-
PDF
- Split View
-
Views
-
Cite
Cite
Laura J. Rojas, Madiha Salim, Eric Cober, Sandra S. Richter, Federico Perez, Robert A. Salata, Robert C. Kalayjian, Richard R. Watkins, Steve Marshall, Susan D. Rudin, T. Nicholas Domitrovic, Andrea M. Hujer, Kristine M. Hujer, Yohei Doi, Keith S. Kaye, Scott Evans, Vance G. Fowler, Robert A. Bonomo, David van Duin, for the Antibacterial Resistance Leadership Group, Colistin Resistance in Carbapenem-Resistant Klebsiella pneumoniae: Laboratory Detection and Impact on Mortality, Clinical Infectious Diseases, Volume 64, Issue 6, 15 March 2017, Pages 711–718, https://doi.org/10.1093/cid/ciw805
Close - Share Icon Share
Abstract
Polymyxins including colistin are an important “last-line” treatment for infections caused by carbapenem-resistant Klebsiella pneumoniae (CRKp). Increasing use of colistin has led to resistance to this cationic antimicrobial peptide.
A cohort nested within the Consortium on Resistance against Carbapenems in Klebsiella pneumoniae (CRACKLE) was constructed of patients with infection, or colonization with CRKp isolates tested for colistin susceptibility during the study period of December, 2011 to October, 2014. Reference colistin resistance determination as performed by broth macrodilution was compared to results from clinical microbiology laboratories (Etest) and to polymyxin resistance testing. Each patient was included once, at the time of their first colistin-tested CRKp positive culture. Time to 30-day in-hospital all-cause mortality was evaluated by Kaplan-Meier curves and Cox proportional hazard modeling.
In 246 patients with CRKp, 13% possessed ColR CRKp. ColR was underestimated by Etest (very major error rate = 35%, major error rate = 0.4%). A variety of rep-PCR strain types were encountered in both the ColS and the ColR groups. Carbapenem resistance was mediated primarily by blaKPC-2 (46%) and blaKPC-3 (50%). ColR was associated with increased hazard for in-hospital mortality (aHR 3.48; 95% confidence interval, 1.73-6.57; P < .001). The plasmid-associated ColR genes, mcr-1 and mcr-2 were not detected in any of the ColR CRKp.
In this cohort, 13% of patients with CRKp presented with ColR CRKp. The apparent polyclonal nature of the isolates suggests de novo emergence of ColR in this cohort as the primary factor driving ColR. Importantly, mortality was increased in patients with ColR isolates.
Carbapenem-resistant Klebsiella pneumoniae (CRKp) infections remain a significant challenge associated with morbidity and mortality worldwide [1, 2]. Few antimicrobials retain activity against CRKp [3]. These include aminoglycosides, tigecycline, and the recently approved ceftazidime/avibactam. In addition, polymyxins including colistin are an important treatment option [3]. After being mostly abandoned in the 1970s, these cationic antimicrobial peptides have reemerged as a “last-line” therapeutic option for several multidrug-resistant organisms [4, 5]. In severe CRKp infections, colistin is often used in combination with other antibiotics including tigecycline, meropenem, gentamicin, or fosfomycin [5].
With the rise in consumption of colistin, cases of colistin-resistant Klebsiella pneumoniae carbapenemase (KPC)–producing strains are reported globally [6]. This emergence of colistin-resistant (ColR) in CRKp creates a therapeutic challenge that threatens to return clinicians and patients to a “pre-antibiotic era.” Here, data from the multicenter Consortium on Resistance against Carbapenems in Klebsiella pneumoniae (CRACKLE) were analyzed to determine risk factors for ColR and to compare outcomes of patients with ColR CRKp vs colistin-susceptible (ColS) CRKp.
METHODS
Patients
CRACKLE is a prospective, multicenter, observational study of hospitalized patients with CRKp in the Great Lakes region of the United States [2, 7–9]. In this nested cohort within CRACKLE, patients were included once at the time of their first culture positive for a CRKp isolate for which in vitro colistin susceptibility testing was performed as a part of clinical care at each study site from 2011 December to 2014 October. All health systems involved in this study had approval from their respective institutional review board.
Microbiology
CRKp were defined as K. pneumoniae isolates with nonsusceptibility to any of the following carbapenems: meropenem, imipenem, or ertapenem, as outlined by the Clinical and Laboratory Standards Institute (CLSI) [10]. Bacterial identification and routine antimicrobial susceptibility testing were performed with MicroScan (Siemens Healthcare Diagnostics) or Vitek2 (bioMérieux), supplemented by GN4F Sensititre tray (Thermo Fisher) or Etest (bioMérieux), as indicated. Colistin minimum inhibitory concentration (MIC) determination was performed in clinical microbiology laboratories using Etest and was reported as “no interpretation” due to a lack of CLSI and Food and Drug Administration (FDA) breakpoints.
Colistin (sulfate salt; Sigma-Aldrich) and polymyxin B (Sigma-Aldrich) MIC determination in the central research laboratory was performed in duplicate using a broth macrodilution method in glass sterile tubes using Escherichia coli ATCC25922 as quality control, according to CLSI guidelines [11]. If the 2 MIC values were >1 dilution apart, further repeats were performed to determine the final MIC. The final colistin MIC thus determined was used to designate ColR vs ColS isolates. In vitro polymyxin resistance was defined per European Committee on Antimicrobial Susceptibility Testing guidelines as MIC of >2 mg/L [12]. Very major and major error rates were defined as described elsewhere [13]. Briefly, very major errors were those in which the clinical laboratory result was susceptible and the reference method result was resistant, and major errors were defined as those in which the clinical laboratory result was resistant and the reference method result was susceptible. Very major and major error rates were calculated using the 31 ColR CRKp isolates and the 215 ColS CRKp isolates as the denominator, respectively.
Detection of carbapenemase genes and repetitive extragenic palindromic (rep)-polymerase chain reaction (PCR) strain typing was performed as previously described [2]. Briefly, PCR amplification of blaKPC, blaNDM, blaVIM, blaIMP, and blaOXA-48 genes was conducted using validated primers and methods; amplicons were sequenced at a commercial sequencing facility (MCLAB, San Francisco, California) and analyzed [14, 15]. rep-PCR was performed using the DiversiLab Strain typing system (Bacterial BarCodes, bioMérieux, Athens, Georgia). Isolates with ≥95% similarity were considered to be of the same rep-PCR type [16]. In addition, all ColR CRKp were tested for the presence of mcr-1 and mcr-2 through PCR using the following primers: mcr-1_F 5’- ATGATGCAGCATACTTCTGTGTGG -3’, mcr-1_R 5’- GTGCGGTCTTTGACTTTGTCC-3’ (amplifying the complete mcr-1 ORF according to GenBank sequence KU341381.1), mcr-2_IF 5’ – TGTTGCTTGTGCCGATTGGA -3’, and mcr-2_IR 5’ - AGATGGTATTGTTGGTTGCTG -3’ [17].
