-
PDF
- Split View
-
Views
-
Cite
Cite
Joost M Costerus, Matthijs C Brouwer, Marieke E S Sprengers, Stefan D Roosendaal, Arie van der Ende, Diederik van de Beek, Cranial Computed Tomography, Lumbar Puncture, and Clinical Deterioration in Bacterial Meningitis: A Nationwide Cohort Study, Clinical Infectious Diseases, Volume 67, Issue 6, 15 September 2018, Pages 920–926, https://doi.org/10.1093/cid/ciy200
Close - Share Icon Share
Abstract
It is unclear how often lumbar puncture (LP) is complicated by cerebral herniation in patients with bacterial meningitis and whether cranial computed tomography (CT) can be used to identify patients at risk for herniation.
We performed a nationwide prospective cohort study of patients with community-acquired bacterial meningitis from 2006 to 2014 and identified patients with clinical deterioration possibly caused by LP. For systematic evaluation of contraindications for LP on cranial CT, these patients were matched to patients in the cohort without deterioration. Four experts, blinded for outcome, scored cranial CT results for contraindications for LP. A Fleiss’ generalized κ for this assessment was determined.
Of 1533 episodes, 47 (3.1%) had deterioration possibly caused by LP. Two patients deteriorated within 1 hour after LP (0.1%). In 43 of 47 patients with deterioration, cranial CT was performed prior to LP, so CT results were matched with 43 patients without deterioration. The interrater reliability of assessment of contraindications for LP on cranial CT was moderate (Fleiss’ generalized κ = 0.47). A contraindication for LP was reported by all 4 raters in 6 patients with deterioration (14%) and in 5 without deterioration (11%).
LP can be performed safely in the large majority of patients with bacterial meningitis, as it is only very rarely complicated by cerebral herniation. Cranial CT can be considered a screening method for contraindications for LP, but the interrater reliability of this assessment is moderate.
Suspected bacterial meningitis is the principal indication for lumbar puncture (LP) [1–3]. One of the issues physicians are faced with in the emergency department is whether cranial computed tomography (CT) should be performed prior to LP. The risk of cerebral herniation following LP has been emphasized in the literature, particularly for bacterial meningitis [4, 5]. Performing an LP in the presence of an occult intracranial mass lesion can possibly lead to increase of brain shift, which may end in herniation and death. Cranial CT is used to evaluate for signs of brain shift contraindicating LP. However, retrospective studies showed that in patients with bacterial meningitis, cranial imaging has been associated with a delay in the initiation of antimicrobial therapy and increased mortality [6, 7]. Cerebral herniation after LP has been described in sporadic patients only [8–14], but the perceived risk incites reluctance to perform LPs without cranial imaging [14, 15].
Clinical features can be used to identify patients who are unlikely to have abnormal findings on cranial CT [16]. A prospective study involving 301 adults with suspected meningitis showed that if 10 items (age >60 years, altered mental status, gaze or facial palsy, abnormal language or inability to answer 2 questions or follow 2 commands, immunocompromised state, history of central nervous system disease, seizure in past week, visual field abnormalities, and arm or leg drift) were absent, there was a negative predictive value of 97% for an intracranial abnormality [16]. It should be noted that this study used CT scan abnormalities as a surrogate marker for increased risk of herniation. Some patients with intracranial abnormalities that were missed using these clinical criteria underwent LP without any apparent complication [14]. Meningitis guidelines of the Infectious Diseases Society of America and the European Society of Clinical Microbiology and Infectious Diseases recommend cranial CT prior to LP in patients with specific clinical features (a severe immunocompromised state, signs that are suspicious for space-occupying lesions, new-onset seizures, or moderate-to-severe impairment of consciousness), and to start antibiotic treatment and dexamethasone before sending the patient for imaging [17, 18].
Important questions remain. What is the actual risk of LP in patients with community-acquired bacterial meningitis? How effective is the use of cranial CT in preventing cerebral herniation after LP, and what is the interrater reliability of assessment of contraindications for LP on cranial CT? In 2006, we started a prospective cohort study to identify and characterize host genetic traits and bacterial genetic factors controlling occurrence and outcome of bacterial meningitis (MeninGene) [3, 19]. Here, we report data from this study, focusing on complications of LP in adults with community-acquired bacterial meningitis and the role of cranial CT prior to LP.
