.73–3.81) | .223 | 1.99 (1.05–3.79) | .053 | - | - | 1.17 (0.47–2.91) | .746 | ||||
| Prosthesis location (knee) | 1.04 (0.86–1.26) | .677 | 0.98 (0.83–1.14) | .753 | 1.18 (0.98–1.41) | .073 | - | - | ||||
| Revision prosthesis | 1.53 (0.83–2.81) | .173 | 1.78 (1.09–2.91) | .027 | 1.77 (1.07–2.93) | .027 | 1.56 (0.90–2.70) | .129 | ||||
| Chronic post-surgical inf.e | 1.212 (0.97–1.23) | .091 | 1.41 (1.10–1.81) | .007 | 1.12 (0.92–1.37) | .256 | 1.47 (1.22–1.77) | <.001 | 2.24 (1.24–4.05) | .008 | ||
| Sinus tract | 0.75 (0.31–1.84) | .529 | 1.05 (0.54–2.06) | .881 | 1.61 (0.84–3.11) | .175 | ||||||
| Bacteremia | 2.17 (1.20–3.92) | .011 | 2.23 (1.80–4.20) | .014 | 1.24 (0.74–2.06) | .420 | 1.23 (0.70–2.19) | .478 | ||||
| Rx signs of infection | 1.16 (0.98–1.39) | .091 | - | - | 0.77 (0.40–1.48) | .421 | 2.21 (1.14–4.30) | .025 | - | - | ||
| Infection by S. pyogenes | 3.10 (1.41–6.85) | .005 | 3.31 (1.41–7.77) | .006 | 0.60 (0.19–1.92) | .357 | 1.11 (0.45–2.78) | .821 | ||||
| Infection by virdidans streptococci | 0.71 (0.32–1.57) | .401 | 1.60 (0.94–2.70) | .094 | … | … | 1.01 (0.51–1.98) | .987 | ||||
| Polymicrobial infection | 0.95 (0.41–2.20) | .896 | 1.33 (0.71–2.47) | .385 | 1.23 (0.61–2.49) | .579 | ||||||
| Time to debridement (>7 days)a | 0.96 (0.54–1.72) | .899 | 1.60 (1.00–2.54)a | .050 | 1.70 (1.05–2.75) | .033 | 1.33 (0.80–2.20) | 0.281 | ||||
| Exchange of polyethylene | 0.56 (0.31–1.02) | .059 | … | … | 0.75 (0.46–1.21) | .234 | 0.45 (0.26–0.77) | .033 | 0.44 (0.26–0.76) | .003 | ||
| Need for ≥2 debridements | 1.16 (0.57–2.36) | .683 | 2.26 (1.63–4.36) | <.001 | 2.45 (1.45–4.15) | .001 | 0.60 (0.26–1.40) | .206 | ||||
| Antimicrobial therapy‡ | ||||||||||||
| Β-lactams (without rifampin) | … | … | 1.41 (0.88–2.27) | .155 | 0.62 (0.37–1.03) | .061 | 0.48 (0.28–0.84) | .010 | ||||
| β-lactams + rifampin | … | … | 0.89 (0.47–1.70) | .724 | 0.42 (0.18–0.98) | .025 | 0.34 (0.12–0.96) | .041 | ||||
| Quinolones + rifampin | … | … | 0.19 (0.03–1.36) | .082 | 0.21 (0.03–1.54) | .125 | 1.03 (0.45–2.40) | .940 | ||||
| Glycopeptides without rifampin | … | … | 3.97 (2.08–7.58) | <.001 | 2.82 (1.43–5.53) | .003 | 4.25 (1.32–13.7) | .015 | … | … | ||
| Duration of therapy >120 days | … | … | … | … | 0.54 (0.29–0.90) | .046 | … | … | ||||
Abbreviations: aHR, adjusted hazard ratio; aOR, adjusted odds ratio; CI, confidence interval; HR, hazard ratio; OR, odds ratio.
