-
PDF
- Split View
-
Views
-
Cite
Cite
Aamer Imdad, Fiona Retzer, Linda S Thomas, Marcy McMillian, Katie Garman, Peter F Rebeiro, Stephen A Deppen, John R Dunn, Amy M Woron, Impact of Culture-Independent Diagnostic Testing on Recovery of Enteric Bacterial Infections, Clinical Infectious Diseases, Volume 66, Issue 12, 15 June 2018, Pages 1892–1898, https://doi.org/10.1093/cid/cix1128
Close - Share Icon Share
Abstract
Culture-independent diagnostic tests (CIDTs) are increasingly used to identify enteric pathogens. However, foodborne illness surveillance systems have relied upon culture confirmation to estimate disease burden and identify outbreaks through molecular subtyping. This study examined the impacts of CIDT and estimated costs for culture verification of Shigella, Salmonella, Shiga toxin–producing Escherichia coli (STEC), and Campylobacter at the Tennessee Department of Health Public Health Laboratory (PHL).
This observational study included laboratory and epidemiological surveillance data collected between years 2013–2016 from patients with the reported enteric illness. We calculated pathogen recovery at PHL based on initial diagnostic test type reported at the clinical laboratory. Adjusted prevalence ratios (PRs) and 95% confidence intervals (CIs) were estimated with modified Poisson regression. Estimates of cost were calculated for pathogen recovery from CIDT-positive specimens compared to recovery from culture-derived isolates.
During the study period, PHL received 5553 specimens from clinical laboratories from patients with the enteric illness. Pathogen recovery was 57% (984/1713) from referred CIDT-positive stool specimens and 95% (3662/3840) from culture-derived isolates (PR, 0.61 [95% CI, .56–.66]). Pathogen recovery from CIDT-positive specimens varied based on pathogen type: Salmonella (72%), Shigella (64%), STEC (57%), and Campylobacter (26%). Compared to stool culture–derived isolates, the cost to recover pathogens from 100 CIDT-positive specimens was higher for Shigella (US $6192), Salmonella (US $18373), and STEC (US $27783).
Pathogen recovery was low from CIDT-positive specimens for enteric bacteria. This has important implications for the current enteric disease surveillance system, outbreak detection, and costs for public health programs.
In the United States, 48 million foodborne enteric illnesses occur annually, resulting in 128000 hospitalizations and 3000 deaths [1, 2]. Multiple public health surveillance systems exist nationally for enteric illnesses to monitor disease burden and trends, investigate individual cases, detect outbreaks, and characterize microorganisms to detect emerging strains [3, 4]. Historically, the foundation of public health surveillance for enteric bacteria has been reliance on accurate identification and recovery of causative agents by stool culture [4, 5]. Increasingly, hospitals and clinical laboratories are adopting culture-independent diagnostic tests (CIDTs) to identify enteric pathogens to guide clinical decision making [6–9] due to their increased sensitivity (80%–100%) [10, 11] and specificity (88%–99%) [10, 11], rapid turnaround time [10], ability to detect multiple pathogens in one test [10, 12], lower cost [13], and less technical expertise needed compared to culture methodology [10, 14]. CIDTs are therefore attractive for clinicians and clinical laboratories. However, increased use of CIDT by clinical laboratories poses a substantial challenge to surveillance programs for enteric illnesses, as CIDT-identified bacteria might not be available for advanced genetic characterization that is used to identify clusters, outbreaks, and emerging infections [4, 6–8, 15, 16] as well as antibiotic resistance testing. Furthermore, when isolates are not available from the clinical laboratory, the burden of isolate recovery is transferred to underresourced public health laboratories (PHLs) [4, 6, 7].
Although a number of previous studies have evaluated CIDTs in the clinical settings [11, 12, 17–22], there are few data regarding performance and impacts of these tests in PHLs. One of the core functions of a PHL is to recover bacteria for further characterization (eg, serotyping) and advanced testing (eg, pulsed-field gel electrophoresis or whole-genome sequencing). Specimens received from clinical laboratories are typically reisolated for pure culture or, in the case of CIDT-positive specimens, primary isolation is performed to replicate clinical laboratory results and recover isolates [4, 14]. Currently, little is known about the recovery of bacteria from newly available CIDT-positive specimens compared to cultured-derived isolates submitted to the PHL from clinical laboratories. Also, it is not well established if various factors such as time to transport the stool specimen to the PHL, CIDT test type, and the number of attempts to recover the bacteria in the PHL affect the recovery of enteric pathogens from CIDT-positive specimens [4, 6, 7]. Furthermore, no budgetary cost estimates are available to assess if there will be an increased cost to PHL to recover the bacteria from CIDT-positive specimens compared to culture-derived isolates. To address these knowledge gaps, we analyzed data from the Tennessee Department of Health (TDH) with the following objectives: (1) to assess the performance of CIDTs for enteric bacterial infections compared to stool culture and (2) to estimate the budgetary cost of recovery of enteric bacteria from CIDT-positive specimens compared to culture-derived isolates for Shigella, Salmonella, Shiga toxin–producing Escherichia coli (STEC), and Campylobacter.