METHODS
Study Population
In a nationwide prospective cohort study, we included patients aged >16 years who had bacterial meningitis and were listed in the database of the Netherlands Reference Laboratory for Bacterial Meningitis (NRLBM) from 1 March 2006 to 31 November 2014 [3]. This laboratory receives cerebrospinal fluid (CSF) of 85%–90% of patients diagnosed with CSF culture– positive bacterial meningitis in Dutch academic and nonacademic hospitals (population of the Netherlands: 16.9 million). The NRLBM provided daily updates of the names of the hospitals where patients with bacterial meningitis had been admitted. The treating physician was contacted or could directly contact the investigators for inclusion of patients. Informed consent was obtained from all participating patients or their legally authorized representatives. As patients pretreated with antibiotics before cranial CT and LP more often yield a negative CSF culture [20], episodes reported by physicians with a clinical presentation compatible with bacterial meningitis but negative CSF cultures or PCR were included if CSF results showed at least 1 individual predictor of bacterial meningitis (defined as a glucose level of <1.9 mmol/L, a ratio of CSF glucose to blood glucose of <0.23, a protein level of >2.20 g/L, or a leukocyte count of >2000 cells/μL) [21]. Patients with hospital-associated meningitis including neurosurgery patients, patients with a neurosurgical device, or patients with a neurotrauma within 1 month of the onset of meningitis were excluded.
Procedures
Data on patient history, symptoms and signs on admission, laboratory findings, radiologic examination, treatment, and outcome were prospectively collected by means of a case record form. To identify patients with possible cerebral herniation after LP, the case record form contained a standard question if patients developed impaired consciousness within 8 hours of LP. This interval was chosen as cerebral herniation has been described to sometimes have a slow progressive nature in the hours after LP due to continuous leakage of CSF, although most case reports describe clinical deterioration within 1 hour of LP [22]. We hypothesized that patients who die of cerebral herniation due to LP die within 7 days after LP. Thus, patients who died within 7 days after admission who initially presented with a Glasgow Coma Scale (GCS) score of ≥8 and had impairment of consciousness noted within 8 hours after LP were included for screening. Furthermore, all patients presenting with a GCS <8 who died within 7 days after admission were included for screening because further impairment of consciousness can be difficult to recognize in these patients.
The case record form, discharge letters, and autopsy report (when available) of these patients were screened to determine a possible causal relationship between LP and deterioration. We identified patients with clinical deterioration, defined as an acute decline in consciousness or cardiorespiratory function. Patients were subsequently excluded if (1) death was preceded by a clinical improvement lasting >48 hours after the LP, (2) cause of death was not cerebral herniation based on clinical grounds or postmortem examination (eg, multiorgan failure), (3) the patient was clinically brain dead prior to LP, or (4) CSF was collected through a ventricular drain or during autopsy. To assess if LP was performed while contraindicated based on cranial imaging, scans performed prior to LP were reanalyzed for the included patients. We individually matched cases to controls consisting of bacterial meningitis patients with similar age and presenting GCS score but a good clinical outcome, defined as a score of 5 on the Glasgow Outcome Scale [23].
Two neurologists specializing in infectious diseases (M. C. B. and D. v. d. B.) and 2 neuroradiologists (M. E. S. S. and S. D. R.) assessed cranial CTs to identify contraindications for LP, unaware of clinical findings and outcome. To represent daily clinical practice, for this study the assessors did not receive any specific instructions on how to interpret a cranial CT for contraindications for LP, or radiological criteria for contraindications. Of all patients 5-mm axial CT images were available. Neuroradiologists were asked to provide reasoning for their decision if they recommended against LP. Cranial imaging after LP (if performed) was reassessed by 2 neurologists (J. M. C. and M. C. B.) and, if imaging was not available for reassessment, the local report was used for analysis.
Statistical Analyses
Categorical variables are expressed as counts (percentage) and continuous variables as median (interquartile range [IQR]). Interrater agreement of all assessors was measured with Fleiss’ κ, and interrater agreement of 2 assessors with Cohen’s κ. Statistical analyses were performed with use of IBM SPSS Statistics, version 24.