aTime to debridement: time from onset of symptoms to the first surgical debridement. Initial models of multivariate analyses were built with variables with a P value < .10 in the univariate analysis and then selected with a stepwise backward process.
bEarly Failure: the initial multivariate model included age, rheumatoid arthritis, late post-surgical infections, Rx signs of infection, infection by S. pyogenes, and bacteremia.
cLate Failure: the initial multivariate model included sex, rheumatoid arthritis, immunosuppressant therapy, chronic renal disease, infection by S. viridians, time to debridement, need for ≥2 debridements, treatment with quinolones plus rifampin, and treatment with glycopeptides without rifampin.
dFailure After Therapy: the initial multivariate model included prosthesis location, late post-surgical infection, Rx signs of infection, exchange of removable components (i.e., polyethylene liner), treatment with beta-lactams (without rifampin), treatment with beta-lactams plus rifampin, and treatment with glycopeptides without rifampin.
e Non-hematogenous infection with symptoms beginning beyond 90 days after the prosthesis placement.
‡Treatments included in the analysis of Late Failure are those received during the first 30 days after debridement and are considered if they were administered for at least 15 days; treatments included in the analysis of Failure After Therapy are those received during the whole period of treatment, both orally and intravenously, and are considered if they were administered for at least 22 days.
Univariate and Multivariate Analysis of Parameters Predicting Early Failure, Late Failure and Failure After Therapy
| . | Early Failure (n = 444, 55 Failures)b . | Late Failure (n = 389, 71 Failures)c . | Failure After Therapy (N = 318, 61 Failures)d . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) . | P . | aOR (95%CI) . | P . | HR (95%CI) . | P . | aHR (95% CI) . | P . | HR (95%CI) . | P . | aHR (95% CI) . | P . | |
| Sex (female) | 1.19 (0.68–2.10) | .540 | 0.50 (0.31–0.81) | .004 | 0.51 (0.30–0.85) | .009 | 1.16 (0.69–1.92) | .572 | ||||
| Age (per year) | 1.03 (0.99–1.01) | .076 | 1.04 (1.00–1.07) | .027 | 1.00 (0.98–1.02) | .995 | 0.99 (0.97–1.01) | .348 | ||||
| Rheumatoid arthritis | 2.98 (1.35–6.56) | .007 | 3.33 (1.40–7.93) | .007 | 2.95 (1.55–5.62) | .004 | - | - | 1.19 (0.37–3.81) | .772 | ||
| Immunosuppressive therapy | 1.49 (0.66–3.66) | .343 | 2.76 (1.56–4.89) | .002 | 2.64 (1.46–4.79) | .001 | 1.51 (0.65–3.51) | .363 | ||||
| Renal chronic disease | 1.67 (0.73–3.81) | .223 | 1.99 (1.05–3.79) | .053 | - | - | 1.17 (0.47–2.91) | .746 | ||||
| Prosthesis location (knee) | 1.04 (0.86–1.26) | .677 | 0.98 (0.83–1.14) | .753 | 1.18 (0.98–1.41) | .073 | - | - | ||||
