-
PDF
- Split View
-
Views
-
Cite
Cite
Maria Elena Tosti, Valeria Alfonsi, Eleonora Lacorte, Alfonso Mele, Cristina Galli, Alessandro Remo Zanetti, Luisa Romanò, for the SEIEVA Collaborating Group, L. Ferrigno, S. Crateri, G. Iantosca, G. Badoni, F. D'Angelo, L. Sudano, M. Ruffier, M. Fischer, M. Augschiller, S. Gamper, A. Foppa, T. Lechthaler, J. Thaler, B. Steinmair, C. Grandi, V. Carraro, S. Franchini, C. Zotti, P. Lanzafame, S. Malaspina, A. Gallone, A. Castella, G. Valenza, V. Silano, MG. Tacca, S. Iodice, AM. Marchisio, AM. Costantino, F. Giovanetti, F. Susani, C. Tagliacarne, A. Donadini, C. Nespoli, L. Trezzi, G. Gennati, A. Monteverdi, L. Boldori, P. De Grada, A. Gattinoni, R. Brugnoli, A. Belloni, M. Binotto, G. Pinciroli, L. Pesci, P. Senegaglia, S. Crippa, G. Altomonte, S. Lodola, I. Aquino, N. Castelli, E. Zecca, M. Nieri, F. Zecca, L. Pasquale, G. Piedacci, E. Giompapa, F. Zorzut, G. Rocco, G. Brianti, T. Gallo, M. Zuliani, A. Breda, O. Feltrin, F. Russo, F. Zanella, R. Mel, M. Soppelsa, F. Russo, R. Zolin, A. Todescato, N. Bacciolo, D. Rizzato, A. Pupo, L. Nicolardi, M. Flora, F. Boin, C. De Sisti, G. D'Ettore, V. Caracciolo, MG. Penon, M. Bellè, L. Cafarra, G. Zivelonghi, S. Soffritti, M. Foroni, AC. Finarelli, BM. Borrini, C. Gualanduzzi, A. Capra, AR. Sacchi, BM. Borrini, G. Mattei, L. Gardenghi, AR. Gianninoni, R. Sancini, E. Dalle Donne, R. Rangoni, M. Cova, L. Bevilacqua, E. Fiumana, B. Bondi, A. Pecci, M. Mela, MP. Briata, P. Michele, V. Turello, A. Opisso, G. Zoppi, P. Torracca, MA. Ricci, A. Capellini, L. Pecori, F. Mazzotta, E. Balocchini, G. Ghiselli, P. Marchini, A. Di Vito, W. Wanderlingh, E. Raso, F. Mazzoli, C. Berti, N. Galletti, E. Grandi, MS. Ferrentino, MG. Marinari, A. Lombardi, A. Barbieri, A. Bagnoli, M. Bandini, I. Lezzi, F. Verdelli, A. Beltrano, R. Bindi, CM. Sansone, G. Boncompagni, F. Zacchini, S. Baretti, O. Baroncini, C. Staderini, P. Filidei, L. Chiapparini, F. Barghini, M. Cadoni, G. Tagliavento, D. Fiacchini, N. Damiani, AR. Pelliccioni, A. Liverani, G. Peccerillo, A. Vaccaro, MR. Spadoni, R. Rossini, F. Pasqualini, A. Priori, N. Burattini, S. Cimica, V. Vitale, F. Laici, F. Migliozzi, G. Moretti, G. Ciarrocchi, S. Impullitti, C. Angelini, A. Tosti, MD. Giaimo, A. Buscosi, A. Pasquale, C. Ciani, F. Santocchia, ML. Proietti, M C. Paoloni, A. Ercole, P. Russo, C. Cerocchi, P. Grillo, M. Loffredo, V. Labriola, A. Pendenza, MR. Nappi, P. Bueti, L. Santucci, F. Mangiagli, D. Varrenti, S. Aquilani, P. Dionette, D. Corpolongo, G. Di Luzio, M. Di Giacomo, M. Graziani, C. Mancini, C. Turchi, C. Granchelli, G. Soldato, F. D'Eugenio, I. Albanesi, MA. Ferrara, A. Citarella, E. Fossi, A. Parlato, R. Alfieri, M. Scotto, A L. Caiazzo, M. Chironna, R. Prato, R. Matera, S. Menolascina, R. Colamaria, N. Azzollini, A. Madaro, G. Scalzo, A. Ancona, P. Pedote, G. Moffa, I. Pagano, R. Angelillis, M. Ferraro, V. Aprile, G L. Turco, S. Minerba, G. Caputi, F. Negrone, M. Maldini, T. Russo, F. Aloia, S. Giuffrida, R. Mangione, R. Consacra, M. Cuccia, S. Rinnone, F. Delogu, D. Fracasso, A. Saba, A. Puggioni, O. Frongia, MV. Marras, MG. Crasta, G. Mereu, GC. Steri, S. Santus, for the SEIEVA Collaborating Group, Acute Hepatitis B After the Implementation of Universal Vaccination in Italy: Results From 22 Years of Surveillance (1993–2014), Clinical Infectious Diseases, Volume 62, Issue 11, 1 June 2016, Pages 1412–1418, https://doi.org/10.1093/cid/ciw162
Close - Share Icon Share
Abstract
Background. Hepatitis B vaccination has proven to be very safe and highly effective. This study assessed the proportion of successfully vaccinated individuals among cases with acute hepatitis B, the proportion of preventable cases if individuals were vaccinated as recommended, and the reasons for failures.
Methods. We analyzed data reported to the Italian Surveillance System for Acute Viral Hepatitis from 1993 to 2014.
