-
PDF
- Split View
-
Views
-
Cite
Cite
Rosalind Foster, John McAllister, Tim R. Read, Anna B. Pierce, Robyn Richardson, Anna McNulty, Andrew Carr, Single-Tablet Emtricitabine-Rilpivirine-Tenofovir as HIV Postexposure Prophylaxis in Men Who Have Sex With Men, Clinical Infectious Diseases, Volume 61, Issue 8, 15 October 2015, Pages 1336–1341, https://doi.org/10.1093/cid/civ511
Close - Share Icon Share
Abstract
Background. Completion rates for human immunodeficiency virus (HIV) postexposure prophylaxis (PEP) are low. We investigated the adherence and safety of coformulated emtricitabine (FTC), rilpivirine (RPV), and tenofovir disoproxil fumarate (TDF) as a 3-drug, single-tablet regimen for PEP in men who have sex with men (MSM).
Methods. In an open-label, single-arm study at 2 public sexual health clinics and 2 hospital emergency departments in urban Australia, 100 HIV-uninfected MSM requiring 3-drug PEP received single-tablet FTC-RPV-TDF once daily for 28 days. The primary endpoint was premature PEP cessation or primary HIV infection through week 12. Additional endpoints were adherence (by self-report of doses missed or not ingested with a meal, by pill count, and by plasma concentrations of tenofovir and FTC at week 4); and safety (clinical and laboratory adverse events [AEs]).
Results. PEP completion was 92% (95% confidence interval, 85%–96%); premature cessation resulted from loss to follow-up (6%), AEs (1%), or study burden (1%). No participant was found to acquire HIV through week 12. Adherence was 98.6% (standard deviation [SD], 2.4) by pill count and 98.5% (SD, 2.7) by self-report; 86% reported taking all doses with food, and 88% of the subset tested had plasma tenofovir levels suggesting full adherence (>40 ng/mL). Eighty-eight participants experienced at least 1 clinical AE; 4 had grade 3 AEs or higher, possibly attributable to study drug. Fifty-six participants experienced at least 1 laboratory AE; 4 had AEs of grade 3 or higher, possibly attributable to study drug.
Conclusions. A single-tablet regimen of FTC-RPV-TDF was well tolerated as once-daily PEP, with high levels of adherence and completion.
Clinical Trials Registration. NCT01715636.
Antiretroviral therapy is widely used and recommended as postexposure prophylaxis (PEP) against human immunodeficiency virus (HIV) infection [1–6]. Evidence for efficacy, timing of initiation, and duration of therapy has been extrapolated from animal and observational human studies. Current Australian guidelines [5] recommend nonoccupational PEP (NPEP) for 28 days with 2 or 3 drugs (based on estimated exposure risk) starting within 72 hours of a transmission risk event. Current guidelines from the World Health Organization (WHO) [3], US Centers for Disease Control and Prevention (CDC) [1], European AIDS Clinical Society [2], and British HIV Association [4] recommend 3-drug PEP as the preferred option for standard of care regardless of exposure risk.
Recommended 3-drug regimens in Australia comprise 2 nucleoside reverse transcriptase inhibitors (NRTIs) plus a nucleotide reverse transcriptase inhibitor (NtRTI), or 2 NRTIs (can include an NtRTI) plus a protease inhibitor. Internationally, raltegravir is commonly prescribed as PEP, and is part of the CDC's occupational PEP guidelines–preferred regimen [6], on the basis of its tolerability and low chance of drug interactions. Raltegravir is, however, a twice-daily drug with the potential for hypersensitivity and acute muscle toxicity [7] and, in one PEP study, adherence to the regimen of tenofovir disoproxil fumarate (TDF), emtricitabine (FTC), and raltegravir was imperfect (only 52% took all 3 pills a day) [8].