Clinical Data
Clinical data were entered into a centralized database using the electronic medical record (EMR). The index hospitalization was recorded as the first hospital stay within the study period during which the CRKp was obtained and tested for colistin susceptibility. CRKp infections were defined by the standardized criteria described previously [2]. Hospital acquisition was defined as timing of the first positive culture more than 2 days into the admission. Critical illness was determined as a Pitt bacteremia score ≥4 points on the day of the index culture [18]. The Charlson comorbidity index was calculated, as described elsewhere [19].
Statistics
Differences between groups were analyzed using Wilcoxon rank sum for continuous variables. Fisher exact and Pearson testing were used for categorical variables where appropriate. Kaplan-Meier curves were constructed to compare time to 30-day in-hospital mortality between groups. Adjusted hazard ratios (aHRs) were calculated using Cox proportional hazards modeling on time to 30-day in-hospital mortality. In the Cox model, all variables were considered that were associated at the P < .1 level with the outcome, followed by backward selection. P values ≤ .05 were considered statistically significant. JMP 10.0.1 software (SAS, Inc, Cary, North Carolina) was used for all analyses.
RESULTS
Colistin Susceptibility Testing
A total of 522 unique patients were included in the CRACKLE study during the study period. Of these 522 patients, 246 (47%) had at least 1 isolate that was shipped to the central research laboratory that had been tested for colistin susceptibility as part of routine clinical care. These 246 patients were included in the study. Colistin resistance per research laboratory determination (“ColR”) was detected in the CRKp isolates for 31 (13%) patients compared to 21 (9%) per clinical laboratory testing. Of note, additional colistin MIC testing beyond duplicates was required in 25 (10%) isolates; the clinical laboratory reported all of these isolates as susceptible. In addition, 25 (10%) of 240 tested isolates were in vitro resistant to polymyxin B as determined by the central research laboratory. In the remaining 6 (2%) isolates, a definitive polymyxin B MIC could not be determined in spite of repetitive testing, due to the observance of “skipped wells” and trailing endpoints.
The distributions of colistin MIC as determined by the central research laboratory compared to the clinical microbiology laboratories and compared to polymyxin B MIC are shown in Figure 1. Testing in the research laboratory resulted in a different interpretation in 12/246 (5%) isolates (Figure 1). The very major error rate was 35% (11/31), and the major error rate was 0.4% (1/215).
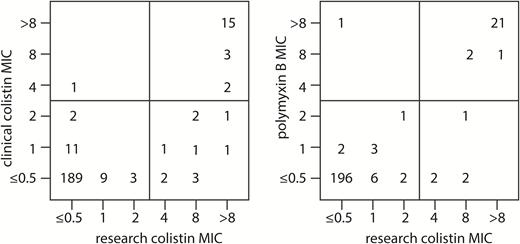
The numbers of isolates that correspond to each minimum inhibitory concentration (MIC) value for the MIC as reported by the clinical microbiology laboratories (“clinical colistin MIC”) and the centralized research laboratory (“research colistin MIC”), as well as the polymyxin B MIC are shown.
Clinical Characteristics
Characteristics of patients with ColR vs ColS CRKp were similar (Table 1). Overall, 111 (45%) patients met criteria for CRKp infection. The anatomic sources of CRKp were urine (59%), blood (18%), respiratory system (11%), wound (5%), and others (7%). Remarkably, time from admission to first positive culture was shorter in patients in the ColR vs ColS group; at a median of 0 days (interquartile range [IQR], 0–3 days) in the ColR group vs 1 day (IQR, 0–9 days) in the ColS group (P = .01). The total length of stay (LOS) was also shorter for patients with ColR isolates; a median of 8 days (IQR, 5–12 days) compared to 13 days (IQR, 7–26 days) in the ColS group (P = .01). When only patients who survived their hospital stay were analyzed, this difference in LOS was similar, albeit no longer significant (median 8 vs 12 days; P = .08). Finally, a marginal association was observed with prehospitalization origin; patients with ColR CRKp were more likely to be admitted from home or a long-term acute care facility (LTAC), whereas patients with ColS CRKp were more likely to be admitted from a skilled nursing facility (SNF) or to be transferred from another hospital (P = .05). No specific SNF or LTAC appeared to be associated with ColR; 12 patients with ColR isolates were admitted from 4 LTACs and 5 SNFs.