METHODS
Study Design
We conducted a retrospective observational study using laboratory and epidemiological surveillance data collected by TDH as part of Foodborne Diseases Active Surveillance Network (FoodNet) from 1 May 2013 to 30 April 2016. State law [19] requires hospitals and clinical laboratories to report enteric infections within a week of diagnosis and an isolate or the positive specimen is required to be submitted to the state PHL. Patients diagnosed with an enteric illness due to a reportable pathogen are interviewed by phone to obtain epidemiologic information.
Selection of Study Population
We included data from patients with enteric illness due to Shigella, Salmonella, STEC, or Campylobacter ascertained in routine surveillance. Patients were excluded if there was a nongastrointestinal infection (eg, blood and urine) or specimens were unsatisfactory for testing upon receipt at the PHL. We also excluded data generated from nonsurveillance activities such as active outbreak investigation, tests for proficiency of laboratory equipment, and test of cure.
Data Collection
Laboratory data were obtained from the TDH laboratory information management system and epidemiological data was obtained from the National Electronic Disease Surveillance System used to manage FoodNet data. The data obtained from these 2 sources were combined into a single dataset using an algorithm (Supplementary Figure 1). Data were de-identified prior to analysis.
Definitions
“Positive CIDT” reports were defined as the detection of the enteric pathogen, or for STEC, Shiga toxin, or the genes that encode Shiga toxin, in a stool specimen or enrichment broth using a CIDT at the clinical laboratory [6]. “Culture-positive isolates” were defined as the microbiologic product obtained by stool culture by clinical laboratory and submitted to public health laboratory. “Transit time” was defined as the number of days between the dates the patient produced the stool specimen to the date of its reception at the PHL. “Work units” was defined as the number of times a broth or isolated colony was subjected to testing at the PHL to recover the pathogen. The term “clinical laboratory” in this study is defined as any clinic, hospital, or reference laboratory that performed the initial testing (CIDT or stool culture) on a stool specimen submitted from a patient with the suspected enteric illness.
Statistical Analysis
The primary outcome was pathogen recovery of Shigella, Salmonella, STEC, or Campylobacter at the PHL, defined as “positive” if the same pathogen was isolated as suspected in the clinical laboratory and “negative” if the suspected pathogen was not identified, a different pathogen was identified compared to the reported pathogen, or the pathogen was not viable (no growth). The conceptual framework in Supplementary Figure 2 provides an explanation for the primary exposure, outcome, and study question. We used modified Poisson regression to assess the probability of pathogen recovery at the PHL based on the type of testing at the clinical laboratory (CIDT vs stool culture). Results were reported in the form of adjusted prevalence ratios (PRs) with 95% confidence intervals (CIs) and can be interpreted as the ratio of the probability of pathogen recovery in the index exposure group (ie, CIDT positive) compared to the reference (ie, culture-derived isolates) [23]. Variables chosen for inclusion in the regression models a priori to control for potential confounding were age, sex, race, type of pathogen, transit time, hospitalization, and the number of work-units. We performed subgroup analyses for primary outcome based on the type of suspected pathogen and type of CIDT. The secondary analyses assessed the association of the recovery of the pathogen at the PHL with respect to transit time and the number of work-units. Logistic regression was used to estimate the association between transit time and pathogen recovery; transit time was modeled using restricted cubic splines with 5 knots to accommodate nonlinearity in the relationship [24].
Medians with interquartile ranges (IQRs) and percentages were used to describe continuous and categorical data, respectively. The χ2 test was used to estimate the statistically significant difference between categorical variables, and Wilcoxon rank-sum test was used for continuous data. For specimens testing positive for ≥2 pathogens in the clinical laboratory, only one result was included to avoid double-counting of demographic data. Multiple imputations were used to estimate missing data when applicable [25]. Details of multiple imputation methods are given in the Supplementary Data. All analyses were performed using Stata software version 14.0 [26].
Institutional review board committees at Vanderbilt University and TDH exempted this study from full review due to lack of human contact during the study.