| Revision prosthesis | 1.53 (0.83–2.81) | .173 | 1.78 (1.09–2.91) | .027 | 1.77 (1.07–2.93) | .027 | 1.56 (0.90–2.70) | .129 | ||||
| Chronic post-surgical inf.e | 1.212 (0.97–1.23) | .091 | 1.41 (1.10–1.81) | .007 | 1.12 (0.92–1.37) | .256 | 1.47 (1.22–1.77) | <.001 | 2.24 (1.24–4.05) | .008 | ||
| Sinus tract | 0.75 (0.31–1.84) | .529 | 1.05 (0.54–2.06) | .881 | 1.61 (0.84–3.11) | .175 | ||||||
| Bacteremia | 2.17 (1.20–3.92) | .011 | 2.23 (1.80–4.20) | .014 | 1.24 (0.74–2.06) | .420 | 1.23 (0.70–2.19) | .478 | ||||
| Rx signs of infection | 1.16 (0.98–1.39) | .091 | - | - | 0.77 (0.40–1.48) | .421 | 2.21 (1.14–4.30) | .025 | - | - | ||
| Infection by S. pyogenes | 3.10 (1.41–6.85) | .005 | 3.31 (1.41–7.77) | .006 | 0.60 (0.19–1.92) | .357 | 1.11 (0.45–2.78) | .821 | ||||
| Infection by virdidans streptococci | 0.71 (0.32–1.57) | .401 | 1.60 (0.94–2.70) | .094 | … | … | 1.01 (0.51–1.98) | .987 | ||||
| Polymicrobial infection | 0.95 (0.41–2.20) | .896 | 1.33 (0.71–2.47) | .385 | 1.23 (0.61–2.49) | .579 | ||||||
| Time to debridement (>7 days)a | 0.96 (0.54–1.72) | .899 | 1.60 (1.00–2.54)a | .050 | 1.70 (1.05–2.75) | .033 | 1.33 (0.80–2.20) | 0.281 | ||||
| Exchange of polyethylene | 0.56 (0.31–1.02) | .059 | … | … | 0.75 (0.46–1.21) | .234 | 0.45 (0.26–0.77) | .033 | 0.44 (0.26–0.76) | .003 | ||
| Need for ≥2 debridements | 1.16 (0.57–2.36) | .683 | 2.26 (1.63–4.36) | <.001 | 2.45 (1.45–4.15) | .001 | 0.60 (0.26–1.40) | .206 | ||||
| Antimicrobial therapy‡ | ||||||||||||
| Β-lactams (without rifampin) | … | … | 1.41 (0.88–2.27) | .155 | 0.62 (0.37–1.03) | .061 | 0.48 (0.28–0.84) | .010 | ||||
| β-lactams + rifampin | … | … | 0.89 (0.47–1.70) | .724 | 0.42 (0.18–0.98) | .025 | 0.34 (0.12–0.96) | .041 | ||||
| Quinolones + rifampin | … | … | 0.19 (0.03–1.36) | .082 | 0.21 (0.03–1.54) | .125 | 1.03 (0.45–2.40) | .940 | ||||
| Glycopeptides without rifampin | … | … | 3.97 (2.08–7.58) | <.001 | 2.82 (1.43–5.53) | .003 | 4.25 (1.32–13.7) | .015 | … | … | ||
| Duration of therapy >120 days | … | … | … | … | 0.54 (0.29–0.90) | .046 | … | … | ||||
| . | Early Failure (n = 444, 55 Failures)b . | Late Failure (n = 389, 71 Failures)c . | Failure After Therapy (N = 318, 61 Failures)d . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) . | P . | aOR (95%CI) . | P . | HR (95%CI) . | P . | aHR (95% CI) . | P . | HR (95%CI) . | P . | aHR (95% CI) . | P . | |
| Sex (female) | 1.19 (0.68–2.10) | .540 | 0.50 (0.31–0.81) | .004 | 0.51 (0.30–0.85) | .009 | 1.16 (0.69–1.92) | .572 | ||||
| Age (per year) | 1.03 (0.99–1.01) | .076 | 1.04 (1.00–1.07) | .027 | 1.00 (0.98–1.02) | .995 | 0.99 (0.97–1.01) | .348 | ||||