| Characteristic . | All . | Colistin Resistant . | Colistin Susceptible . | P Valuea . |
|---|---|---|---|---|
| N | 246 | 31 | 215 | |
| Age in years, median (IQR) | 67 (54–77) | 62 (53–73) | 67 (54–78) | .43 |
| Female | 139 (57) | 20 (65) | 119 (55) | .44 |
| Race/Ethnicity | .22 | |||
| White | 135 (55) | 13 (42) | 122 (57) | |
| Black | 95 (39) | 16 (52) | 79 (37) | |
| Hispanic | 7 (3) | 0 | 7 (3) | |
| Other | 9 (4) | 2 (6) | 7 (3) | |
| Charlson comorbidity index, median (IQR) | 3 (2–5) | 3 (2–4) | 3 (2–5) | .78 |
| Diabetes mellitus | 118 (48) | 15 (48) | 103 (48) | 1.0 |
| Renal failureb | 75 (30) | 7 (23) | 68 (32) | .40 |
| Heart disease | 121 (49) | 14 (45) | 107 (50) | .70 |
| Chronic obstructive pulmonary disease | 62 (25) | 8 (26) | 54 (25) | 1.0 |
| Malignancy | 41 (17) | 8 (26) | 33 (15) | .19 |
| Infection | 111 (45) | 13 (42) | 98 (46) | .85 |
| Source | .22 | |||
| Urine | 144 (59) | 16 (52) | 128 (60) | |
| Blood | 45 (18) | 5 (16) | 40 (19) | |
| Respiratory system | 28 (11) | 4 (13) | 24 (11) | |
| Wound | 13 (5) | 1 (3) | 12 (6) | |
| Other | 16 (7) | 5 (16) | 11 (5) | |
| Days to first positive culture, median (IQR) | 1 (0–7) | 0 (0–3) | 1 (0–9) | .01 |
| Hospital acquisition | 115 (47) | 10 (32) | 105 (49) | .12 |
| Origin | .05 | |||
| Home | 90 (37) | 14 (45) | 76 (35) | |
| Skilled nursing facility | 76 (31) | 6 (19) | 70 (33) | |
| Hospital transfer | 59 (24) | 5 (16) | 54 (25) | |
| Long-term acute care | 21 (9) | 6 (19) | 15 (7) | |
| Critical illnessc | 86 (35) | 7 (23) | 79 (37) | .16 |
| Length of stay in days, median (IQR) | 12 (6–24) | 8 (5–12) | 13 (7–26) | .01 |
| Characteristic . | All . | Colistin Resistant . | Colistin Susceptible . | P Valuea . |
|---|---|---|---|---|
| N | 246 | 31 | 215 | |
| Age in years, median (IQR) | 67 (54–77) | 62 (53–73) | 67 (54–78) | .43 |
| Female | 139 (57) | 20 (65) | 119 (55) | .44 |
| Race/Ethnicity | .22 | |||
| White | 135 (55) | 13 (42) | 122 (57) | |
| Black | 95 (39) | 16 (52) | 79 (37) | |
| Hispanic | 7 (3) | 0 | 7 (3) | |
| Other | 9 (4) | 2 (6) | 7 (3) | |
| Charlson comorbidity index, median (IQR) | 3 (2–5) | 3 (2–4) | 3 (2–5) | .78 |
| Diabetes mellitus | 118 (48) | 15 (48) | 103 (48) | 1.0 |
| Renal failureb | 75 (30) | 7 (23) | 68 (32) | .40 |
| Heart disease | 121 (49) | 14 (45) | 107 (50) | .70 |
| Chronic obstructive pulmonary disease | 62 (25) | 8 (26) | 54 (25) | 1.0 |
| Malignancy | 41 (17) | 8 (26) | 33 (15) | .19 |
| Infection | 111 (45) | 13 (42) | 98 (46) | .85 |
| Source | .22 | |||
| Urine | 144 (59) | 16 (52) | 128 (60) | |
| Blood | 45 (18) | 5 (16) | 40 (19) | |
| Respiratory system | 28 (11) | 4 (13) | 24 (11) | |
| Wound | 13 (5) | 1 (3) | 12 (6) | |
| Other | 16 (7) | 5 (16) | 11 (5) | |
| Days to first positive culture, median (IQR) | 1 (0–7) | 0 (0–3) | 1 (0–9) | .01 |
| Hospital acquisition | 115 (47) | 10 (32) | 105 (49) | .12 |
| Origin | .05 | |||
| Home | 90 (37) | 14 (45) | 76 (35) | |
| Skilled nursing facility | 76 (31) | 6 (19) | 70 (33) | |
| Hospital transfer | 59 (24) | 5 (16) | 54 (25) | |
| Long-term acute care | 21 (9) | 6 (19) | 15 (7) | |
| Critical illnessc | 86 (35) | 7 (23) | 79 (37) | .16 |
| Length of stay in days, median (IQR) | 12 (6–24) | 8 (5–12) | 13 (7–26) | .01 |
All data expressed as n (%), unless otherwise indicated.
Abbreviation: IQR, interquartile range.
aUnivariable relationship between variable of interest and colistin resistance.
bRenal failure defined as creatinine >2 mg/dL on admission.
cCritical illness defined as Pitt bacteremia score ≥4 at the time of index culture.
| Characteristic . | All . | Colistin Resistant . | Colistin Susceptible . | P Valuea . |
|---|---|---|---|---|
| N | 246 | 31 | 215 | |
| Age in years, median (IQR) | 67 (54–77) | 62 (53–73) | 67 (54–78) | .43 |
| Female | 139 (57) | 20 (65) | 119 (55) | .44 |
| Race/Ethnicity | .22 | |||
| White | 135 (55) | 13 (42) | 122 (57) | |
| Black | 95 (39) | 16 (52) | 79 (37) | |
| Hispanic | 7 (3) | 0 | 7 (3) | |
| Other | 9 (4) | 2 (6) | 7 (3) | |
| Charlson comorbidity index, median (IQR) | 3 (2–5) | 3 (2–4) | 3 (2–5) | .78 |
| Diabetes mellitus | 118 (48) | 15 (48) | 103 (48) | 1.0 |
| Renal failureb | 75 (30) | 7 (23) | 68 (32) | .40 |
| Heart disease | 121 (49) | 14 (45) | 107 (50) | .70 |
| Chronic obstructive pulmonary disease | 62 (25) | 8 (26) | 54 (25) | 1.0 |
| Malignancy | 41 (17) | 8 (26) | 33 (15) | .19 |
| Infection | 111 (45) | 13 (42) | 98 (46) | .85 |
| Source | .22 | |||
| Urine | 144 (59) | 16 (52) | 128 (60) | |
| Blood | 45 (18) | 5 (16) | 40 (19) | |
| Respiratory system | 28 (11) | 4 (13) | 24 (11) | |
| Wound | 13 (5) | 1 (3) | 12 (6) | |
| Other | 16 (7) | 5 (16) | 11 (5) | |
| Days to first positive culture, median (IQR) | 1 (0–7) | 0 (0–3) | 1 (0–9) | .01 |
| Hospital acquisition | 115 (47) | 10 (32) | 105 (49) | .12 |
| Origin | .05 | |||