Cost Calculation
The procedures to recover a pathogen at PHL from stool specimens differ based on the suspected pathogen and type of specimen received. We performed a budgetary impact analysis for the PHL to assess the cost to recover the suspected bacteria when it processed CIDT-positive specimens compared to culture-derived isolates. We considered the cost of growing the bacteria and that of testing to characterize the pathogen such as serotyping (but not the advanced testing such as whole-genome sequencing) that is conditional on the recovery of bacteria. We considered factors including the cost of reagents, labor cost, the number of work-units, and percentage recovery of the pathogen by type from CIDT-positive specimen and culture-derived isolates. We calculated the cost per 100 specimens for each pathogen and an overall estimated cost to process all CIDT-positive specimens. Specific cost methods are found in Supplementary Figure 3.
RESULTS
Study Population
During the study period, a total of 7707 specimens were submitted to PHL for reportable pathogen testing, and 5553 (72%) were included in our analysis. The reasons for exclusion of specimens are shown in Figure 1. Among specimens included in the analyses, clinical laboratories used a CIDT for 1713 (31%) specimens, with the remainder tested by stool culture (Figure 1). Among CIDT-positive specimens, polymerase chain reaction (PCR) testing was reported more commonly (73%) than enzyme immunoassay (EIA) testing (27%). Numbers of males and females were similar and about half of the study population was pediatric (age <18 years) (Table 1). CIDT was used in a higher proportion of pediatric patients compared to adults and for white compared to nonwhite patients (Table 1).
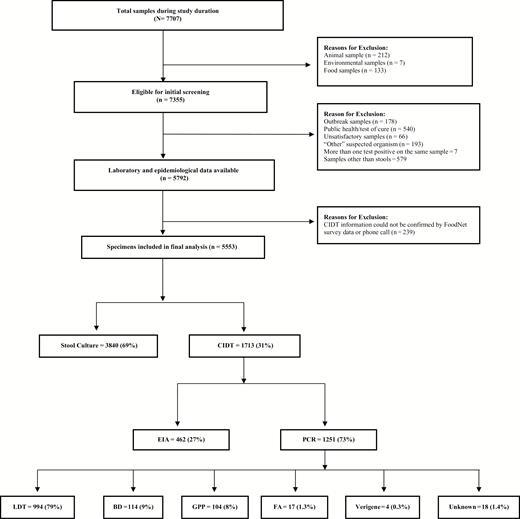
Selection of study population. Flow diagram showing selection of study samples and description of culture-independent diagnostic test distribution; the percentages in each box are with respect to the box linked above them by the arrow.
Abbreviations: BD, BD MAX; CIDT, culture-independent diagnostic test; EIA, enzyme-linked immunoassay; FA, FilmArray; GPP, xTAG gastrointestinal pathogen panel; LDT, locally developed diagnostic test; PCR, polymerase chain reaction.
| Characteristic . | Total (N = 5553) . | Testing at Clinical Laboratory . | |
|---|---|---|---|
| CIDT (n = 1713) . | Stool Culture (n = 3840) . | ||
| Age <18 y | 2862 (51) | 1052 (61) | 1810 (47) |
| Male sex | 2750 (49) | 836 (49) | 1914 (50) |
| Race/ethnicity | |||
| Whitea | 3357 (60) | 1137 (66) | 2220 (57) |
| Blacka | 731 (13) | 172 (10) | 559 (14) |
| Hispanicb | 267 (4) | 100 (6) | 167 (4) |
| Travel: yesc | 146 (3) | 42 (2) | 104 (2) |
| Hospitalization: yesd | 1272 (23) | 244 (14) | 1028 (27) |
| Out-of-state specimens | 500 (9) | 71 (4) | 429 (11) |
| Year of study | |||
| 2013 | 1403 (25) | 344 (20) | 1059 (27) |
| 2014 | 2119 (38) | 620 (36) | 1499 (39) |
| 2015 | 1598 (29) | 529 (31) | 1069 (28) |
| 2016 | 433 (8) | 220 (13) | 213 (6) |
| Characteristic . | Total (N = 5553) . | Testing at Clinical Laboratory . | |
|---|---|---|---|
| CIDT (n = 1713) . | Stool Culture (n = 3840) . | ||
| Age <18 y | 2862 (51) | 1052 (61) | 1810 (47) |
| Male sex | 2750 (49) | 836 (49) | 1914 (50) |
| Race/ethnicity | |||
| Whitea | 3357 (60) | 1137 (66) | 2220 (57) |
| Blacka | 731 (13) | 172 (10) | 559 (14) |
| Hispanicb | 267 (4) | 100 (6) | 167 (4) |
| Travel: yesc | 146 (3) | 42 (2) | 104 (2) |
| Hospitalization: yesd | 1272 (23) | 244 (14) | 1028 (27) |
| Out-of-state specimens | 500 (9) | 71 (4) | 429 (11) |
| Year of study | |||
| 2013 | 1403 (25) | 344 (20) | 1059 (27) |
| 2014 | 2119 (38) | 620 (36) | 1499 (39) |
| 2015 | 1598 (29) | 529 (31) | 1069 (28) |
| 2016 | 433 (8) | 220 (13) | 213 (6) |
Data are presented as No. (%). The percentages in the column are relative to total numbers given at the top of each column. All characteristics were statistically significantly different with P < .0 except that for sex and travel history.
