-
PDF
- Split View
-
Views
-
Cite
Cite
Qiulei Wang, Shaodan Chen, Zhenhong Guo, Sheng Xia, Minghui Zhang, NK-like CD8 T cell: one potential evolutionary continuum between adaptive memory and innate immunity, Clinical and Experimental Immunology, Volume 217, Issue 2, August 2024, Pages 136–150, https://doi.org/10.1093/cei/uxae038
Close - Share Icon Share
Summary
CD8 T cells are crucial adaptive immune cells with cytotoxicity to fight against pathogens or abnormal self-cells via major histocompatibility complex class I-dependent priming pathways. The composition of the memory CD8 T-cell pool is influenced by various factors. Physiological aging, chronic viral infection, and autoimmune diseases promote the accumulation of CD8 T cells with highly differentiated memory phenotypes. Accumulating studies have shown that some of these memory CD8 T cells also exhibit innate-like cytotoxicity and upregulate the expression of receptors associated with natural killer (NK) cells. Further analysis shows that these NK-like CD8 T cells have transcriptional profiles of both NK and CD8 T cells, suggesting the transformation of CD8 T cells into NK cells. However, the specific induction mechanism underlying NK-like transformation and the implications of this process for CD8 T cells are still unclear. This review aimed to deduce the possible differentiation model of NK-like CD8 T cells, summarize the functions of major NK-cell receptors expressed on these cells, and provide a new perspective for exploring the role of these CD8 T cells in health and disease.
Introduction
Natural killer (NK) and CD8 T cells are two major cytotoxic lymphocytes, representing cytotoxic arms of innate and adaptive immunity, respectively. Their primary role is the direct elimination of target cells, such as infected, stressed, or transformed cells, accomplished mainly through the action of perforin and granzymes, but they adhere to different paradigms for recognition [1]. In addition, the ability to form long-term immune memory after antigen clearance is traditionally considered one of the main differences between them. In recent years, increasing evidence has revealed that immune cells exhibit a high degree of developmental plasticity and are no longer strictly confined to the boxes of “innate” or “adaptive.” For NK cells, the concept of “adaptive NK cells” challenges the aforementioned stereotype and enlightens us about the possession of some traits of adaptive immunity by NK cells [2]. During viral infection, adaptive NK cells act as CD8 T cells, performing functions such as undergoing clonal-like expansion, generating a long-term memory population, and mediating recall response [3]. Correspondingly, innate-like T cells appear to be at the blurred boundary between adaptive and innate immunity. Innate-like T cells are often referred to several groups of T cells expressing semi-invariant T-cell receptors (TCRs) and recognizing unconventional antigen-presenting molecules, including CD1d-restricted invariant NKT (iNKT) cells, MR1-restricted mucosal-associated invariant T (MAIT) cells, major histocompatibility complex (MHC) class Ib-reactive T cells, and γδ T cells [4]. Their thymic development, biology, and therapeutic potential have been well reviewed previously [4–6].
However, the “innateness” of conventional T cells with a diverse αβ TCR repertoire has not received the attention it deserves. In this review, we focused on a heterogeneous CD8αβ T-cell population exhibiting NK cell-like properties. Some of the overlapping features of these cells were identified in a previous review article as follows [3]: (i) they display an effector memory/terminally differentiated phenotype, (ii) they express numerous NK cell-associated receptors (NKRs) and perform rapid effector functions dependent on cytokines, and (iii) they accumulate due to aging and prolonged antigen exposure. It is not appropriate to call these NKR-expressing cells as “NKT cells.” Based on the classification strategy by Godfrey et al., NKT cells in the broad sense can be divided into type I (iNKT), type II, and NKT-like cells [7]. Both iNKT and type II NKT cells are CD1d restricted, indicative of their ability to recognize glycolipid antigens. However, NKT-like T cells are CD1d-unrestricted T cells with an oligoclonal TCR. Accumulated data show that a vast majority of these NKT-like cells are CD8 single positive. To explore the exact role of these cytotoxic lymphocytes that have innate properties but are different from innate-like CD8 T cells, we accurately name these cells as “NK-like CD8 T cells” in this review.
The molecular clues that lead to the generation of NK-like CD8 T cells are still debated. However, it is clear that they are not a novel population independent of the developmental lineages of NK and T cells, but rather a conditional intermediate of the transformation from T cells into NK cells [8]. In addition, NK-like CD8 T cells preferentially share some features with senescent CD8 T cells, such as loss of co-stimulatory receptors CD27/CD28, increased expression of maturation markers CD57 and killer cell lectin-like receptor G1 (KLRG1), and impaired proliferative capacity [9]. However, instead of being dysfunctional, they use acquired functional NK-cell receptors as an alternative mechanism to mediate rapid effector functions [10–12]. Driven by aging, chronic viral infection, and autoimmune diseases, these cells gradually accumulate and emerge as a key component of the memory cell pool [9]. Thus, it is essential to elucidate the position of these cells during the transformation from adaptive memory into innate immunity.
Paradox between conventional memory T and virtual memory T cells
The generation and efficient maintenance of memory T cells are essential for long-lasting immunological protection. The phenotypic analysis of NKR-expressing αβCD8 T cells revealed that a majority of these cells exhibited a terminally differentiated memory phenotype [12, 13]. Conventional memory CD8 T cells are generated by antigen stimulation in the periphery and play a critical role in secondary immune responses against infection. Following an acute antigen exposure, naive CD8 T cells (TN; CD45RO-CD45RA+CCR7+) are activated and undergo robust clonal expansion. Most of these differentiate into short-lived effector cells (SLECs; CD127dim KLRG1bright) and die weeks after pathogen clearance [14]. A small subset, known as memory precursor effector cells (MPECs; CD127bright KLRG1dim), has the potential to differentiate into long-lived memory T (Tmem) cells [14], including central memory T cells (TCM; CD45RO+CD45RA−CCR7+) located in lymphoid organs that can proliferate after reencountering antigen; effector memory T cells (TEM; CD45RO+CD45RA−CCR7−) that circulate in various tissues and retain the ability to rapidly perform effector functions against reinfection; and tissue-resident memory T cells (TRM; CD45RO+CD45RA−CCR7−) that persist in tissues without circulating [14, 15]. Terminally differentiated effector memory T cells regain the expression of CD45RA (TEMRA; CD27−CD28−CD45RO−CD45RA+CCR7−) following multiple rounds of exposure to cognate antigens together with the presence of homeostatic cytokines [16, 17]. In addition, memory phenotype T cells can be generated directly from a proportion of naive T cells undergoing steady-state proliferation and differentiation [18]. Among these, virtual memory T (TVM) cells were first described in unchallenged mice as a specialized subset of CD8 T cells that were semi-differentiated, antigen-naive, and characterized by a memory phenotype and functionality [19]. TVM cells constitute a majority of memory‐phenotype CD8 T cells in unimmunized mice and about 5-10% and 10-20% of the CD8 T cells in the blood and spleen, respectively [20]. A putative TVM cell subset has been identified in the cord blood of humans, indicating that it does not necessarily have to be antigen experienced [21].
TVM cells share certain characteristics with TEMRA cells stimulated by conventional antigens: (i) they mainly exhibit terminally differentiated phenotype and replicative cell senescence [8, 22]; (ii) they express NK-cell-associated molecules, such as NKG2D, killer immunoglobulin (Ig)-like receptor (KIR), and NKG2A, performing innate-like rapid effector functions mediated by NKRs [10, 23]; (iii) they exert rapid cytotoxicity and secrete high levels of proinflammatory cytokines such as TNF-α and IFN-γ in response to innate stimuli, but are not sensitive to TCR stimulation [24]; and (iv) they accumulate with aging and even comprise a dominant proportion of circulating peripheral CD8 T cells bearing memory phenotype [19, 25]. These findings suggest that TVM cells may be a subset of or perhaps equivalent to the TEMRA cell population, especially in older individuals. However, an apparent paradox exists about their generation conditions: TVM cells are antigen inexperienced, which is directly proved by the presence of TVM in germ-free mice and human cord blood [19, 21]. However, the generation of TEMRA cells requires persistent antigenic stimulation [10]. This indicates the presence of more critical key factors in their formation that cause them to exhibit such similar properties. It is now clear that, besides antigens, the differentiation status of memory CD8 T cells are influenced by multiple factors, including co-receptors, nutrients, and cytokines [26]. This review attempted to explain the formation of NK-like cells in terms of a pivotal cytokine: interleukin (IL)-15.