| Rheumatoid arthritis | 2.98 (1.35–6.56) | .007 | 3.33 (1.40–7.93) | .007 | 2.95 (1.55–5.62) | .004 | - | - | 1.19 (0.37–3.81) | .772 | ||
| Immunosuppressive therapy | 1.49 (0.66–3.66) | .343 | 2.76 (1.56–4.89) | .002 | 2.64 (1.46–4.79) | .001 | 1.51 (0.65–3.51) | .363 | ||||
| Renal chronic disease | 1.67 (0.73–3.81) | .223 | 1.99 (1.05–3.79) | .053 | - | - | 1.17 (0.47–2.91) | .746 | ||||
| Prosthesis location (knee) | 1.04 (0.86–1.26) | .677 | 0.98 (0.83–1.14) | .753 | 1.18 (0.98–1.41) | .073 | - | - | ||||
| Revision prosthesis | 1.53 (0.83–2.81) | .173 | 1.78 (1.09–2.91) | .027 | 1.77 (1.07–2.93) | .027 | 1.56 (0.90–2.70) | .129 | ||||
| Chronic post-surgical inf.e | 1.212 (0.97–1.23) | .091 | 1.41 (1.10–1.81) | .007 | 1.12 (0.92–1.37) | .256 | 1.47 (1.22–1.77) | <.001 | 2.24 (1.24–4.05) | .008 | ||
| Sinus tract | 0.75 (0.31–1.84) | .529 | 1.05 (0.54–2.06) | .881 | 1.61 (0.84–3.11) | .175 | ||||||
| Bacteremia | 2.17 (1.20–3.92) | .011 | 2.23 (1.80–4.20) | .014 | 1.24 (0.74–2.06) | .420 | 1.23 (0.70–2.19) | .478 | ||||
| Rx signs of infection | 1.16 (0.98–1.39) | .091 | - | - | 0.77 (0.40–1.48) | .421 | 2.21 (1.14–4.30) | .025 | - | - | ||
| Infection by S. pyogenes | 3.10 (1.41–6.85) | .005 | 3.31 (1.41–7.77) | .006 | 0.60 (0.19–1.92) | .357 | 1.11 (0.45–2.78) | .821 | ||||
| Infection by virdidans streptococci | 0.71 (0.32–1.57) | .401 | 1.60 (0.94–2.70) | .094 | … | … | 1.01 (0.51–1.98) | .987 | ||||
| Polymicrobial infection | 0.95 (0.41–2.20) | .896 | 1.33 (0.71–2.47) | .385 | 1.23 (0.61–2.49) | .579 | ||||||
| Time to debridement (>7 days)a | 0.96 (0.54–1.72) | .899 | 1.60 (1.00–2.54)a | .050 | 1.70 (1.05–2.75) | .033 | 1.33 (0.80–2.20) | 0.281 | ||||
| Exchange of polyethylene | 0.56 (0.31–1.02) | .059 | … | … | 0.75 (0.46–1.21) | .234 | 0.45 (0.26–0.77) | .033 | 0.44 (0.26–0.76) | .003 | ||
| Need for ≥2 debridements | 1.16 (0.57–2.36) | .683 | 2.26 (1.63–4.36) | <.001 | 2.45 (1.45–4.15) | .001 | 0.60 (0.26–1.40) | .206 | ||||
| Antimicrobial therapy‡ | ||||||||||||
| Β-lactams (without rifampin) | … | … | 1.41 (0.88–2.27) | .155 | 0.62 (0.37–1.03) | .061 | 0.48 (0.28–0.84) | .010 | ||||
| β-lactams + rifampin | … | … | 0.89 (0.47–1.70) | .724 | 0.42 (0.18–0.98) | .025 | 0.34 (0.12–0.96) | .041 | ||||
| Quinolones + rifampin | … | … | 0.19 (0.03–1.36) | .082 | 0.21 (0.03–1.54) | .125 | 1.03 (0.45–2.40) | .940 | ||||
| Glycopeptides without rifampin | … | … | 3.97 (2.08–7.58) | <.001 | 2.82 (1.43–5.53) | .003 | 4.25 (1.32–13.7) | .015 | … | … | ||
| Duration of therapy >120 days | … | … | … | … | 0.54 (0.29–0.90) | .046 | … | … | ||||
Abbreviations: aHR, adjusted hazard ratio; aOR, adjusted odds ratio; CI, confidence interval; HR, hazard ratio; OR, odds ratio.