Results. A total of 362 of 11 311 (3.2%) cases with acute hepatitis B were vaccinated. Of the 277 cases for whom immunization data were available, 50 (18%) received a complete vaccination course according to the correct schedule and before exposure to hepatitis B virus. Molecular characterization of 17 of these cases showed that 6 were infected with S-gene mutants. Among the 10 949 unvaccinated cases, 213 (1.9%) escaped mandatory vaccination and 2821 (25.8%) were not vaccinated despite being at increased risk of infection. Among the latter, the most common risk factors were cohabitation with hepatitis B surface antigen (HBsAg) carriers, intravenous drug use, and homosexual/bisexual practices. Thirty-seven percent of the unvaccinated households with HBsAg carriers were aware of their risk. Lack of trust in the vaccination, negative attitude, and inaccurate beliefs followed by lack of or poor communication and low perceived severity of the disease were the most frequent reasons for vaccine hesitancy.
Conclusions. Development of acute disease in successfully vaccinated individuals is a rare event. Further efforts are needed to enhance the vaccine coverage rate in individuals at increased risk of infection.
Hepatitis B virus (HBV) infection and HBV-related diseases are a major public health issue worldwide. Currently about 240 million people are estimated to be chronically infected with HBV, and more than 780 000 individuals die each year due to hepatitis B complications, including cirrhosis and liver cancer [1].
Vaccination is the most effective and economically favorable measure to control and prevent hepatitis B on a global scale [2–4]. Italy is one of the first countries in the world to implement a vaccination policy, starting in 1983 with a program targeted to individuals at increased risk of infection [5–6]. Vaccination became mandatory in 1991 for all infants and all 12-year-olds. This program also includes the mandatory screening of pregnant women for hepatitis B surface antigen (HBsAg) in order to identify babies in need of treatment with hepatitis B immune globulin and vaccine at birth. The program also includes recommendations for vaccination of groups at higher risk of infection. At the end of 2003, those in the first infant cohort vaccinated in 1991 were age 12 years. Thus, the vaccination of 12-year-olds was stopped, as all children at that age were covered, while the vaccination of infants was maintained. Because of the Italian vaccination delivery system's effectiveness and public awareness of the disease, the take-up of vaccination was rapid and reached a coverage rate of >95% within a few years [7]. To maintain the highest possible coverage rate and, in turn, guarantee an effective protection at a social level through herd immunity, parents/legal guardians who miss vaccination are required to have their children vaccinated. Opponents to vaccination are invited to discuss their objections with the staff of vaccination centers (medical doctors, trained nurses) who encourage vaccination for the benefit of both individuals and the community at large. Noneligible children are exonerated. Parents/legal guardians who choose to opt out despite having been repeatedly invited are asked to sign a waiver. As a consequence of this policy, approximately 20 million people (34 birth cohorts) have been vaccinated against hepatitis B with an outstanding record of safety and efficacy. This resulted in a substantial decrease in the burden of disease, carrier rate, and HBV-related morbidity and mortality [8, 9]. Despite this success, there are still cases of acute hepatitis B that are reported to the national surveillance system for acute viral hepatitis (SEIEVA [Sistema Epidemiologico Integrato dell′Epatite Virale Acuta], Istituto Superiore di Sanità, Rome), even among individuals who have been vaccinated or who should have been vaccinated as they were eligible for vaccination.
Our aim in this study was to assess the proportion of successfully vaccinated individuals in cases with acute hepatitis B, the proportion of cases that could have been avoided if fully and timely vaccinated, and reasons of failures.
METHODS
National Surveillance System for Acute Viral Hepatitis
SEIEVA is a national passive surveillance system that is a supplement to the Italian Official Surveillance System for Infectious Diseases. It was implemented in 1984 [10]; over 3 decades, SEIEVA has included a network of Italian local health units (LHUs), covering a mean of 60% of the Italian population (approximately 36 million people). As described by Tosti et al [11] and according to the ongoing protocol, clinical acute cases are reported by physicians in charge of the diagnosis to the pertaining LHU. Participation is voluntary. Demographic, clinical, epidemiological, and laboratory data as well as information on risk factors and hepatitis B vaccination status are collected and maintained in a dedicated database. Information about vaccination schedule (timing, number of doses, and type of vaccine administered) is further confirmed for each case by reviewing the vaccination registries of the LHUs. All SEIEVA information can be found on their website (www.iss.it/seieva/).
Study Design and Population
This was a retrospective descriptive study of acute hepatitis B cases reported to SEIEVA between 1993 and 2014. Within the SEIEVA surveillance system, the definition of an acute hepatitis B case is based on clinical and serological criteria including acute symptoms consistent with viral hepatitis, significant (more than 10-fold) increase in serum alanine amino transferase (ALT), immunoglobulin M anti-HBc antibody positivity.
Cases with a previous hepatitis B vaccination were analyzed to assess whether they were properly vaccinated. For the purposes of this study, a properly vaccinated case was defined as a diagnosis of acute hepatitis B (clinical breakthrough) in an individual who had been previously immunized according to our nationally recommended schedule of vaccination (3 doses given at 3, 5, and 11 months of age in infants and at 0, 3, and 6 months in children and adults) and had symptom onset at least 6 months (maximum incubation period) after completing a full vaccination course. This definition of HBV breakthrough does not take into account whether the vaccine recipient developed a post-vaccination immune response (anti-HBs antibody ≥10 mIU/mL as measured 1–3 months after administration of the last dose of the initial series), since post-vaccination testing is not routinely performed in Italy.
All cases with missing information on hepatitis B vaccination status were excluded from the analysis. Moreover, unvaccinated cases were studied to determine whether they belonged to high-risk groups or to groups for which vaccination is mandatory. To gather information about missing vaccinations, an ad hoc questionnaire was administered to households with HBsAg chronic carriers who were aware of the chronic infectious status of their relatives and acquired acute disease.