IL-15 plays a pivotal role in the generation and maintenance of CD8 memory T cells
IL-15 is a member of the common γ-chain family of cytokines, largely produced by monocytes/macrophages and dendritic cells in response to microbial infection, inflammation, and stress signals [27]. The heterotrimeric receptor of IL-15 shares the IL-2R/IL-15Rβ (CD122) and common gamma (γc) chain (CD132) with the IL-2 receptor. The IL-15Rα chain (CD125) expressed on the myeloid cells surface tightly binds to IL-15 and then presents it to IL-2/15Rβγ heterodimers. The engagement subsequently transmits stimulatory signals through three parallel downstream pathways: the JAK-STAT, Ras-Raf-MEK-mitogen-activated protein kinase (MAPK), and phosphoinositide 3-kinase (PI3K)-Akt-mTOR pathway [27].
Functionally, IL-15 is involved in maintaining diverse subsets of memory CD8 T cells by promoting their generation, proliferation, and survival [17]. First, IL-15 per se is sufficient to convert naive CD8 T cells into memory phenotype cells [28]. In IL-15/IL-15Rα-deficient mice, the number of memory CD8 T cells substantially decreases, a condition that can be reversed by administering exogenous IL-15 [29]. Second, IL-15 induces strong proliferative signals via JAK/STAT and Ras/MAPK signaling pathways [30]. Adoptively transferred memory phenotype CD8 T cells into IL-15-deficient hosts failed to undergo homeostatic proliferation and died rapidly [31]. Although virus-specific memory CD8 T cells could be generated in both IL-15–/– and IL-15Rα–/– mice, their homeostatic proliferation was severely impaired [32]. Finally, IL-15 plays a critical role in maintaining the survival of memory CD8 T cells [33]. Histologically, TVM cells mainly settle in and preferentially home to the liver, where IL-15 is abundant, indicating that IL-15 provides a suitable cytokine environment for TVM survival [34]. Mechanistically, IL-15 prevents activation-induced cell death (AICD) of CD8 T cells by increasing the levels of antiapoptotic proteins Bcl-2 and Bcl-xL and decreasing the expression of proapoptotic proteins Bim and Puma by activating the PI3K pathway [35].
Both antigen-induced memory and virtual memory CD8 T cells can be activated by innate stimuli in the absence of TCR stimulation, which is called bystander activation [24]. IL-15, as an important driver of bystander response, can enhance the multiple effector functions of memory CD8 T cells, including producing IFN-γ and cytotoxic molecules such as perforin and granzyme B [36]. Besides several myeloid cells, the inflamed tissue cells (e.g. intestinal epithelium, hepatocytes) also release excessive IL-15, and therefore the level of IL-15 increases significantly following infections or various forms of inflammatory challenges [37–39]. Prolonged exposure to IL-15 enables memory CD8 T cells to acquire upregulated expression of NK-cell receptors [40]. Also, IL-15-stimulated memory CD8 T cells can kill target cells in an NK-cell-like manner [37], which is discussed in the remaining part of this review. Collectively, the data indicate that IL-15 may serve as a key mediator in the formation and maintenance of NK-like CD8 T cells.
Differentiation model of NK-like CD8 T cells
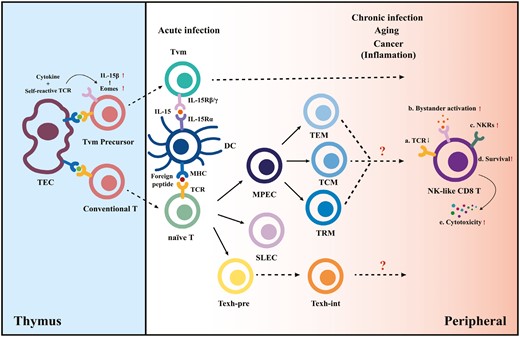
We speculate about the differentiation pathways of memory CD8 T cells biased toward NK-cell-like phenotypic transitions mediated by cognate antigen and cytokine signaling (Fig. 1). We hypothesize that CD8 T cells have two “chances” to acquire NK-cell properties during their lifetime.
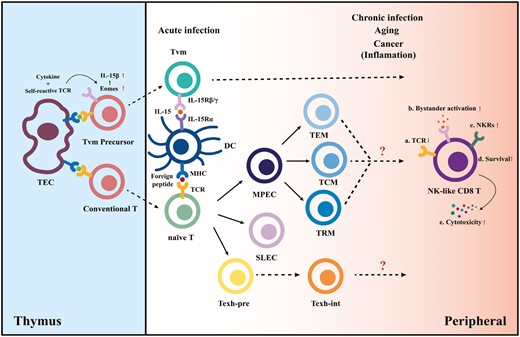
Differentiation model of NK-like CD8 T cells. In the thymus, the combined effects of high cytokine levels and modestly self-reactive TCR signaling lead CD8 single-positive (SP) T cells to upregulate Eomes and IL-15Rβ expression, permitting their cytokine-driven differentiation into virtual memory T-cell (TVM) precursors. In the periphery, conventional naive cytotoxic T lymphocytes differentiate into different subsets upon acute antigen exposure. However, the inflammatory environment created by aging and sustained antigen exposure together with the presence of cytokines generates NK-like CD8 T cells and drives the TVM maturation.
The combined effects of cytokine and TCR signaling in the thymus lead double-positive T cells to the first crossroad. The positive selection in the thymus cortex ensures a T-cell repertoire that can react modestly to self-peptide + MHC ligands on thymic epithelial cells [41]. These MHC-restricted thymocytes are delivered peripherally after negative selection and have the potential to differentiate into conventional antigen-specific memory cells. However, virtual memory T-cell differentiation is initially programmed by high self-peptide + MHC class I reactivity [20], driving elevated CD5 (a marker of self-reactivity), and Eomesodermin expression (Eomes; a T-box transcription factor that promotes the expression of CD122). The upregulated Eomes may explain the sensitivity of TVM precursor cells to IL-15 [42]. After TVM-cell precursors are transported to the periphery, IL-15Rα expressed on dendritic cells drive the maturation of TVM through the interaction of IL-15 [43]. Eomes is also required for NK-cell development, homeostasis, and function, so it may be a potential fate checkpoint of the formation of NK-like T cells. Further, TVM generation is influenced by exposure to IL-4. IL-4 production by iNKT cells was deficient in patients with chronic myeloid leukemia, reducing the number of virtual memory CD8 T cells and making them functionally impaired [44].
The second “chance” is given to conventional antigen-experienced CD8 T cells. Accumulating evidence confirms that persistent infection, aging, and chronic inflammation lead to an expansion of NKR-expressing T cells; however, clear mechanisms driving those events remain unclear [25, 45]. This review focuses on the combined effect of cognate antigen + cytokine signaling. Chronic antigen exposure due to persistent viral infections is one of the main drivers contributing to the accumulation of highly differentiated antigen-specific CD8 T lymphocytes with replicative senescence [46]. In addition, a striking upregulation of NKR has been demonstrated in virus-specific CD8 T cells after virus reactivation [47]. Cytokine IL-15 signaling induces an increase in the expression levels of NKRs on memory CD8 T cells, as noted earlier [37, 40]. Moreover, the cytokine-activated killer cells have a terminally differentiated memory phenotype [17], which supports the possibility that bystander activation of T cells may induce both senescence and NK receptor expression.
Moreover, persistent stimulation induces differentiation of exhausted T cells (Texh) during chronic infection, cancer, and autoimmunity. A recent study has identified several intermediate exhausted T cells (Texh-int) possessing effector function, including a subset expressing NK-cell-associated genes (Klrs and Fcgr2b), cytotoxic genes (Gzma/b), and migration-related genes (S1pr5 and Itgb7), indicating that these cells have more cytolytic potential than other Texh subsets [48]. However, the time when the development of these Texh cells diverges from that of Teff/Tmem cells and what signals drive the fate of different CD8 T-cell subsets have not yet been clarified.