aTime to debridement: time from onset of symptoms to the first surgical debridement. Initial models of multivariate analyses were built with variables with a P value < .10 in the univariate analysis and then selected with a stepwise backward process.
bEarly Failure: the initial multivariate model included age, rheumatoid arthritis, late post-surgical infections, Rx signs of infection, infection by S. pyogenes, and bacteremia.
cLate Failure: the initial multivariate model included sex, rheumatoid arthritis, immunosuppressant therapy, chronic renal disease, infection by S. viridians, time to debridement, need for ≥2 debridements, treatment with quinolones plus rifampin, and treatment with glycopeptides without rifampin.
dFailure After Therapy: the initial multivariate model included prosthesis location, late post-surgical infection, Rx signs of infection, exchange of removable components (i.e., polyethylene liner), treatment with beta-lactams (without rifampin), treatment with beta-lactams plus rifampin, and treatment with glycopeptides without rifampin.
e Non-hematogenous infection with symptoms beginning beyond 90 days after the prosthesis placement.
‡Treatments included in the analysis of Late Failure are those received during the first 30 days after debridement and are considered if they were administered for at least 15 days; treatments included in the analysis of Failure After Therapy are those received during the whole period of treatment, both orally and intravenously, and are considered if they were administered for at least 22 days.
Characteristics associated with Late Failure were male sex, immunosuppressant therapy, revision prosthesis, debridement delay >7 days, and the need for >1 debridement to control the infection. Failure was also associated with the early use of glycopeptides during >14 days. However, the addition of rifampin to treatment with glycopeptides neutralized this poor prognosis. The early use of rifampin plus fluoroquinolones also showed a trend toward a favorable outcome in the univariate analysis (HR, 0.19; P = .082).
Late post-surgical infection was an independent predictor of Failure after Therapy, whereas the exchange of removable components was associated with a favorable outcome. The use of β-lactams for >21 days, both alone and combined with rifampin, were independently associated with better outcomes (HR, 0.48 and 0.34, respectively) (Figure 2).
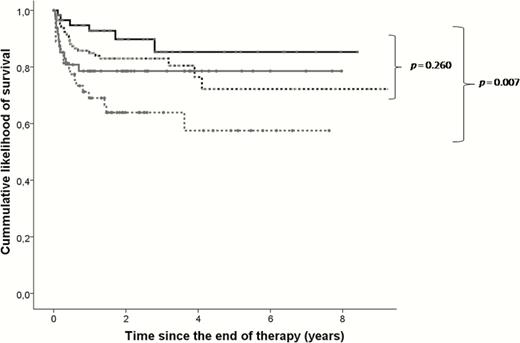
Prognostic after the end of therapy according to the antibiotic treatment.
Analysis performed in cases that did not fail during treatment (n = 318, failures = 61). Black continuous line: patients treated during >21 days with β-lactams + rifampin (n = 60, failures = 6); black dotted line: patients treated during >21 days with β-lactams, but no rifampin (n = 154, failures = 26); gray continuous line: patients treated >21 days with a rifampin-based combination other than β-lactams plus rifampin (n = 48; failures = 10); gray dotted line: patients who did not receive either β-lactams or rifampin for >21 days (n = 56; failures = 19). Comparisons calculated with the Long-rank test. The comparison of these 4 treatment regimes showed similar trends when the analysis was stratified for patients meeting and not meeting IDSA criteria and for patients who did and did not undergo exchange of removable components during debridement. Abbreviation: IDSA, Infectious Diseases Society of America.
The benefits of early treatment with rifampin were also observed for patients when treatment did not fail within the first 30 days after debridement (HR, 0.98 per day of treatment, P = .034) (Table 3).
DISCUSSION
This is the largest series to our knowledge assessing the management of streptococcal PJI by DAIR. Our results show an overall long-term likelihood of curing the infection and keeping the prosthesis of 57%. The large sample used in our study, the diversity of streptococcal species, and the high number of participating hospitals increase the external validity of our results.