All data were anonymously analyzed in accordance with Italian privacy legislation.
Virological Testing
Molecular characterization of the specific viral strain causing hepatitis was possible in only a few cases. In particular, the amplification of the S gene of HBV DNA was performed in sera collected during the course of acute hepatitis B. Then, the HBV genotypes and the presence of mutations in the “a” determinant, that is, the neutralizing epitope within the major hydrophilic region of the HBsAg, were determined by direct sequence analysis [12].
Statistical Analyses
Pearson χ2 test or Fisher exact test, when necessary, were used to assess differences in discrete characteristics between groups, such as vaccinated/unvaccinated cases, geographical areas, and year of diagnosis. The Kruskal–Wallis test was used to investigate the significance of by-group differences in continuous variables.
P values <.05 were considered statistically significant. All statistical procedures were performed using the STATA statistical package, version 13.1.
RESULTS
As shown in Figure 1, 12 472 cases of acute hepatitis B were reported to SEIEVA between 1993 and 2014. Information on hepatitis B vaccination status was available for 11 311 (90.7%) cases, which were included in the study population. The median age was 36 years (range, 0–100) and 74.9% were males. During this period, the median age of individuals with hepatitis B significantly rose from 26 years in 1993 to 46 years in 2014 (P < .001).
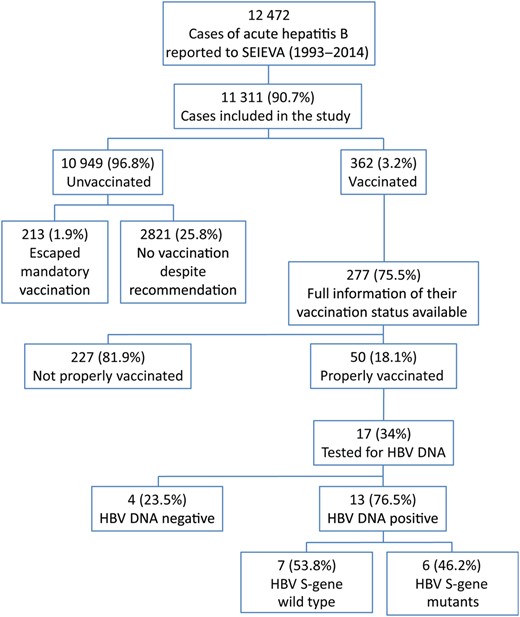
Study design. Abbreviations: HBV, hepatitis B virus; SEIEVA, Sistema Epidemiologico Integrato dell'Epatite Virale Acuta.
Within the study population, 362 cases (3.2%) occurred in individuals who had received at least 1 dose of vaccine. The yearly percentage of cases in vaccinated individuals did not significantly change during the study period. Complete information on vaccination history (ie, number of doses of vaccine administered, time between doses) was available for 277 (76.5%) vaccinees. In most of these (n = 227; 81.9%), the vaccination schedule was not completed properly. Vaccine was administered post-exposure in 151 cases, with a median time lapse between vaccination and onset of disease of 31 days (range, 5–175). Moreover, 40 individuals were administered an incomplete schedule (24 received 1 dose, 16 received 2 doses), and 36 individuals received 3 doses but with an incorrect interval between them (1 individual received the second dose 25 months after the first dose and 35 individuals had a median span of 19 months [range, 16–41] between the second and the third doses). The remaining 50 (18.1%) individuals (74% males; median age 30 years, range, 2–79 years) developed acute hepatitis B after a median of 7.5 years (range, 223 days–21.5 years), despite having been given a full course of vaccination according to the correct schedule and before exposure to HBV. These cases were uniformly distributed throughout the observation period. Table 1 shows a comparison of demographic characteristics, risk factors, clinical characteristics, and liver function tests between correctly vaccinated and unvaccinated cases. Vaccinated individuals were significantly younger, less frequently hospitalized, and had lower levels of ALT and aspartate amino transferase compared with unvaccinated cases. The percentage of intravenous drug users (IVDUs) and households with HBsAg chronic carriers was significantly higher among vaccinated than among unvaccinated individuals.
