-
PDF
- Split View
-
Views
-
Cite
Cite
Amy L Sandul, Nwabunie Nwana, J Mike Holcombe, Mark N Lobato, Suzanne Marks, Risa Webb, Shu-Hua Wang, Brock Stewart, Phil Griffin, Garrett Hunt, Neha Shah, Asween Marco, Naveen Patil, Leonard Mukasa, Ruth N Moro, John Jereb, Sundari Mase, Terence Chorba, Sapna Bamrah-Morris, Christine S Ho, High Rate of Treatment Completion in Program Settings With 12-Dose Weekly Isoniazid and Rifapentine for Latent Mycobacterium tuberculosis Infection, Clinical Infectious Diseases, Volume 65, Issue 7, 1 October 2017, Pages 1085–1093, https://doi.org/10.1093/cid/cix505
Close - Share Icon Share
Abstract
Randomized controlled trials have demonstrated that the newest latent tuberculosis (LTBI) regimen, 12 weekly doses of directly observed isoniazid and rifapentine (3HP), is as efficacious as 9 months of isoniazid, with a greater completion rate (82% vs 69%); however, 3HP has not been assessed in routine healthcare settings.
Observational cohort of LTBI patients receiving 3HP through 16 US programs was used to assess treatment completion, adverse drug reactions, and factors associated with treatment discontinuation.
Of 3288 patients eligible to complete 3HP, 2867 (87.2%) completed treatment. Children aged 2–17 years had the highest completion rate (94.5% [155/164]). Patients reporting homelessness had a completion rate of 81.2% (147/181). In univariable analyses, discontinuation was lowest among children (relative risk [RR], 0.44 [95% confidence interval {CI}, .23–.85]; P = .014), and highest in persons aged ≥65 years (RR, 1.72 [95% CI, 1.25–2.35]; P < .001). In multivariable analyses, discontinuation was lowest among contacts of patients with tuberculosis (TB) disease (adjusted RR [ARR], 0.68 [95% CI, .52–.89]; P = .005) and students (ARR, 0.45 [95% CI, .21–.98]; P = .044), and highest with incarceration (ARR, 1.43 [95% CI, 1.08–1.89]; P = .013) and homelessness (ARR, 1.72 [95% CI, 1.25–2.39]; P = .001). Adverse drug reactions were reported by 1174 (35.7%) patients, of whom 891 (76.0%) completed treatment.
Completion of 3HP in routine healthcare settings was greater overall than rates reported from clinical trials, and greater than historically observed using other regimens among reportedly nonadherent populations. Widespread use of 3HP for LTBI treatment could accelerate elimination of TB disease in the United States.
Recommendations for improving prevention and control of tuberculosis (TB) in the United States include testing and treatment of persons for latent TB (LTBI) [1]. Reactivation of LTBI accounts for the majority of TB disease cases; therefore, finding and treating LTBI is a key component of the US strategy for TB elimination [2].
Daily self-administered isoniazid (INH) has been the standard therapy for LTBI for ≥50 years, but its effectiveness is limited by drug toxicity and low rates of treatment completion because of the long duration of INH monotherapy regimens [3]. Adoption of shorter, safer regimens is important for increasing treatment completion rates. Randomized controlled trials have demonstrated that, compared to 6–9 months of daily INH, the 3HP regimen of 12 once-weekly doses (3 months) of rifapentine (RPT) plus INH by directly observed therapy (DOT) is statistically noninferior to longer INH regimens for preventing TB and is more likely to be completed [4–6].
Although 3HP is recommended as an alternative to daily INH and preferred for patients who cannot or are unlikely to complete a longer regimen [2], acceptance and benefits of 3HP that have been observed in controlled clinical trials merit confirmation among diverse populations and programmatic settings. We sought to measure rates of 3HP treatment completion in US nonresearch settings, determine factors associated with treatment discontinuation, and conduct surveillance for adverse drug reactions (ADRs).
METHODS
Project Design
This postmarketing project was an observational cohort assessment of directly observed 3HP (weight-adjusted, maximal dose of 900 mg each of RPT [P] and INH [H] for patients weighing ≥50 kg), implemented through clinics across the United States, including health departments, student health centers, correctional facilities, and shelters for persons experiencing homelessness. Solicitations for participation were sent to TB controllers in all US jurisdictions, and a meeting was convened with interested clinicians and program managers to develop standard data collection instruments and assess site-specific needs for data collection. Participating project sites agreed that programs would share de-identified patient data on the basis of their standard practices. Data were observational, dose adherence was assessed by DOT, and ADR surveillance was based on patient self-report; the methods were designed to be minimally intrusive for participating sites and required only routine practices in their programs. Project sites were self-selected and most participating programs used 3HP for all LTBI treatment. Local standards and indications for treatment were used at participating sites for LTBI diagnosis, and programs developed their own clinical protocols and training for 3HP treatment implementation and monitoring. Monitoring of patients who took 3HP included a dose and symptom review checklist. A database with 3 nested tiers (basic, standard, and comprehensive) for data collection was developed to accommodate differing capacities at the volunteer sites. Basic data collection included patient demographics, weekly dose and symptom review, and final disposition. Standard data collection consisted of basic data collection elements plus selected medical conditions and population risk factors for TB, and concomitant medications. Comprehensive data collection included all data collected in the standard data collection plus any additional patient medications or medical conditions. Standard and comprehensive tier patient care data included comorbid medical conditions, medication use, behavioral risk factors, and population risk factors for TB [1]. Comorbid medical conditions included conditions that impair the immune system or involve use of medications that suppress immune response and predispose to active TB disease (including diabetes, chronic renal disease, immunocompromised [unspecified], hepatitis, chronic lung disease, malnutrition, gastrectomy, or jejunoileal bypass) and common conditions that involve concomitant use of medications that pose potential drug interactions (including hypertension, seizure disorder, and mental health disorder). Any medical condition was defined as a patient reporting a comorbid medical condition or any other medical condition. Behavioral risk factors were alcohol use defined as >2 drinks per day, current or past smoking, intravenous drug use (IDU), and nonintravenous drug use (non-IDU). Any substance use risk was defined as a patient reporting current or past history of smoking, use of alcohol, IDU, or non-IDU. Population risk factors for TB included being a migrant worker, healthcare worker, or an employee or resident in a TB-risk setting (eg, homeless shelter, correctional facility, long-term-care facility).
Six sites were in the basic data collection tier, 4 in the standard tier, and 6 in the comprehensive tier. Sites were geographically representative across the United States: 1 in the Northeast, 4 in the West, 5 in the Midwest, and 6 in the South. One participating site was federal with 7 health facilities in 6 states. Each site was provided a database shell corresponding to its respective specific tier of data collection. Data were collected for patients who received 1 or more 3HP treatment doses during June 2011 through December 2013, in accordance with Centers for Disease Control and Prevention (CDC) recommendations [2].
ADRs were defined as any symptoms reported since receipt of the last 3HP dose [7]. Throughout treatment, patients were asked at each weekly DOT visit, or any interim visits or calls, to report any ADRs or symptoms experienced since administration of the prior week’s medication dose.
Outcome Measures and Associated Factors
Patient care data collected at all sites included demographics, treatment reason, and history of homelessness or incarceration anytime during the 12 months before 3HP initiation. Reasons for treatment initiation with 3HP were per local policy, not mutually exclusive, and included risk for TB exposure or infection, foreign birth in a high-prevalence country, and persons at high risk for progression to TB disease if infected (eg, persons with human immunodeficiency virus [HIV] or other immunosuppressive medical conditions, persons taking medications known to increase the risk for disease, and persons with prior inadequately treated TB).
Treatment outcomes were completion, temporary discontinuation due to ADRs with subsequent continuation and treatment completion, discontinuation due to ADRs (ie, provider or patient decision), discontinuation as a result of loss to follow-up, and substitution of an alternate regimen. Treatment completion for 3HP was defined as documented receipt of 11 or 12 doses of 3HP, each at least 3 days apart with no more than 5 doses given within a 28-day period, within 16 weeks of initiating treatment. Patient final disposition deemed “completion” included treatment without interruption, and temporary halting with subsequent continuation and completion. Patient final disposition deemed “discontinuation” included stopping due to ADRs, switching to an alternate regimen, and loss to follow-up.
Data Management and Statistical Analysis
Patient data were entered into Microsoft Access 2010 databases (Microsoft Corporation, Redmond, Washington) developed by CDC. In April 2014, participating sites exported their data files to an encrypted, password-protected secure file transport protocol site at CDC. After files were downloaded from CDC’s secure site, data for each participating site were cleaned; quality and validation checks were run; and incomplete and missing data points for individual patients were queried with site collaborators. After review for internal consistency and completeness, data from all 16 sites were collated into a single data set. All tiers were combined for calculating overall completion rates, and the standard and comprehensive tiers were combined for subgroup analyses.
Statistical analyses were performed using SAS 9.3 (SAS Institute, Cary, North Carolina). We described patient characteristics of the entire cohort and calculated discontinuation percentages for demographic, treatment indication, medical conditions, and behavioral risk factors. We modeled the association of 3HP discontinuation in univariable and multivariable log-binomial regression models by calculating relative risk (RR). Each univariable model looked at the association of 3HP discontinuation with potential risk factors individually; age was assessed as a continuous variable and as a categorical variable in separate univariable models. RR values, 95% confidence intervals (CIs), and P values were calculated for each potential risk factor. Independent risk factors were tested for significance at a level of P ≤ .05.
