-
Views
-
Cite
Cite
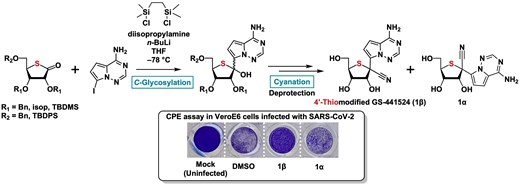
Nana Mihara, Yuhei Nogi, Noriko Saito-Tarashima, Takaaki Koma, Masako Nomaguchi, Noriaki Minakawa, Synthesis of 4′-thiomodified GS-441524, a nucleoside unit of Remdesivir, as an anti-SARS-CoV-2 agent, Chemistry Letters, Volume 54, Issue 5, May 2025, upaf080, https://doi.org/10.1093/chemle/upaf080
Close - Share Icon Share
Abstract
The COVID-19 pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), created an urgent need for effective antiviral treatments. While Remdesivir (GS-5734) and its parent nucleoside GS-441524 have been explored as anti-SARS-CoV-2 agents, their use is limited by toxicity concerns. Here we synthesized a 4′-thiomodified derivative of GS-441524 (1β) using a refined C-glycosylation strategy. In VeroE6 cells infected with SARS-CoV-2, 1β demonstrated modest antiviral activity without detectable cytotoxicity. Although its potency was lower than Remdesivir, its favorable safety profile suggests 1β has potential as a safer antiviral for RNA viruses.