Abbreviation: CIDT, culture-independent diagnostic test.
aTotal missing values: 1245 (22%), CIDT = 332 (19%), stool culture = 913 (23%); 220 (4%) were other races that included Asian, Pacific Islander, multirace, etc.
bTotal missing values: 1316 (24%), CIDT = 342 (20%), stool culture = 974 (25%).
cTotal missing values: 1763 (32%), CIDT = 478 (27%), stool culture = 1285 (33%).
dTotal missing values: 1159 (21%), CIDT = 297 (17%), stool culture = 862 (22%).
| Characteristic . | Total (N = 5553) . | Testing at Clinical Laboratory . | |
|---|---|---|---|
| CIDT (n = 1713) . | Stool Culture (n = 3840) . | ||
| Age <18 y | 2862 (51) | 1052 (61) | 1810 (47) |
| Male sex | 2750 (49) | 836 (49) | 1914 (50) |
| Race/ethnicity | |||
| Whitea | 3357 (60) | 1137 (66) | 2220 (57) |
| Blacka | 731 (13) | 172 (10) | 559 (14) |
| Hispanicb | 267 (4) | 100 (6) | 167 (4) |
| Travel: yesc | 146 (3) | 42 (2) | 104 (2) |
| Hospitalization: yesd | 1272 (23) | 244 (14) | 1028 (27) |
| Out-of-state specimens | 500 (9) | 71 (4) | 429 (11) |
| Year of study | |||
| 2013 | 1403 (25) | 344 (20) | 1059 (27) |
| 2014 | 2119 (38) | 620 (36) | 1499 (39) |
| 2015 | 1598 (29) | 529 (31) | 1069 (28) |
| 2016 | 433 (8) | 220 (13) | 213 (6) |
| Characteristic . | Total (N = 5553) . | Testing at Clinical Laboratory . | |
|---|---|---|---|
| CIDT (n = 1713) . | Stool Culture (n = 3840) . | ||
| Age <18 y | 2862 (51) | 1052 (61) | 1810 (47) |
| Male sex | 2750 (49) | 836 (49) | 1914 (50) |
| Race/ethnicity | |||
| Whitea | 3357 (60) | 1137 (66) | 2220 (57) |
| Blacka | 731 (13) | 172 (10) | 559 (14) |
| Hispanicb | 267 (4) | 100 (6) | 167 (4) |
| Travel: yesc | 146 (3) | 42 (2) | 104 (2) |
| Hospitalization: yesd | 1272 (23) | 244 (14) | 1028 (27) |
| Out-of-state specimens | 500 (9) | 71 (4) | 429 (11) |
| Year of study | |||
| 2013 | 1403 (25) | 344 (20) | 1059 (27) |
| 2014 | 2119 (38) | 620 (36) | 1499 (39) |
| 2015 | 1598 (29) | 529 (31) | 1069 (28) |
| 2016 | 433 (8) | 220 (13) | 213 (6) |
Data are presented as No. (%). The percentages in the column are relative to total numbers given at the top of each column. All characteristics were statistically significantly different with P < .0 except that for sex and travel history.
Abbreviation: CIDT, culture-independent diagnostic test.
aTotal missing values: 1245 (22%), CIDT = 332 (19%), stool culture = 913 (23%); 220 (4%) were other races that included Asian, Pacific Islander, multirace, etc.
bTotal missing values: 1316 (24%), CIDT = 342 (20%), stool culture = 974 (25%).
cTotal missing values: 1763 (32%), CIDT = 478 (27%), stool culture = 1285 (33%).
dTotal missing values: 1159 (21%), CIDT = 297 (17%), stool culture = 862 (22%).
Recovery of Pathogens in PHL
Recovery of the target pathogen occurred in 95% (3662/3840) from specimens that tested positive by stool culture at the originating clinical laboratory but only 57% (984/1713) of CIDT-positive specimens (Figure 2). The adjusted PRs showed that compared to culture-derived isolates, CIDT-positive specimens yielded a pathogen in only 61% of cases (PR, 0.61 [95% CI, .56–.66]).