PEP does not always prevent HIV acquisition, and failure has been linked to suboptimal adherence or discontinuation [9]. A recent meta-analysis of 97 studies reporting on 21 462 PEP initiations from 1988 to 2013 found that only 56.6% (95% confidence interval [CI], 50.9%–62.2%) completed a 28-day course of PEP. Completion was higher in the context of NPEP but was still only 65.6% (95% CI, 55.6%–75.6%) [10].
The use of a regimen that is well tolerated, safe, and easy to use could improve PEP adherence and completion. Whereas there is evidence that single-tablet regimens and lower pill burden regimens improve adherence in HIV-infected populations [11, 12], this has not been studied in the context of PEP. Measurement of plasma drug levels is a direct marker of adherence. A number of factors can contribute to variability in plasma concentrations, but an undetectable level suggests nonadherence [13].
Coformulated FTC-RPV-TDF (emtricitabine 200 mg, rilpivirine 25 mg, TDF 300 mg) is a once-daily single-tablet candidate PEP regimen. It has superior tolerability and similar efficacy to efavirenz-FTC-TDF in adults initiating antiretroviral therapy [14]. FTC, RPV, and TDF are absorbed rapidly, with Tmax of 1–2 hours, 4–5 hours, and 1 hour, respectively, postdose, potentially reducing time to acquisition of therapeutic drug levels. This is important, as PEP failure has been linked to delayed initiation [9]. FTC, RPV, and TDF all block HIV replication preintegration into the host cell genome, and thus make greater biological sense as prophylaxis than a protease inhibitor. A potential disadvantage of RPV is that it must be taken with food [15].
The key objectives of this study were to describe the completion rates, adherence, and safety of FTC-RPV-TDF as a 28-day PEP single-tablet regimen.
METHODS
Design
This was a multicenter, open-label, nonrandomized trial. One hundred eligible men who have sex with men (MSM) were assigned to receive FTC-RPV-TDF, 1 tablet, once daily with food for 28 days according to Australian NPEP guidelines for the use of 3-drug NPEP. Participants attended for up to 7 study visits over 12 weeks: visit 1 at baseline; visit 2 at days 3–5; visit 3 at days 12–14; visit 4 at days 26–28; visit 5 during week 5; and visits 6 and 7 during week 12. FTC-RPV-TDF was dispensed at visits 1, 2, and 3. Visits 5 and 7 (to give HIV serology and other key results) could be conducted by phone.
Study Settings
The study was conducted in 4 centers to which adults self-refer for possible PEP: Sydney Sexual Health Centre; St Vincent's Hospital, Sydney; Melbourne Sexual Health Centre; and The Alfred Hospital, Melbourne. Both sexual health clinics offer NPEP during normal working hours, and both hospital sites provide NPEP 24 hours a day. Patients eligible for 3-drug PEP were offered the option of participating in the study. St Vincent's Hospital Human Research Ethics Committee granted ethical and scientific approval of the study (approval number HREC/12/SVH/142). The protocol is registered at ClinicalTrials.gov (NCT01715636).
Eligibility Criteria
Patients were eligible for the study if they were HIV uninfected, healthy MSM aged ≥18 years; eligible for 3-drug PEP because of unprotected insertive or receptive anal intercourse with a known HIV-infected male source, or unprotected receptive anal intercourse with a male of unknown HIV status in the previous 72 hours; and provided written informed consent. The study was restricted to MSM because they are the principal target group for PEP in Australia, as they account for the overwhelming majority of PEP presentations and represent the majority of incident HIV infections. Patients were ineligible if they were taking any medication contraindicated with FTC-RPV-TDF, were on medication for hepatitis B, or if they had taken FTC-RPV-TDF previously for PEP. Participants would be subsequently withdrawn from the study if baseline screening showed serological evidence of HIV infection (including indeterminate serology consistent with possible primary HIV infection) or chronic/active hepatitis B, serum alanine aminotransaminase (ALT) >5 times the upper limit of normal, or serum estimated glomerular filtration rate (eGFR) <60 mL/minute/1.73 m2. Ineligible patients, participants who tested positive for HIV at baseline, those who withdrew after their “source” partner was found to be HIV-negative, and those who declined to participate were followed up as per usual site-specific protocols.