| Home | 90 (37) | 14 (45) | 76 (35) | |
| Skilled nursing facility | 76 (31) | 6 (19) | 70 (33) | |
| Hospital transfer | 59 (24) | 5 (16) | 54 (25) | |
| Long-term acute care | 21 (9) | 6 (19) | 15 (7) | |
| Critical illnessc | 86 (35) | 7 (23) | 79 (37) | .16 |
| Length of stay in days, median (IQR) | 12 (6–24) | 8 (5–12) | 13 (7–26) | .01 |
| Characteristic . | All . | Colistin Resistant . | Colistin Susceptible . | P Valuea . |
|---|---|---|---|---|
| N | 246 | 31 | 215 | |
| Age in years, median (IQR) | 67 (54–77) | 62 (53–73) | 67 (54–78) | .43 |
| Female | 139 (57) | 20 (65) | 119 (55) | .44 |
| Race/Ethnicity | .22 | |||
| White | 135 (55) | 13 (42) | 122 (57) | |
| Black | 95 (39) | 16 (52) | 79 (37) | |
| Hispanic | 7 (3) | 0 | 7 (3) | |
| Other | 9 (4) | 2 (6) | 7 (3) | |
| Charlson comorbidity index, median (IQR) | 3 (2–5) | 3 (2–4) | 3 (2–5) | .78 |
| Diabetes mellitus | 118 (48) | 15 (48) | 103 (48) | 1.0 |
| Renal failureb | 75 (30) | 7 (23) | 68 (32) | .40 |
| Heart disease | 121 (49) | 14 (45) | 107 (50) | .70 |
| Chronic obstructive pulmonary disease | 62 (25) | 8 (26) | 54 (25) | 1.0 |
| Malignancy | 41 (17) | 8 (26) | 33 (15) | .19 |
| Infection | 111 (45) | 13 (42) | 98 (46) | .85 |
| Source | .22 | |||
| Urine | 144 (59) | 16 (52) | 128 (60) | |
| Blood | 45 (18) | 5 (16) | 40 (19) | |
| Respiratory system | 28 (11) | 4 (13) | 24 (11) | |
| Wound | 13 (5) | 1 (3) | 12 (6) | |
| Other | 16 (7) | 5 (16) | 11 (5) | |
| Days to first positive culture, median (IQR) | 1 (0–7) | 0 (0–3) | 1 (0–9) | .01 |
| Hospital acquisition | 115 (47) | 10 (32) | 105 (49) | .12 |
| Origin | .05 | |||
| Home | 90 (37) | 14 (45) | 76 (35) | |
| Skilled nursing facility | 76 (31) | 6 (19) | 70 (33) | |
| Hospital transfer | 59 (24) | 5 (16) | 54 (25) | |
| Long-term acute care | 21 (9) | 6 (19) | 15 (7) | |
| Critical illnessc | 86 (35) | 7 (23) | 79 (37) | .16 |
| Length of stay in days, median (IQR) | 12 (6–24) | 8 (5–12) | 13 (7–26) | .01 |
All data expressed as n (%), unless otherwise indicated.
Abbreviation: IQR, interquartile range.
aUnivariable relationship between variable of interest and colistin resistance.
bRenal failure defined as creatinine >2 mg/dL on admission.
cCritical illness defined as Pitt bacteremia score ≥4 at the time of index culture.
Previous Antibiotic Exposure
Antibiotic exposures in the 14 days preceding the first positive culture were analyzed (Table 2). In 82 (33%) patients, antibiotic use was not documented. In the remaining 164 (67%), vancomycin was the single most common drug exposure and was given to 80 (33%) patients. Twenty-seven (11%) patients received more than 3 classes of antibiotics in the 14-day period before the positive culture. Exposure to fluoroquinolones was less common in the ColR group compared to the ColS group. Otherwise, antibiotic exposures in this time period were not different between patients with or without ColR CRKp.
| Variable . | All (n = 246) . | Colistin Resistant (n = 31) . | Colistin Susceptible (n = 215) . | P Value . |
|---|---|---|---|---|
| Classes of antibiotics | .68 | |||
| None | 82 (33) | 10 (32) | 72 (33) | |
| 1 | 51 (21) | 9 (29) | 42 (20) | |
| 2 | 51 (21) | 5 (16) | 46 (21) | |
| 3 | 35 (14) | 5 (16) | 30 (14) | |
| >3 | 27 (11) | 2 (6) | 25 (12) | |
| Tigecycline | 7 (3) | 2 (6) | 5 (2) | .22 |
| Carbapenem | 36 (15) | 4 (13) | 32 (15) | 1.0 |
| Fluoroquinolone | 44 (18) | 1 (3) | 43 (20) | .02 |
| Colistin | 2 (1) | 0 | 2 (1) | 1.0 |
| Vancomycin | 80 (33) | 8 (26) | 72 (33) | .54 |
| β-lactam/β-lactamase inhibitor | 52 (21) | 7 (23) | 45 (21) | .82 |
| Cephalosporin | 25 (10) | 5 (16) | 20 (9) | .22 |
| Aminoglycoside | 14 (6) | 2 (6) | 12 (6) | .69 |
| Daptomycin | 10 (4) | 2 (6) | 8 (4) | .37 |
| Metronidazole | 19 (8) | 4 (13) | 15 (7) | .27 |
| Other | 90 (37) | 11 (35) | 79 (37) | 1.0 |
| Variable . | All (n = 246) . | Colistin Resistant (n = 31) . | Colistin Susceptible (n = 215) . | P Value . |
|---|---|---|---|---|
| Classes of antibiotics | .68 | |||
| None | 82 (33) | 10 (32) | 72 (33) | |
| 1 | 51 (21) | 9 (29) | 42 (20) | |
| 2 | 51 (21) | 5 (16) | 46 (21) | |
| 3 | 35 (14) | 5 (16) | 30 (14) | |
| >3 | 27 (11) | 2 (6) | 25 (12) | |
| Tigecycline | 7 (3) | 2 (6) | 5 (2) | .22 |
| Carbapenem | 36 (15) | 4 (13) | 32 (15) | 1.0 |
| Fluoroquinolone | 44 (18) | 1 (3) | 43 (20) | .02 |
| Colistin | 2 (1) | 0 | 2 (1) | 1.0 |
| Vancomycin | 80 (33) | 8 (26) | 72 (33) | .54 |
| β-lactam/β-lactamase inhibitor | 52 (21) | 7 (23) | 45 (21) | .82 |
| Cephalosporin | 25 (10) | 5 (16) | 20 (9) | .22 |
| Aminoglycoside | 14 (6) | 2 (6) | 12 (6) | .69 |
| Daptomycin | 10 (4) | 2 (6) | 8 (4) | .37 |
| Metronidazole | 19 (8) | 4 (13) | 15 (7) | .27 |
| Other | 90 (37) | 11 (35) | 79 (37) | 1.0 |
Shown is the documented exposure to antibiotics in 14 days prior to first positive culture. All data expressed as n (%).
