-
Views
-
Cite
Cite
Yunxia Wang, Yanlong Ma, Yifa Du, Fayan Zhu, Guosheng Shi, Yongquan Zhou, Min Wang, Unveiling the structure of aqueous calcium nitrate solutions by x-ray scattering, Chemistry Letters, Volume 54, Issue 4, April 2025, upaf070, https://doi.org/10.1093/chemle/upaf070
Close - Share Icon Share
Abstract
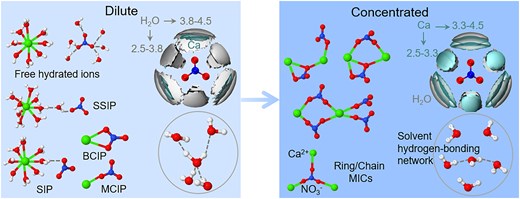
This study investigates Ca(NO3)2 solutions across varying concentrations, revealing that Ca2+ maintains a coordination number of ∼8 whereas NO3⁻ hydration decreases with concentration. At high concentrations, NO3⁻ partially replaces water molecules in the first coordination shell of Ca2+, forming ion pairs and clusters via bidentate and monodentate coordination modes. Ion aggregation disrupts and restructures the hydrogen-bond network. These findings offer essential insights into ion–solvent interactions in concentrated electrolytes.