Predictors of a poor outcome in this series were similar to those found in previous studies of PJI by staphylococci and GNB managed by DAIR. In previous reports, patients with bacteremia, needing >1 debridement, or with high CRP levels have shown to have a bad prognosis [24–29]. In our series, bacteremia and infection by S. pyogenes were independent predictors of Early Failure.
Otherwise, the streptococcal species presented a very similar pattern regarding clinical presentation and outcome, though S. pneumoniae presented more frequently as a hematogenous infection, and was usually associated with a better prognosis (non-significant).
The percentage of hematogenous infection in this series was notably high, when compared with PJI by S. aureus (52% vs. 15%) [25]. Moreover, we cannot rule out that some late post-surgical infections were actually hematogenous. Although staphylococcal hematogenous PJI has been reported to carry a poor prognosis [25, 30, 31], in this study we did not find an association with failure, despite the higher association of hematogenous infection with bacteremia, fever, high levels of CRP, and a high leukocyte count. It is possible that the ability of β-lactams to clear bacteremia and planktonic infection in hematogenous PJI could be higher for streptococci than for staphylococci.
Univariate and multivariate analyses have shown that some debilitating baseline conditions are associated with a worse outcome. Taken together with our previous large series, rheumatoid arthritis, immunosuppressant therapy, and chronic renal insufficiency seem to be associated with a higher risk of treatment failure when attempting DAIR [25, 27]. The exchange of removable components was associated with a favorable outcome, something that has also been observed in previous studies [25, 32]. This is consistent with the physical removal of the biofilm and probably stands as a surrogate marker of an exhaustive surgical debridement. Of note, this benefit was particularly observed in patients not fulfilling IDSA criteria for DAIR.
Unfortunately, the possibility of performing an accurate analysis of antimicrobial efficacy is impaired by the retrospective nature of this study, along with the heterogeneity of the therapeutic schedules. Still, the large size of our series allows for some interesting considerations.
Β-lactams have classically been the preferred therapy for streptococcal infections, including PJI, providing very good activity for the initial planktonic phase of these infections [33]. However, once this initial phase has passed, the antibiofilm profile of these antimicrobials is questionable because, as with any antibiotic with a mechanism of action dependent on cell wall synthesis, they will become less effective against biofilm-embedded bacteria [34]. There is now strong evidence that β-lactams have poor efficacy for staphylococcal and GNB PJI, especially when contrasted with other antibiotics that have superior antibiofilm profiles, such as rifampin against staphylococci or fluoroquinolones against GNB [25–27, 35, 36]. However, these findings have not been demonstrated in streptococcal PJI, which has been disregarded in those studies.
Our patients were mostly treated with β-lactams, in line with classic recommendations and routine clinical practice. The multivariate analysis concerning Failure after Therapy showed that this therapy was beneficial, with superiority over less effective alternatives like glycopeptides. This beneficial effect probably depended, in part, on the activity of β-lactams against planktonic bacteria in the first weeks of treatment [37]. Therefore, this contribution may be relevant to the outcome of PJI.
However, other data could indicate the suboptimal antibiofilm activity of β-lactams in our series, along with some evidence of a possible beneficial effect of rifampin. Among patients who completed a long course of treatment with β-lactams, we did not observe statistical differences among those also receiving rifampin or not, but a tendency toward a better prognosis was found in those treated with combined therapy (10.0% failure rate vs. 16.8%, Figure 2). In addition, the initial treatment with rifampin was also proved as an independent predictor of a favorable outcome (Table 4).