Functional NKRs expressed on NK-like CD8 T cells
The functional status of NK cells is precisely regulated by germline-encoded activating and inhibitory receptors on the cell surface [49]. Inhibitory receptors maintain the tolerance of NK cells to normal cells by binding to MHC class I molecules, whereas the MHC class I loss or downregulation of abnormal cells, such as virus-infected, transformed, and stressed cells, weakens the inhibitory signals. In contrast, activating receptors detect stress-induced ligands on target cells and then provide activating signals for NK cells, initiating NK-cell-mediated cytotoxicity and cytokine production [49]. In this section, we discuss the types of functional NKRs expressed on human NK-like CD8 T cells and the way they are induced (Fig. 2). Some other NKRs primarily used as signature markers, including CD56, CD161, and KLRG1, may also be involved in the signal regulation of NK cells, but they are not further discussed herein.
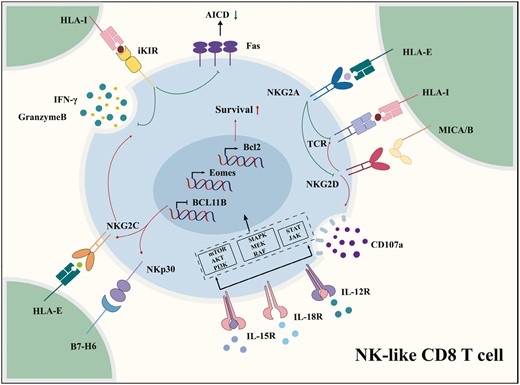
Natural killer-associated receptors expressed on NK-like CD8 T cells. The IL-15 signaling pathways trigger NK-cell-like reprogramming and induce the upregulation of functional NKRs. Inhibitory NKRs can inhibit activation-induced cell death and repress TCR activation. The ligations of activating NKRs, including NKG2D, NKG2C, and NKp30, increase the production of IFN-γ and multiple cytotoxicity granules, such as granzymes, from NK-like CD8 T cells in a TCR-independent manner.
Inhibitory receptors
KIR/NKG2A
NK-cell tolerance to self is ensured by the ligation of inhibitory cell surface receptors and self-MHC class I molecules. A majority of these receptors are also expressed on CD8 T cells, including inhibitory KIRs and NKG2A [50]. A common characteristic of inhibitory receptors specific for MHC class I signal is immunoreceptor tyrosine-based inhibitory motifs (ITIMs) in their cytoplasmic domains that enable them to recruit and activate SHP-1 and SHP-2 phosphatases [51]. KIRs are polygenic and polymorphic Ig superfamily receptors that recognize different allelic groups of HLA-A, HLA-B, and HLA-C molecules. NKG2A, also known as killer cell lectin-like receptor C1, is a type II glycoprotein with a C-type lectin scaffold structure. The heterodimeric complex NKG2A-CD94 recognizes HLA-E, a nonclassical HLA class I molecule with a limited polymorphism. HLA-E binds to conserved self-peptides derived from the leader sequences of various HLA-I class molecules [52]. It is poorly expressed by normal cells, but its expression is elevated on the surface of several tumor cell types. This may be one of the strategies of immune escape because induced cascade inhibitory signals can block the activation of NK and CD8 T cells [53].
KIR/NKG2A is preferentially expressed on late effector memory CD8 T cells but scarcely on their naive counterparts. The KIR-expressing CD8 T cells have a highly restricted KIR repertoire, more than 90% of which are dominated by a single activating or inhibitory KIR [54]. Moreover, the KIR’s expression on CD8 T cells in vivo seems to be maintained through continuous antigen encounters. In the absence of TCR engagement, KIRs expressed on CD8 T cells are slowly downregulated by interacting with KIR ligands expressed on antigen-presenting cells [55]. TCR engagement can sustain KIR expression and even reinduce the functional levels of KIR expression after ligand-induced downregulation of KIR [55]. In contrast, the expression kinetics of NKG2A is late but stable. Unlike the classical checkpoint PD-1, whose expression is rapidly induced by the initial TCR triggering but subsequently downregulated, the expression of NKG2A is only induced under repeated cognate antigen stimulations but maintained at a stable level in each resting phase [56]. However, the mechanism of how KIR/NKG2A can be induced or upregulated on activated CD8 T cells has not been clarified. Some evidence proves that transforming growth factor beta (TGF-β) can directly strengthen the increased expression of CD94/NKG2A receptors on CD8 memory T cells [57, 58]. TGF-β is an important functional regulator of various immune cells. It can suppress cytotoxicity and reduce the apoptosis of CD8 T-cell significantly in vitro [59, 60]. It can also induce the NK cell conversion toward the less cytolytic type 1 innate lymphoid cells, upregulating the surface expression of several functional receptors, including NKG2A, NKG2D, KIR2DL1, KIR3DL1, NKp30, NKp44, and NKp46 [56, 61]. This process can be enhanced by IL-15 through Ras signaling or via the activation of MEK/ERK [61]. In addition, KIR expression is induced in CD8 T cells with increasing age [62, 63], with one possible explanation being epigenetic control. For example, the complete demethylation of the minimal KIR2DL3 promoter is a characteristic feature of CD158b-expressing cells. However, with age, partial demethylation of the KIR promoter occurs in CD8 T cells that did not express the CD158b protein, which lowers the threshold for transcriptional activation and renders CD8 T cells more susceptible to express KIR [63].
However, KIR/NKG2A+ CD8 T cells exhibiting a memory phenotype are also identified in human cord blood, suggesting that their generation does not depend on cognate antigens as a necessary prerequisite [21]. A large number of these KIR/NKG2A+ CD8 T cells accumulate with increasing age, showing severely diminished proliferative capacity to TCR signals [64]. In addition, KIR/NKG2A together with Eomes have been identified as a signature to distinguish between virtual memory and antigen-experienced memory CD8 T cells [23]. Eomes can be a lineage marker of this population of cells because their Eomes− counterpart share functional and phenotypic features but with a lower expression of senescence markers and a proportional decrease with age, which may represent a less differentiated stage of the NK-like CD8 T-cell subset [65]. Recent findings have suggested that KIR+CD8 and NKG2A+CD8 T cells are distinct innate-like populations [66]. KIR+CD8 T cells are more terminally differentiated and replicatively senescent than NKG2A+CD8 T cells [67]. In terms of cytokine responsiveness, NKG2A+CD8 T cells are more sensitive to IL-12/IL-18-induced production of IFN-γ, whereas IL-15-induced NK-like cytotoxicity is prominent in KIR+CD8 T cells [66]. This suggests that more accurate markers should be used when exploring the characteristics of heterogeneous cell subsets.
Activating receptors
NKG2D
NKG2D is a type II transmembrane protein with a C-type lectin-like extracellular domain, which is extensively expressed on the surface of NK cells, CD8 T cells, and subsets of CD4 T cells [68]. In humans, NKG2D conveys signals by associating with the DAP10 adapter, which then transmits activation signals through the downstream PI3K signaling pathway [69]. The ligands of NKG2D include six members of the MHC class I chain-related protein A/B (MICA/B) and UL16-binding proteins (ULBP) families, which are usually less abundant on the surface of normal cells but can be induced during infection and tumorigenesis [70].
The NKG2D-mediated response can be modulated by cytokine milieu [37, 71]. Despite constitutive expression on almost all CD8 αβ T cells in humans, NKG2D alone cannot trigger cell-mediated cytotoxicity or cytokine production of resting CD8 T cells [68]. Nevertheless, in long-term cultures with high concentrations of IL-2 or IL-15 secreted by inflamed tissue cells, CD8 αβ T cells acquire upregulated NKG2D expression and consequential NKG2D-mediated cytolytic function [37]. Whether the level of NKG2D ligand affects NKG2D expression on T cells is debatable. It was initially observed in breast and lung cancer tissues that some membrane-bound NKG2D ligands were hydrolyzed into a soluble form, accompanied by the inhibition of NKG2D-mediated cell killing. Some believed that the downregulated NKG2D expression was due to the increased endocytic degradation of NKG2D after binding to these sMICs [72]. Others, however, proposed that this tumor-derived functional alteration of CTLs was mainly caused by a decrease in the density of membrane-bound NKG2DL on the surface of tumor cells [73]. Recently, the involvement of sestrins (a family of stress-sensing proteins induced by low glucose concentration, oxidative stress, and cellular senescence) in the reprogramming of nonproliferative senescent T cells to acquire an innate-like killing activity has been reported. The genetic inhibition of sestrin 2 resulted in decreased expression of NKG2D and DAP12 in CD8 T cells [74].