Comparison Between Properly Vaccinated Cases and Unvaccinated Cases, by Demographic Characteristics, Risk Factors, Hospitalization Rate, and Clinical and Biochemical Parameters
| Characteristic . | Properly Vaccinated Cases . | Unvaccinated Cases . | P Valuea . | ||
|---|---|---|---|---|---|
| N = 50 . | N = 10 949 . | ||||
| N . | % . | N . | % . | ||
| Gender | |||||
| Male | 37 | 74.0 | 8176 | 75.2 | ns |
| Female | 13 | 26.0 | 2703 | 24.8 | |
| Age (y) | |||||
| 0–14 | 5 | 10.0 | 109 | 1.0 | <.001 |
| 15–24 | 13 | 26.0 | 1552 | 14.3 | |
| 25–34 | 13 | 26.0 | 3377 | 31.0 | |
| 35–54 | 17 | 34.0 | 4217 | 38.7 | |
| ≥55 | 2 | 4.0 | 1632 | 15.0 | |
| Risk factorb | |||||
| Intravenous drug use | 10 | 20.4 | 1139 | 10.6 | .035 |
| Nosocomial exposurec | 8 | 16.0 | 1863 | 17.1 | ns |
| Parenteral exposured | 15 | 30.6 | 3488 | 32.1 | ns |
| Dental therapy | 16 | 32.7 | 3312 | 30.8 | ns |
| Household Hepatitis B surface antigen + | 8 | 21.6 | 871 | 10.4 | .026 |
| >2 sexual partner | 7 | 22.6 | 2190 | 27.7 | ns |
| Unsafe sexual practicese | 7 | 17.1 | 2076 | 20.9 | ns |
| Clinical characteristics and liver function tests | |||||
| Hospitalization | 43 | 86.0 | 10 176 | 93.5 | .042 |
| Jaundice | 42 | 84.0 | 9239 | 85.2 | ns |
| Aspartate amino transferase: mean (SD) | 1060 (812) | 1453 (1059) | .039 | ||
| Alanine amino transferase: mean (SD) | 1730 (1213) | 2357 (1375) | .011 | ||
| Total bilirubin: mean (SD) | 8.3 (6.7) | 11.9 (11.4) | .057 | ||
| Direct bilirubin: mean (SD) | 10.0 (20.0) | 9.0 (10.1) | ns | ||
| International Normalized Ratio: mean (SD) | 1.2 (0.1) | 1.5 (1.0) | ns | ||
| Characteristic . | Properly Vaccinated Cases . | Unvaccinated Cases . | P Valuea . | ||
|---|---|---|---|---|---|
| N = 50 . | N = 10 949 . | ||||
| N . | % . | N . | % . | ||
| Gender | |||||
| Male | 37 | 74.0 | 8176 | 75.2 | ns |
| Female | 13 | 26.0 | 2703 | 24.8 | |
| Age (y) | |||||
| 0–14 | 5 | 10.0 | 109 | 1.0 | <.001 |
| 15–24 | 13 | 26.0 | 1552 | 14.3 | |
| 25–34 | 13 | 26.0 | 3377 | 31.0 | |
| 35–54 | 17 | 34.0 | 4217 | 38.7 | |
| ≥55 | 2 | 4.0 | 1632 | 15.0 | |
| Risk factorb | |||||
| Intravenous drug use | 10 | 20.4 | 1139 | 10.6 | .035 |
| Nosocomial exposurec | 8 | 16.0 | 1863 | 17.1 | ns |
| Parenteral exposured | 15 | 30.6 | 3488 | 32.1 | ns |
| Dental therapy | 16 | 32.7 | 3312 | 30.8 | ns |
| Household Hepatitis B surface antigen + | 8 | 21.6 | 871 | 10.4 | .026 |
| >2 sexual partner | 7 | 22.6 | 2190 | 27.7 | ns |
| Unsafe sexual practicese | 7 | 17.1 | 2076 | 20.9 | ns |
| Clinical characteristics and liver function tests | |||||
| Hospitalization | 43 | 86.0 | 10 176 | 93.5 | .042 |
| Jaundice | 42 | 84.0 | 9239 | 85.2 | ns |
| Aspartate amino transferase: mean (SD) | 1060 (812) | 1453 (1059) | .039 | ||
| Alanine amino transferase: mean (SD) | 1730 (1213) | 2357 (1375) | .011 | ||
| Total bilirubin: mean (SD) | 8.3 (6.7) | 11.9 (11.4) | .057 | ||
| Direct bilirubin: mean (SD) | 10.0 (20.0) | 9.0 (10.1) | ns | ||
| International Normalized Ratio: mean (SD) | 1.2 (0.1) | 1.5 (1.0) | ns | ||
Data from SEIEVA (Sistema Epidemiologico Integrato dell′Epatite Virale Acuta) 1993–2014.
Abbreviations: ns, not statistically significant; SD, standard deviation.
a Obtained using Mann–Whitney test for continuous variables and χ2 test (or Fisher exact test) for discrete characteristics.
b Each case may report more than 1 risk factor.
c Hospitalization, hemodialysis, surgical intervention, endoscopy, blood transfusion.
d Piercing, tattooing, acupuncture, manicurist/chiropodist attendance, barber-shop shaving.
e Condom use (occasional/never vs always) during occasional sexual intercourses.
Comparison Between Properly Vaccinated Cases and Unvaccinated Cases, by Demographic Characteristics, Risk Factors, Hospitalization Rate, and Clinical and Biochemical Parameters
| Characteristic . | Properly Vaccinated Cases . | Unvaccinated Cases . | P Valuea . | ||
|---|---|---|---|---|---|
| N = 50 . | N = 10 949 . | ||||
| N . | % . | N . | % . | ||
| Gender | |||||
| Male | 37 | 74.0 | 8176 | 75.2 | ns |
| Female | 13 | 26.0 | 2703 | 24.8 | |
| Age (y) | |||||
| 0–14 | 5 | 10.0 | 109 | 1.0 | <.001 |
| 15–24 | 13 | 26.0 | 1552 | 14.3 | |
| 25–34 | 13 | 26.0 | 3377 | 31.0 | |
| 35–54 | 17 | 34.0 | 4217 | 38.7 | |
| ≥55 | 2 | 4.0 | 1632 | 15.0 | |
| Risk factorb | |||||
| Intravenous drug use | 10 | 20.4 | 1139 | 10.6 | .035 |
| Nosocomial exposurec | 8 | 16.0 | 1863 | 17.1 | ns |
| Parenteral exposured | 15 | 30.6 | 3488 | 32.1 | ns |
| Dental therapy | 16 | 32.7 | 3312 | 30.8 | ns |
| Household Hepatitis B surface antigen + | 8 | 21.6 | 871 | 10.4 | .026 |