| Variable . | All (n = 246) . | Colistin Resistant (n = 31) . | Colistin Susceptible (n = 215) . | P Value . |
|---|---|---|---|---|
| Classes of antibiotics | .68 | |||
| None | 82 (33) | 10 (32) | 72 (33) | |
| 1 | 51 (21) | 9 (29) | 42 (20) | |
| 2 | 51 (21) | 5 (16) | 46 (21) | |
| 3 | 35 (14) | 5 (16) | 30 (14) | |
| >3 | 27 (11) | 2 (6) | 25 (12) | |
| Tigecycline | 7 (3) | 2 (6) | 5 (2) | .22 |
| Carbapenem | 36 (15) | 4 (13) | 32 (15) | 1.0 |
| Fluoroquinolone | 44 (18) | 1 (3) | 43 (20) | .02 |
| Colistin | 2 (1) | 0 | 2 (1) | 1.0 |
| Vancomycin | 80 (33) | 8 (26) | 72 (33) | .54 |
| β-lactam/β-lactamase inhibitor | 52 (21) | 7 (23) | 45 (21) | .82 |
| Cephalosporin | 25 (10) | 5 (16) | 20 (9) | .22 |
| Aminoglycoside | 14 (6) | 2 (6) | 12 (6) | .69 |
| Daptomycin | 10 (4) | 2 (6) | 8 (4) | .37 |
| Metronidazole | 19 (8) | 4 (13) | 15 (7) | .27 |
| Other | 90 (37) | 11 (35) | 79 (37) | 1.0 |
| Variable . | All (n = 246) . | Colistin Resistant (n = 31) . | Colistin Susceptible (n = 215) . | P Value . |
|---|---|---|---|---|
| Classes of antibiotics | .68 | |||
| None | 82 (33) | 10 (32) | 72 (33) | |
| 1 | 51 (21) | 9 (29) | 42 (20) | |
| 2 | 51 (21) | 5 (16) | 46 (21) | |
| 3 | 35 (14) | 5 (16) | 30 (14) | |
| >3 | 27 (11) | 2 (6) | 25 (12) | |
| Tigecycline | 7 (3) | 2 (6) | 5 (2) | .22 |
| Carbapenem | 36 (15) | 4 (13) | 32 (15) | 1.0 |
| Fluoroquinolone | 44 (18) | 1 (3) | 43 (20) | .02 |
| Colistin | 2 (1) | 0 | 2 (1) | 1.0 |
| Vancomycin | 80 (33) | 8 (26) | 72 (33) | .54 |
| β-lactam/β-lactamase inhibitor | 52 (21) | 7 (23) | 45 (21) | .82 |
| Cephalosporin | 25 (10) | 5 (16) | 20 (9) | .22 |
| Aminoglycoside | 14 (6) | 2 (6) | 12 (6) | .69 |
| Daptomycin | 10 (4) | 2 (6) | 8 (4) | .37 |
| Metronidazole | 19 (8) | 4 (13) | 15 (7) | .27 |
| Other | 90 (37) | 11 (35) | 79 (37) | 1.0 |
Shown is the documented exposure to antibiotics in 14 days prior to first positive culture. All data expressed as n (%).
Documented previous colistin use in the 14 days leading up to the day of first positive culture was uncommon in the cohort as a whole, and patients in the ColR group did not receive colistin during this limited time period. Colistin exposure data beyond the 14-day period were available for 27 patients with ColR isolates, based on EMR of the healthcare system of the index hospitalization. In total, 6/27 (22%) patients were found to have previous colistin exposure at any time prior to first positive culture with a ColR CRKp.
Microbiology
The susceptibilities for antibiotics other than colistin were determined as clinically indicated by clinical microbiology laboratories (Figure 2). ColR CRKp were significantly less likely to be amikacin susceptible; 8/15 (53%) of tested ColR CRKp were amikacin susceptible compared to 104/126 (83%) of tested ColS CRKp (P = .02). In contrast, ColR CRKp were more likely to be gentamicin susceptible; 21/31 (68%) of tested ColR CRKp were gentamicin susceptible compared to 97/212 (46%) of tested ColS CRKp (P = .03). Furthermore, ColR isolates were also more likely to be tigecycline nonsusceptible; 13/21 (62%) of tested ColR CRKp were tigecycline susceptible compared to 138/168 (82%) of tested ColS CRKp (P = .04).
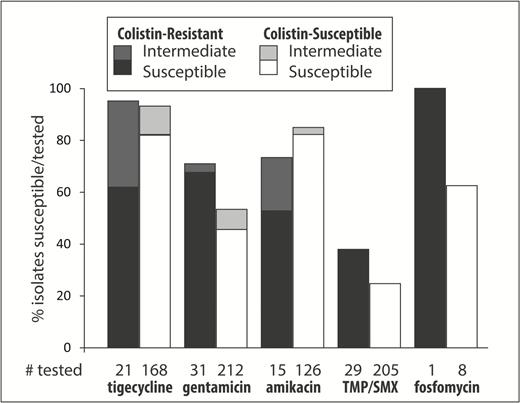
The percentages of susceptible and intermediate isolates for each drug are shown separately for colistin-resistant (ColR) and colistin-susceptible (ColS) carbapenem-resistant Klebsiella pneumoniae (CRKp). Underneath each bar is the number of CRKp tested for each drug in the ColR and ColS groups. The black and white bars represent the percentage of susceptible/tested CRKp isolates in the ColR and ColS groups, respectively. The dark-gray and light-gray bars on top of the black and white bars represent the percentage of intermediate/tested CRKp isolates in the ColR and ColS groups, respectively. Abbreviation: TMP/SMX, trimethoprim/sulfamethoxazole.
Overall, a nonclonal distribution was observed. rep-PCR suggested that most strains belonged to clades ST258A (n = 76, 31%) and ST258B (n = 102, 41%). The remaining 68 (28%) isolates belonged to 28 other rep-PCR types. Likewise in the ColR group, a nonclonal pattern was observed (Figure 3). rep-PCR types corresponding to clades ST258A and ST258B together accounted for 77% of ColR isolates.
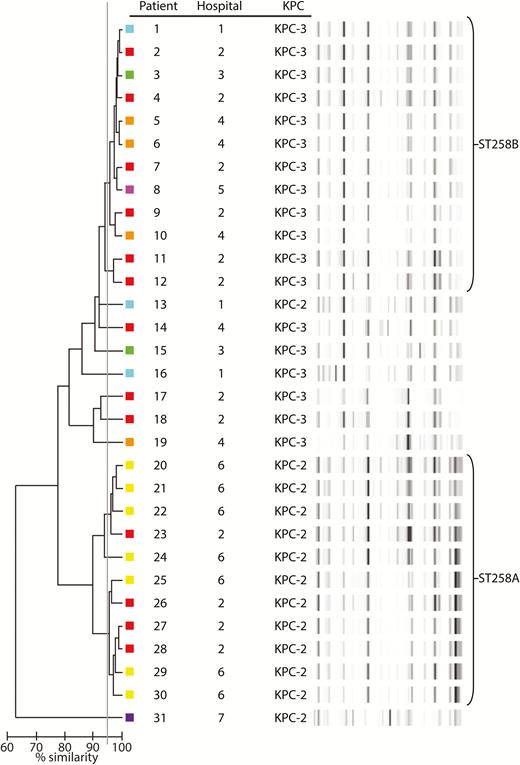
Repetitive extragenic palindromic–polymerase chain reaction (rep-PCR) dendrogram of colistin-resistant carbapenem-resistant Klebsiella pneumoniae isolates. A ≥95% similarity cutoff (gray line) was used to consider isolates of the same rep-PCR type. Colored boxes next to each strain represent the various hospitals from which isolates were recovered. Abbreviation: KPC, klebsiella pneumoniae carbapenemase.