NKG2C
Similar to NKG2A, NKG2C also forms a heterodimer with CD94, binding to HLA-E as ligands but with lower affinity. NKG2A/CD94 heterodimer transduces signals through the immunoreceptor tyrosine activation motif (ITAM)-bearing adapter molecule DAP12. NKG2C is more often expressed on highly differentiated T cells along with other typical NK cell receptors, such as NKG2D, KIR2DL2/DL3, CD16, and maturation marker CD57 [75]. The expansion of NKG2C+CD8 T-cell populations has been reported under several pathologic conditions, including celiac disease, hepatitis virus infection, and especially human cytomegalovirus infection (HCMV) [76–78]. Recently, a small transcriptionally distinct subpopulation of NKG2C-exprssing CD8Tmem cells (identified as NKG2C+GZMB−) was confirmed with age-associated decline [79]. HCMV is a widespread and lifetime-latent pathogen causing poor outcomes in immunocompromised hosts such as transplant recipients and human immunodeficiency virus (HIV)-infected patients [76]. HCMV infection in the elderly people has some correlation with immunosenescence, and may affect host immunity and aggravate the aging process. In the context of cytomegalovirus infection, both NK and T cells can exhibit rather unconventional phenotypes, sharing characteristics of innate and adaptive immune cells [80]. Some peptides of the UL40 protein from certain HCMV strains have homology with HLA-I-derived peptides and can also be presented by HLA-E. On the one hand, HCMV infection triggers the expansion of NKG2Cbright NK cells, which are thought to be a group of adaptive NK cells that can recognize specific viral protein UL40 and exert cytotoxic functions against HCMV-infected cells. Moreover, NKG2Cbright NK cells persist at higher frequencies after initial infection in response to HCMV reactivation [81]. On the other hand, HCMV infection causes the activation and preferential expansion of NKG2C+CD8 T cells (identified as CD56+ CD94+ DAP12+ NKG2C+ CD45RA+ CCR7− PD-1−/low) [82]. The transcriptomic analysis revealed that BCL11B, an essential transcription factor of T-cell lineage commitment and identity maintenance, was downregulated in these NKG2C+CD8 T cells [76]. The deletion of BCL11B in mature T cells could cause increased expression of genes associated with NK cells (e.g. Il2rb, Nfil3, and GZMB) and acquired NK-cell receptors (e.g. NKp46, NKG2A, and NKG2C) [83], thus inducing their reprogramming into induced T-to-NK cells (ITNKs) [84]. A recent study found that BCL11B may promote the formation of human adaptive NK cells, suggesting that BCL11B also played a direct mechanistic role in NK-cell reprogramming, for example, by suppressing ZBTB16, which happened to be a specific transcription factor for nearly all of the innate-like T cells [85].
NKp30
Natural cytotoxicity receptors (NCRs), including NKp46, NKp30, and NKp44, are members of the Ig superfamily (IgSF) but have no homology to each other. NKp46 and NKp30 are expressed on the surface of all NK cells (including mature, immature, inactive, and activated NK cells), whereas NKp44 is expressed only on the surface of activated NK cells, which is a specific marker of activated NK cells [86]. Due to the short cytoplasmic region, NCRs noncovalently couple with CD3ζ/FcRγ or DAP12 homodimers containing ITAM motif in the cytoplasmic region for activating signal transduction [86]. The co-expression of NKp30 and NKG2D has been frequently detected under the bystander activation of several hepatitis virus-specific CD8 T cells [39, 87]. Although these studies focused on the NKG2D-dependent cytotoxic effects, the function of NKp30 is often overlooked. NKp30 is also involved in the TCR-independent target cell recognition and killing [88]. The expression level of NKp30 on CTL is also affected by IL-15 induction. Margareta et al. proposed a differentiation program model of IL-15-induced NKp30+ CD8 T cells: IL-15 drove reprogramming on NKp30−/int CD8 T cells. This induced FcεRIγ promoter demethylation and concurrent expression of the transcription factor PLZF, resulting in the generation of NKp30+ CD8 T cells. These cytokine-induced NKp30+ CD8 T cells displayed potent NK-like cytotoxicity in vitro and antitumor activity in a xenograft tumor model [89]. With a parallel elevated serum IL-15 concentration, patients with coronavirus disease 2019 (COVID-19) displayed an increased proportion of NKp30+ CD8 T cells, accompanied by a more differentiated process of cytotoxic CD8 T cells [90]. The use of CRISPR/Cas9-mediated knockout technology to deplete BCL11B in CD8 T cells also induced NKp30 expression, which mechanistically replicated the signal transduction of IL-15 because IL-15 itself had the ability to inhibit BCL11B expression in vivo [91].
Role of NKRs on NK-like CD8 T cells
We have discussed earlier the expression of several NK-cell receptors on NK-like CD8 T cells. The functionality of these receptors has been well understood for NK cells, but not for CD8 T cells. In this section, we discuss how these NK-cell receptors are involved in regulating TCR signaling and how they influence the phenotypic and functional transitions of NK-like CD8 T cells.
NK-like CD8 T cells use NKRs in a different manner
Inhibitory NKRs of NK-like CD8 T cells also transmit negative signals and dampen the immune response but in a totally different manner. Further, although multiple effect functions of NK-like CD8 T cells in response to a TCR-dependent stimuli were inhibited by KIR, KIR+CD8 T cells exhibited a surprisingly stronger response on stimulation with PMA/ionomycin compared with KIR-CD8 T cells. This phenomenon demonstrated that KIR+CD8 T cells were not exhausted but were unable to respond to stimuli via TCR [92]. The presence of inhibitory receptors endows NK cells with higher effector capabilities, which is referred to NK-cell “education” or “licensing” [93]. Whether the increased responsiveness to PMA/ionomycin is due to T-cell “education” of KIRs or a result of the highly differentiated status of KIR+CD8 T cells remains to be explored. However, the “education” of the other αβ CD8 T-cell subpopulations, intestinal intraepithelial lymphocytes (iIELs), is lacking, although iIELs express multiple inhibitory receptors specific for self-MHC class I molecules [94]. Furthermore, the activation of NK-like CD8 T cells can be blocked even in the absence of KIR ligands, indicating that these inhibitory receptors may constitutively repress TCR activation [67, 92]. One possible explanation for this is the recruitment of KIR into the TCR synapse when bound to MHC class I, blocking downstream signal transduction events through phosphatases SHP-1/2; this association can occur constitutionally with KIR in the absence of ligand interaction [95]. Last but not least, the expression of inhibitory KIRs on CD8 T cells in vivo may be maintained through continuous exposure to antigens. In the absence of TCR engagement, KIRs are slowly downregulated by KIR ligands on antigen-presenting cells so that the resulting expression levels of KIR can no longer inhibit T-cell activation [55]. The mechanism by which activating NKRs function on NK-like T cells is similarly flexible. Activating NKRs can either act as a co-stimulatory receptor of TCR, compensating for the weakened stimulus signal caused by the loss of CD27/CD28, or act independently of TCR stimuli [96, 97]. Overall, NK-like CD8 T cells use NKR signaling differently from NK cells, thus fully demonstrating the plasticity of functional NKRs during the transition from CTL to NK-like properties.
KIRs are involved in the survival of NK-like CD8 T cells
As mentioned earlier, inhibitory KIR-expressing CD8 T cells harbor senescent-like phenotype, including replicative senescence and impaired proliferative capacity, but their frequency in the periphery still increases with age or chronic viral infections. One explanation for this phenomenon is that inhibitory KIR expression directly or indirectly affects the lifespan of memory CD8 T cells. It is proved that the engagement of transgenic KIRs in mice selectively drives the accumulation of memory phenotype CD8 T cells expressing IL-2Rβ chain in vivo, which may be achieved by inhibiting AICD because the binding of KIR to homologous HLA molecules does not alter antigen-stimulated cell proliferation [98]. Further studies confirmed this view, demonstrating that KIR engagement reduced tumor-mediated CTL apoptosis by interfering with two Fas downstream signaling pathway: sustained c-FLIP-L induction and decreased caspase 8 activity [99]. It seems as if KIR-promoted cell survival does not interfere with its impaired expansion because engagement of KIR by MHC ligands diminishes early TCR signaling and the reduced AICD cannot rescue lost proliferation capacity [100]. Besides, a recent study proposed a new perspective: it was not the presence of inhibitory KIR, but the number of KIR-HLA pairs, which regulated the survival of memory CD8 T cells. In this model, KIR+CD8 T cells exhibited regulatory activity and indirectly influenced CD8 T cell longevity via KIR-HLA [101].