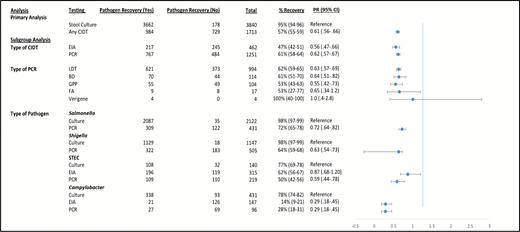
Pathogen recovery and adjusted prevalence ratios (PRs) for primary and subgroup analyses. The PRs are adjusted for age, sex, race, type of pathogen, transit time, hospitalization, and a number of work-units. The confidence intervals for percentage recovery of pathogen were calculated by exact methods. Abbreviations: BD, BD MAX; CI, confidence interval; CIDT, culture-independent diagnostic test; EIA, enzyme-linked immunoassay; FA, FilmArray; GPP, xTAG gastrointestinal pathogen panel; LDT, locally developed diagnostic test; PCR, polymerase chain reaction; PR, prevalence ratio; STEC, Shiga toxin–producing Escherichia coli.
A subgroup analysis based on the type of CIDT (for all pathogens) showed that the pathogen recovery was 61% (767/1251) for PCR and 47% (217/462) for EIA (Figure 2). Recovery was similar across different types of PCR panels; however, 2 PCR panels (FilmArray and Verigene) did not have sufficient numbers of CIDT-positive submissions to the PHL to draw meaningful conclusions (Figure 2).
Analyses based on the type of pathogen and type of CIDT showed that pathogen recovery for PCR testing ranged from 72% for Salmonella to 28% for Campylobacter (Figure 2). For EIA, recovery for STEC and Campylobacter was 62% and 14%, respectively (Figure 2). Adjusted PRs for this subgroup analysis are shown in Figure 2.
Transit Time and Pathogen Recovery
Median transit time for the study population and for culture-positive specimens was 8 (IQR, 6–11) days and 6 (IQR, 6–11) days for CIDT-positive specimens. Pathogen recovery decreased with increasing transit time and the rate of decrease was more pronounced for CIDT-positive specimens compared to culture-positive specimens (Figure 3A and 3B). A logistic regression model adjusted for age, sex, type of pathogen, and type of testing at clinical laboratory showed that with each day of transit time, the probability of pathogen recovery from CIDT-positive specimens decreased by 3% (95% CI, 1%–4%).
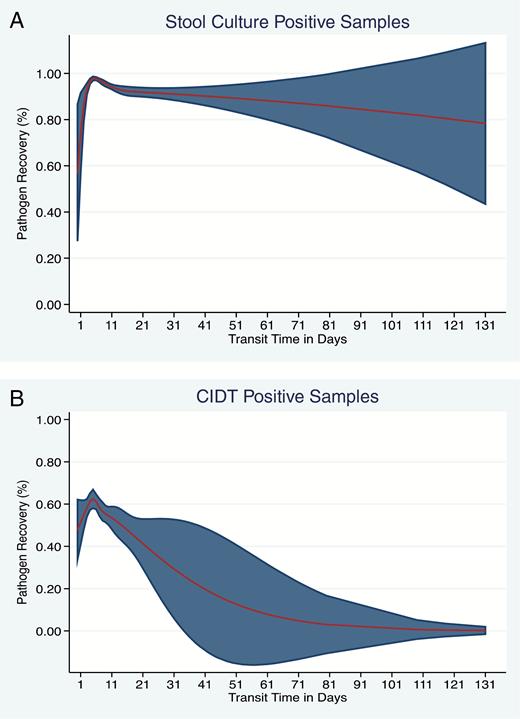
A, Transit time in days and recovery of pathogen in the state laboratory—specimen tested by stool culture at clinical laboratory. B, Transit time in days and recovery of pathogen in the state laboratory—specimen tested by a culture-independent diagnostic test (CIDT) at the clinical laboratory. The red line shows the trend in recovery with increasing transit time and the shaded area indicates the 95% confidence interval.
Number of Work-Units and Pathogen Recovery
The median number of work-units required to recover a pathogen from specimens submitted by clinical laboratories was 2, except for specimens tested positive by a CIDT for STEC, which required a median of 3 work-units (Supplementary Table 1).