Two multivariable regression analyses using backward elimination were performed to calculate adjusted relative risks (ARRs) using log-binomial regression. Factors considered for inclusion in the models were selected a priori and chosen based on subject-domain judgement. Age was modeled as a continuous variable in multivariable analyses.
The first model examined the effect of demographic and population factors on 3HP treatment discontinuation while controlling for the effect of individual treatment sites for all patients starting treatment (n = 3288). The second model examined the association of medical conditions and behavioral risk factors with 3HP treatment discontinuation among the subgroup of patients (n = 2389) from standard and comprehensive tier sites, while controlling for age. In both multivariable models, associations between covariates and 3HP treatment discontinuation were considered significant at P < .05. Treatment reasons, medical conditions, and behavioral risk factors were dichotomous variables. Potential interactions were hypothesized a priori; interaction terms were entered into the models with all covariates to assess their effect. Interaction terms significant at P ≤ .10 were explored [8]. We found no evidence of interaction among variables in the model.
Ethics Oversight
The CDC determined that this assessment of postmarketing experiences was public health practice and the project did not involve an investigational intervention, clinical research activities, or procedures for which written consent was required. Each participating site obtained ethical review and approval for participation in accordance with local requirements.
LTBI treatment was not a US Food and Drug Administration (FDA)–approved indication for RPT when this assessment began, although 3HP use was recommended by CDC [2]. The FDA approved a labeling change (RPT in combination with INH for LTBI) on 2 December 2014 [9].
RESULTS
Of 3327 patients who received ≥1 dose of 3HP, treatment was stopped for 39 (1.2%) when a contraindication developed or was identified. Of those 39 patients, 2 had negative QuantiFERON results (lack of LTBI diagnosis), and 37 had contraindications for 3HP: 20 were contacts of INH-resistant index cases; 14 were women who became pregnant; 2 had HIV infection and were taking highly active antiretroviral therapy (HAART); and 1 had TB disease. Among the remaining 3288 patients, 421 (12.8%) discontinued treatment (Figure 1). Discontinuation rates across the sites ranged from 0.0% to 19.2% (Supplementary Table 1).
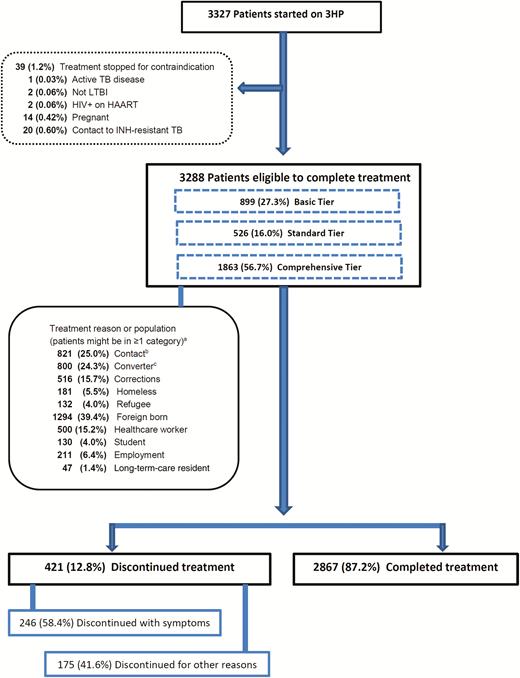
Profile of persons with latent tuberculosis (LTBI) who took a 3-month regimen of isoniazid and rifapentine. aTreatment reasons per local policy are dichotomous factors: therefore, n does not equal 3288. bRecent contact of person with contagious TB. cPerson with baseline tuberculin skin test who has ≥10-mm increase in induration, or positive interferon-γ release assay with a documented negative TB test, within a 2-year period. Abbreviations: 3HP, 12-week regimen of directly observed isoniazid and rifapentine; HAART, highly active antiretroviral therapy; HIV, human immunodeficiency virus; INH, isoniazid; LTBI, latent tuberculosis; TB, tuberculosis.
Of the 3288 patients who could have completed treatment, 2928 (89.1%) were aged 18–64 years and 1994 (60.6%) had been born in the United States. Demographic characteristics of the patient cohort are presented in Table 1. Children and adolescents aged 2–17 years had the highest treatment completion rates (155/164 [94.5%]), and patients aged ≥65 years had the lowest (154/196 [78.6%]). Twelve children were aged 2–11 years, and 11 completed the regimen. Eight of 155 (5.2%) children aged 12–17 years discontinued treatment: 4 had ADRs; 1 was lost to follow-up; 1 refused DOT and requested self-administered therapy but did not return for follow-up; and 2 were siblings whose parents disallowed further treatment.
Characteristics Among Patients Started on 12 Weeks of Directly Observed Isoniazid and Rifapentine (N = 3288)
| Characteristic . | Total No. (%) . | Completed 3HP, No. (%) . |
|---|---|---|
| Sex | ||
| Male | 1759 (53.5) | 1550 (88.1) |
| Female | 1529 (46.5) | 1317 (86.1) |
| Age, y | ||
| 2–17 | 164 (5.0) | 155 (94.5) |
| 18–30 | 1034 (31.5) | 921 (89.1) |
| 31–44 | 953 (29.0) | 834 (87.5) |
| 45–64 | 941 (28.6) | 803 (85.3) |
| ≥65 | 196 (6.0) | 154 (78.6) |
| Race/ethnicity | ||
| Hispanic | 751 (22.8) | 682 (90.8) |
| Non-Hispanic white | 721 (21.9) | 595 (82.5) |
| Non-Hispanic black | 1195 (36.3) | 1035 (86.6) |
| Asian | 540 (16.4) | 481 (89.1) |
| Other a | 81 (2.5) | 74 (91.4) |
| Country of birth | ||
| United States | 1994 (60.6) | 1699 (85.2) |
| Foreign-born | 1294 (39.4) | 1168 (90.3) |
| Treatment reasonb | ||
| Contactc | 821 (25.0) | 751 (91.5) |
| Converterd | 800 (24.3) | 670 (83.8) |
| Corrections during last 12 mo | 516 (15.7) | 451 (87.4) |
| Homeless during last 12 mo | 181 (5.5) | 147 (81.2) |
| Foreign-born | 1294 (39.4) | 1168 (90.3) |
| Refugee | 132 (4.0) | 113 (85.6) |
| Healthcare worker | 500 (15.2) | 416 (83.2) |
| Studente | 130 (4.0) | 123 (94.6) |
| Employment | 211 (6.4) | 180 (85.3) |
| Long-term-care resident | 47 (1.4) | 41 (87.2) |
| Any population riskf | 1534 (46.7) | 1315 (85.7) |
| Medical conditionsg | ||
| Diabetes | 176 (7.4) | 147 (83.5) |
| Chronic renal disease | 30 (1.3) | 24 (80.0) |
| Immunocompromised | 91 (3.8) | 82 (90.1) |
| Hepatitis | 58 (2.4) | 45 (77.6) |
| Chronic lung disease | 78 (3.3) | 59 (75.6) |
| Mental health problems | 127 (5.3) | 99 (78.0) |
| Hypertension | 304 (12.7) | 260 (85.5) |
| Other medical conditionh | 572 (23.9) | 482 (84.3) |
| Any medical conditioni | 775 (32.4) | 649 (83.7) |
| Behavioral risk factorsf | ||
| Alcohol use | 211 (8.8) | 176 (83.4) |
| Current or past smoker | 534 (22.4) | 433 (81.1) |
| Injection drug use | 23 (1.0) | 19 (82.6) |
| Noninjection drug use | 157 (6.6) | 129 (82.2) |
| Any substance use riskj | 660 (27.6) | 542 (82.1) |
| Characteristic . | Total No. (%) . | Completed 3HP, No. (%) . |
|---|---|---|
| Sex | ||
| Male | 1759 (53.5) | 1550 (88.1) |
| Female | 1529 (46.5) | 1317 (86.1) |
| Age, y | ||
| 2–17 | 164 (5.0) | 155 (94.5) |
| 18–30 | 1034 (31.5) | 921 (89.1) |
| 31–44 | 953 (29.0) | 834 (87.5) |
| 45–64 | 941 (28.6) | 803 (85.3) |
| ≥65 | 196 (6.0) | 154 (78.6) |
| Race/ethnicity | ||
| Hispanic | 751 (22.8) | 682 (90.8) |
| Non-Hispanic white | 721 (21.9) | 595 (82.5) |
| Non-Hispanic black | 1195 (36.3) | 1035 (86.6) |
| Asian | 540 (16.4) | 481 (89.1) |
| Other a | 81 (2.5) | 74 (91.4) |
| Country of birth | ||
| United States | 1994 (60.6) | 1699 (85.2) |
| Foreign-born | 1294 (39.4) | 1168 (90.3) |
| Treatment reasonb | ||
| Contactc | 821 (25.0) | 751 (91.5) |
| Converterd | 800 (24.3) | 670 (83.8) |
| Corrections during last 12 mo | 516 (15.7) | 451 (87.4) |
| Homeless during last 12 mo | 181 (5.5) | 147 (81.2) |
| Foreign-born | 1294 (39.4) | 1168 (90.3) |
| Refugee | 132 (4.0) | 113 (85.6) |
| Healthcare worker | 500 (15.2) | 416 (83.2) |
| Studente | 130 (4.0) | 123 (94.6) |
| Employment | 211 (6.4) | 180 (85.3) |
| Long-term-care resident | 47 (1.4) | 41 (87.2) |
| Any population riskf | 1534 (46.7) | 1315 (85.7) |
| Medical conditionsg | ||
| Diabetes | 176 (7.4) | 147 (83.5) |
| Chronic renal disease | 30 (1.3) | 24 (80.0) |
| Immunocompromised | 91 (3.8) | 82 (90.1) |
| Hepatitis | 58 (2.4) | 45 (77.6) |
| Chronic lung disease | 78 (3.3) | 59 (75.6) |
| Mental health problems | 127 (5.3) | 99 (78.0) |
| Hypertension | 304 (12.7) | 260 (85.5) |
| Other medical conditionh | 572 (23.9) | 482 (84.3) |
| Any medical conditioni | 775 (32.4) | 649 (83.7) |
| Behavioral risk factorsf | ||
| Alcohol use | 211 (8.8) | 176 (83.4) |
| Current or past smoker | 534 (22.4) | 433 (81.1) |
| Injection drug use | 23 (1.0) | 19 (82.6) |
| Noninjection drug use | 157 (6.6) | 129 (82.2) |
| Any substance use riskj | 660 (27.6) | 542 (82.1) |
Abbreviation: 3HP, 3-month regimen of isoniazid and rifapentine.