| >2 sexual partner | 7 | 22.6 | 2190 | 27.7 | ns |
| Unsafe sexual practicese | 7 | 17.1 | 2076 | 20.9 | ns |
| Clinical characteristics and liver function tests | |||||
| Hospitalization | 43 | 86.0 | 10 176 | 93.5 | .042 |
| Jaundice | 42 | 84.0 | 9239 | 85.2 | ns |
| Aspartate amino transferase: mean (SD) | 1060 (812) | 1453 (1059) | .039 | ||
| Alanine amino transferase: mean (SD) | 1730 (1213) | 2357 (1375) | .011 | ||
| Total bilirubin: mean (SD) | 8.3 (6.7) | 11.9 (11.4) | .057 | ||
| Direct bilirubin: mean (SD) | 10.0 (20.0) | 9.0 (10.1) | ns | ||
| International Normalized Ratio: mean (SD) | 1.2 (0.1) | 1.5 (1.0) | ns | ||
| Characteristic . | Properly Vaccinated Cases . | Unvaccinated Cases . | P Valuea . | ||
|---|---|---|---|---|---|
| N = 50 . | N = 10 949 . | ||||
| N . | % . | N . | % . | ||
| Gender | |||||
| Male | 37 | 74.0 | 8176 | 75.2 | ns |
| Female | 13 | 26.0 | 2703 | 24.8 | |
| Age (y) | |||||
| 0–14 | 5 | 10.0 | 109 | 1.0 | <.001 |
| 15–24 | 13 | 26.0 | 1552 | 14.3 | |
| 25–34 | 13 | 26.0 | 3377 | 31.0 | |
| 35–54 | 17 | 34.0 | 4217 | 38.7 | |
| ≥55 | 2 | 4.0 | 1632 | 15.0 | |
| Risk factorb | |||||
| Intravenous drug use | 10 | 20.4 | 1139 | 10.6 | .035 |
| Nosocomial exposurec | 8 | 16.0 | 1863 | 17.1 | ns |
| Parenteral exposured | 15 | 30.6 | 3488 | 32.1 | ns |
| Dental therapy | 16 | 32.7 | 3312 | 30.8 | ns |
| Household Hepatitis B surface antigen + | 8 | 21.6 | 871 | 10.4 | .026 |
| >2 sexual partner | 7 | 22.6 | 2190 | 27.7 | ns |
| Unsafe sexual practicese | 7 | 17.1 | 2076 | 20.9 | ns |
| Clinical characteristics and liver function tests | |||||
| Hospitalization | 43 | 86.0 | 10 176 | 93.5 | .042 |
| Jaundice | 42 | 84.0 | 9239 | 85.2 | ns |
| Aspartate amino transferase: mean (SD) | 1060 (812) | 1453 (1059) | .039 | ||
| Alanine amino transferase: mean (SD) | 1730 (1213) | 2357 (1375) | .011 | ||
| Total bilirubin: mean (SD) | 8.3 (6.7) | 11.9 (11.4) | .057 | ||
| Direct bilirubin: mean (SD) | 10.0 (20.0) | 9.0 (10.1) | ns | ||
| International Normalized Ratio: mean (SD) | 1.2 (0.1) | 1.5 (1.0) | ns | ||
Data from SEIEVA (Sistema Epidemiologico Integrato dell′Epatite Virale Acuta) 1993–2014.
Abbreviations: ns, not statistically significant; SD, standard deviation.
a Obtained using Mann–Whitney test for continuous variables and χ2 test (or Fisher exact test) for discrete characteristics.
b Each case may report more than 1 risk factor.
c Hospitalization, hemodialysis, surgical intervention, endoscopy, blood transfusion.
d Piercing, tattooing, acupuncture, manicurist/chiropodist attendance, barber-shop shaving.
e Condom use (occasional/never vs always) during occasional sexual intercourses.
Molecular characterization was performed on sera collected from 17 of the 50 (34%) individuals who were successfully vaccinated and showed that 13 of them were HBV-DNA positive. Genotype F was found in 5 cases, genotype D in 4 cases, and genotype A in 2 cases, while genotypes B and E were found in 1 case each. Sequence analysis of the viral “a” determinant showed that 7 of these cases were infected with wild-type HBV, while 6 cases carried S-gene mutations (Table 2). Three of the 6 cases infected with mutant viruses had cocirculating seroprotective concentrations of anti-HBs antibody (≥10 mIU/mL).
Molecular Characteristics of 6 Cases of Acute Hepatitis B in Individuals Properly Vaccinated and Infected With Mutant Hepatitis B Viruses
| Case Number . | HBV Genotype . | Mutation . | Anti-HBs . |
|---|---|---|---|
| 1 | D | G145R | ≥10 mIU/mL |
| 2 | F | T118K | ≥10 mIU/mL |
| 3 | F | T126A | ≥10 mIU/mL |
| 4 | D | Q129H | Negative |
| 5 | D | D144E | Negative |
| 6 | B | T143M | Negative |
| Case Number . | HBV Genotype . | Mutation . | Anti-HBs . |
|---|---|---|---|
| 1 | D | G145R | ≥10 mIU/mL |
| 2 | F | T118K | ≥10 mIU/mL |
| 3 | F | T126A | ≥10 mIU/mL |
| 4 | D | Q129H | Negative |
| 5 | D | D144E | Negative |
| 6 | B | T143M | Negative |
Abbreviation: HBV, hepatitis B virus.
Molecular Characteristics of 6 Cases of Acute Hepatitis B in Individuals Properly Vaccinated and Infected With Mutant Hepatitis B Viruses
| Case Number . | HBV Genotype . | Mutation . | Anti-HBs . |
|---|---|---|---|
| 1 | D | G145R | ≥10 mIU/mL |
| 2 | F | T118K | ≥10 mIU/mL |
| 3 | F | T126A | ≥10 mIU/mL |
| 4 | D | Q129H | Negative |
| 5 | D | D144E | Negative |
| 6 | B | T143M | Negative |
| Case Number . | HBV Genotype . | Mutation . | Anti-HBs . |
|---|---|---|---|
| 1 | D | G145R | ≥10 mIU/mL |
| 2 | F | T118K | ≥10 mIU/mL |
| 3 | F | T126A | ≥10 mIU/mL |
| 4 | D | Q129H | Negative |
| 5 | D | D144E | Negative |
| 6 | B | T143M | Negative |
Abbreviation: HBV, hepatitis B virus.