Assessments and Outcome Measures
The primary endpoint was the proportion of participants with premature cessation of PEP (prior to day 28—except for cessation if their “source” partner was subsequently found to be HIV uninfected or the participant was found to be HIV-infected at baseline) or primary HIV infection up to week 12.
The secondary endpoints were adherence (by self-report of doses missed, by self-report of doses not ingested with a meal, by pill count, and by plasma concentrations of tenofovir and FTC at week 4) and safety (proportion of participants with clinical adverse events [AEs], proportion of participants with laboratory AEs, and proportion of participants ceasing PEP due to AEs).
Baseline data on demographics, medical and sexual health, concomitant medication use, HIV risk behavior, and context of the risk event were collected. Data regarding subjective AEs were collected at each subsequent visit and graded according to the Division of AIDS Table for Grading the Severity of Adult and Pediatric Adverse Events (version 1.0). Adherence was assessed by pill counts and self-report at visits 2, 3, 4, and 5. Concomitant medication and HIV risk behavior were recorded at visits 1, 2, 3, 4, and 6. All participants were given standardized education and counseling, according to the treating center's standard of care, regarding HIV risk, the risks and benefits of PEP, and adherence. All participants were contacted if an appointment was missed, and received SMS (text messaging) reminders prior to appointments.
Blood was sampled and tested at baseline (pre-PEP), week 2, week 4, and week 12. Standard of care baseline serological screening for HIV, hepatitis B, and hepatitis C was performed. Repeat HIV serology was performed at weeks 4 and 12, along with sexually transmitted infection screening at week 2, and repeat serology for hepatitis C and syphilis at week 12. Study-specific testing included baseline, week 2, and week 4 biochemistry (urea, sodium, potassium, calcium, phosphate, creatinine, eGFR), liver function tests (total protein, albumin, ALT, alkaline phosphatase, γ-glutamyl transferase, bilirubin), glucose, amylase, lipase, creatine kinase, and lactate. In addition, baseline plasma was stored for pre-NPEP HIV genotype/RNA testing in the event that baseline or subsequent HIV serology returned a positive result. Freshly voided urine was collected at baseline and week 4 for dipstick analysis.
The protocol was amended to include plasma sampling for FTC and tenofovir concentration levels using high-performance liquid chromatographic separation in the final 50 participants at week 4 while on treatment (Clinical Pharmacology Analytical Laboratory, Johns Hopkins University School of Medicine, Baltimore, Maryland). A plasma tenofovir level >40 ng/mL is thought to indicate recent full adherence [16], and a level <10 ng/mL suggests no dose for at least 7 days. Absence of FTC in a plasma sample is thought to represent no drug in at least 1 week [17].
Sample Size and Data Analysis
For an estimated completion rate of 95%, 100 patients would give a precision around the completion rate of approximately ±4.3% with 95% CIs ranging from 90.7% to 99.3%. Analyses were performed on the intention-to-treat population unless otherwise stated. Descriptive statistics were calculated. Tests of significance included tests of correlation using Pearson ρ, interrater agreement between self-report and pill count using Cohen κ statistic, and variability in laboratory parameters over follow-up using a mixed-effects/random-effects longitudinal regression model with a last-observation-carried-forward approach, and an available data sensitivity analysis. Significance was set at .05 and CIs at 95%.