aOther race/ethnicity includes American Indian or Alaska Native, Native Hawaiian or other Pacific Islander, biracial, and unknown.
bTreatment reasons per local policy are dichotomous factors.
cRecent contact of person with infectious tuberculosis (TB).
dPerson with baseline tuberculin skin test who has ≥10-mm increase in induration, or positive interferon-γ release assay with a documented negative TB test, within a 2-year period.
eMost students were of foreign birth from high-prevalence countries, or were contacts or converters; 11 patients not identified as foreign-born had no treatment indication other than student status.
fAny population risk was defined as being a migrant worker, healthcare worker, employee or resident in TB-risk setting (eg, homeless shelter or correctional facility), or resident in a long-term-care facility.
gMedical conditions and behavioral risks are dichotomous factors reported for a subgroup of 2389 patients.
hOther medical condition was defined as a patient reporting any medical condition other than diabetes, chronic renal disease, immunocompromised, hepatitis, chronic lung disease, mental health problems, or hypertension.
iAny medical condition was defined as a patient reporting diabetes, chronic renal disease, immunocompromised, hepatitis, chronic lung disease, mental health problems, hypertension, and any other medical condition.
jAny substance use was defined as a patient reporting current or past history of smoking, use of alcohol, injection drug use, or noninjection drug use.
Characteristics Among Patients Started on 12 Weeks of Directly Observed Isoniazid and Rifapentine (N = 3288)
| Characteristic . | Total No. (%) . | Completed 3HP, No. (%) . |
|---|---|---|
| Sex | ||
| Male | 1759 (53.5) | 1550 (88.1) |
| Female | 1529 (46.5) | 1317 (86.1) |
| Age, y | ||
| 2–17 | 164 (5.0) | 155 (94.5) |
| 18–30 | 1034 (31.5) | 921 (89.1) |
| 31–44 | 953 (29.0) | 834 (87.5) |
| 45–64 | 941 (28.6) | 803 (85.3) |
| ≥65 | 196 (6.0) | 154 (78.6) |
| Race/ethnicity | ||
| Hispanic | 751 (22.8) | 682 (90.8) |
| Non-Hispanic white | 721 (21.9) | 595 (82.5) |
| Non-Hispanic black | 1195 (36.3) | 1035 (86.6) |
| Asian | 540 (16.4) | 481 (89.1) |
| Other a | 81 (2.5) | 74 (91.4) |
| Country of birth | ||
| United States | 1994 (60.6) | 1699 (85.2) |
| Foreign-born | 1294 (39.4) | 1168 (90.3) |
| Treatment reasonb | ||
| Contactc | 821 (25.0) | 751 (91.5) |
| Converterd | 800 (24.3) | 670 (83.8) |
| Corrections during last 12 mo | 516 (15.7) | 451 (87.4) |
| Homeless during last 12 mo | 181 (5.5) | 147 (81.2) |
| Foreign-born | 1294 (39.4) | 1168 (90.3) |
| Refugee | 132 (4.0) | 113 (85.6) |
| Healthcare worker | 500 (15.2) | 416 (83.2) |
| Studente | 130 (4.0) | 123 (94.6) |
| Employment | 211 (6.4) | 180 (85.3) |
| Long-term-care resident | 47 (1.4) | 41 (87.2) |
| Any population riskf | 1534 (46.7) | 1315 (85.7) |
| Medical conditionsg | ||
| Diabetes | 176 (7.4) | 147 (83.5) |
| Chronic renal disease | 30 (1.3) | 24 (80.0) |
| Immunocompromised | 91 (3.8) | 82 (90.1) |
| Hepatitis | 58 (2.4) | 45 (77.6) |
| Chronic lung disease | 78 (3.3) | 59 (75.6) |
| Mental health problems | 127 (5.3) | 99 (78.0) |
| Hypertension | 304 (12.7) | 260 (85.5) |
| Other medical conditionh | 572 (23.9) | 482 (84.3) |
| Any medical conditioni | 775 (32.4) | 649 (83.7) |
| Behavioral risk factorsf | ||
| Alcohol use | 211 (8.8) | 176 (83.4) |
| Current or past smoker | 534 (22.4) | 433 (81.1) |
| Injection drug use | 23 (1.0) | 19 (82.6) |
| Noninjection drug use | 157 (6.6) | 129 (82.2) |
| Any substance use riskj | 660 (27.6) | 542 (82.1) |
| Characteristic . | Total No. (%) . | Completed 3HP, No. (%) . |
|---|---|---|
| Sex | ||
| Male | 1759 (53.5) | 1550 (88.1) |
| Female | 1529 (46.5) | 1317 (86.1) |
| Age, y | ||
| 2–17 | 164 (5.0) | 155 (94.5) |
| 18–30 | 1034 (31.5) | 921 (89.1) |
| 31–44 | 953 (29.0) | 834 (87.5) |
| 45–64 | 941 (28.6) | 803 (85.3) |
| ≥65 | 196 (6.0) | 154 (78.6) |
| Race/ethnicity | ||
| Hispanic | 751 (22.8) | 682 (90.8) |
| Non-Hispanic white | 721 (21.9) | 595 (82.5) |
| Non-Hispanic black | 1195 (36.3) | 1035 (86.6) |
| Asian | 540 (16.4) | 481 (89.1) |
| Other a | 81 (2.5) | 74 (91.4) |
| Country of birth | ||
| United States | 1994 (60.6) | 1699 (85.2) |
| Foreign-born | 1294 (39.4) | 1168 (90.3) |
| Treatment reasonb | ||
| Contactc | 821 (25.0) | 751 (91.5) |
| Converterd | 800 (24.3) | 670 (83.8) |
| Corrections during last 12 mo | 516 (15.7) | 451 (87.4) |
| Homeless during last 12 mo | 181 (5.5) | 147 (81.2) |
| Foreign-born | 1294 (39.4) | 1168 (90.3) |
| Refugee | 132 (4.0) | 113 (85.6) |
| Healthcare worker | 500 (15.2) | 416 (83.2) |
| Studente | 130 (4.0) | 123 (94.6) |
| Employment | 211 (6.4) | 180 (85.3) |
| Long-term-care resident | 47 (1.4) | 41 (87.2) |
| Any population riskf | 1534 (46.7) | 1315 (85.7) |
| Medical conditionsg | ||
| Diabetes | 176 (7.4) | 147 (83.5) |
| Chronic renal disease | 30 (1.3) | 24 (80.0) |
| Immunocompromised | 91 (3.8) | 82 (90.1) |
| Hepatitis | 58 (2.4) | 45 (77.6) |
| Chronic lung disease | 78 (3.3) | 59 (75.6) |
| Mental health problems | 127 (5.3) | 99 (78.0) |
| Hypertension | 304 (12.7) | 260 (85.5) |
| Other medical conditionh | 572 (23.9) | 482 (84.3) |
| Any medical conditioni | 775 (32.4) | 649 (83.7) |
| Behavioral risk factorsf | ||
| Alcohol use | 211 (8.8) | 176 (83.4) |
| Current or past smoker | 534 (22.4) | 433 (81.1) |
| Injection drug use | 23 (1.0) | 19 (82.6) |
| Noninjection drug use | 157 (6.6) | 129 (82.2) |
| Any substance use riskj | 660 (27.6) | 542 (82.1) |
Abbreviation: 3HP, 3-month regimen of isoniazid and rifapentine.
aOther race/ethnicity includes American Indian or Alaska Native, Native Hawaiian or other Pacific Islander, biracial, and unknown.
bTreatment reasons per local policy are dichotomous factors.
cRecent contact of person with infectious tuberculosis (TB).
dPerson with baseline tuberculin skin test who has ≥10-mm increase in induration, or positive interferon-γ release assay with a documented negative TB test, within a 2-year period.
eMost students were of foreign birth from high-prevalence countries, or were contacts or converters; 11 patients not identified as foreign-born had no treatment indication other than student status.
fAny population risk was defined as being a migrant worker, healthcare worker, employee or resident in TB-risk setting (eg, homeless shelter or correctional facility), or resident in a long-term-care facility.
gMedical conditions and behavioral risks are dichotomous factors reported for a subgroup of 2389 patients.
hOther medical condition was defined as a patient reporting any medical condition other than diabetes, chronic renal disease, immunocompromised, hepatitis, chronic lung disease, mental health problems, or hypertension.
iAny medical condition was defined as a patient reporting diabetes, chronic renal disease, immunocompromised, hepatitis, chronic lung disease, mental health problems, hypertension, and any other medical condition.
jAny substance use was defined as a patient reporting current or past history of smoking, use of alcohol, injection drug use, or noninjection drug use.