During the study period, 10 949 cases of acute hepatitis B in unvaccinated individuals were reported to SEIEVA (Figure 1). Of these, 213 (1.9%) escaped mandatory vaccination. Their distribution showed a significant geographical gradient, with 1.2% and 1.7% in northern and central Italy, respectively, compared with 4.4% in southern Italy (P < .001). A total of 187 (87.8%) of 213 cases escaped mandatory vaccination as adolescents and 26 (12.2%) escaped as infants.
Acute hepatitis B was diagnosed in 2821 (25.8%) cases who were at increased risk of exposure to HBV and for whom hepatitis B vaccination was strongly recommended and offered free of charge. The trend of these cases showed a significant yearly decrease (150–250 cases/year in 1993–2001 vs 40–60 cases/year in 2010–2014; P < .001). As shown in Figure 2, most of the 2821 cases that were in high-risk groups were IVDUs (n = 1047; 37.1%), including 135 (12.9%) who referred to centers for the treatment of addictions, 843 (29.9%) households with HBsAg chronic carriers, and 800 (28.4%) homosexual/bisexual men. Among the households with HBsAg chronic carriers, 310 (36.8%) were aware of his/her relative's infectious status. SEIEVA collected information about the reasons for missed vaccination from 285 of these 310 (91.9%) cases. As shown in Table 3, the main reasons were lack of trust in the vaccination, negative attitude, and inaccurate beliefs (67.7%) followed by lack of or poor communication (10.5%) and low perceived severity of the disease.
Reasons for Nonvaccination Among Households With Hepatitis B Surface Antigen Chronic Carriers Who Were Aware of Their Relative's Infective Status
| Reason for Nonvaccination . | Number of Cases . | % . |
|---|---|---|
| Lack of trust in the vaccination, negative attitude, and inaccurate beliefs | 193 | 67.7 |
| Lack of or poor communication | 30 | 10.5 |
| Low perceived severity of the disease | 25 | 8.8 |
| Doubts about the vaccine's efficacy | 24 | 8.4 |
| Fear of side effects | 13 | 4.6 |
| Total | 285 | 100.0 |
| Reason for Nonvaccination . | Number of Cases . | % . |
|---|---|---|
| Lack of trust in the vaccination, negative attitude, and inaccurate beliefs | 193 | 67.7 |
| Lack of or poor communication | 30 | 10.5 |
| Low perceived severity of the disease | 25 | 8.8 |
| Doubts about the vaccine's efficacy | 24 | 8.4 |
| Fear of side effects | 13 | 4.6 |
| Total | 285 | 100.0 |
Data from SEIEVA (Sistema Epidemiologico Integrato dell′Epatite Virale Acuta) 1993–2014.
Reasons for Nonvaccination Among Households With Hepatitis B Surface Antigen Chronic Carriers Who Were Aware of Their Relative's Infective Status
| Reason for Nonvaccination . | Number of Cases . | % . |
|---|---|---|
| Lack of trust in the vaccination, negative attitude, and inaccurate beliefs | 193 | 67.7 |
| Lack of or poor communication | 30 | 10.5 |
| Low perceived severity of the disease | 25 | 8.8 |
| Doubts about the vaccine's efficacy | 24 | 8.4 |
| Fear of side effects | 13 | 4.6 |
| Total | 285 | 100.0 |
| Reason for Nonvaccination . | Number of Cases . | % . |
|---|---|---|
| Lack of trust in the vaccination, negative attitude, and inaccurate beliefs | 193 | 67.7 |
| Lack of or poor communication | 30 | 10.5 |
| Low perceived severity of the disease | 25 | 8.8 |
| Doubts about the vaccine's efficacy | 24 | 8.4 |
| Fear of side effects | 13 | 4.6 |
| Total | 285 | 100.0 |
Data from SEIEVA (Sistema Epidemiologico Integrato dell′Epatite Virale Acuta) 1993–2014.
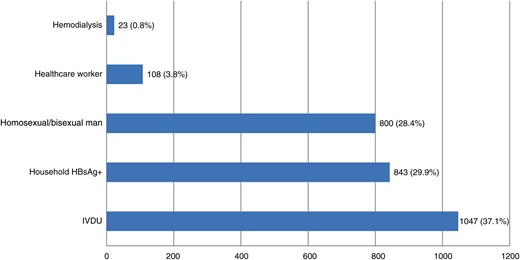
Indication for vaccination in 2821 unvaccinated individuals with acute hepatitis B belonging to high-risk group. Abbreviations: HBsAg, hepatitis B surface antigen; IVDU, intravenous drug users.