RESULTS
From March 2006 to November 2014, 1533 episodes of bacterial meningitis were included in the cohort; 58 patients presented with a GCS score ≥8, had clinical deterioration within the first 8 hours of LP, and died within 7 days, and 42 patients presented with a GCS score <8 and died within 7 days (Figure 1). From these 100 patients, 53 patients were excluded because of alternative cause of death (n = 31), no signs of cerebral herniation at autopsy (n = 10), absence of brain stem reflexes prior to LP (n = 4), no LP performed (n = 4), or initial recovery after LP (n = 4). The remaining 47 patients (3.1%) had possible deterioration after LP (29 presented in noncomatose state and 18 patients in comatose state). Of 18 patients with a comatose state, deterioration within 8 hours of LP was reported in 10 and reported to be absent in 4 (4 unknown).
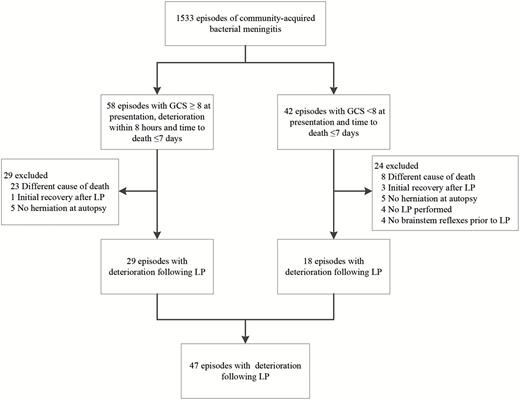
Flowchart of patient selection. Abbreviations: GCS, Glasgow Coma Scale score; LP, lumbar puncture.
Median age of the 47 patients was 63 years (IQR, 49–68 years; Table 1) and 45% were male. Fifteen patients were reported to be immunocompromised (diabetes mellitus [n = 7], immunosuppressive medication [n = 5], alcoholism [n = 5], and active cancer [n = 5]). Papilledema was evaluated in 10 patients and was present in 2 (20%). The median GCS score at presentation was 9 (IQR, 6–11). CSF opening pressure was evaluated in 19 patients and exceeded 400 mm of water in 15 (79%). CSF cultures were positive for Streptococcus pneumoniae in 77% of patients.
Characteristics of 47 Patients With Clinical Deterioration Following Lumbar Puncture
| Characteristic . | Patients With Deterioration (n = 47) . |
|---|---|
| Age, y, median (IQR) | 63 (49–68) |
| Male sex | 21/47 (45) |
| New-onset seizures | 7/47 (15) |
| Score on GCS, median (IQR) | 9 (6–11) |
| GCS score <10 | 29/47 (62) |
| Streptococcus pneumoniae as causative organism | 37/47 (79) |
| Guideline indication for CT prior to LPa | 38/47 (81) |
| Empiric treatment started prior to cranial CT | 11/38 (28) |
| Local report of cranial CT | |
| Normal | 17/43 (40) |
| Generalized edema | 13/43 (30) |
| Hydrocephalus | 2/43 (5) |
| Generalized edema and hydrocephalus | 4/43 (9) |
| Intracranial air | 2/43 (5) |
| Other (metastasis, old vascular abnormalities, focal edema) | 5/43 (12) |
| Characteristic . | Patients With Deterioration (n = 47) . |
|---|---|
| Age, y, median (IQR) | 63 (49–68) |
| Male sex | 21/47 (45) |
| New-onset seizures | 7/47 (15) |
| Score on GCS, median (IQR) | 9 (6–11) |
| GCS score <10 | 29/47 (62) |
| Streptococcus pneumoniae as causative organism | 37/47 (79) |
| Guideline indication for CT prior to LPa | 38/47 (81) |
| Empiric treatment started prior to cranial CT | 11/38 (28) |
| Local report of cranial CT | |
| Normal | 17/43 (40) |
| Generalized edema | 13/43 (30) |
| Hydrocephalus | 2/43 (5) |
| Generalized edema and hydrocephalus | 4/43 (9) |
| Intracranial air | 2/43 (5) |
| Other (metastasis, old vascular abnormalities, focal edema) | 5/43 (12) |
Data are presented as no./ No. (%) with data available unless otherwise indicated.