Hispanic patients had the highest rate of treatment completion, and non-Hispanic white patients had the lowest (90.8% vs 82.5%). Treatment completion was higher for foreign-born patients than for US-born patients (90.3% vs 85.2%). Patients with diabetes mellitus comprised 7.4% (176/2389) of the patient subgroup with medical conditions, and 83.5% completed treatment.
Approximately one-third (1174/3288 [35.7%]) of patients reported ≥1 ADR during treatment. Among all patients who reported any reaction temporally associated with taking a 3HP dose, the most common reactions were nausea, fatigue, sore muscles, headache, fever or chills, dizziness, and abdominal pain (Figure 2). Of patients reporting ADRs, 246 (21%) discontinued treatment; of those patients, 3HP was most frequently stopped after the third dose. Among patients who discontinued treatment (n = 246), the most frequently reported reactions were nausea, fever or chills, fatigue, sore muscles, rash or hives, dizziness, and headache. Of the 3327 who began 3HP, 26 (0.8%) were hospitalized and 19 (0.6%) patients were examined in emergency departments during their treatment course. No deaths or long-term sequela were reported.
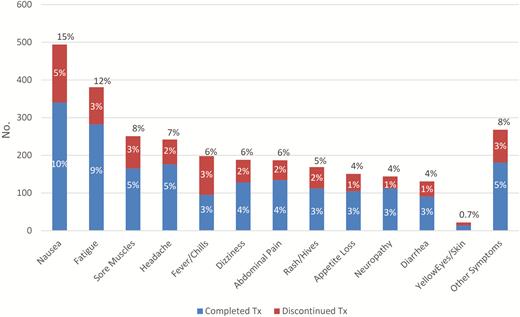
Number and percentage of patients with medication reactions after any 3HP dose (n = 1174). Patients could report ≥1 reaction, after ≥1 dose. Other symptoms reported by ≥1 patient includes dermatological-related symptoms, gastrointestinal symptoms, cough, mental health symptoms, weight loss, blurred vision, flu-like symptoms, breathing issues, back pain, gynecologic symptoms, chest discomfort, diaphoresis, angioedema, bleeding, palpitations, easy bruising, swelling, neurologic symptoms, hypotension/near syncope and syncope, flushing, red eyes, upper respiratory infection, genitourinary symptoms, other eye symptoms, black stool, pain, pneumonia, and sepsis. Abbreviations: Tx, treatment; 3HP, 3-month regimen of isoniazid and rifapentine.
Most patients (2793/3288 [85%]) completed treatment without interruption. Among patients with treatment interruption, 14.9% (74/495) temporarily halted but subsequently completed; 23.2% (115/495) discontinued 3HP and were reported to have switched to an alternative regimen; 26.5% (131/495) of patients discontinued 3HP and stopped all LTBI treatment; and 35.4% (175/495) of patients were lost to follow-up (Supplementary Table 2).
Factors Associated With Treatment Discontinuation
In univariable analysis (Tables 2 and 3), the following characteristics were significantly associated with increased likelihood of discontinuation of 3HP treatment: age ≥65 years, non-Hispanic white race/ethnicity, recent converter (ie, person with baseline tuberculin skin test who has ≥10-mm increase in induration, or positive interferon-γ release assay with a documented previous negative TB test, within the past 2 years), homelessness in the 12 months before treatment, healthcare worker, or having any population risk factor. Patient factors significantly associated with lower likelihood of discontinuation of 3HP treatment included the following: age 2–17 years, being the contact of a TB case, foreign birth, or being a student.
Univariable and Multivariable Analysis of Factors Associated With Discontinuation of 12 Weeks Directly Observed Isoniazid and Rifapentine (N = 3288)
| Factor . | Discontinued No. (%) . | Univariable Analysisa RR (95% CI)b . | P Value . | Multivariable Analysisc,d ARR (95% CI)b . | P Value . |
|---|---|---|---|---|---|
| Sex | |||||
| Male | 208 (11.8) | 0.85 (.71–1.02) | .074 | 0.82 (.69–.99) | .041 |
| Female | 213 (13.9) | Reference | |||
| Age | 421 (12.8) | 1.01 (1.01–1.02) | <.001 | 1.01 (1.01–1.02) | <.001 |
| Age categories, y | |||||
| 2–17 | 9 (5.5) | 0.44 (.23–.85) | .014 | Not done | |
| 18–30 | 113 (10.9) | 0.88 (.69–1.11) | .280 | Not done | |
| 31–44 | 119 (12.5) | Reference | Not done | ||
| 45–64 | 138 (14.7) | 1.17 (.94–1.48) | .167 | Not done | |
| ≥65 | 42 (21.4) | 1.72 (1.25–2.35) | <.001 | Not done | |
| Race/ethnicitye | |||||
| Hispanic | 69 (9.2) | 0.84 (.61–1.17) | .307 | 0.89 (.63–1.26) | .519 |
| Non-Hispanic white | 126 (17.5) | 1.60 (1.20–2.13) | .001 | 1.22 (.90–1.66) | .199 |
| Non-Hispanic black | 160 (13.4) | 1.23 (.93–1.63) | .141 | 0.88 (.65–1.88) | .396 |
| Asian | 59 (10.9) | Reference | Reference | ||
| Otherf | 7 (8.6) | 0.79 (.37–1.67) | .715 | 0.72 (.34–1.53) | .396 |
| Treatment reasong | |||||
| Contact | 70 (8.5) | 0.60 (.47–.76) | <.001 | 0.68 (.52–.89) | .005 |
| Converter | 130 (16.3) | 1.39 (1.15–1.68) | <.001 | ||
| Corrections within past year | 65 (12.6) | 0.98 (.77–1.26) | .878 | 1.43 (1.08–1.89) | .013 |
| Homeless within past year | 34 (18.8) | 1.51 (1.10–2.07) | .011 | 1.72 (1.25–2.39) | .001 |
| Foreign-born | 126 (9.7) | 0.66 (.54–.80) | <.001 | ||
| Refugee | 19 (14.4) | 1.13 (.74–1.73) | .574 | ||
| Healthcare worker | 84 (16.8) | 1.39 (1.12–1.73) | .003 | ||
| Studenth | 7 (5.4) | 0.41 (.20–.85) | .016 | 0.45 (.21–.98) | .044 |
| Employment | 31 (15.0) | 1.16 (.83–1.63) | .392 | ||
| Long-term-care resident | 6 (12.8) | 1.00 (.47–2.12) | .994 | ||
| Any population riski | 219 (14.3) | 1.24 (1.04–1.48) | .018 | ||
| Factor . | Discontinued No. (%) . | Univariable Analysisa RR (95% CI)b . | P Value . | Multivariable Analysisc,d ARR (95% CI)b . | P Value . |
|---|---|---|---|---|---|
| Sex | |||||
| Male | 208 (11.8) | 0.85 (.71–1.02) | .074 | 0.82 (.69–.99) | .041 |
| Female | 213 (13.9) | Reference | |||
| Age | 421 (12.8) | 1.01 (1.01–1.02) | <.001 | 1.01 (1.01–1.02) | <.001 |
| Age categories, y | |||||
| 2–17 | 9 (5.5) | 0.44 (.23–.85) | .014 | Not done | |
| 18–30 | 113 (10.9) | 0.88 (.69–1.11) | .280 | Not done | |
| 31–44 | 119 (12.5) | Reference | Not done | ||
| 45–64 | 138 (14.7) | 1.17 (.94–1.48) | .167 | Not done | |
| ≥65 | 42 (21.4) | 1.72 (1.25–2.35) | <.001 | Not done | |
| Race/ethnicitye | |||||
| Hispanic | 69 (9.2) | 0.84 (.61–1.17) | .307 | 0.89 (.63–1.26) | .519 |
| Non-Hispanic white | 126 (17.5) | 1.60 (1.20–2.13) | .001 | 1.22 (.90–1.66) | .199 |
| Non-Hispanic black | 160 (13.4) | 1.23 (.93–1.63) | .141 | 0.88 (.65–1.88) | .396 |
| Asian | 59 (10.9) | Reference | Reference | ||
| Otherf | 7 (8.6) | 0.79 (.37–1.67) | .715 | 0.72 (.34–1.53) | .396 |
| Treatment reasong | |||||
| Contact | 70 (8.5) | 0.60 (.47–.76) | <.001 | 0.68 (.52–.89) | .005 |
| Converter | 130 (16.3) | 1.39 (1.15–1.68) | <.001 | ||
| Corrections within past year | 65 (12.6) | 0.98 (.77–1.26) | .878 | 1.43 (1.08–1.89) | .013 |
| Homeless within past year | 34 (18.8) | 1.51 (1.10–2.07) | .011 | 1.72 (1.25–2.39) | .001 |
| Foreign-born | 126 (9.7) | 0.66 (.54–.80) | <.001 | ||
| Refugee | 19 (14.4) | 1.13 (.74–1.73) | .574 | ||
| Healthcare worker | 84 (16.8) | 1.39 (1.12–1.73) | .003 | ||
| Studenth | 7 (5.4) | 0.41 (.20–.85) | .016 | 0.45 (.21–.98) | .044 |
| Employment | 31 (15.0) | 1.16 (.83–1.63) | .392 | ||
| Long-term-care resident | 6 (12.8) | 1.00 (.47–2.12) | .994 | ||
| Any population riski | 219 (14.3) | 1.24 (1.04–1.48) | .018 | ||
Abbreviations: ARR, adjusted relative risk; CI, confidence interval; RR, relative risk.
aUnivariable analyses test the association between the indicated factor and discontinuation of the 12-week regimen of isoniazid and rifapentine (3HP).
bBolded confidence intervals indicate a statistically significant association at P ≤ .05.
cMultivariable analyses for demographic factors and treatment reasons show the association between the indicated factor and 3HP discontinuation while controlling for all other factors listed in the model and the potential effect of the site; treats age as continuous.
dAll covariates were put in the model at the beginning of the backward elimination procedure; multivariable ARRs with P > .1 are not reported.
eRace/ethnicity was overall P < .1.
f Other race/ethnicity includes American Indian or Alaska Native, Native Hawaiian or other Pacific Islander, biracial, or unknown.
gTreatment reasons per local policy are dichotomous factors.
hMost students were of foreign birth from high-prevalence countries, or were contacts or converters; 11 patients not identified as foreign-born had no treatment indication other than student status.
iAny population risk was defined as being a migrant worker, healthcare worker, employee or resident in tuberculosis-risk setting (eg, homeless shelter or correctional facility), or resident in a long-term-care facility.