The presence of carbapenemase genes was confirmed in 238 (97%) isolates. The carbapenemase genes blaKPC-2 and blaKPC-3 were the most common and were found in 113 (46%) and 124 (50%) of CRKp isolates, respectively. The distribution of carbapenemase genes did not differ between ColR and ColS CRKp. Of note, blaNDM-1 was found in only 1 isolate. This ColS isolate was extensively described previously using whole genome sequencing [2]. In the remaining 8 (3%) isolates, carbapenemase genes were not detected. The plasmid-mediated colistin resistance genes, mcr-1 and mcr-2, were not detected in any of the ColR CRKp.
Treatment and Mortality
In the first 7 days after the first positive culture, 143 (58%) patients received directed treatment. Treatment regimens used in these 143 patients are summarized in Table 3. Treatment with colistin was uncommon; colistin, either alone or in combination with other antibiotics, was given to 31 (22%) of treated patients. Colistin treatment was given to 30 of 127 (24%) treated patients in the ColS group compared to 1/16 (6%) treated patients in the ColR group. Other treatment regimens were similar between the 2 groups. The most commonly used antibiotics were aminoglycosides and tigecycline. Aminoglycosides were given to 72 (50%) of treated patients; 36 (25%) patients received only aminoglycosides in the first 7 days. Similarly, 63 (44%) of treated patients received tigecycline, of whom 21 (15%) received tigecycline monotherapy in the first 7 days.
| Characteristic . | All . | Colistin Resistant . | Colistin Susceptible . |
|---|---|---|---|
| n | 143 | 16 | 127 |
| Any colistin in first 7 days | 31 (22) | 1 (6) | 30 (24) |
| One antibiotic in first 7 daysa | 90 (63) | 10 (63) | 80 (63) |
| Aminoglycoside | 36 (25) | 5 (31) | 31 (24) |
| Colistin | 10 (7) | 1 (6) | 9 (7) |
| Tigecycline | 21 (15) | 3 (19) | 18 (14) |
| Trimethoprim/sulfamethoxazole | 13 (9) | 0 | 13 (10) |
| fosfomycin | 10 (7) | 1 (6) | 9 (7) |
| >1 antibiotic in first 7 days | 53 (37) | 6 (38) | 47 (37) |
| 2 antibiotics | 38 (27) | 5 (31) | 33 (26) |
| 3 antibiotics | 13 (9) | 1 (6) | 12 (9) |
| 4 antibiotics | 2 (1) | 0 | 2 (2) |
| Characteristic . | All . | Colistin Resistant . | Colistin Susceptible . |
|---|---|---|---|
| n | 143 | 16 | 127 |
| Any colistin in first 7 days | 31 (22) | 1 (6) | 30 (24) |
| One antibiotic in first 7 daysa | 90 (63) | 10 (63) | 80 (63) |
| Aminoglycoside | 36 (25) | 5 (31) | 31 (24) |
| Colistin | 10 (7) | 1 (6) | 9 (7) |
| Tigecycline | 21 (15) | 3 (19) | 18 (14) |
| Trimethoprim/sulfamethoxazole | 13 (9) | 0 | 13 (10) |
| fosfomycin | 10 (7) | 1 (6) | 9 (7) |
| >1 antibiotic in first 7 days | 53 (37) | 6 (38) | 47 (37) |
| 2 antibiotics | 38 (27) | 5 (31) | 33 (26) |
| 3 antibiotics | 13 (9) | 1 (6) | 12 (9) |
| 4 antibiotics | 2 (1) | 0 | 2 (2) |
Treatment is shown in the subset of patients who received at least 1 antibiotic with potential in vitro anti–carbapenem-resistant Klebsiella pneumoniae (CRKp) activity in the first 7 days after first positive CRKp culture.
aOnly antibiotics with potential in vitro anti-CRKp activity were included.
| Characteristic . | All . | Colistin Resistant . | Colistin Susceptible . |
|---|---|---|---|
| n | 143 | 16 | 127 |
| Any colistin in first 7 days | 31 (22) | 1 (6) | 30 (24) |
| One antibiotic in first 7 daysa | 90 (63) | 10 (63) | 80 (63) |
| Aminoglycoside | 36 (25) | 5 (31) | 31 (24) |
| Colistin | 10 (7) | 1 (6) | 9 (7) |
| Tigecycline | 21 (15) | 3 (19) | 18 (14) |
| Trimethoprim/sulfamethoxazole | 13 (9) | 0 | 13 (10) |
| fosfomycin | 10 (7) | 1 (6) | 9 (7) |
| >1 antibiotic in first 7 days | 53 (37) | 6 (38) | 47 (37) |
| 2 antibiotics | 38 (27) | 5 (31) | 33 (26) |
| 3 antibiotics | 13 (9) | 1 (6) | 12 (9) |
| 4 antibiotics | 2 (1) | 0 | 2 (2) |
| Characteristic . | All . | Colistin Resistant . | Colistin Susceptible . |
|---|---|---|---|
| n | 143 | 16 | 127 |
| Any colistin in first 7 days | 31 (22) | 1 (6) | 30 (24) |
| One antibiotic in first 7 daysa | 90 (63) | 10 (63) | 80 (63) |
| Aminoglycoside | 36 (25) | 5 (31) | 31 (24) |
| Colistin | 10 (7) | 1 (6) | 9 (7) |
| Tigecycline | 21 (15) | 3 (19) | 18 (14) |
| Trimethoprim/sulfamethoxazole | 13 (9) | 0 | 13 (10) |
| fosfomycin | 10 (7) | 1 (6) | 9 (7) |
| >1 antibiotic in first 7 days | 53 (37) | 6 (38) | 47 (37) |
| 2 antibiotics | 38 (27) | 5 (31) | 33 (26) |
| 3 antibiotics | 13 (9) | 1 (6) | 12 (9) |
| 4 antibiotics | 2 (1) | 0 | 2 (2) |
Treatment is shown in the subset of patients who received at least 1 antibiotic with potential in vitro anti–carbapenem-resistant Klebsiella pneumoniae (CRKp) activity in the first 7 days after first positive CRKp culture.
aOnly antibiotics with potential in vitro anti-CRKp activity were included.