Abbreviations: CT, computed tomography; GCS, Glasgow Coma Scale score; IQR, interquartile range; LP, lumbar puncture.
aNew-onset seizures, papilledema, severe immunocompromised state, focal neurologic deficits (excluding cranial nerve palsy), or GCS score <10 [20].
bLowest GCS score after clinical deterioration was available in 46 of 47 patients (98%).
Characteristics of 47 Patients With Clinical Deterioration Following Lumbar Puncture
| Characteristic . | Patients With Deterioration (n = 47) . |
|---|---|
| Age, y, median (IQR) | 63 (49–68) |
| Male sex | 21/47 (45) |
| New-onset seizures | 7/47 (15) |
| Score on GCS, median (IQR) | 9 (6–11) |
| GCS score <10 | 29/47 (62) |
| Streptococcus pneumoniae as causative organism | 37/47 (79) |
| Guideline indication for CT prior to LPa | 38/47 (81) |
| Empiric treatment started prior to cranial CT | 11/38 (28) |
| Local report of cranial CT | |
| Normal | 17/43 (40) |
| Generalized edema | 13/43 (30) |
| Hydrocephalus | 2/43 (5) |
| Generalized edema and hydrocephalus | 4/43 (9) |
| Intracranial air | 2/43 (5) |
| Other (metastasis, old vascular abnormalities, focal edema) | 5/43 (12) |
| Characteristic . | Patients With Deterioration (n = 47) . |
|---|---|
| Age, y, median (IQR) | 63 (49–68) |
| Male sex | 21/47 (45) |
| New-onset seizures | 7/47 (15) |
| Score on GCS, median (IQR) | 9 (6–11) |
| GCS score <10 | 29/47 (62) |
| Streptococcus pneumoniae as causative organism | 37/47 (79) |
| Guideline indication for CT prior to LPa | 38/47 (81) |
| Empiric treatment started prior to cranial CT | 11/38 (28) |
| Local report of cranial CT | |
| Normal | 17/43 (40) |
| Generalized edema | 13/43 (30) |
| Hydrocephalus | 2/43 (5) |
| Generalized edema and hydrocephalus | 4/43 (9) |
| Intracranial air | 2/43 (5) |
| Other (metastasis, old vascular abnormalities, focal edema) | 5/43 (12) |
Data are presented as no./ No. (%) with data available unless otherwise indicated.
Abbreviations: CT, computed tomography; GCS, Glasgow Coma Scale score; IQR, interquartile range; LP, lumbar puncture.
aNew-onset seizures, papilledema, severe immunocompromised state, focal neurologic deficits (excluding cranial nerve palsy), or GCS score <10 [20].
bLowest GCS score after clinical deterioration was available in 46 of 47 patients (98%).
Cranial CT was performed prior to LP in 43 of 47 patients (91%), including 35 of 38 patients with a guideline indication for cranial CT (92%) [20]. The most common guideline indication for cranial CT was a GCS score <10 (n = 30 [79%]); other indications included focal neurologic deficits (n = 15 [39%]), new-onset seizures (n = 7 [18%]), immunocompromised state (n = 5 [14%]), and papilledema (n = 2 [6%]). Two guideline indications were present in 13 patients (34%), and 3 guideline indications were present in 4 patients (11%). A GCS score <10 was the only guideline indication for cranial CT in 13 patients (34%), of whom 12 patients (92%) underwent cranial CT prior to LP. The reason why cranial CT was not performed prior to LP in the 3 patients with a guideline indication but no imaging prior to LP was unknown. A normal cranial CT was initially described in 17 of 43 patients (40%; Table 1). The most common reported abnormality was generalized cerebral edema (30%). Cranial CT was performed in 8 of 9 patients without a guideline indication, yielding a normal CT in 5, generalized edema in 2, and generalized edema with a hydrocephalus in 1 patient [17]. Presenting clinical characteristics and treatment of the 43 patients with deterioration and cranial CT prior to LP and their matched controls were similar (median, age 63 years [IQR, 49–68 years] vs 62 years [IQR, 47–67 years]; median presenting GCS score, 9 [IQR, 6–11] vs 9 [IQR, 7–11]; antibiotic treatment was started before cranial CT in 28% of patients with deterioration and 13% of matched controls, and S. pneumoniae was the causative organism in 77% of patients with deterioration and in 84% of controls [Supplementary Appendix]).