RESULTS
Baseline Characteristics
Between 23 December 2012 and 12 June 2014, 736 men received 3-drug PEP across the 4 centers, of whom 100 were enrolled (mean age, 31 years [standard deviation {SD}, 9 years]) (Figure 1). The majority of nonrecruitment in the 2 emergency department settings was because of presentation at a time that a study coordinator was not available. Presentation was a mean of 30 hours (range, 2–73; SD, 21) after anal sex, of which 88% was receptive. Sixty-five percent had not used a condom, 29% used a condom that broke or slipped off, and 6% reported the source partner had removed the condom. Thirty-nine participants reported that the source was HIV infected; of these participants, 5 (13%) were reported to have a detectable viral load and 17 (44%) had an unknown viral load. Thirty-four participants reported HIV risk in the preceding 12 weeks, and 48% reported having received previous PEP with a mean of 1 (range, 0–6; SD, 1) prior episode.
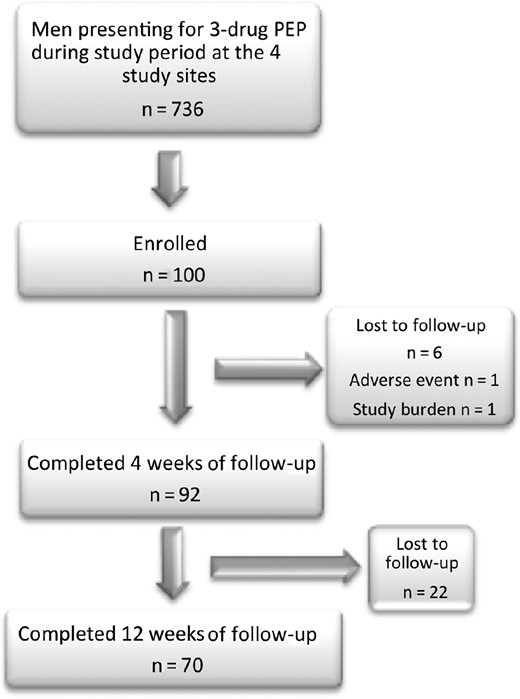
Consolidated Standards of Reporting Trials (CONSORT) diagram. Abbreviation: PEP, postexposure prophylaxis.
Treatment Outcomes
PEP was commenced a mean of 2 hours (SD, 2.3 hours) after presentation. No participant withdrew because he was found to be HIV infected at enrollment or was withdrawn later because his sexual “source” partner was found to be HIV uninfected.
PEP completion was 92% (95% CI, 85%–96%). Premature cessations occurred at a median of 14 days (range, 2–22 days) due to loss to follow-up (6%), AE (1%), and study burden (1%). The 1 participant who withdrew due to AEs experienced grade 3 clinical depression, difficulty concentrating, and fatigue, plus grade 1 diarrhea and acne. Previous PEP use was not associated with noncompletion (data not shown). No participant was found to acquire HIV through week 12 (n = 70).
Adherence
PEP adherence was 98.5% (range, 89%–100%; SD, 2.7) by self-report in the 92 participants who completed 28 days of follow-up, of whom 67 (73%) reported no missed doses, and 86 of 91 (95%) for whom data was available reported all doses were taken with food. No participant reported missing >3 doses. In the 78 participants who returned their pill bottles at day 28, adherence by pill count was 98.6% (range, 93–100; SD, 2.4). Seventy-eight paired assessments for percentage of adherence by pill count and by self-report showed 100% agreement (expected agreement, 57.4%; κ statistic 1.0; P < .0001). Greater than 90% adherence was self-reported by 98% of the 92 participants for whom data were available, and by 90% of participants in intention-to-treat analysis.
From the final 50 participants, plasma tenofovir and FTC levels were measured at week 4 within 48 hours (mean, 16 hours; SD, 10) of the last dose in 41 (82%) participants (Figure 2). Of these, 36 participants (88%) had tenofovir levels >40 ng/mL, 4 (10%) 20–40 ng/mL, and 1 (2%) <10 ng/mL; all 41 participants (100%) had FTC levels >0 ng/mL. Correlation between plasma tenofovir and FTC levels was high (Pearson ρ = 0.89; P < .0001; Figure 2). Despite their suboptimal plasma levels, all 5 participants with tenofovir levels <40 ng/mL reported 100% adherence; 3 of the 4 with levels between 20 ng/mL and 40 ng/mL had been sampled ≥20 hours after the final dose.