Cost
The estimated budgetary cost to process 100 CIDT-positive specimens was US $6192 for Shigella, US $18373 for Salmonella, and US $27783 for STEC compared to pathogen recovery from culture-derived isolates (Table 2). The same cost for Campylobacter was, however, lower as additional testing such as serotyping was not required in many cases due to low recovery rates of the pathogen from CIDT-positive specimens. Overall, it cost an estimated US $230000 to process the 1713 CIDT-positive specimens during the study period.
| Type of Pathogen . | Clinical Laboratory Testing . | Cost per Work-Unit for Pathogen Positive, US $ . | Cost per Work-Unit for Pathogen Negative, US $ . | Median No. of Picks . | Total Cost per 100 Samples, US $ . | Net Cost Difference per 100 Samples: CIDT – Stool Culture . | Total Cost . |
|---|---|---|---|---|---|---|---|
| Shigella | Stool culture | 10.8 | 2.99 | 2 | 2128.76 | 6192.88 | 231807 |
| CIDT | 41.66 | 2.99 | 3 | 8321.64 | |||
| Salmonella | Stool culture | 34.59 | 2.52 | 2 | 6789.72 | 18373.88 | |
| CIDT | 173.25 | 3.85 | 2 | 25163.6 | |||
| STEC | Stool culture | 1.26 | 2.68 | 2 | 370.92 | 27783.75 | |
| CIDT | 161.26 | 4.49 | 3 | 28154.67 | |||
| Campylobacter | Stool culture | 96.6 | 2.4 | 2 | 15175.2 | –11115.6 | |
| CIDT | 96.6 | 2.4 | 2 | 4059.6 |
| Type of Pathogen . | Clinical Laboratory Testing . | Cost per Work-Unit for Pathogen Positive, US $ . | Cost per Work-Unit for Pathogen Negative, US $ . | Median No. of Picks . | Total Cost per 100 Samples, US $ . | Net Cost Difference per 100 Samples: CIDT – Stool Culture . | Total Cost . |
|---|---|---|---|---|---|---|---|
| Shigella | Stool culture | 10.8 | 2.99 | 2 | 2128.76 | 6192.88 | 231807 |
| CIDT | 41.66 | 2.99 | 3 | 8321.64 | |||
| Salmonella | Stool culture | 34.59 | 2.52 | 2 | 6789.72 | 18373.88 | |
| CIDT | 173.25 | 3.85 | 2 | 25163.6 | |||
| STEC | Stool culture | 1.26 | 2.68 | 2 | 370.92 | 27783.75 | |
| CIDT | 161.26 | 4.49 | 3 | 28154.67 | |||
| Campylobacter | Stool culture | 96.6 | 2.4 | 2 | 15175.2 | –11115.6 | |
| CIDT | 96.6 | 2.4 | 2 | 4059.6 |
Cost calculations show the amount spent per 100 specimens to grow a pathogen in state laboratory when an isolate (from stool culture) or broth (from CIDT testing) from the clinical laboratory was received. Cost per work-unit was calculated by estimating the cost related to equipment, chemicals used, and the time taken by the technician to read the stool culture in the state laboratory. The cost per 100 samples for each row in the table was calculated with the following formula: (cost per workup for pathogen-positive sample × median number of work-units × percentage recovery) + [(cost per workup for pathogen-negative sample × median number of work-units) × (1 – percentage recovery)]. Data for percentage recovery was taken from Figure 2. The third-to-last column shows the extra cost that was spent on CIDT-positive samples compared to current standard practice (ie, stool culture). This did not include cost related to advanced testing such as pulsed-field gel electrophoresis or whole-genome sequencing. The second-to-last column shows the amount of money that was spent on CIDT-positive specimens that did not yield a pathogen compared to stool culture. The last column shows the extra amount of money that was spent on all the CIDT-positive samples (n = 1713) included in this study.
Abbreviations: CIDT, culture-independent diagnostic test; STEC, Shiga toxin–producing Escherichia coli.