IDSA criteria for instituting DAIR were not met by all cases in this study. Consistent with previous studies, this allowed us to confirm the usefulness of these criteria for selecting suitable candidates for DAIR [6, 7, 25, 27]. We were also able to test the effect of each of these criteria on the outcomes. In this regard, the duration of symptoms may be difficult to establish, especially in postoperative cases where pain and inflammation may overlap those of the post-surgical period. The age of the prosthesis may therefore be a more objective measure in such cases, consistent with the IDSA recommendation that patients undergo DAIR only if there is a short time between the prosthesis placement and debridement [4]. The definition of early postoperative PJI has varied over time in several landmark publications, ranging from 1 to 3 months [2, 11, 36], with the IDSA recommending that DAIR should be performed within 1 month after placing the prosthesis [4]. However, we have observed a similar prognosis for patients with postoperative infection whose symptoms began within the first month after prosthesis placement and those whose symptoms started between the first and third month (Figure 2). A similar finding was also observed for staphylococcal PJI [25], and it would emphasize this 3-month time limit over a more strict cutoff.
As mentioned, our analysis has the inherent limitations of retrospective studies. For instance, the influence of antibiotics was evaluated with continuous variables (i.e., days of antibiotics) but also after arbitrarily categorizing these parameters (i.e., >21 days of treatment). Also, the possible relevance of endocarditis was not evaluated in this study. Finally, it has been already mentioned the significant heterogeneity of patients included across the participating institutions, especially regarding their management: the fulfillment of the IDSA criteria, the participation of different surgical teams, or the decision on whether to use or not rifampin are all examples of this variability (supplementary Figure 2). Still, these cases form a large cohort of patients with streptococcal PJI, all treated by DAIR. This has given us the opportunity to study their prognosis in the best and the worst possible clinical scenario, thus providing an overall perspective of the clinical problem.
In summary, we analyzed the largest series of streptococcal PJI managed by DAIR to date and showed a modest prognosis of curing the infection and retaining the prosthesis. We conclude that classical treatment with β-lactams is probably ideal for fighting the planktonic component of the infection. We found a piece of evidence suggesting that addition of rifampin some days or weeks after debridement could improve the outcome, but this should be confirmed in further studies. IDSA criteria are a valid clinical tool for deciding DAIR, late post-surgical infection (i.e., symptoms beginning >3 months since prosthesis placement) being the most important contra-indication. The exchange of removable components during debridement stands as an independent predictor of a favorable outcome.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank Michael Maudsley (University of Barcelona) for reviewing the English manuscript.
Financial support. J. L.-T holds a clinical research contract “Sara Borrell” (CD14/00176) from the Instituto de Salud Carlos III (Spanish Ministry of Economy and Competitiviness). CRIOGO is funded by the French Ministry of Health. A. R. was supported by a research grant from the Bellvitge Biomedical Research Institute (IDIBELL). REIPI is supported by the Spanish Ministry of Economy and Competititviness, Instituto de Salud Carlos III, and by the European Development Regional Fund “A way to achieve Europe.”
Potential conflicts of interest. All author certifies no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
Author notes
See listing after the references.
Correspondence: J. Lora-Tamayo, Unit of Infectious Diseases, Department of Internal Medicine, Hospital Universitario 12 de Octubre, Avenida de Córdoba s/n, 28041 Madrid ([email protected]).
LIST OF COLLABORATORS/GROUP OF INVESTIGATORS FOR STREPTOCOCCAL PROSTHETIC JOINT INFECTION
This is a multicenter study. In each institution there are many researchers that have helped to make this study possible. We are deeply indebted to these collaborators, who are:
Fernando Chaves, José Alberto Moreno-Beamud, Rafael Navarro Arribas (Hospital Universitario 12 de Octubre, Madrid, Spain).
Sophie Nguyen (Gustave Dron Hospital of Tourcoing, France).
Oscar Murillo, Xavier Cabo, Salvador Pedrero (Hospital Universitari de Bellvitge, Barcelona, Spain).
Frédéric Dauchy, Hervé Dutronc, Bertille de Barbeyrac (Centre correspondant de prise en charge des Infections Ostéo-articulaires Complexes Du Grand Sud-Ouest—CHU Bordeaux—France).
Matthew Scarborough, Martin McNally, Bridget Atkins (Bone Infection Unit, Nuffield Orthopaedic Centre, Oxford, United Kingdom).