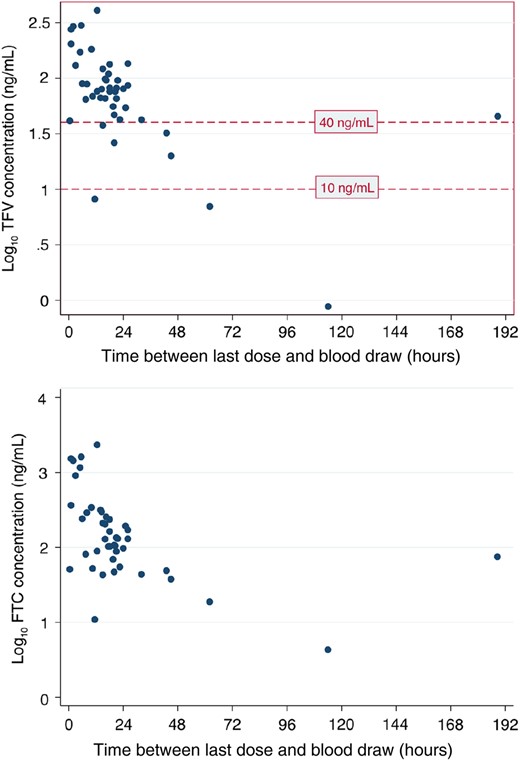
Log plasma concentrations of tenofovir (TFV) and emtricitabine (FTC), by elapsed time since previous dose.
Safety
Eighty-eight participants experienced 1 or more clinical AEs. The most common clinical AEs were fatigue (34%) and nausea (23%). Twenty participants experienced a clinical grade 2 or higher AE, and 4 participants experienced grade 3 or higher clinical AEs possibly attributable to study drug.
Fifty-six participants experienced 1 or more laboratory AEs. Twenty-nine participants experienced a grade 2 or higher laboratory AE, and 4 patients experienced grade 3 or higher laboratory AEs possibly attributable to study drug.
One participant developed grade 2 symptoms (acute abdominal pain and vomiting) and grade 4 laboratory evidence (lipase 872 IU/L) of acute pancreatitis within 1 week of completing PEP. The episode resolved within 21 days, without the need for hospitalization. There were no recognized underlying risk factors for acute pancreatitis.
Serum creatinine rose modestly from 83 µmol/L to 88 µmol/L from baseline to week 4 (P < .001) by both intention-to-treat and available-data analyses, with no grade 1 or higher toxicities, and no significant change in mean serum phosphate level. One participant tested positive for protein on urinalysis at week 4, which had not been present at baseline. The proportion of participants with eGFR 60–90 mL/minute/1.73 m2 and 30–60 mL/minute/1.73 m2 at week 4 increased from 30% to 43% and from 0% to 2%, respectively. There was no significant correlation between tenofovir plasma concentration and change in eGFR or creatinine from baseline.
DISCUSSION
In this population, the proportion of participants with premature cessation of FTC-RPV-TDF PEP was low (8%), and there were no cases of primary HIV infection up to week 12 in those tested (70/92 [76%]).
The completion rate of 92% (95% CI, 85%–96%) is favorable compared with completion rates from recently published studies: 65.6% (95% CI, 55.6%–75.6%) for NPEP, and 67.2% (95% CI, 59.5%–74.9%) for PEP in MSM in a recently published meta-analysis [10] of PEP in 1998–2012; 89% (SD, 29) in a similar study of MSM taking FTC-TDF-RAL NPEP [8]; and 63.4%–68.5% for FTC-TDF-lopinavir/ritonavir, 88.4% for FTC-TDF-maraviroc, and 76.3% for FTC-TDF-raltegravir in 2 recent randomized controlled trials [18].