DISCUSSION
Viral hepatitis B is a vaccine-preventable disease. According to World Health Organization recommendations, more than 183 countries have implemented hepatitis B vaccination into their national childhood immunization programs [1]. Evidence shows that after completing a routine 3-dose schedule of vaccination, seroconversion to protective concentrations (≥10 mIU/mL) of anti-HBs is reached in more than 95% of healthy vaccine recipients, and that protection lasts more than 15–20 years after vaccination, with no need for booster doses [13–17]. The introduction of hepatitis B vaccine led to a global decrease in the incidence of disease, HBV carrier rate, and HBV-related mortality due to cirrhosis or hepatocellular carcinoma (HCC). In Taiwan, after the implementation of universal hepatitis B vaccination in 1984, the HBsAg seropositive rate in children decreased from 10% to 0.9% in 2012 [18]. Concurrent with this decrease, a significant reduction was observed in the incidence of HCC among children and teenagers, indicating that the hepatitis B vaccine was the first vaccine used for a major human cancer [19, 20]. Similar results have been achieved in other previously hyperendemic locations such as Alaska, Gambia, China, and South Africa [21–24]. In Italy, where the vaccination of individuals at increased risk of HBV exposure started in 1983 and universal vaccination started in 1991, data from SEIEVA show a substantial decline in the overall incidence of acute hepatitis B from 5 per 100 000 inhabitants in 1990 to <0.8 in 2014 [25]. The decrease was even more evident in individuals aged 15–24 years and in those aged 0–14 years in whom morbidity rates (per 100 000) decreased, in the same period, from 17 to 0.3 (98% decrease) and from 1 to 0.03 (97% decrease), respectively. Moreover, the prevalence of HBV markers also substantially decreased in children and young adults after vaccination [8, 9]. A further benefit, due to the biological dependence of hepatitis delta virus from HBV, was that hepatitis delta also decreased significantly in Italy as a consequence of the implementation of hepatitis B vaccination [26–28].
Some long-term studies have documented HBV breakthrough, proven by seroconversion to anti-HBc antibody, while virtually no clinical disease or carriage has been reported to date [29, 30].
Between 1993 and 2014, 96.8% of the 11 311 cases reported to SEIEVA and included in this study occurred in unvaccinated individuals; in the remaining 362 (3.2%) cases, acute disease occurred in those who were administered at least 1 dose of vaccine. Complete information on vaccination status was collected for 277 of the 362 (75%) vaccinated individuals, showing that only 50 cases out of 227 (18.1%) had received a full primary course of vaccination according to the proper schedule and before exposure to HBV. Recalculation of the 18.1% of the total 362 cases in vaccinated individuals results in an estimated 66 breakthrough cases (correctly vaccinated before exposure) reported over a 22-year period to SEIEVA. Considering that during the period of surveillance, SEIEVA covered a mean of 60% of the entire Italian population (approximately 60 million), we estimate that 5 vaccination failures could occur in Italy each year. This clearly indicates that breakthrough infections are rare in Italy, where approximately 20 million individuals, or one third of the entire population, have been vaccinated. In this context, the herd immunity that is secondary to the large number of vaccinated individuals may have played a crucial role in the control and prevention of hepatitis B.
Correctly vaccinated cases were younger, hospitalized less frequently, and had lower ALT values than those who were not vaccinated. This could suggest some effect of vaccination in slowing the natural course of the disease, but this interpretation needs further confirmation. The most common risk factors identified among fully vaccinated individuals included intravenous drug use and a household contact with an HBsAg carrier; however, the reason for this association needs to be further investigated. The molecular characterization of the samples collected from 13 of the vaccinated individuals with breakthrough infection who were HBV-DNA positive showed that 7 of them were infected with HBV wild-type and 6 with S-gene mutants potentially able to evade the vaccine-induced immunity. Three of these cases developed acute hepatitis B despite the presence of protective levels of anti-HBs (≥ 10 mIU/mL). It has been reported that vaccination based on recombinant HBsAg from HBV genotypes A and D (the vaccines currently used worldwide) may be less effective toward genotypes E and F since they display marked differences from the other genotypes in the “a” determinant domain toward which neutralizing (protective) antibodies are largely targeted [31]. The fact that 6 (46.2%) cases of breakthrough infection were caused by genotype F (n = 5) or genotype E (n = 1), which are both quite rare in Italy, is intriguing even though is important to say that in Gambia, where genotype E is predominant [32], and in Alaska, where genotype F is highly diffused [33], vaccination had great success.
Despite the high coverage rate achieved over time in Italy, cases of acute hepatitis B continue to be reported among individuals who should have been vaccinated but were not, including a number of cases (213; 1.9%) who escaped mandatory vaccination. The majority of these (87.8%) were adolescents, most of whom lived in southern Italy.
In 25.8% of unvaccinated cases, acute hepatitis B was diagnosed in individuals at increased risk of exposure to HBV for whom hepatitis B vaccination is strongly recommended and offered free of charge. Grouping those who escaped mandatory vaccination with those who were not vaccinated despite being at high risk of infection, the disease could have been prevented in 27.7% (3034 of 10 949) of cases if duly vaccinated. However, the trend of such cases showed a significant yearly decrease (150–250 cases/year in 1993–2001 vs 40–60 cases/year in 2010–2014; P < .001), attributable to an increase in the proportion of vaccinated individuals and possibly to an increasing herd immunity effect.
Cohabitation with chronic HBsAg carriers, IVDU, and homosexual/bisexual practices were the major risk factors associated with acute hepatitis B. Here, we report an alarming number of missed opportunities for immunization in 13% of IVDUs who were being seen at treatment centers before the onset of acute hepatitis and in at least 37% of households with HBsAg carriers who were not vaccinated despite being aware of the condition of their cohabitant(s). For this latter group, data collected using an ad hoc questionnaire showed that lack of trust in the vaccination, negative attitude, and inaccurate beliefs followed by lack of or poor communication and low perceived severity of the disease were the most frequent reasons for vaccine hesitancy.
In conclusion, data from this study show that the Italian program of vaccination resulted in substantial progress toward the prevention and control of hepatitis B infection. Cases of acute hepatitis B reported in successfully vaccinated individuals are currently infrequent and rarely caused by vaccine-escape S gene viral mutants. Further efforts to achieve and maintain a high level of public confidence in the safety and efficacy of hepatitis B vaccination are essential to reaching high coverage rates, especially among individuals at increased risk of HBV infection.