Pierre Tattevin, Marie Ghéno, Enora Ouamara-Digue (Rennes University Hospital, Rennes, France).
Bernhard Kessler (Kantosspital Baselland, Liestal, Switzerland).
Sébastien Lustig, Florent Valour, Christian Chdiac (Hôpital de la Croix-Rousse, Hospices Civils de Lyon. Lyon, France).
Miguel Ángel Goenaga, Asier Mitxelena, Enrique Moreno (Hospital Universitario Donostia, San Sebastián, Spain).
Maja Bombek Ihan, Zmago Krajnc (University Clinical Center, Maribor, Slovenia).
Carles Pigrau, Pablo S. Corona Pérez-Cardona (Hospital Universitari Vall d’Hebron, Barcelona, Spain).
Cecilia Peñas Espinar, Ana Isabel Suárez, Miguel Muniain Ezcurra (University Hospitals Virgen Macarena y Virgen del Rocío. Sevilla, Spain).
María Carmen Fariñas (Hospital Universitario Marqués de Valdecilla, Santander, Spain).
Markku Vuorinen, Jarkko Leskinen (Helsinki University Hospital, Helsinki, Finland).
Tristan Yolland, Mark Lowenthal (John Hunter Hospital, Newcastel, NSW, Australia).
Julia Praena, Salvador Fornell, María-José Gómez (Hospital Universitario Virgen del Rocío, Sevilla, Spain).
Paul C. Jutte (University Medical Center Groningen, Groningen, The Netherlands).
Anže Mihelič, Rene Mihalič (Valdoltra Orthopaedic Hospital, Ankaran, Slovenia).
Guillem Bori, Laura Morata, Eduard Tornero (Hospital Clínic, Barcelona, Spain).
Carlos Fuster Foz, Susana García Villabrille, Marta Novoa (Hospital de El Bierzo, Ponferrada, Spain).
Emerson K. Honda, Ricardo de Paula Leite Cury (Santa Casa de Misericórdia, São Paulo, Brazil).
Juan Corredoira (Hospital Universitario Lucus Augusti, Lugo, Spain).
Pere Coll, Isabel Mur, Xavier Crusi (Hospital de la Santa Creu i Sant Pau, Barcelona, Spain).
Antonio Ramírez, Francisco Montaner (Hospital Universitario Son Espasses, Palma de Mallorca, Spain).
Eva Cuchí (Catlab, Viladecavalls, Barcelona, Spain), Alfredo Matamala (Hospital Universitari Mútua de Terrassa, Spain).
Antonio Blanco, Joaquín García-Cañete, Raúl Parrón (Fundación Jiménez Díaz, Madrid, Spain).
Luisa Sorlí, Lluis Puig, Nuria Prim (Hospital del Mar, Barcelona, Spain).
Botond Lakatos, Gyula Prinz (Orthopedic Clinic, Semmelweis University Budapest, Hungary).
Gema Gresco, Patricia Ruiz-Garbajosa (Hospital Universitario Ramón y Cajal, Madrid, Spain).
Mercedes Marín Arriaza (Hospital Universitario Gregorio Marañón, Madrid, Spain).
Isabel Sánchez-Romero, Miguel Ángel García Viejo, Jesús Campo Loarte (Hospital Universitario Puerta de Hierro, Madrid, Spain).
Antonios Papadopoulos (ATTIKON University General Hospital, Athens, Greece).
María Fernanda Ramírez-Hidalgo, Laura Prats-Gispert, Ferran Pérez-Villar (Hospital Universitari Arnau de Vilanova, Lleida, Spain).
Juan Romanyk (Hospital Universitario Príncipe de Asturias, Alcalá de Henares, Madrid, Spain).
Guido Grappiolo, Mattia Loppini, Marco Scardino (Humanitas Research Hospital, Milan, Italy).
Elaine Cheong, Genevieve McKew, Amarita Ronnachit (Concord Hospital, Concord, NSW, Australia).