Adherence rates compare favorably to other published PEP studies; the percentage of participants with ≥90% adherence by self-report was 98% by available data, and there was excellent correlation between different measures of adherence (pill count and self-report). For the participants who completed the PEP course, dosing with food did not appear to be problematic; 95% reported that all doses were taken with food. Only 1 of 36 (2%) participants with relevant plasma tenofovir level data had a level <10 ng/mL, and all participants had detectable plasma FTC levels.
The proportion of participants ceasing PEP due to AEs was low (1%), as was the proportion of participants with documented clinical (4%) and laboratory (4%) grade 3 or higher AEs possibly attributed to study drug. There was no serious AE recorded.
It is worth noting that study drug was commenced a mean of 2 hours (SD, 2.3) after presentation, whereas in a similar study using raltegravir plus FTC/TDF in Sydney, the mean was 1 hour (SD, 1.2) [8]. This could have been related to the fact that RPV needs to be dosed with food, and so could not always be taken immediately at the time of clinical presentation.
Our study has limitations. This was an open-label study, so participants knew what side effects they might experience. We only studied MSM, so our findings may not apply to other populations receiving PEP. We did not exclude those who had previously received PEP, which could have biased uptake, completion, and tolerance of this PEP regimen based on their prior experience (good or bad) of PEP including TDF and FTC. We studied FTC-RPV-TDF in a population with a low background of transmitted NRTI (4.1%) and nonnucleoside reverse transcriptase inhibitor (3.1%) resistance [19]; use of this combination in other settings with higher rates of resistance might need to be considered more carefully. The study was not powered to measure efficacy in preventing infection. Selection bias should be considered in view of the fact that only 100 of a potential 736 eligible men were enrolled. Participants received more intensive follow-up than is standard of care in the participating centers, with greater frequency of follow-up and more intensive efforts to recall participants. Despite this, 24% (22/92) of those who completed 28 days of PEP did not attend 12-week follow-up; this compares favorably with published data on follow-up [10], but could introduce bias and must be considered when interpreting the 100% noninfection rate in this study.
In conclusion, a single-tablet regimen of FTC-RPV-TDF was well tolerated as once-daily PEP, with high levels of adherence and completion. Other single-tablet regimens should be evaluated as PEP.
Notes
Acknowledgments. The authors acknowledge the 100 men who took part in this study; Dr Stephen Kerr from Thai Red Cross AIDS Research Centre, St Vincent's Hospital Centre for Applied Medical Research, for his statistical analysis of the study data; Mark Marzinke, Director, Johns Hopkins Clinical Pharmacology Analytical Laboratory for plasma drug level measurement; and Professor Craig Hendrix for advice and interpretation of plasma drug level results.
Disclaimer. Gilead Sciences had no input into study design or analyses.
Financial support. This work was supported in part by a Gilead Sciences educational grant, including study drug provision.
Potential conflicts of interest. A. C. has received research funding from Gilead Sciences, MSD, Pfizer, and ViiV Healthcare; has received consultancy fees from Gilead Sciences, MSD, and ViiV Healthcare; has received lecture and travel sponsorships from Bristol-Myers Squibb, Gilead Sciences, Janssen, MSD, and ViiV Healthcare; and has served on advisory boards for Gilead Sciences, MSD, and ViiV Healthcare. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- plasma drug concentration
- hiv
- australia
- emergency service, hospital
- follow-up
- food
- plasma
- safety
- tablet dosage form
- arm
- tenofovir
- hiv infection, primary
- emtricitabine
- ingestion
- hiv postexposure prophylaxis
- post-exposure prophylaxis
- rilpivirine
- health clinic
- surrogate endpoints
- adverse event
- emtricitabine/rilpivirine /tenofovir disoproxil
- men who have sex with men
- self-report