Univariable and Multivariable Analysis of Factors Associated With Discontinuation of 12 Weeks Directly Observed Isoniazid and Rifapentine (N = 3288)
| Factor . | Discontinued No. (%) . | Univariable Analysisa RR (95% CI)b . | P Value . | Multivariable Analysisc,d ARR (95% CI)b . | P Value . |
|---|---|---|---|---|---|
| Sex | |||||
| Male | 208 (11.8) | 0.85 (.71–1.02) | .074 | 0.82 (.69–.99) | .041 |
| Female | 213 (13.9) | Reference | |||
| Age | 421 (12.8) | 1.01 (1.01–1.02) | <.001 | 1.01 (1.01–1.02) | <.001 |
| Age categories, y | |||||
| 2–17 | 9 (5.5) | 0.44 (.23–.85) | .014 | Not done | |
| 18–30 | 113 (10.9) | 0.88 (.69–1.11) | .280 | Not done | |
| 31–44 | 119 (12.5) | Reference | Not done | ||
| 45–64 | 138 (14.7) | 1.17 (.94–1.48) | .167 | Not done | |
| ≥65 | 42 (21.4) | 1.72 (1.25–2.35) | <.001 | Not done | |
| Race/ethnicitye | |||||
| Hispanic | 69 (9.2) | 0.84 (.61–1.17) | .307 | 0.89 (.63–1.26) | .519 |
| Non-Hispanic white | 126 (17.5) | 1.60 (1.20–2.13) | .001 | 1.22 (.90–1.66) | .199 |
| Non-Hispanic black | 160 (13.4) | 1.23 (.93–1.63) | .141 | 0.88 (.65–1.88) | .396 |
| Asian | 59 (10.9) | Reference | Reference | ||
| Otherf | 7 (8.6) | 0.79 (.37–1.67) | .715 | 0.72 (.34–1.53) | .396 |
| Treatment reasong | |||||
| Contact | 70 (8.5) | 0.60 (.47–.76) | <.001 | 0.68 (.52–.89) | .005 |
| Converter | 130 (16.3) | 1.39 (1.15–1.68) | <.001 | ||
| Corrections within past year | 65 (12.6) | 0.98 (.77–1.26) | .878 | 1.43 (1.08–1.89) | .013 |
| Homeless within past year | 34 (18.8) | 1.51 (1.10–2.07) | .011 | 1.72 (1.25–2.39) | .001 |
| Foreign-born | 126 (9.7) | 0.66 (.54–.80) | <.001 | ||
| Refugee | 19 (14.4) | 1.13 (.74–1.73) | .574 | ||
| Healthcare worker | 84 (16.8) | 1.39 (1.12–1.73) | .003 | ||
| Studenth | 7 (5.4) | 0.41 (.20–.85) | .016 | 0.45 (.21–.98) | .044 |
| Employment | 31 (15.0) | 1.16 (.83–1.63) | .392 | ||
| Long-term-care resident | 6 (12.8) | 1.00 (.47–2.12) | .994 | ||
| Any population riski | 219 (14.3) | 1.24 (1.04–1.48) | .018 | ||
| Factor . | Discontinued No. (%) . | Univariable Analysisa RR (95% CI)b . | P Value . | Multivariable Analysisc,d ARR (95% CI)b . | P Value . |
|---|---|---|---|---|---|
| Sex | |||||
| Male | 208 (11.8) | 0.85 (.71–1.02) | .074 | 0.82 (.69–.99) | .041 |
| Female | 213 (13.9) | Reference | |||
| Age | 421 (12.8) | 1.01 (1.01–1.02) | <.001 | 1.01 (1.01–1.02) | <.001 |
| Age categories, y | |||||
| 2–17 | 9 (5.5) | 0.44 (.23–.85) | .014 | Not done | |
| 18–30 | 113 (10.9) | 0.88 (.69–1.11) | .280 | Not done | |
| 31–44 | 119 (12.5) | Reference | Not done | ||
| 45–64 | 138 (14.7) | 1.17 (.94–1.48) | .167 | Not done | |
| ≥65 | 42 (21.4) | 1.72 (1.25–2.35) | <.001 | Not done | |
| Race/ethnicitye | |||||
| Hispanic | 69 (9.2) | 0.84 (.61–1.17) | .307 | 0.89 (.63–1.26) | .519 |
| Non-Hispanic white | 126 (17.5) | 1.60 (1.20–2.13) | .001 | 1.22 (.90–1.66) | .199 |
| Non-Hispanic black | 160 (13.4) | 1.23 (.93–1.63) | .141 | 0.88 (.65–1.88) | .396 |
| Asian | 59 (10.9) | Reference | Reference | ||
| Otherf | 7 (8.6) | 0.79 (.37–1.67) | .715 | 0.72 (.34–1.53) | .396 |
| Treatment reasong | |||||
| Contact | 70 (8.5) | 0.60 (.47–.76) | <.001 | 0.68 (.52–.89) | .005 |
| Converter | 130 (16.3) | 1.39 (1.15–1.68) | <.001 | ||
| Corrections within past year | 65 (12.6) | 0.98 (.77–1.26) | .878 | 1.43 (1.08–1.89) | .013 |
| Homeless within past year | 34 (18.8) | 1.51 (1.10–2.07) | .011 | 1.72 (1.25–2.39) | .001 |
| Foreign-born | 126 (9.7) | 0.66 (.54–.80) | <.001 | ||
| Refugee | 19 (14.4) | 1.13 (.74–1.73) | .574 | ||
| Healthcare worker | 84 (16.8) | 1.39 (1.12–1.73) | .003 | ||
| Studenth | 7 (5.4) | 0.41 (.20–.85) | .016 | 0.45 (.21–.98) | .044 |
| Employment | 31 (15.0) | 1.16 (.83–1.63) | .392 | ||
| Long-term-care resident | 6 (12.8) | 1.00 (.47–2.12) | .994 | ||
| Any population riski | 219 (14.3) | 1.24 (1.04–1.48) | .018 | ||
Abbreviations: ARR, adjusted relative risk; CI, confidence interval; RR, relative risk.
aUnivariable analyses test the association between the indicated factor and discontinuation of the 12-week regimen of isoniazid and rifapentine (3HP).
bBolded confidence intervals indicate a statistically significant association at P ≤ .05.
cMultivariable analyses for demographic factors and treatment reasons show the association between the indicated factor and 3HP discontinuation while controlling for all other factors listed in the model and the potential effect of the site; treats age as continuous.
dAll covariates were put in the model at the beginning of the backward elimination procedure; multivariable ARRs with P > .1 are not reported.
eRace/ethnicity was overall P < .1.
f Other race/ethnicity includes American Indian or Alaska Native, Native Hawaiian or other Pacific Islander, biracial, or unknown.
gTreatment reasons per local policy are dichotomous factors.
hMost students were of foreign birth from high-prevalence countries, or were contacts or converters; 11 patients not identified as foreign-born had no treatment indication other than student status.
iAny population risk was defined as being a migrant worker, healthcare worker, employee or resident in tuberculosis-risk setting (eg, homeless shelter or correctional facility), or resident in a long-term-care facility.