Activating NKRs are helpers or substitutes for TCR
The activation of T cells requires the participation of a dual signaling system. Besides MHC/antigenic peptide-TCR providing the first signal, co-stimulatory molecules are required to provide the second signal. NK-like CD8 T cells typically lose the co-stimulatory molecule CD28, which is not conducive to reactivation [102]. Several activating NKRs appear compensatory to the activity of co-stimulatory molecules to enhance TCR signaling. It was once thought that NKG2D functioned as a co-stimulatory receptor that could substitute for CD28. Although NKG2D is present in both naive T and memory T cells, only the effector function of CD8 T cells with low CD28 expression levels is enhanced by NKG2D stimulation [96, 102]. Besides promoting T-cell activation, proliferation, and secretion of the basic cytokine IL-2, the co-stimulation of CD8 T cells by NKG2D also induces the production of CD8 T cells with multi-cytokine (IL-2, IFN-γ, and TFN-α) capabilities [103]. For HLA-E-restricted CD8 T cells under HCMV infection, NKG2C can cooperate with TCR activation or act alone in degranulation and IFN-γ production [52, 76]. NKp30 expressed by IL-15-induced CD8 T cells can synergize in inducing T-cell function via degranulation, IFN-γ release, redirected killing, and apoptosis, demonstrating a role for NCRs in decreasing the TCR activation threshold [89, 104].
Even in the absence of TCR stimulation, NKG2C ligation per se can mediate NK-like CD8 T-cell proliferation and kill virus-transfected target cells that do not express the other MHC class I molecules [77, 105]. A distinct CD56hiCD16−CD8 T-cell population amplified in the liver of HBV-infected patients was highly expressed in various NK receptors. These cells not only exerted NKG2C-mediated NK-like effector functions but also responded to innate cytokines, such as IL-12/18 and IL-15, in the absence of TCR stimulation [78]. A recent study found that NKp30+CD8 T cell subpopulation exhibited high NKp30-dependent antitumor activity in vitro or in vivo during the extensive reprogramming of CD8 T cells derived by IL-15 [89, 104].
NKG2D engagement on NK-like CD8 T cells plays a more significant role alone, contributing to the response to bystander activation; this can serve as an innate-like killing mechanism [106]. Bystander activation can not only produce antiviral protection but also induce body disease. Interferon type I, IL-18, and IL-15 are cytokines essential for inducing the bystander activation of T cells [36]. In the absence of TCR involvement, the signal transduction of IL-15 receptors induces the upregulation of NKG2D expression, which can mediate cell lysis by promoting CD8 T-cell degranulation and releasing cytotoxic molecules [107]. However, the simultaneous activation of TCR can eliminate IL-15-induced upregulation of NKG2D on the surface of memory CD8 T cells. This result supports the idea that NKG2D preferentially functions without TCR stimulation [39]. This NKG2D-mediated bystander activation can provide early protection against pathogens and help control tumor progression [106, 108]. Compared with antigen-specific T-cell response, this engagement of the NKG2D-NKG2DL activation mode has its own drawbacks sometimes leading to serious pathological reactions [39, 109]. Thus, further investigation is required to avoid the lysis of normal tissues by NK-like CD8 T cells when harnessing NKG2D as a potential immunotherapy target.
Balance between the inhibitory and activating signals on NK-like CD8 T cells
The response of NK-like CD8 T cells can also be regulated by the balance of signals between cell surface-activating receptors and inhibitory receptors. HCMV provides UL40-derived peptides that are structurally similar to HLA-E ligands and stimulates robust HLA-E-restricted CD8 T-cell responses. One recent study evaluated the auto-peptide responsiveness of HCMV-specific T cells [52]. The results showed that CD8 T cells bearing TCRs with high affinity for HLA-E exerted the weakest functional response due to increased expression of inhibitory KIRs. However, HLA-E-restricted T cells bearing lower-affinity TCRs expressed the activating receptor NKG2C, allowing NKG2C-mediated cell activation, lysis, and cytokine secretion. This result illustrated the balancing role of NK-cell receptor calibration in helping T cells fight viruses and prevent autoimmunity [52]. The engagement of NKG2A-CD94 with peptide-loaded HLA-E leads to the phosphorylation of the cytoplasmic ITIMs in NKG2A, resulting in the transmission of an inhibitory signal that suppresses competing signals from TCR or activating receptor NKG2D [110]. Thus, the inhibition of the NKG2A immune checkpoint restores T-cell effector function in preclinical cancer models.
NK-like CD8 T cells are involved in diseases
NK-like CD8 T cells accumulate in healthy individuals during aging or in patients with chronic virus infection, cancers, and autoimmune diseases. Instead of being dysfunctional, NK-like CD8 T cells begin to express the molecular machinery, which enables them to behave more like NK cells. Unlike in vitro experiments, the behavior of NKRs on NK-like CD8 T cells is influenced by the pathological tissue microenvironment in vivo. Therefore, we summarized information on several diseases related to NK-like CD8 T cells and expounded the role of NKRs on the onset and progression of these diseases as comprehensively as possible (Table 1).
Summary of previous studies evaluating the role of NK-like CD8 T cells in diseases
| Disease type . | Disease . | Functional NKRs involved . | Mechanisms and impact on disease . | References . |
|---|---|---|---|---|
| Infection | Hepatitis virus infection | NKG2D/NKp30 | IL-15-induced NKG2D-dependent cytotoxicity of liver CD8 T cells may contribute to inflammation and liver injury. | [39, 87] |
| NKG2C | The distinct CD56hiCD161-CD8 T cell population characterized by NK-like activation via TCR-independent NKG2C ligation was significantly increased in patients with HBV-associated CLD. | [78] | ||
| KIR/NKG2A | Both NKG2A and KIRs could inhibit the response of HCV-specific CD8 T cells ex vivo, which could be responsible for liver lesions. | [52] | ||
| Leishmania major infection | NKG2D | NKG2D engagement on LCMV-specific memory CD8 T cells induces immunopathology in Leishmania major infection. | [107] | |
| Hantaan virus infection | NKG2D | Bystander-activated CD8 T cells could exert cytotoxicity effects against HTNV-infected HUVECs, which could be enhanced by IL-15 stimulation and blocked by NKG2D antibody. | [109] | |
| Respiratory viral infection | KIR | KIR+ CD8 T suppresses the proliferation of effector CD8 T cells associated with a longer duration of respiratory symptoms in old age. | [62] | |
| NKG2A | Elevated NKG2A expression on cytotoxic lymphocytes correlates with disease severity in COVID-19 patients. | [111] | ||
| Polyomavirus | NKG2A | Antiviral CD8 T cells upregulate CD94-NKG2A, which is responsible for downregulating their antigen-specific cytotoxicity during viral clearance and virus-induced tumorigenesis. | [112] | |
| Cytomegalovirus infection | NKG2C | NKG2C+ CD8 T cells exhibited strong effector function against leukemia cells and HCMV-infected fibroblasts, which was dictated by both NKG2C and TCR specificity. | [76] | |
| NKG2D | Infection by cytomegalovirus resulted in substantial increases in MIC on cultured fibroblast as well as endothelial cells, and induced MIC expression was associated with interstitial pneumonia. | [96] | ||
| HIV infection | Pan-KIR, and/or NKG2A | KIR was associated with a profound inhibition of cytokine secretion, degranulation, proliferation, and activation by CD8 T cells in HIV-1-infected patients. | [92, 113] | |
| Autoimmune disease | Celiac disease | NKG2C/NKp44/NKp46 | The NK transformation of CTLs may underlie the self-perpetuating, gluten-independent tissue damage in celiac disease. | [77] |
| KIR | KIR+ CD8 T cells efficiently eliminated pathogenic gliadin-specific CD4 T cells from the leukocytes of celiac disease patients in vitro. | [114] | ||
| Multiple sclerosis | NKG2D | CD8 T cells promote MS progression by degranulation by ligation to the NKG2D receptor ULBP4 expressed by astrocytes. | [115] | |