We evaluated time to all-cause in-hospital mortality, censored at 30 days (Figure 4). In univariable Kaplan-Meier survival curves, patients with ColR CRKp were at increased hazard of 30-day mortality (P < .0001). After adjustment, the aHR of ColR vs ColS was 3.48 (95% confidence interval [CI] 1.73–6.57; P < .001). Other variables that impacted survival included age, pre-hospitalization origin, and anatomical source of the positive CRKp culture (Table 4). Treatment variables and infection vs colonization status were not associated with mortality, and inclusion of these into the model did not significantly alter the association between colistin and mortality. Of note, when the colistin resistance as determined by the clinical microbiology laboratory was entered into this model instead of ColR as determined by the research laboratory, this was still associated with increased mortality but to a lesser extent (aHR, 2.78; 95% CI, 1.22–5.71; P = .02; data not shown).

Kaplan-Meier curve showing the 30-day in-hospital survival for patients with colistin-resistant carbapenem-resistant Klebsiella pneumoniae (CRKp) as compared to colistin-susceptible CRKp. Patients were censored at the time of hospital discharge.
| Variable . | Adjusted Hazard Ratio . | 95% Confidence Interval . | P Value . |
|---|---|---|---|
| Colistin resistance | 3.48 | 1.73–6.57 | < .001 |
| Age (by decade) | 1.24 | 1.03–1.53 | .02 |
| Origin | < .01 | ||
| Home (reference) | — | — | |
| Skilled nursing facility | 2.52 | 1.12–6.03 | |
| Hospital transfer | 2.64 | 1.18–6.35 | |
| Long-term acute care | 5.18 | 2.17–12.82 | |
| Source | < .01 | ||
| Blood (reference) | — | — | |
| Urine | 0.35 | 0.19–0.66 | |
| Other | 0.29 | 0.13–0.60 |
| Variable . | Adjusted Hazard Ratio . | 95% Confidence Interval . | P Value . |
|---|---|---|---|
| Colistin resistance | 3.48 | 1.73–6.57 | < .001 |
| Age (by decade) | 1.24 | 1.03–1.53 | .02 |
| Origin | < .01 | ||
| Home (reference) | — | — | |
| Skilled nursing facility | 2.52 | 1.12–6.03 | |
| Hospital transfer | 2.64 | 1.18–6.35 | |
| Long-term acute care | 5.18 | 2.17–12.82 | |
| Source | < .01 | ||
| Blood (reference) | — | — | |
| Urine | 0.35 | 0.19–0.66 | |
| Other | 0.29 | 0.13–0.60 |
| Variable . | Adjusted Hazard Ratio . | 95% Confidence Interval . | P Value . |
|---|---|---|---|
| Colistin resistance | 3.48 | 1.73–6.57 | < .001 |
| Age (by decade) | 1.24 | 1.03–1.53 | .02 |
| Origin | < .01 | ||
| Home (reference) | — | — | |
| Skilled nursing facility | 2.52 | 1.12–6.03 | |
| Hospital transfer | 2.64 | 1.18–6.35 | |
| Long-term acute care | 5.18 | 2.17–12.82 | |
| Source | < .01 | ||
| Blood (reference) | — | — | |
| Urine | 0.35 | 0.19–0.66 | |
| Other | 0.29 | 0.13–0.60 |
| Variable . | Adjusted Hazard Ratio . | 95% Confidence Interval . | P Value . |
|---|---|---|---|
| Colistin resistance | 3.48 | 1.73–6.57 | < .001 |
| Age (by decade) | 1.24 | 1.03–1.53 | .02 |
| Origin | < .01 | ||
| Home (reference) | — | — | |
| Skilled nursing facility | 2.52 | 1.12–6.03 | |
| Hospital transfer | 2.64 | 1.18–6.35 | |
| Long-term acute care | 5.18 | 2.17–12.82 | |
| Source | < .01 | ||
| Blood (reference) | — | — | |
| Urine | 0.35 | 0.19–0.66 | |
| Other | 0.29 | 0.13–0.60 |
DISCUSSION
In this nested cohort of patients from CRACKLE—a prospective, ongoing, multicenter evaluation of hospitalized patients with carbapenem-resistant Enterobacteriaceae (CRE)—a ColR rate of 13% was observed in CRKp isolates. This rate was underestimated by testing as performed by clinical microbiology laboratories. In addition, even testing in optimal research conditions resulted in the need for repeat colistin MIC testing in 10% of isolates. Furthermore, polymyxin B MIC testing was also fraught with difficulties; a definitive polymyxin B MIC could not be determined in 6 isolates, even with repetitive testing. The difficulty in determining colistin MIC has been previously reported by Hindler et al [20]. Broth macrodilution, as used here, was found to be the most reliable [20]. The American Society for Microbiology recently released a white paper on the issue of colistin testing, recommending that “laboratories that choose to test for colistin susceptibility should validate a broth microdilution method and report MIC values only” [21]. Clearly, the absence of FDA breakpoints and the resulting impossibility of an FDA-approved test for colistin susceptibility remains a major issue.
The association between ColR and mortality was also seen in an Italian case-control study on CRKp bloodstream infections [22]. In that study 30-day all-cause mortality rates were 51% in the ColR group (n = 142) vs 39% in the ColS group (n = 284; P = .02). The increased mortality may be directly related to decreased colistin susceptibility of the organism. Alternatively, the observed mortality differences may be secondary to either variance in baseline characteristics of patients with ColR vs ColS CRKp or to an alteration in the virulence of the pathogen [23]. Regardless of causality, it is clear that patients with ColR CRKp are at increased risk of hospital mortality.