Subgroup Univariable and Multivariable Analysis of Factors Associated With Discontinuation of 12 Weeks Directly Observed Isoniazid and Rifapentine (n = 2389)
| Factor . | Discontinued No. (%) . | Univariable Analysisa RR (95% CI)b . | P Value . | Multivariable Analysisc,d,e ARR (95% CI)b . | P Value . |
|---|---|---|---|---|---|
| Subgroup analysisf | |||||
| Sex | |||||
| Male | 161 (13.0) | 0.88 (.72–1.08) | .219 | 0.82 (.67–1.01) | .062 |
| Female | 170 (14.8) | Reference | |||
| Age | 331 (13.9) | 1.02 (1.01–1.02) | <.001 | 1.01 (1.01–1.02) | <.001 |
| Age categories, y | |||||
| 2–17 | 8 (8.0) | 0.57 (.29–1.14) | .112 | Not done | |
| 18–30 | 80 (10.6) | 0.76 (.57–1.00) | .051 | Not done | |
| 31–44 | 92 (14.0) | Reference | Not done | ||
| 45–64 | 111 (15.6) | 1.11 (.86–1.44) | .404 | Not done | |
| ≥65 | 40 (24.2) | 1.73 (1.25–2.41) | ≤.001 | Not done | |
| Race/ethnicityg | |||||
| Hispanic | 49 (10.4) | 0.97 (.66–1.41) | .857 | 0.84 (.57–1.24) | .382 |
| Non-Hispanic white | 114 (19.5) | 1.81 (1.32–2.49) | ≤.001 | 1.25 (.89–1.75) | .201 |
| Non-Hispanic black | 117 (13.9) | 1.29 (.94–1.78) | .120 | 0.83 (.58–1.19) | .317 |
| Asian | 46 (10.8) | Reference | Reference | ||
| Otherh | 5 (7.8) | 0.73 (.30–1.76) | .477 | 0.64 (.26–1.54) | .315 |
| Treatment reasoni | |||||
| Contact | 52 (8.3) | 0.53 (.40–.70) | ≤.001 | 0.64 (.47–.88) | .006 |
| Converter | 112 (16.7) | 1.31 (1.06–1.61) | .012 | ||
| Corrections within past year | 42 (18.2) | 1.36 (1.01–1.82) | .041 | 1.36 (.99–1.87) | .055 |
| Homeless within past year | 32 (27.4) | 2.08 (1.52–2.84) | <.001 | 1.80 (1.30–2.50) | <.001 |
| Foreign-born | 85 (10.3) | 0.65 (.52–.82) | <.001 | ||
| Refugee | 4 (20.0) | 1.45 (.60–3.50) | .410 | ||
| Healthcare worker | 76 (16.0) | 1.20 (.95–1.52) | .133 | ||
| Studentj | 6 (4.7) | 0.33 (.15–.72) | .005 | 0.39 (.17–.90) | .027 |
| Employment | 30 (14.5) | 1.05 (.74–1.49) | .780 | ||
| Long-term-care resident | 6 (12.8) | 0.92 (.43–1.96) | .828 | ||
| Any population riskk | 184 (7.7) | 1.34 (1.09–1.63) | .005 | ||
| Medical conditionsf,i | |||||
| Diabetes | 29 (16.5) | 1.21 (.85–1.71) | .290 | ||
| Chronic renal disease | 6 (20.0) | 1.45 (.70–2.99) | .312 | ||
| Immunocompromised | 9 (9.9) | 0.71 (.38–1.32) | .277 | ||
| Hepatitis | 13 (22.4) | 1.64 (1.01–2.68) | .047 | ||
| Chronic lung disease | 19 (24.4) | 1.80 (1.20–2.70) | .004 | ||
| Mental health problems | 28 (22.0) | 1.65 (1.17–2.32) | .005 | ||
| Hypertension | 44 (14.5) | 1.05 (.78–1.41) | .738 | ||
| Other medical conditionl | 90 (15.7) | 1.19 (.95–1.48) | .134 | ||
| Any medical conditionm | 126 (16.3) | 1.28 (1.04–1.57) | .018 | ||
| Behavioral risk factorsf,i | |||||
| Alcohol use | 35 (16.6) | 1.22 (.89–1.68) | .223 | ||
| Current or past smoker | 101 (18.9) | 1.53 (1.23–1.89) | <.001 | 1.41 (1.14–1.75) | .002 |
| IDU drug use | 4 (17.4) | 1.26 (.51–3.08) | .615 | ||
| Non-IDU drug use | 28 (17.8) | 1.31 (.92–1.87) | .128 | ||
| Any substance riskn | 118 (17.9) | 1.45 (1.18–1.78) | <.001 | ||
| Factor . | Discontinued No. (%) . | Univariable Analysisa RR (95% CI)b . | P Value . | Multivariable Analysisc,d,e ARR (95% CI)b . | P Value . |
|---|---|---|---|---|---|
| Subgroup analysisf | |||||
| Sex | |||||
| Male | 161 (13.0) | 0.88 (.72–1.08) | .219 | 0.82 (.67–1.01) | .062 |
| Female | 170 (14.8) | Reference | |||
| Age | 331 (13.9) | 1.02 (1.01–1.02) | <.001 | 1.01 (1.01–1.02) | <.001 |
| Age categories, y | |||||
| 2–17 | 8 (8.0) | 0.57 (.29–1.14) | .112 | Not done | |
| 18–30 | 80 (10.6) | 0.76 (.57–1.00) | .051 | Not done | |
| 31–44 | 92 (14.0) | Reference | Not done | ||
| 45–64 | 111 (15.6) | 1.11 (.86–1.44) | .404 | Not done | |
| ≥65 | 40 (24.2) | 1.73 (1.25–2.41) | ≤.001 | Not done | |
| Race/ethnicityg | |||||
| Hispanic | 49 (10.4) | 0.97 (.66–1.41) | .857 | 0.84 (.57–1.24) | .382 |
| Non-Hispanic white | 114 (19.5) | 1.81 (1.32–2.49) | ≤.001 | 1.25 (.89–1.75) | .201 |
| Non-Hispanic black | 117 (13.9) | 1.29 (.94–1.78) | .120 | 0.83 (.58–1.19) | .317 |
| Asian | 46 (10.8) | Reference | Reference | ||
| Otherh | 5 (7.8) | 0.73 (.30–1.76) | .477 | 0.64 (.26–1.54) | .315 |
| Treatment reasoni | |||||
| Contact | 52 (8.3) | 0.53 (.40–.70) | ≤.001 | 0.64 (.47–.88) | .006 |
| Converter | 112 (16.7) | 1.31 (1.06–1.61) | .012 | ||
| Corrections within past year | 42 (18.2) | 1.36 (1.01–1.82) | .041 | 1.36 (.99–1.87) | .055 |
| Homeless within past year | 32 (27.4) | 2.08 (1.52–2.84) | <.001 | 1.80 (1.30–2.50) | <.001 |
| Foreign-born | 85 (10.3) | 0.65 (.52–.82) | <.001 | ||
| Refugee | 4 (20.0) | 1.45 (.60–3.50) | .410 | ||
| Healthcare worker | 76 (16.0) | 1.20 (.95–1.52) | .133 | ||
| Studentj | 6 (4.7) | 0.33 (.15–.72) | .005 | 0.39 (.17–.90) | .027 |
| Employment | 30 (14.5) | 1.05 (.74–1.49) | .780 | ||
| Long-term-care resident | 6 (12.8) | 0.92 (.43–1.96) | .828 | ||
| Any population riskk | 184 (7.7) | 1.34 (1.09–1.63) | .005 | ||
| Medical conditionsf,i | |||||
| Diabetes | 29 (16.5) | 1.21 (.85–1.71) | .290 | ||
| Chronic renal disease | 6 (20.0) | 1.45 (.70–2.99) | .312 | ||
| Immunocompromised | 9 (9.9) | 0.71 (.38–1.32) | .277 | ||
| Hepatitis | 13 (22.4) | 1.64 (1.01–2.68) | .047 | ||
| Chronic lung disease | 19 (24.4) | 1.80 (1.20–2.70) | .004 | ||
| Mental health problems | 28 (22.0) | 1.65 (1.17–2.32) | .005 | ||
| Hypertension | 44 (14.5) | 1.05 (.78–1.41) | .738 | ||
| Other medical conditionl | 90 (15.7) | 1.19 (.95–1.48) | .134 | ||
| Any medical conditionm | 126 (16.3) | 1.28 (1.04–1.57) | .018 | ||
| Behavioral risk factorsf,i | |||||
| Alcohol use | 35 (16.6) | 1.22 (.89–1.68) | .223 | ||
| Current or past smoker | 101 (18.9) | 1.53 (1.23–1.89) | <.001 | 1.41 (1.14–1.75) | .002 |
| IDU drug use | 4 (17.4) | 1.26 (.51–3.08) | .615 | ||
| Non-IDU drug use | 28 (17.8) | 1.31 (.92–1.87) | .128 | ||
| Any substance riskn | 118 (17.9) | 1.45 (1.18–1.78) | <.001 | ||
Abbreviations: ARR, adjusted relative risk; CI, confidence interval; IDU, intravenous drug use; RR, relative risk.
aUnivariable analyses test the association between the indicated factor and discontinuation of the 12-week regimen of isoniazid and rifapentine (3HP).
bBolded confidence intervals indicate a statistically significant association at P ≤ .05.
cMultivariable analyses for demographic factors and treatment reasons show the association between the indicated factor and 3HP discontinuation while controlling for all other factors listed in the model and the potential effect of the site; treats age as continuous.
dAll covariates were put in the model at the beginning of the backward elimination procedure; multivariable ARRs with P > .1 are not reported.
eMultivariable analyses for medical conditions and behavioral risk factors show the association between the indicated factor and 3HP discontinuation while controlling for age as a continuous variable.
fAnalysis includes only patients from the standard and comprehensive tiers including medical conditions and behavioral risk factor data (n = 2389); interaction and corresponding main effects terms are kept.
gRace/ethnicity was overall P < .1.
hOther race/ethnicity includes American Indian or Alaska Native, Native Hawaiian or other Pacific Islander, biracial, or unknown.
iTreatment reasons per local policy, medical conditions, and behavioral risks are dichotomous factors.
jMost students were of foreign birth from high-prevalence countries, or were contacts or converters; 11 patients not identified as foreign-born had no treatment indication other than student status.
kAny population risk was defined as being a migrant worker, healthcare worker, employee or resident in tuberculosis-risk setting (eg, homeless shelter or correctional facility), or resident in a long-term-care facility.
lOther medical condition was defined as a patient reporting any medical condition other than diabetes, chronic renal disease, immunocompromised, hepatitis, chronic lung disease, mental health problems, or hypertension.
mAny medical condition was defined as a patient reporting diabetes, chronic renal disease, immunocompromised, hepatitis, chronic lung disease, mental health problems, hypertension, and any other medical condition.
nAny substance use risk was defined as a patient reporting current or past history of smoking, use of alcohol, injection drug use, or noninjection drug use.