| Type of Pathogen . | Clinical Laboratory Testing . | Cost per Work-Unit for Pathogen Positive, US $ . | Cost per Work-Unit for Pathogen Negative, US $ . | Median No. of Picks . | Total Cost per 100 Samples, US $ . | Net Cost Difference per 100 Samples: CIDT – Stool Culture . | Total Cost . |
|---|---|---|---|---|---|---|---|
| Shigella | Stool culture | 10.8 | 2.99 | 2 | 2128.76 | 6192.88 | 231807 |
| CIDT | 41.66 | 2.99 | 3 | 8321.64 | |||
| Salmonella | Stool culture | 34.59 | 2.52 | 2 | 6789.72 | 18373.88 | |
| CIDT | 173.25 | 3.85 | 2 | 25163.6 | |||
| STEC | Stool culture | 1.26 | 2.68 | 2 | 370.92 | 27783.75 | |
| CIDT | 161.26 | 4.49 | 3 | 28154.67 | |||
| Campylobacter | Stool culture | 96.6 | 2.4 | 2 | 15175.2 | –11115.6 | |
| CIDT | 96.6 | 2.4 | 2 | 4059.6 |
| Type of Pathogen . | Clinical Laboratory Testing . | Cost per Work-Unit for Pathogen Positive, US $ . | Cost per Work-Unit for Pathogen Negative, US $ . | Median No. of Picks . | Total Cost per 100 Samples, US $ . | Net Cost Difference per 100 Samples: CIDT – Stool Culture . | Total Cost . |
|---|---|---|---|---|---|---|---|
| Shigella | Stool culture | 10.8 | 2.99 | 2 | 2128.76 | 6192.88 | 231807 |
| CIDT | 41.66 | 2.99 | 3 | 8321.64 | |||
| Salmonella | Stool culture | 34.59 | 2.52 | 2 | 6789.72 | 18373.88 | |
| CIDT | 173.25 | 3.85 | 2 | 25163.6 | |||
| STEC | Stool culture | 1.26 | 2.68 | 2 | 370.92 | 27783.75 | |
| CIDT | 161.26 | 4.49 | 3 | 28154.67 | |||
| Campylobacter | Stool culture | 96.6 | 2.4 | 2 | 15175.2 | –11115.6 | |
| CIDT | 96.6 | 2.4 | 2 | 4059.6 |
Cost calculations show the amount spent per 100 specimens to grow a pathogen in state laboratory when an isolate (from stool culture) or broth (from CIDT testing) from the clinical laboratory was received. Cost per work-unit was calculated by estimating the cost related to equipment, chemicals used, and the time taken by the technician to read the stool culture in the state laboratory. The cost per 100 samples for each row in the table was calculated with the following formula: (cost per workup for pathogen-positive sample × median number of work-units × percentage recovery) + [(cost per workup for pathogen-negative sample × median number of work-units) × (1 – percentage recovery)]. Data for percentage recovery was taken from Figure 2. The third-to-last column shows the extra cost that was spent on CIDT-positive samples compared to current standard practice (ie, stool culture). This did not include cost related to advanced testing such as pulsed-field gel electrophoresis or whole-genome sequencing. The second-to-last column shows the amount of money that was spent on CIDT-positive specimens that did not yield a pathogen compared to stool culture. The last column shows the extra amount of money that was spent on all the CIDT-positive samples (n = 1713) included in this study.
Abbreviations: CIDT, culture-independent diagnostic test; STEC, Shiga toxin–producing Escherichia coli.
DISCUSSION
This large observational analysis demonstrated that recovery of Shigella, Salmonella, STEC, and Campylobacter at PHL was low from CIDT-positive specimens compared to culture-derived isolates. Adjusted analyses showed that, compared to the culture-derived isolates, CIDT-positive specimens yielded a pathogen in only 61% of the cases. We identified “transit time” as an important factor and showed increased transit time significantly reduced recovery of pathogens for CIDT-positive specimens. Additionally, we describe significant budgetary costs to process CIDT-positive specimens compared to culture-derived isolates.
The performance of CIDT varied based on the type of CIDT and type of suspected pathogen. The most noticeable results were for Campylobacter, where recovery of pathogens was only 14% for EIA and 28% for PCR. There could be multiple reasons for these results. The first consideration is that culturing for Campylobacter is complex and this pathogen is hard to grow on culture media [27]. This observation is evident from this study as recovery rates from stool cultures positive for Campylobacter was 78% compared to Shigella and Salmonella (98%). Second is that recovery of Campylobacter by stool culture seems to depend on bacterial load as shown by Wohlwend et al [22]. This means that a CIDT could have picked up the pathogen resulting in a CIDT-positive sample and negative stool culture [22, 28]. The third is the potential low specificity of various CIDTs, meaning that the antibody used in CIDTs may cross-react with antigens other than target antigens in the stool resulting in false-positive tests [6–8, 10, 11, 27, 28]. The recovery of the pathogen from culture-derived isolates was also not 100% for any of the pathogen. The potential reasons for this include loss of isolate viability during transport, misidentification by the submitting laboratory, and laboratory error in handling the specimen at PHL.
The budgetary cost to PHL to recover Shigella, Salmonella, and STEC from CIDT-positive specimens was higher compared to culture-positive specimens. Costs to achieve an isolate were actually lower for Campylobacter and there are at least 2 potential reasons. First, cost per work-unit to the state laboratory was the same if an isolate or stool broth was received for Campylobacter. The second was that because very few Campylobacter CIDT-positive specimens yield a pathogen (ie, 28% for PCR and 14% for EIA), costs were avoided for additional characterization (ie, serotyping) of the pathogen that is conditional on the pathogen recovery. Overall, we estimated that >US $230000 was spent to process 1713 CIDT-positive specimens compared to the cost to process culture-derived isolates.