| Cancer | Lung cancer | NKG2D | IL-15 treatment in MCMV-infected mice upregulated TNF-α and IFN-γ responsive genes to control tumor growth. | [108] |
| NKG2A | Cytokines and cytotoxic molecules secreted by tumor-infiltrating NKG2A+ CD8 T cells were significantly lower. | [116] | ||
| Colorectal cancer | NKG2D/NKp44/NKp46 | There was a significantly lower number of NKG2D+ CD8 T cells and reduced expression level of the NKp44 and NKp46 on NKT-like cells in the peripheral blood of patients with metastatic colorectal cancer. | [117] | |
| NKG2A | Inhibition of anti-tumor reactivity of CD8 T cells could be overcome by blocking NKG2A. | [118] | ||
| Melanoma | KIR/NKG2A/ NKG2C | The increased expression of NKRs on CD8 T cells may contribute to the final outcome of the immune response against melanoma. | [12] | |
| Neuroblastoma | NKG2D | NB cell‑derived sNKG2DL induced degradation of NKG2D on CD8 T cells and impaired CD8 T cell proliferation, IFN‑γ production, and CD107a translocation. | [119] | |
| Cervical carcinoma | NKG2A | Cervical cancer cells could promote the expression of iNKRs via an IL-15- and possibly TGF-β-mediated mechanism and abrogate the antitumor cytotoxicity of TILs. | [58] |
| Disease type . | Disease . | Functional NKRs involved . | Mechanisms and impact on disease . | References . |
|---|---|---|---|---|
| Infection | Hepatitis virus infection | NKG2D/NKp30 | IL-15-induced NKG2D-dependent cytotoxicity of liver CD8 T cells may contribute to inflammation and liver injury. | [39, 87] |
| NKG2C | The distinct CD56hiCD161-CD8 T cell population characterized by NK-like activation via TCR-independent NKG2C ligation was significantly increased in patients with HBV-associated CLD. | [78] | ||
| KIR/NKG2A | Both NKG2A and KIRs could inhibit the response of HCV-specific CD8 T cells ex vivo, which could be responsible for liver lesions. | [52] | ||
| Leishmania major infection | NKG2D | NKG2D engagement on LCMV-specific memory CD8 T cells induces immunopathology in Leishmania major infection. | [107] | |
| Hantaan virus infection | NKG2D | Bystander-activated CD8 T cells could exert cytotoxicity effects against HTNV-infected HUVECs, which could be enhanced by IL-15 stimulation and blocked by NKG2D antibody. | [109] | |
| Respiratory viral infection | KIR | KIR+ CD8 T suppresses the proliferation of effector CD8 T cells associated with a longer duration of respiratory symptoms in old age. | [62] | |
| NKG2A | Elevated NKG2A expression on cytotoxic lymphocytes correlates with disease severity in COVID-19 patients. | [111] | ||
| Polyomavirus | NKG2A | Antiviral CD8 T cells upregulate CD94-NKG2A, which is responsible for downregulating their antigen-specific cytotoxicity during viral clearance and virus-induced tumorigenesis. | [112] | |
| Cytomegalovirus infection | NKG2C | NKG2C+ CD8 T cells exhibited strong effector function against leukemia cells and HCMV-infected fibroblasts, which was dictated by both NKG2C and TCR specificity. | [76] | |
| NKG2D | Infection by cytomegalovirus resulted in substantial increases in MIC on cultured fibroblast as well as endothelial cells, and induced MIC expression was associated with interstitial pneumonia. | [96] | ||
| HIV infection | Pan-KIR, and/or NKG2A | KIR was associated with a profound inhibition of cytokine secretion, degranulation, proliferation, and activation by CD8 T cells in HIV-1-infected patients. | [92, 113] | |
| Autoimmune disease | Celiac disease | NKG2C/NKp44/NKp46 | The NK transformation of CTLs may underlie the self-perpetuating, gluten-independent tissue damage in celiac disease. | [77] |
| KIR | KIR+ CD8 T cells efficiently eliminated pathogenic gliadin-specific CD4 T cells from the leukocytes of celiac disease patients in vitro. | [114] | ||
| Multiple sclerosis | NKG2D | CD8 T cells promote MS progression by degranulation by ligation to the NKG2D receptor ULBP4 expressed by astrocytes. | [115] | |
| Cancer | Lung cancer | NKG2D | IL-15 treatment in MCMV-infected mice upregulated TNF-α and IFN-γ responsive genes to control tumor growth. | [108] |
| NKG2A | Cytokines and cytotoxic molecules secreted by tumor-infiltrating NKG2A+ CD8 T cells were significantly lower. | [116] | ||
| Colorectal cancer | NKG2D/NKp44/NKp46 | There was a significantly lower number of NKG2D+ CD8 T cells and reduced expression level of the NKp44 and NKp46 on NKT-like cells in the peripheral blood of patients with metastatic colorectal cancer. | [117] | |
| NKG2A | Inhibition of anti-tumor reactivity of CD8 T cells could be overcome by blocking NKG2A. | [118] | ||
| Melanoma | KIR/NKG2A/ NKG2C | The increased expression of NKRs on CD8 T cells may contribute to the final outcome of the immune response against melanoma. | [12] | |
| Neuroblastoma | NKG2D | NB cell‑derived sNKG2DL induced degradation of NKG2D on CD8 T cells and impaired CD8 T cell proliferation, IFN‑γ production, and CD107a translocation. | [119] | |
| Cervical carcinoma | NKG2A | Cervical cancer cells could promote the expression of iNKRs via an IL-15- and possibly TGF-β-mediated mechanism and abrogate the antitumor cytotoxicity of TILs. | [58] |
Summary of previous studies evaluating the role of NK-like CD8 T cells in diseases
| Disease type . | Disease . | Functional NKRs involved . | Mechanisms and impact on disease . | References . |
|---|---|---|---|---|
| Infection | Hepatitis virus infection | NKG2D/NKp30 | IL-15-induced NKG2D-dependent cytotoxicity of liver CD8 T cells may contribute to inflammation and liver injury. | [39, 87] |
| NKG2C | The distinct CD56hiCD161-CD8 T cell population characterized by NK-like activation via TCR-independent NKG2C ligation was significantly increased in patients with HBV-associated CLD. | [78] | ||
| KIR/NKG2A | Both NKG2A and KIRs could inhibit the response of HCV-specific CD8 T cells ex vivo, which could be responsible for liver lesions. | [52] | ||
| Leishmania major infection | NKG2D | NKG2D engagement on LCMV-specific memory CD8 T cells induces immunopathology in Leishmania major infection. | [107] | |
| Hantaan virus infection | NKG2D | Bystander-activated CD8 T cells could exert cytotoxicity effects against HTNV-infected HUVECs, which could be enhanced by IL-15 stimulation and blocked by NKG2D antibody. | [109] | |
| Respiratory viral infection | KIR | KIR+ CD8 T suppresses the proliferation of effector CD8 T cells associated with a longer duration of respiratory symptoms in old age. | [62] | |
| NKG2A | Elevated NKG2A expression on cytotoxic lymphocytes correlates with disease severity in COVID-19 patients. | [111] | ||
| Polyomavirus | NKG2A | Antiviral CD8 T cells upregulate CD94-NKG2A, which is responsible for downregulating their antigen-specific cytotoxicity during viral clearance and virus-induced tumorigenesis. | [112] | |
| Cytomegalovirus infection | NKG2C | NKG2C+ CD8 T cells exhibited strong effector function against leukemia cells and HCMV-infected fibroblasts, which was dictated by both NKG2C and TCR specificity. | [76] | |
| NKG2D | Infection by cytomegalovirus resulted in substantial increases in MIC on cultured fibroblast as well as endothelial cells, and induced MIC expression was associated with interstitial pneumonia. | [96] | ||
| HIV infection | Pan-KIR, and/or NKG2A | KIR was associated with a profound inhibition of cytokine secretion, degranulation, proliferation, and activation by CD8 T cells in HIV-1-infected patients. | [92, 113] | |
| Autoimmune disease | Celiac disease | NKG2C/NKp44/NKp46 | The NK transformation of CTLs may underlie the self-perpetuating, gluten-independent tissue damage in celiac disease. | [77] |
| KIR | KIR+ CD8 T cells efficiently eliminated pathogenic gliadin-specific CD4 T cells from the leukocytes of celiac disease patients in vitro. | [114] | ||
| Multiple sclerosis | NKG2D | CD8 T cells promote MS progression by degranulation by ligation to the NKG2D receptor ULBP4 expressed by astrocytes. | [115] | |
| Cancer | Lung cancer | NKG2D | IL-15 treatment in MCMV-infected mice upregulated TNF-α and IFN-γ responsive genes to control tumor growth. | [108] |