The 2 neurologists reported a contraindication for LP in 29 of 86 (34%) and 34 of 86 (40%) patients, and the 2 neuroradiologists in 14 of 86 (16%) and 28 of 86 (33%) patients. Cranial CT was judged as unsafe for LP by 1 or more assessors in 46 of 86 patients (53%), and by 1 or more neuroradiologists in 31 of 86 patients (36%; most commonly due to generalized cerebral edema and/or obliteration of the basal cisterns [n = 21] and movement artefacts or insufficient slides of the lower fossa posterior [n = 10]). Cranial CT was judged as unsafe to perform LP by all 4 assessors in 6 patients with clinical deterioration and in 5 controls (Table 2, Figure 2). The Fleiss’ generalized κ for all assessors was 0.47 (95% confidence interval [CI], .38–.55], P < .0001) indicating moderate agreement only between assessors. Cohen’s κ was 0.39 (95% CI, .19–.60) for the 2 neuroradiologists and 0.53 (95% CI, .34–.71) for the 2 neurologists specializing in infectious diseases. Of the 12 patients who underwent cranial CT prior to LP and had impaired consciousness as the only guideline indication for cranial CT, a contraindication for LP was reported by 1 assessor in 2 patients, by 2 assessors in 5 patients, and by all assessors in 1 patient.
Results of Assessment for Contraindications for Lumbar Puncture on Cranial Computed Tomography
| No. of Raters Reporting a Contraindication . | Possible Herniation (n = 43), No. (%) . | Controls (n = 43), No. (%) . |
|---|---|---|
| 0/4 | 22 (51) | 18 (42) |
| 1/4 | 6 (14) | 10 (23) |
| 2/4 | 8 (19) | 4 (9) |
| 3/4 | 1 (2) | 6 (14) |
| 4/4 | 6 (14) | 5 (12) |
| No. of Raters Reporting a Contraindication . | Possible Herniation (n = 43), No. (%) . | Controls (n = 43), No. (%) . |
|---|---|---|
| 0/4 | 22 (51) | 18 (42) |
| 1/4 | 6 (14) | 10 (23) |
| 2/4 | 8 (19) | 4 (9) |
| 3/4 | 1 (2) | 6 (14) |
| 4/4 | 6 (14) | 5 (12) |
Results of Assessment for Contraindications for Lumbar Puncture on Cranial Computed Tomography
| No. of Raters Reporting a Contraindication . | Possible Herniation (n = 43), No. (%) . | Controls (n = 43), No. (%) . |
|---|---|---|
| 0/4 | 22 (51) | 18 (42) |
| 1/4 | 6 (14) | 10 (23) |
| 2/4 | 8 (19) | 4 (9) |
| 3/4 | 1 (2) | 6 (14) |
| 4/4 | 6 (14) | 5 (12) |
| No. of Raters Reporting a Contraindication . | Possible Herniation (n = 43), No. (%) . | Controls (n = 43), No. (%) . |
|---|---|---|
| 0/4 | 22 (51) | 18 (42) |
| 1/4 | 6 (14) | 10 (23) |
| 2/4 | 8 (19) | 4 (9) |
| 3/4 | 1 (2) | 6 (14) |
| 4/4 | 6 (14) | 5 (12) |
Repeated cranial CT after deterioration was performed in 22 of 47 patients, with a median time between CTs of 11.6 hours (IQR, 7.8–23.2 hours). Radiology report of the local hospital was available for all repeated CTs, and 20 of 22 scans (91%) were available for reassessment. Repeated cranial CT showed generalized edema in 15 patients (68%), hypodense lesions consistent with cerebral infarctions in 3 (14%), and hydrocephalus and no abnormalities each in 2 patients (9%). In 10 of 22 patients (45%), cerebral herniation was described on the repeated CT.

Brain imaging of episodes with contraindication for lumbar puncture as assessed by all 4 raters. Reported contraindication by the neuroradiologists: generalized edema (A), artefacts in posterior fossa (B), generalized edema (C), artefacts in posterior fossa (D), obliteration of basal cisterns (E), generalized edema and obliteration of basal cisterns (F).