Subgroup Univariable and Multivariable Analysis of Factors Associated With Discontinuation of 12 Weeks Directly Observed Isoniazid and Rifapentine (n = 2389)
| Factor . | Discontinued No. (%) . | Univariable Analysisa RR (95% CI)b . | P Value . | Multivariable Analysisc,d,e ARR (95% CI)b . | P Value . |
|---|---|---|---|---|---|
| Subgroup analysisf | |||||
| Sex | |||||
| Male | 161 (13.0) | 0.88 (.72–1.08) | .219 | 0.82 (.67–1.01) | .062 |
| Female | 170 (14.8) | Reference | |||
| Age | 331 (13.9) | 1.02 (1.01–1.02) | <.001 | 1.01 (1.01–1.02) | <.001 |
| Age categories, y | |||||
| 2–17 | 8 (8.0) | 0.57 (.29–1.14) | .112 | Not done | |
| 18–30 | 80 (10.6) | 0.76 (.57–1.00) | .051 | Not done | |
| 31–44 | 92 (14.0) | Reference | Not done | ||
| 45–64 | 111 (15.6) | 1.11 (.86–1.44) | .404 | Not done | |
| ≥65 | 40 (24.2) | 1.73 (1.25–2.41) | ≤.001 | Not done | |
| Race/ethnicityg | |||||
| Hispanic | 49 (10.4) | 0.97 (.66–1.41) | .857 | 0.84 (.57–1.24) | .382 |
| Non-Hispanic white | 114 (19.5) | 1.81 (1.32–2.49) | ≤.001 | 1.25 (.89–1.75) | .201 |
| Non-Hispanic black | 117 (13.9) | 1.29 (.94–1.78) | .120 | 0.83 (.58–1.19) | .317 |
| Asian | 46 (10.8) | Reference | Reference | ||
| Otherh | 5 (7.8) | 0.73 (.30–1.76) | .477 | 0.64 (.26–1.54) | .315 |
| Treatment reasoni | |||||
| Contact | 52 (8.3) | 0.53 (.40–.70) | ≤.001 | 0.64 (.47–.88) | .006 |
| Converter | 112 (16.7) | 1.31 (1.06–1.61) | .012 | ||
| Corrections within past year | 42 (18.2) | 1.36 (1.01–1.82) | .041 | 1.36 (.99–1.87) | .055 |
| Homeless within past year | 32 (27.4) | 2.08 (1.52–2.84) | <.001 | 1.80 (1.30–2.50) | <.001 |
| Foreign-born | 85 (10.3) | 0.65 (.52–.82) | <.001 | ||
| Refugee | 4 (20.0) | 1.45 (.60–3.50) | .410 | ||
| Healthcare worker | 76 (16.0) | 1.20 (.95–1.52) | .133 | ||
| Studentj | 6 (4.7) | 0.33 (.15–.72) | .005 | 0.39 (.17–.90) | .027 |
| Employment | 30 (14.5) | 1.05 (.74–1.49) | .780 | ||
| Long-term-care resident | 6 (12.8) | 0.92 (.43–1.96) | .828 | ||
| Any population riskk | 184 (7.7) | 1.34 (1.09–1.63) | .005 | ||
| Medical conditionsf,i | |||||
| Diabetes | 29 (16.5) | 1.21 (.85–1.71) | .290 | ||
| Chronic renal disease | 6 (20.0) | 1.45 (.70–2.99) | .312 | ||
| Immunocompromised | 9 (9.9) | 0.71 (.38–1.32) | .277 | ||
| Hepatitis | 13 (22.4) | 1.64 (1.01–2.68) | .047 | ||
| Chronic lung disease | 19 (24.4) | 1.80 (1.20–2.70) | .004 | ||
| Mental health problems | 28 (22.0) | 1.65 (1.17–2.32) | .005 | ||
| Hypertension | 44 (14.5) | 1.05 (.78–1.41) | .738 | ||
| Other medical conditionl | 90 (15.7) | 1.19 (.95–1.48) | .134 | ||
| Any medical conditionm | 126 (16.3) | 1.28 (1.04–1.57) | .018 | ||
| Behavioral risk factorsf,i | |||||
| Alcohol use | 35 (16.6) | 1.22 (.89–1.68) | .223 | ||
| Current or past smoker | 101 (18.9) | 1.53 (1.23–1.89) | <.001 | 1.41 (1.14–1.75) | .002 |
| IDU drug use | 4 (17.4) | 1.26 (.51–3.08) | .615 | ||
| Non-IDU drug use | 28 (17.8) | 1.31 (.92–1.87) | .128 | ||
| Any substance riskn | 118 (17.9) | 1.45 (1.18–1.78) | <.001 | ||
| Factor . | Discontinued No. (%) . | Univariable Analysisa RR (95% CI)b . | P Value . | Multivariable Analysisc,d,e ARR (95% CI)b . | P Value . |
|---|---|---|---|---|---|
| Subgroup analysisf | |||||
| Sex | |||||
| Male | 161 (13.0) | 0.88 (.72–1.08) | .219 | 0.82 (.67–1.01) | .062 |
| Female | 170 (14.8) | Reference | |||
| Age | 331 (13.9) | 1.02 (1.01–1.02) | <.001 | 1.01 (1.01–1.02) | <.001 |
| Age categories, y | |||||
| 2–17 | 8 (8.0) | 0.57 (.29–1.14) | .112 | Not done | |
| 18–30 | 80 (10.6) | 0.76 (.57–1.00) | .051 | Not done | |
| 31–44 | 92 (14.0) | Reference | Not done | ||
| 45–64 | 111 (15.6) | 1.11 (.86–1.44) | .404 | Not done | |
| ≥65 | 40 (24.2) | 1.73 (1.25–2.41) | ≤.001 | Not done | |
| Race/ethnicityg | |||||
| Hispanic | 49 (10.4) | 0.97 (.66–1.41) | .857 | 0.84 (.57–1.24) | .382 |
| Non-Hispanic white | 114 (19.5) | 1.81 (1.32–2.49) | ≤.001 | 1.25 (.89–1.75) | .201 |
| Non-Hispanic black | 117 (13.9) | 1.29 (.94–1.78) | .120 | 0.83 (.58–1.19) | .317 |
| Asian | 46 (10.8) | Reference | Reference | ||
| Otherh | 5 (7.8) | 0.73 (.30–1.76) | .477 | 0.64 (.26–1.54) | .315 |
| Treatment reasoni | |||||
| Contact | 52 (8.3) | 0.53 (.40–.70) | ≤.001 | 0.64 (.47–.88) | .006 |
| Converter | 112 (16.7) | 1.31 (1.06–1.61) | .012 | ||
| Corrections within past year | 42 (18.2) | 1.36 (1.01–1.82) | .041 | 1.36 (.99–1.87) | .055 |
| Homeless within past year | 32 (27.4) | 2.08 (1.52–2.84) | <.001 | 1.80 (1.30–2.50) | <.001 |
| Foreign-born | 85 (10.3) | 0.65 (.52–.82) | <.001 | ||
| Refugee | 4 (20.0) | 1.45 (.60–3.50) | .410 | ||
| Healthcare worker | 76 (16.0) | 1.20 (.95–1.52) | .133 | ||
| Studentj | 6 (4.7) | 0.33 (.15–.72) | .005 | 0.39 (.17–.90) | .027 |
| Employment | 30 (14.5) | 1.05 (.74–1.49) | .780 | ||
| Long-term-care resident | 6 (12.8) | 0.92 (.43–1.96) | .828 | ||
| Any population riskk | 184 (7.7) | 1.34 (1.09–1.63) | .005 | ||
| Medical conditionsf,i | |||||
| Diabetes | 29 (16.5) | 1.21 (.85–1.71) | .290 | ||
| Chronic renal disease | 6 (20.0) | 1.45 (.70–2.99) | .312 | ||
| Immunocompromised | 9 (9.9) | 0.71 (.38–1.32) | .277 | ||
| Hepatitis | 13 (22.4) | 1.64 (1.01–2.68) | .047 | ||
| Chronic lung disease | 19 (24.4) | 1.80 (1.20–2.70) | .004 | ||
| Mental health problems | 28 (22.0) | 1.65 (1.17–2.32) | .005 | ||
| Hypertension | 44 (14.5) | 1.05 (.78–1.41) | .738 | ||
| Other medical conditionl | 90 (15.7) | 1.19 (.95–1.48) | .134 | ||
| Any medical conditionm | 126 (16.3) | 1.28 (1.04–1.57) | .018 | ||
| Behavioral risk factorsf,i | |||||
| Alcohol use | 35 (16.6) | 1.22 (.89–1.68) | .223 | ||
| Current or past smoker | 101 (18.9) | 1.53 (1.23–1.89) | <.001 | 1.41 (1.14–1.75) | .002 |
| IDU drug use | 4 (17.4) | 1.26 (.51–3.08) | .615 | ||
| Non-IDU drug use | 28 (17.8) | 1.31 (.92–1.87) | .128 | ||
| Any substance riskn | 118 (17.9) | 1.45 (1.18–1.78) | <.001 | ||
Abbreviations: ARR, adjusted relative risk; CI, confidence interval; IDU, intravenous drug use; RR, relative risk.