| NKG2A | Cytokines and cytotoxic molecules secreted by tumor-infiltrating NKG2A+ CD8 T cells were significantly lower. | [116] | ||
| Colorectal cancer | NKG2D/NKp44/NKp46 | There was a significantly lower number of NKG2D+ CD8 T cells and reduced expression level of the NKp44 and NKp46 on NKT-like cells in the peripheral blood of patients with metastatic colorectal cancer. | [117] | |
| NKG2A | Inhibition of anti-tumor reactivity of CD8 T cells could be overcome by blocking NKG2A. | [118] | ||
| Melanoma | KIR/NKG2A/ NKG2C | The increased expression of NKRs on CD8 T cells may contribute to the final outcome of the immune response against melanoma. | [12] | |
| Neuroblastoma | NKG2D | NB cell‑derived sNKG2DL induced degradation of NKG2D on CD8 T cells and impaired CD8 T cell proliferation, IFN‑γ production, and CD107a translocation. | [119] | |
| Cervical carcinoma | NKG2A | Cervical cancer cells could promote the expression of iNKRs via an IL-15- and possibly TGF-β-mediated mechanism and abrogate the antitumor cytotoxicity of TILs. | [58] |
| Disease type . | Disease . | Functional NKRs involved . | Mechanisms and impact on disease . | References . |
|---|---|---|---|---|
| Infection | Hepatitis virus infection | NKG2D/NKp30 | IL-15-induced NKG2D-dependent cytotoxicity of liver CD8 T cells may contribute to inflammation and liver injury. | [39, 87] |
| NKG2C | The distinct CD56hiCD161-CD8 T cell population characterized by NK-like activation via TCR-independent NKG2C ligation was significantly increased in patients with HBV-associated CLD. | [78] | ||
| KIR/NKG2A | Both NKG2A and KIRs could inhibit the response of HCV-specific CD8 T cells ex vivo, which could be responsible for liver lesions. | [52] | ||
| Leishmania major infection | NKG2D | NKG2D engagement on LCMV-specific memory CD8 T cells induces immunopathology in Leishmania major infection. | [107] | |
| Hantaan virus infection | NKG2D | Bystander-activated CD8 T cells could exert cytotoxicity effects against HTNV-infected HUVECs, which could be enhanced by IL-15 stimulation and blocked by NKG2D antibody. | [109] | |
| Respiratory viral infection | KIR | KIR+ CD8 T suppresses the proliferation of effector CD8 T cells associated with a longer duration of respiratory symptoms in old age. | [62] | |
| NKG2A | Elevated NKG2A expression on cytotoxic lymphocytes correlates with disease severity in COVID-19 patients. | [111] | ||
| Polyomavirus | NKG2A | Antiviral CD8 T cells upregulate CD94-NKG2A, which is responsible for downregulating their antigen-specific cytotoxicity during viral clearance and virus-induced tumorigenesis. | [112] | |
| Cytomegalovirus infection | NKG2C | NKG2C+ CD8 T cells exhibited strong effector function against leukemia cells and HCMV-infected fibroblasts, which was dictated by both NKG2C and TCR specificity. | [76] | |
| NKG2D | Infection by cytomegalovirus resulted in substantial increases in MIC on cultured fibroblast as well as endothelial cells, and induced MIC expression was associated with interstitial pneumonia. | [96] | ||
| HIV infection | Pan-KIR, and/or NKG2A | KIR was associated with a profound inhibition of cytokine secretion, degranulation, proliferation, and activation by CD8 T cells in HIV-1-infected patients. | [92, 113] | |
| Autoimmune disease | Celiac disease | NKG2C/NKp44/NKp46 | The NK transformation of CTLs may underlie the self-perpetuating, gluten-independent tissue damage in celiac disease. | [77] |
| KIR | KIR+ CD8 T cells efficiently eliminated pathogenic gliadin-specific CD4 T cells from the leukocytes of celiac disease patients in vitro. | [114] | ||
| Multiple sclerosis | NKG2D | CD8 T cells promote MS progression by degranulation by ligation to the NKG2D receptor ULBP4 expressed by astrocytes. | [115] | |
| Cancer | Lung cancer | NKG2D | IL-15 treatment in MCMV-infected mice upregulated TNF-α and IFN-γ responsive genes to control tumor growth. | [108] |
| NKG2A | Cytokines and cytotoxic molecules secreted by tumor-infiltrating NKG2A+ CD8 T cells were significantly lower. | [116] | ||
| Colorectal cancer | NKG2D/NKp44/NKp46 | There was a significantly lower number of NKG2D+ CD8 T cells and reduced expression level of the NKp44 and NKp46 on NKT-like cells in the peripheral blood of patients with metastatic colorectal cancer. | [117] | |
| NKG2A | Inhibition of anti-tumor reactivity of CD8 T cells could be overcome by blocking NKG2A. | [118] | ||
| Melanoma | KIR/NKG2A/ NKG2C | The increased expression of NKRs on CD8 T cells may contribute to the final outcome of the immune response against melanoma. | [12] | |
| Neuroblastoma | NKG2D | NB cell‑derived sNKG2DL induced degradation of NKG2D on CD8 T cells and impaired CD8 T cell proliferation, IFN‑γ production, and CD107a translocation. | [119] | |
| Cervical carcinoma | NKG2A | Cervical cancer cells could promote the expression of iNKRs via an IL-15- and possibly TGF-β-mediated mechanism and abrogate the antitumor cytotoxicity of TILs. | [58] |
Role of NK-like CD8 T cells in infectious diseases
NK-like CD8 T cells may play a protective role occasionally. However, findings from many studies imply that these cells are involved in generating exacerbated inflammation, contributing to the progression of immunopathology and tissue injury. Viral hepatitis refers to a class of liver infectious diseases caused by hepatitis viruses (HAV, HBV, HCV, HEV, and HGV) and is pathologically characterized by acute hepatocellular necrosis and chronic inflammatory response. Chronic stimulation promotes the accumulation of NKR-expressing liver-infiltrating CD8 T cells. In HCV-infected patients, the increased frequency of NKG2A+/KIR+ CD8 T cells in the liver was positively correlated with histological activity. Both NKG2A and KIRs could inhibit the response of HCV-specific CD8 T cells ex vivo [67]. During HAV, the virus-infected hepatocytes upregulated NKG2DL and produced IL-15 to induce TCR-independent activation of CD8 T cells specific to unrelated viruses. Bystander-activated CD8 T cells exerted NKG2D- and NKp30-dependent cytolytic activity, which was associated with liver injury in patients with AHA [39, 87]. Furthermore, CD8 MAIT cells were not excluded from CD8 T cells in these studies. MAIT cells are the most abundant innate-like T cells in the liver that can also elicit potent TCR/MR1-independent cytotoxicity in response to IL-15 during acute hepatitis A [120]. In chronic cutaneous leishmaniasis, the recruitment of cytolytic T cells to cutaneous lesions usually leads to increased inflammation without effectively controlling the parasites. NKG2DLs are upregulated on stromal cells within Leishmania major-infected lesions, making them susceptible to NKG2D-mediated killing. Coincidentally, IL-15 is enriched in infected lesions, driving NKG2D expression on CD8 T cells. NKG2D blockade on CD8 T cells effectively reduces lysis mediated by NKG2D–NKG2D ligand interactions and alleviates disease severity [121]. The same is true for hantavirus infection. Bystander-activated CD8 T cells can exert cytotoxicity effects against HTNV-infected HUVECs, which can be enhanced by IL-15 stimulation and blocked by NKG2D antibody [109]. The KIR expression on memory HIV-specific CD8 T cells is correlated with the level of HIV-1 replication in HIV-infected individuals. A significant upregulation of KIR on CD8 T cells was associated with impaired degranulation, reduced cytokine secretion, and inhibited cell proliferation in response to TCR-mediated stimulation [92, 113]. A clinical study on patients with COVID-19 patients revealed that the percentages of NK and CD8 T cells expressing NKG2A receptor and the mean fluorescence intensity of NKG2A were significantly correlated with disease progression [111]. These cytotoxic lymphocytes with upregulated NKG2A exhibited a reduced ability to produce effector molecules CD107a, IFN-γ, IL-2, granzyme B, and TNF-α, strongly supporting the finding that elevated NKG2A expression might be involved in the functional exhaustion of T and NK cells [122]. CD94-NKG2A is upregulated by antiviral CD8 T cells during acute polyoma infection and is responsible for downregulating its antigen-specific cytotoxicity during viral clearance and virus-induced tumorigenesis [112].