Clinical deterioration occurred within 1 hour after LP in 2 of 47 patients (4% of 47 patients; 0.1% of total 1533 episodes). A 68-year-old man presenting with fever, headache, and neck stiffness, a GCS score of 13, and right-side gaze palsy developed bradycardia followed by cardiac arrest directly after LP and died. A 33-year-old woman presenting with headache, fever, and a GCS score of 7 deteriorated to a GCS score of 3 within 30 minutes after LP, with subsequent wide unresponsive pupils and respiratory failure, and died. For both patients, cranial CT prior to LP was initially read as normal, but showed generalized edema in retrospective evaluation.
Median lowest GCS score after clinical deterioration following LP for the 47 patients was 3 (IQR, 3–3), and 35 of 47 (74%) underwent mechanical ventilation. Median time between LP and death for the 47 patients was 1 day (IQR, 1–2 days). The treating physician noted the cause of death in 44 of 47 patients (94%), most commonly cerebral herniation on clinical grounds (in 20 patients [45%]), brain death or brain edema (9 patients [20%]), meningitis (6 patients [14%]), or withdrawal of supportive treatment because of poor prognosis (6 patients [14%]). Postmortem brain examination was performed in 1 of 47 episodes (4%), showing severe bilateral flattening of the cerebral gyri with a pressure imprint in the gyri parahippocampii and cerebellar tonsils consistent with cerebral herniation.
DISCUSSION
Our study shows that cerebral herniation following LP in bacterial meningitis is a rare event (range, 0.1%–3%, but likely to be closer to 0.1%). Cerebral herniation was described in almost half of patients with clinical deterioration after LP who had repeat CT, but it remains unclear whether herniation was caused by fulminant meningitis, by direct consequence of the LP, or by the combination of both. Previous studies reported rates of cerebral herniation following LP ranging from 1.0% to 6.0%, but these studies were small retrospective cohort studies in children with bacterial meningitis [9, 11, 24]. In a retrospective cohort study, 40 of 296 adults with community-acquired bacterial meningitis died shortly after LP [25]. Autopsy report was available for 27 of these patients and showed cerebral herniation in 8 (2.7%). We performed the first nationwide prospective study on cerebral herniation after lumbar puncture in bacterial meningitis and show that LP is a relative safe procedure in this high-risk population.
Cranial imaging can be considered as a way to evaluate for signs of brain shift as a precaution in selected patients before LP [17, 18, 26]. Naturally, CT scan abnormalities present as surrogate markers because not all abnormalities on cranial CT pose a real contraindication for LP. A retrospective case series using historical controls showed that the deletion of impaired consciousness as an indication for cranial CT before LP was associated with favorable outcome [27]. However, this study did not include patients suspected of bacterial meningitis in whom another diagnosis was made after LP. In our study, we identified 13 patients with possible clinical deterioration after LP in whom impaired consciousness was the only indication for cranial CT prior to LP, including a 33-year-old woman with generalized edema on cranial CT who deteriorated within 30 minutes after LP and subsequently died. Our results therefore do not support deletion of impaired consciousness as indication for cranial CT prior to LP.
Case reports and a retrospective cohort study including 39 patients with acute bacterial meningitis showed that cranial CT often fails to identify signs of raised intracranial pressure [28, 29]. However, there is no evidence that raised intracranial pressure without brain shift—that is, raised intracranial pressure without a pressure gradient—is a contraindication for LP. Guidelines describe that performing LP in a patient with an intracranial space-occupying lesion can precipitate cerebral herniation, but do not provide a detailed protocol for which abnormalities the LP should be deferred [17, 18]. Interestingly, abnormalities on cranial imaging were reported by the local radiologist in 60% of patients with clinical deterioration after LP in this study, most commonly brain edema. In the urgency of this illness, neurologists apparently decided to perform LP notwithstanding abnormal imaging results. Nevertheless, we identified only 2 of 1533 patients (0.1%) with clinical deterioration within 1 hour after LP. For both patients, cranial CT prior to LP was initially read as normal by radiologists, but showed generalized edema in our retrospective evaluation.