We observed that—similar to patients with tigecycline-resistant CRKp—isolation of ColR CRKp tended to occur earlier during hospitalization compared to ColS CRKp [7]. Our findings suggest that the introduction of ColR isolates may be present on admission to acute care hospitals. In the majority of patients, colistin exposure prior to the positive culture was not documented. This may be explained in a number of ways. First, some patients may have had unrecorded colistin exposure at nonstudy hospitals. The intuitive link between colistin exposure and ColR has been previously documented for CRKp and carbapenem-resistant Acinetobacter baumannii (CRAB) [4, 22]. Second, patients with ColR CRKp without colistin exposure may have received ColR CRKp from either another person exposed to colistin or potentially from a food source. The latter option would appear less likely as no such food-related transmission has been reported in the United States for K. pneumoniae. In contrast, person-to-person transmission of ColR CRKp without further colistin exposure of subsequently infected patients was shown to be an important mechanism of spread in an outbreak-related report [24]. The lack of clonality in ColR CRKp described in that report argues against a single clonal outbreak but obviously does not rule out multiple person-to-person transmission events.
Previously we described various chromosomally mediated mechanisms of ColR for a subset of isolates in this cohort [25]. Of concern, 2 recently described plasmid-mediated, readily transmissible ColR mechanisms—mcr-1 and mcr-2—are being recognized in Enterobacteriaceae from animals and humans around the world [17, 26]. Even though mcr-1 has already been reported in the United States, this gene was not detected in any of the isolates described here [27, 28]. Reproducibility issues in polymyxin/colistin MIC testing, as well as variability between testing methods illustrated here and in other reports, may complicate tracking of mcr-1– and mcr-2–carrying isolates [20]. If screening of mcr carriage is only performed on isolates based on colistin MIC by Etest, the most common method used by clinical labs, the spread of mcr is likely to be underestimated.
There are a number of limitations to this work. We purposefully selected isolates in which colistin susceptibility testing was performed by the clinical microbiology laboratories as clinically indicated, and patients with CRKp isolates that were not tested are not included in this study. Therefore, our estimate of 13% ColR at first test may not necessarily be representative of all patients with CRKp. Furthermore, follow-up for patients in this observational study is limited to the duration of hospitalization. Consequently, we cannot comment on long-term outcomes. Another potential limitation of our work is that we included both patients who met standardized criteria for infection as well as those who did not. Nevertheless, standardized criteria have limitations in distinguishing patients with infection from those who are colonized. In addition, patients who do not meet criteria for infection often receive antibiotic treatment and are an important source of transmission. Finally, our study population was predominantly derived from the Great Lakes region of the United States. Therefore, these data may not accurately reflect incidence of ColR in other CRKp in the United States. In a study performed by the Centers for Disease Control and Prevention in 2012–2013 in 7 US communities, 12 CRE isolates were tested for colistin susceptibility. Nine of these were reported to be nonsusceptible, based on testing performed in clinical laboratories [29]. Additional data from other US regions are expected from ongoing surveillance programs and from the recently started national expansion of CRACKLE. Additionally, there are important differences in ColR rates in CRE isolates outside of the United States as well [17, 26].
In summary, we observed a 13% ColR rate in this multicenter, observational cohort of hospitalized patients with CRKp in US hospitals. Colistin in vitro susceptibility testing is characterized by reproducibility issues. Patients with ColR CRKp were at higher risk for mortality. They also tended to present earlier during admission. This, in combination with the polyclonality of the observed ColR CRKp isolates, suggests that direct patient-to-patient transmission of CRKp isolates within short-term acute care hospitals is unlikely to be the primary mechanism driving resistance rates. Alternative, not mutually exclusive explanations include within-patient development of ColR and transmission in healthcare settings outside of short-term acute care hospitals, such as LTACs or SNFs.
Note
Disclaimer. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH; UM1AI104681 and R21AI114508) and by funding to D. v. D. and F. P. from the Clinical and Translational Science Collaborative of Cleveland (UL1TR000439) from the National Center for Advancing Translational Sciences component of the NIH and NIH Roadmap for Medical Research. V. G. F. was supported by a Mid-Career Mentoring Award (K24-AI093969) from NIH. In addition, research reported in this publication was supported in part by the NIAID of the NIH (R21AI114508 to D. v. D. and R. A. B. and R01AI100560, R01AI063517, and R01AI072219 to R. A. B.). This study was supported in part by funds and/or facilities provided by the (Cleveland Department of Veterans Affairs 1I01BX001974 to R. A. B.) from the Biomedical Laboratory Research & Development Service of the Veterans Affairs Office of Research and Development and the Geriatric Research Education and Clinical Center VISN 10 (R. A. B.). Further support came from the Research Program Committees of the Cleveland Clinic and an unrestricted research grant from the STERIS Corporation (D. v. D.). Y. D. was supported by research awards from the NIH (R01AI104895 and R21AI107302). K. S. K. is supported by the NIAID, Division of Microbiology and Infectious Diseases (protocol 10–0065 and R01AI119446-01).
Potential conflicts of interest.S. S .R.: research support: bioMerieux, BD Diagnostics, BioFire, OpGen, Forest Laboratories, Achaogen, Nanosphere, Pocared; honorarium: bioMerieux. Y. D.: grant support: the Medicines Company, NIH; advisory board: Meiji, Tetraphase, Achaogen. K. K: consultant, grant investigator, speaker’s bureau, consulting fee, grant recipient, speaker honorarium: Allergan; grant recipient, consultant: Merck; consultant: Xellia, Achaogen. R. R. W.: grant support, Allergan. R. A. B.: grant investigator, grant recipient: AstraZeneca, Merck, Melinta, Steris, NIH, VA Merit Review. V.G.F.: grant and research support, Advanced Liquid Logic, Cubist, Cerexa, MedImmune, Merck, NIH, Novartis, Pfizer, Theravance; paid consultant, Affinium, Baxter, Cerexa, Cubist, Debiopharm, Durata, Merck, Novartis, NovaDigm, the Medicines Company, MedImmune, Pfizer, Theravance, Trius; honoraria: Arpida, Astellas, Cubist, Inhibitex, Merck, Pfizer, Targanta, Theravance, Wyeth, Ortho-McNeil, Novartis, Vertex Pharmaceuticals; membership: Merck co-chair V710 Vaccine. D. v. D.: advisory board: Allergan, Shionogi, Achaogen, Actavis, Tetraphase, Sanofi-Pasteur, MedImmune, Astellas, Steris Inc.; research funding: Scynexis Research. All other authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
Author notes
Presented in part: IDWeek. San Diego, California. 7–11 October 2015.
Correspondence: D. van Duin, MD, PhD, Division of Infectious Diseases, CB 7030, University of North Carolina, 130 Mason Farm Road, Chapel Hill, North Carolina 27599 ([email protected])