aUnivariable analyses test the association between the indicated factor and discontinuation of the 12-week regimen of isoniazid and rifapentine (3HP).
bBolded confidence intervals indicate a statistically significant association at P ≤ .05.
cMultivariable analyses for demographic factors and treatment reasons show the association between the indicated factor and 3HP discontinuation while controlling for all other factors listed in the model and the potential effect of the site; treats age as continuous.
dAll covariates were put in the model at the beginning of the backward elimination procedure; multivariable ARRs with P > .1 are not reported.
eMultivariable analyses for medical conditions and behavioral risk factors show the association between the indicated factor and 3HP discontinuation while controlling for age as a continuous variable.
fAnalysis includes only patients from the standard and comprehensive tiers including medical conditions and behavioral risk factor data (n = 2389); interaction and corresponding main effects terms are kept.
gRace/ethnicity was overall P < .1.
hOther race/ethnicity includes American Indian or Alaska Native, Native Hawaiian or other Pacific Islander, biracial, or unknown.
iTreatment reasons per local policy, medical conditions, and behavioral risks are dichotomous factors.
jMost students were of foreign birth from high-prevalence countries, or were contacts or converters; 11 patients not identified as foreign-born had no treatment indication other than student status.
kAny population risk was defined as being a migrant worker, healthcare worker, employee or resident in tuberculosis-risk setting (eg, homeless shelter or correctional facility), or resident in a long-term-care facility.
lOther medical condition was defined as a patient reporting any medical condition other than diabetes, chronic renal disease, immunocompromised, hepatitis, chronic lung disease, mental health problems, or hypertension.
mAny medical condition was defined as a patient reporting diabetes, chronic renal disease, immunocompromised, hepatitis, chronic lung disease, mental health problems, hypertension, and any other medical condition.
nAny substance use risk was defined as a patient reporting current or past history of smoking, use of alcohol, injection drug use, or noninjection drug use.
In the final multivariable model (Tables 2 and 3), the following factors were associated with significantly greater risk for discontinuing 3HP treatment: having been incarcerated anytime during the 12 months before treatment or having been homeless anytime during the 12 months before treatment. Patients who were significantly less likely to discontinue treatment were contacts of a TB case, or students.
Subgroup analyses of 2389 patients with data collected regarding comorbid medical conditions and behavioral risks demonstrated the following factors associated with significantly greater risk for discontinuation of 3HP treatment in univariable analysis (Table 3): hepatitis, chronic lung disease, mental health problems, any medical condition, current or past smoking, or any substance use. In multivariable analyses, after controlling for all other medical conditions and behavioral factors, patients who were current or past smokers were significantly more likely to discontinue 3HP.
DISCUSSION
In this nationwide observational cohort assessment of treatment completion and associated ADRs to 3HP for treating LTBI, we determined that completion of the 3HP regimen among a diverse patient cohort treated at different types of clinical settings (87.2%) was at least as good as that observed in the clinical trial setting (82.1%) and substantively greater than most reported rates of completion for the standard 6–9 months of daily self-administered therapy with INH (30%–64%) [5, 6, 10–13]. CDC recommendations for 3HP [2] were issued on the basis of findings from clinical trials; in contrast, this project assessed the frequency of ADR and treatment completion outcomes of 3HP offered in routine healthcare settings. Indications for treatment varied across participating sites, with the largest site offering 3HP to all persons with LTBI who did not have contraindications.
Approximately two-thirds of patients who took 3HP reported no ADRs during treatment. Of those who did report ≥1 reaction, 1 in 5 discontinued treatment. Children, adolescents, and younger adults were most likely to complete treatment, but even patients aged ≥65 years had higher completion rates for 3HP than observed with standard INH regimens overall [4, 10, 11]. The preferred treatment for children age 2–11 years is 9 months of INH because data and experience with the 3HP regimen among this age group is limited [2]. However, our data are consistent with findings in 1 clinical trial: children aged 2–17 years were more likely to complete treatment than any other age group, and the regimen was well-tolerated [11].
High treatment completion rates were achieved for certain patient groups who historically have had low completion rates with INH-only regimens. This assessment included persons who were recently or currently homeless or in a correctional facility, persons known to be challenging to treat, especially with regimens that require daily dosing [12], and college students from countries with high TB prevalence who may be lost to follow-up before completion of 6–9 months of INH [13]. Although these patient groups have previously been associated with greater risk of treatment discontinuation than other patient groups, their completion rates were still higher than traditionally seen with 6–9 months of INH. We attribute high rates of treatment completion among these populations to the practical advantages of a shorter, once-weekly regimen.
Treatment completion was also high among groups with chronic disease conditions. This is important because diabetes mellitus, HIV infection, and certain other conditions are risk factors for progression to TB disease [14]. The majority of participating sites offered 3HP to all patients except those for whom 3HP was not recommended (ie, children aged <2 years, HIV-infected patients receiving HAART, pregnant women, and patients with presumed INH- or rifampin-resistant LTBI). We examined ≥10 medical conditions and determined that only persons who were current or past smokers were less likely to complete treatment. Patients with diabetes mellitus, HIV infection not on HAART, or other immunocompromising conditions did well with the regimen; these groups did not have more ADRs or adverse events reported, and completed treatment at rates similar to other patient groups (Table 1).
One limitation of this project was that it was not a funded study; therefore, standardized training was not provided to staff. Another limitation was that data capture was tiered for selected variables (eg, diabetes mellitus or substance use) and data were not solicited uniformly across sites. This project was conducted within routine program operations. As such, participating sites and local standards varied in how patients were selected. For example, at most sites, 3HP was used for all patients who did not have contraindications; at a small number of sites, the regimen was offered to specific patient cohorts (eg, homeless persons, correctional population). Programs also varied in how patient histories were taken, how dose and symptom review checklists were completed, and how patients were counseled regarding reporting of ADRs in association with medication doses. No additional guidance was provided for patient clinical assessment; participating providers might have had varying experience with the 3HP regimen and differed in their thresholds for discontinuing therapy when patients reported symptoms (eg, abdominal pain) or signs (eg, rash or fever) that might be construed as antecedent to a severe adverse event.
This project, reflecting the translation of research and guidelines into routine clinical practice, demonstrates that wider adoption of 3HP is possible and should be implemented [2, 4–6, 14]. Rates of 3HP completion were high for groups with historically low rates of completing LTBI regimens, such as homeless persons and students. The consistently high rates of completion in programmatic settings, in contrast with ≥6 months of INH, hold promise of better returns for the investment of treating persons with LTBI to prevent TB disease.
The 3HP regimen for LTBI treatment was well tolerated by patients in routine healthcare settings, with high rates of completion and low rates of ADRs resulting in discontinuation, among all patient cohorts. Using the new regimen required preparatory and startup efforts, but sites reported that long-term efforts after rollout were comparable with operating procedures for other LTBI regimens and had the benefit of higher rates of treatment completion. It is possible these findings underestimate the true effect of 3HP and other short-course regimens on acceptability, uptake, and completion of treatment for LTBI. Expanded use of 3HP could facilitate successful treatment of more persons with LTBI to accelerate achieving the goal of TB elimination in the United States.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank the patients and providers who participated in this project.
3HP Post-Marketing Assessment Group Investigators. Christine Ho, Nwabunie N. Nwana, Amy L. Sandul, Mark Lobato, Suzanne Marks, Sapna Bamrah-Morris, Terence Chorba, Sundari Mase, John Jereb, Ruth Moro, Brock Stewart, Neha Shah, and Vernard Green (Division of TB Elimination, National Centers for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, Centers for Disease Control and Prevention (CDC), Atlanta, Georgia); Mike Holcombe and Risa Webb (Mississippi State Department of Health, Jackson); Shu-Hua Wang (Columbus Public Health, Columbus, Ohio); Garrett Hunt (Nationwide Children’s Hospital, Columbus , Ohio); Simona Lang and Lynn Sosa (Connecticut Department of Public Health, Hartford); Asween Marco, Leonard Mukasa, and Naveen Patil (Arkansas Department of Health, Little Rock); Phil Griffin (Kansas Department of Health and Environment, Topeka); Jane Moore (Virginia Department of Health, Richmond); Seth Edmunds and Tammy McKenna (South Carolina Department of Health and Environmental Control, Columbia); Rose Sales (Georgia Department of Public Health, Atlanta); Elaine Darnall and Arlene Ryndak (Kane County Health Department, Illinois); Angela Goodbody and Richard Brostrom (Hawaii Department of Health, Honolulu); Diana Fortune (New Mexico Department of Health, Santa Fe); Maria Galvis (Southern Nevada Health District, Las Vegas); Deborah Sodt and Dean Tsukiyama (Minnesota Department of Health, Minneapolis); Neha Shah (California Department of Public Health, Richmond); Sherri Wheeler and Sarah Bur (Federal Bureau of Prisons, Washington, DC).
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC or other authors’ affiliated institutions.
Financial support. This work was supported by intramural CDC funding for coordination of planning meetings and multiyear support for an Oak Ridge Institute for Science and Education fellow for project coordination and data analyses. Participating sites received no additional funding for postmarketing assessment activities.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References