Some authors advocate the use of intracranial pressure–guided treatment for patients with bacterial meningitis, for example, through external lumbar CSF drains [30]. A retrospective Canadian study evaluating lumbar CSF drains in patients with bacterial meningitis showed a lower mortality in patients treated with adjuvant lumbar CSF drainage compared to conventional therapy [30]. In this study, mean time from admission to insertion of the lumbar drain was 37 hours, suggesting that late lumbar drainage is not harmful. Although our current data now show that, in most patients, CSF drainage through LP earlier in the disease is mostly safe, continuous drainage through lumbar CSF drains early in the disease may have higher risks. Especially when cerebral edema develops during the first days of disease, continuous CSF drainage may invoke cerebral herniation in a higher proportion of meningitis patients than we currently observe in patients undergoing LP.
The interrater reliability of contraindications for LP on cranial CT was moderate. Main contraindications noted by the raters were generalized cerebral edema and/or obliteration of the basal cisterns or insufficient slides of the lower fossa posterior. No patients with space-occupying lesions causing lateral shift on cranial imaging were identified. Because all patients underwent LP, this might indicate that space-occupying lesions with surrounding edema or lateral shift are relatively easy to recognize and led to deferral of LP. Generalized cerebral edema with obliteration of basal cisterns may be more difficult to recognize on CT.
Our study has several limitations. First, only patients with proven bacterial meningitis were included in the study. We do not have data on patients who were suspected of bacterial meningitis but in whom the LP excluded this diagnosis. Data on the rate of incidence of acute deterioration and cerebral herniation in this group of patients are lacking. Patients with expanding masses (eg, brain abscess, or necrotic temporal lobe in herpes simplex encephalitis) may present with symptoms that appear to be identical with those of meningitis, and in these patients LP may be complicated by cerebral herniation. Second, the majority of our patients had positive CSF cultures. As negative CSF cultures have been reported in 11%–30% of patients with bacterial meningitis, this could have led to a selection bias [31]. However, no differences in clinical presentation or outcome have been reported between patients with culture-positive bacterial meningitis and culture-negative bacterial meningitis [25]. Therefore this is unlikely to affect the results of our study. As our study was nationwide, we were able to study a representative sample of adults with acute bacterial meningitis with clinical deterioration after LP. Last, clinical signs of cerebral herniation were not prospectively recorded.
Results of our study do not argue against LP in patients with suspected or proven bacterial meningitis. An LP should be carried out in patients when a suspicion of bacterial meningitis remains after a history and physical examination. CSF examination confirms the diagnosis, identifies the causative pathogens allowing antibiotic sensitivity testing, and therefore rationalizes treatment [26, 31]. We also do not plead against performing CT scan prior to LP. Our data show that there is possibly a very small subset of patients whose clinical condition could acutely worsen after LP has been performed. Our data, however, also show that the interpretation of cranial CT for potential contraindications in the acute setting of a patient with bacterial meningitis is difficult, as shown by the moderate interrater reliability.
We conclude that the risk of cerebral herniation caused by LP is low, and performing an LP is a safe procedure in the large majority of patients. Cranial CT can be used as a screening method for contraindications in selected patients with suspected bacterial meningitis. If imaging is performed before LP, images should be carefully evaluated for contraindications for LP and therapy should be initiated before the patient is sent for neuroimaging [17].
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. J. M. C.: substantial contribution to conception and design, acquisition of data, analysis and interpretation of data; drafted the manuscript. M. C. B.: substantial contribution to conception and design, acquisition of data, analysis and interpretation of data; revised the manuscript for important intellectual content. M. E. S. S.: analysis and interpretation of data; revised the manuscript for important intellectual content. S. D. R.: analysis and interpretation of data; revised the manuscript for important intellectual content. A. v. d. E.: acquisition of data; revised the manuscript for important intellectual content. D. v. d. B.: substantial contribution to conception and design, acquisition of data, analysis, and interpretation of data; revised the manuscript for important intellectual content. All authors provided final approval of the version to be published.
Acknowledgments. The authors are indebted to all the Dutch physicians and patients who participated in the MeninGene study.
Financial support. This work was supported by the Netherlands Organization for Health Research and Development (ZonMw; NWO-Vidi-Grant [grant number 917.17.308 to M. B.], NWO-Vidi-Grant [grant number 016.116.358 to D. B.]); the Academic Medical Center (AMC Fellowship to D. B.); and the European Research Council (ERC starting grant to D. B.).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.