This study had a few limitations of note. Data were only from the state of Tennessee and results may not be generalizable to the rest of the United States. However, increased use of CIDT is occurring nationally [8] and public health infrastructure is similar to other state health departments. About 58% of CIDT testing was based on a locally developed test at the clinical laboratories, and details of locally developed test methods are generally not available publicly compared to US Food and Drug Administration–approved syndromic panels. We did not have information on the use of antibiotics in patients, so their impact on final recovery could not be assessed. The cost analysis was a budgetary impact analysis and not a cost-effectiveness analysis, as that would consider potential impacts of CIDTs on clinical care of patients such as hospitalization, antibiotic stewardship, mortality, and public health impact such as estimation of the burden of disease and prevention, detection, and investigation of outbreaks. Even though surveillance under FoodNet requires reporting of 9 enteric pathogens [26], we included only the 4 most common bacterial pathogens reported annually across the United States [2].
The findings from these analyses have important implications related to public health practice and can be considered in 3 different aspects related to surveillance of foodborne illnesses and outbreak detection. First, increased use of CIDTs by clinical laboratories is impacting the current methods to define incidence estimates and trends of enteric pathogens [6, 8]. Second, isolates will not be available for an increasing proportion of CIDT-positive specimens for laboratory networks such as PulseNet. These networks continue to need isolates for molecular subtyping that identify clusters facilitating outbreak investigation [4]. The third aspect is that the burden of recovery of enteric pathogens creates a substantial increase in cost to public health departments and strain on limited infrastructure.
Potential solutions to address these concerns include clinical laboratories performing reflex culture when a positive CIDT test for Shigella, Salmonella, STEC, or Campylobacter occurs as recommended by interim guidelines by the Association of Public Health Laboratories [29]. If the clinical laboratory is not able to perform the reflex stool culture, they should work with their PHL to comply with submission regulations in their jurisdiction and transport the stool sample/broth to state laboratory rapidly to maximize the chances of pathogen recovery. Costs for reflex culture at the clinical laboratory or PHL, however, should be evaluated further in regard to costs to patients.
CIDT is an advanced testing method with rapid uptake in clinical practice [8, 9]. The advantages of these methods should be evaluated further to assess their potential impact on both clinical and public health practice. In the clinical settings, evaluation is needed to establish if CIDT improves patient care in terms of antibiotic stewardship, secondary prevention of hospitalization, decreasing length of hospital stay, and reducing mortality. Additionally, clinical guidelines for specimens that test positive for multiple pathogens are not currently available. Similarly, additional evaluations of surveillance systems such as FoodNet are needed to understand the impact of CIDT. CIDT may increase the ability to detect more cases of enteric and foodborne illness as stool culture is comparatively less sensitive than some CIDTs. Similarly, further evaluation is needed to assess the public health impact of CIDT on outbreak detection and investigation, and detection of emerging pathogens. Finally, future evaluation and improved methods are needed to enhance the performance of culture-independent testing facilitating molecular characterization for serotyping, antimicrobial resistance testing, and cluster evaluation.
CONCLUSIONS
In summary, the recovery of Shigella, Salmonella, STEC, and Campylobacter was low from CIDT-positive specimens. This has important implications for the current enteric disease surveillance systems and outbreak detection. We described increased costs to PHLs as a result of CIDT uptake. More assessments are needed to define the exact role of CIDTs in disease detection and outbreak investigation with the development of advanced methods that might not depend on culture isolation of the bacteria. A cost analysis that considers both clinical and public health impact of CIDT is needed to decide if the use of CIDTs is cost-effective to investigate the bacterial enteric infections.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. A. I., A. M. W., K. G., and J. R. D. conceptualized the study. F. R., A. M. W., K. G., M. M., and A. I. helped with the extraction of data. A. I. and P. F. R. conducted the statistical analysis. A. I., L. S. T., and S. A. D. helped with cost calculations. A. I. wrote the first draft of the manuscript. All the authors reviewed the manuscript and contributed regarding their respective expertise. All the authors approved the final manuscript before submission. A, M. W. is responsible for the data.
Acknowledgments. We thank Dr Kathryn M. Edwards and Dr Marie Griffin, who provided helpful feedback to improve the manuscript.
Financial support. This study was supported by an Epidemiology Laboratory Capacity Grant to the Tennessee Department of Health.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
Author notes
Present affiliations: aState University of New York, Upstate Medical University, Syracuse, NY; bState Laboratories Division, 2725 Waimano Home Road, Pearl City, HI 96782.