A limitation of this study is that SEIEVA does not collect data on the follow-up of vaccinated individuals with hepatitis B, thus missing the opportunity to determine whether, in case of infection, vaccination can protect against the development of a chronic carrier state. However, since the risk of becoming a chronic carrier is known to be largely age dependent, with a higher frequency in younger individuals compared with adults [34], the fact that the median age of our cases increased from 26 years in 1993 to 46 years in 2014 is reassuring. The vaccine-induced shifting of infection to those who are older, in fact, favors a reduction in the rate of infected individuals who can develop a chronic carrier state. Further studies are needed to better clarify this issue.
Notes
Acknowledgments. We thank the study team and nurses whose collaboration was crucial to making this study possible.
The SEIEVA collaborating group included the following: Ferrigno L., Crateri S., Iantosca G., Badoni G., D′Angelo F (ISS); Sudano L., Ruffier M (Valle d'Aosta); Fischer M., Augschiller M., Gamper S., Foppa A., Lechthaler T., Thaler J., Steinmair B. (Prov Aut Bolzano); Grandi C., Carraro V., Franchini S. (Prov Aut Trento); Zotti C., Lanzafame P., Malaspina S., Gallone A., Castella A., Valenza G., Silano V., Tacca M.G., Iodice S., Marchisio A.M., Costantino A.M., Giovanetti F., Susani F. (Piemonte); Tagliacarne C., Donadini A., Nespoli C., Trezzi L., Gennati G., Monteverdi A., Boldori L., De Grada P., Gattinoni A., Brugnoli R., Belloni A., Binotto M., Pinciroli G., Pesci L., Senegaglia P., Crippa S., Altomonte G., Lodola S., Aquino I., Castelli N., Zecca E., Nieri M., Zecca F., Pasquale L., Piedacci G., Giompapa E. (Lombardia); Zorzut F., Rocco G., Brianti G., Gallo T., Zuliani M., Breda A., Feltrin O. (Friuli Venezia Giulia); Russo F., Zanella F., Mel R., Soppelsa M., Russo F., Zolin R., Todescato A., Bacciolo N., Rizzato D., Pupo A., Nicolardi L., Flora M., Boin F., De Sisti C., D'Ettore G., Caracciolo V., Penon M.G., Bellè M., Cafarra L., Zivelonghi G., Soffritti S., Foroni M. (Veneto); Finarelli A.C., Borrini B.M., Gualanduzzi C., Capra A., Sacchi A.R., Borrini B.M., Mattei G., Gardenghi L., Gianninoni A.R., Sancini R., Dalle Donne E., Rangoni R., Cova M., Bevilacqua L., Fiumana E., Bondi B., Pecci A. (Emilia Romagna); Mela M., Briata M.P., Michele P., Turello V., Opisso A., Zoppi G., Torracca P., Ricci M.A., Capellini A. (Liguria); Pecori L., Mazzotta F., Balocchini E., Ghiselli G., Marchini P., Di Vito A., Wanderlingh W., Raso E., Mazzoli F., Berti C., Galletti N., Grandi E., Ferrentino M.S., Marinari M.G., Lombardi A., Barbieri A., Bagnoli A., Bandini M., Lezzi I., Verdelli F., Beltrano A., Bindi R., Sansone C.M., Boncompagni G., Zacchini F., Baretti S., Baroncini O., Staderini C., Filidei P., Chiapparini L., Barghini F., Cadoni M. (Toscana); Tagliavento G., Fiacchini D., Damiani N., Pelliccioni A.R., Liverani A., Peccerillo G., Vaccaro A., Spadoni M.R., Rossini R., Pasqualini F., Priori A., Burattini N., Cimica S., Vitale V., Laici F., Migliozzi F., Moretti G., Ciarrocchi G., Impullitti S., Angelini C. (Marche); Tosti A., Giaimo M.D., Buscosi A., Pasquale A., Ciani C., Santocchia F., Proietti M.L., Paoloni M.C. (Umbria); Ercole A., Russo P., Cerocchi C., Grillo P., Loffredo M., Labriola V., Pendenza A., Nappi M.R., Bueti P., Santucci L., Mangiagli F., Varrenti D., Aquilani S., Dionette P., Corpolongo D., Di Luzio G. (Lazio); Di Giacomo M., Graziani M., Mancini C., Turchi C., Granchelli C., Soldato G., D'Eugenio F. Albanesi I. (Abruzzo); Ferrara M.A., Citarella A., Fossi E., Parlato A., Alfieri R., Scotto M., Caiazzo A.L. (Campania); Chironna M., Prato R., Matera R., Menolascina S., Colamaria R., Azzollini N., Madaro A., Scalzo G., Ancona A., Pedote P., Moffa G., Pagano I., Angelillis R., Ferraro M., Aprile V., Turco G.L., Minerba S., Caputi G. (Puglia); Negrone F., Maldini M., Russo T. (Basilicata); Aloia F., Giuffrida S. (Calabria); Mangione R., Consacra R., Cuccia M., Rinnone S. (Sicilia); Delogu F., Fracasso D., Saba A., Puggioni A., Frongia O., Marras M.V., Crasta M.G., Mereu G., Steri G.C., Santus S. (Sardegna).
Financial support. This work was supported by the Italian Ministry of Health.
Potential conflicts of interest. A. R. Z. has received a consulting honorarium from GlaxoSmithKline and from Sanofi Pasteur MSD. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
Author notes
Members of the SEIEVA Collaborating Group are listed in the Acknowledgments.