NK-like CD8 T cells exhibit immunomodulation in autoimmune diseases
Our previous study suggested that NK-like CD8 T cells can function as antigen-specific suppressive cells by killing antigen-bearing DCs, providing an active and precise method for constraining an excessive immune response [123]. Increasing evidence shows that NK-like T cells are involved in regulating the immune response. CD8 T cells expressing inhibitory KIRs exhibit immunomodulatory functional properties and may represent the human equivalent of Ly49+CD8 regulatory T cells in mice. Ly49+CD8 T cell-selective ablation made virus-infected mice more susceptible to various autoimmune diseases [114]. During COVID-19 infection, KIR+CD8 T cells are induced to inhibit CD4 T cells with strong reactivity to self, which causes autoimmunity, without interfering with the immune responses to pathogens [114]. Multiple sclerosis (MS) is an immune-mediated disease in which the immune system attacks the myelin enveloping nerve fibers in the brain and spinal cord, causing permanent damage or degeneration of nerve fibers. The upregulated level of soluble ULBP4 was found in the cerebrospinal fluid of patients with MS. Also, the functional assays demonstrated its capacity to boost inflammatory cytokine secretion by CD8 T lymphocytes. In addition, the live imaging analysis revealed a long-lasting interaction for NKG2D and ULBP4 between astrocytes and CD8 T cells, which promoted MS progression by degranulating ULBP4-expressing astrocytes [115]. However, whether and how NKRs are involved in mediating autoimmune diseases, leading to opposite contribution of CD8 T cells expressing distinct NKRs in one disease type, is unclear. For example, celiac disease is an inherited autoimmune disorder in which the ingestion of gluten causes damage to the small intestine. On the one hand, the uncontrolled expansion of intraepithelial CD8 T cells aberrantly expressing cytolytic NK receptors was detected in patients with celiac disease; further, this NK transformation of CTLs might underlie gluten-independent tissue damage [77]. On the other hand, KIR+CD8 T cells efficiently eliminated pathogenic gliadin-specific CD4+ T cells from the leukocytes of patients with celiac disease, proving that these regulatory CD8 T cells helped control pathology in celiac disease [114]. Collectively, the heterogeneity of NK-like CD8 T cells should be fully taken into account while exploring their immunomodulation in autoimmune diseases.
Role of NK-like CD8 T cells in cancers
NK-like CD8 T cells can lyse target cells in an innate-like manner in vitro, indicating their strong antitumor potential [108]. A recently identified FCER1G-expressing innate-like T-cell population was highly similar to NK-like CD8 T cells, with enhanced tissue residency, preferentially tracked to tumor sites where cytokine IL-15 was abundant. These cells were broadly reactive to unmutated self-antigens and almost did not upregulate PD-1 expression like conventional tumor-infiltrating T cells, which was important for tumor types that did not respond to immune checkpoint therapy [124]. Our previous study found that NK-like CD8 T cells could kill myeloid-derived suppressor cells (MDSCs) in a granzyme B-dependent manner, suggesting their potential role in clearing tumor antigen-bearing MDSCs to improve the antitumor microenvironment [125]. In line with the hypothesis that the increase in the number of NK-like CD8 T cells in aged healthy people may represent a beneficial remodeling of the T-cell compartment [9], one recent study indicated that NK-like CD8 T cells might contribute to tumor immunity in the elderly people [126]. A murine model with B16 melanoma was applied in this study. Unexpectedly, lung metastasis was significantly suppressed in aged mice compared with young mice. CD8 T cells of aged mice activated in vitro had higher cytotoxic activity precisely because CD8 T cells with senescent T-cell phenotypes acquired NK-cell-like function and participated in tumor elimination [12, 126].
Many clinical studies have been conducted on the use of genetically modified or in vitro-induced expansion of NK-like CD8 T cells for antitumor therapy. For example, NKp30+CD8 T cells equipped with TCRs or chimeric antigen receptors (CARs) targeting epidermal growth factor receptor 2 (HER2), a tumor-associated target overexpressed in several malignancies, not only killed HER2-expressing target cell lines but also eliminated tumor cells without MHC class I molecules or tumor-associated antigens [127]. ITNKs (induced by inactivation of BCL11B in human T cells, as mentioned earlier) and CAR-transduced ITNKs selectively lysed various cancer cells in culture and suppressed the growth of solid tumors in xenograft models. Also, tumor stabilization along with one partial remission was observed in more than 60% patients in a preliminary clinical study [77, 128]. Combining multiple treatment methods has become the trend in antitumor immunotherapy. The chemotherapy pretreatment of cancer cells directly activate NK-like CD8 T cells to mediate tumor cytotoxicity in an antigen-independent manner [129]. The cytotoxic effects of cytokine-induced killer cells can be improved through co-culture with breast cancer cells first treated with carboplatin [130]. We conducted one clinical trial using allogeneic NK-like CD8 T cells combined with gefitinib, which significantly reduced gefitinib-acquired resistance and prolonged progression-free survival of patients with advanced non-small-cell lung carcinoma [131].
Conversely, tumor cells can also escape immune surveillance by modulating the expression of NKRs on CD8 T cells. Tumor-infiltrating CD8 T lymphocytes expressing upregulated CD94/NKG2A were observed in various cancer types [58, 116], which might be driven by inflammatory cytokine milieu and continuous antigen exposure in the tumor lesion [58, 132], as discussed earlier. The functional analyses revealed that the levels of cytokines and cytotoxic molecules secreted by CD8 T cells were significantly lower on the upregulation of CD94/NKG2A [58, 116, 118]. NKG2A blockade could reactivate the cytotoxic activity of these cancer-infiltrating NKG2A+CD8 T cells; therefore, CD94/NKG2A has recently been recognized as an immune checkpoint [53, 118, 133]. In addition, releasing soluble NKG2D ligands is another strategy of tumors to escape immunological surveillance. Tumor-derived sMICA and sULBP-2 not only downregulated the NKG2D expression on CD8 T cells but also impaired cell proliferation, IFN-γ production, and CD107a translocation [119]. Colorectal cancer (CRC) is one of the deadliest malignancies globally. NK-like CD8 T cells were expanded and activated after tumor resection in patients with CRC who did not receive any adjuvant therapy, indicating that the effect of NK-like CD8 T cells was inhibited by tumor microenvironment [134]. Decreased expression levels of NKG2D, NKp44, and NKp46 from NK-like T cells in these patients were associated with tumor progression and poor survival [117, 135]. In summary, targeting NK-like T cells present a promising approach for future antitumor immunotherapy. However, a deeper understanding of the underlying mechanisms is needed.
Conclusions
Human beings have developed sophisticated immune systems in the fight against intracellular and extracellular challenges. The capacity to develop immune memory has been considered the signature feature distinguishing adaptive immune cells from their innate counterparts. However, growing evidence revealed the convergence of innate and adaptive lymphocytes [10]. Maria et al. proposed an interesting concept: an “innateness gradient” placing adaptive cells on one end and NK cells on the other, with innate T-cell populations clustering between the adaptive and innate cells. Thus, they suggested that the growth potential and rapid effector function should, respectively, be hallmarks of adaptive and innate cells [136]. Consistent with this, Yuki et al. proposed a hypothesis suggesting that, by sharing rapid effector function with innate immune cells, the memory/effector T-cell state may represent a default innate-like response to antigen recognition [41]. In this review, we discussed the “innateness” of memory CD8 T-cell populations expressing NKRs and how it could be induced by inflammatory microenvironment and persistent antigenic stimulation. We speculate that the conditional transition from CD8 T cells to NK-like CD8 T cells can reveal a new immune surveillance mechanism in which the adaptive immune cells acquire the properties of the innate immune cells in the face of uncontrolled stress. Nevertheless, further studies are needed to confirm the validity of this theory.
More closely related to practical application is how to effectively use present technology to harness the full potential of NK-like CD8 T cells. The regulatory capacity and dual cytotoxicity of NK-like CD8 T cells hold significant application potential, especially in the elderly people. In this review, we summarized the categories and functions of NKRs on NK-like CD8 T cells, providing ideas for the identification of new immunotherapy targets. Given the nonspecific recognition and bystander activation of NK-like CD8 T cells, measures should be taken to prevent the occurrence of autoimmune diseases.
In conclusion, NK-like CD8 T cells may represent the evolutionary continuum from adaptive immunity to innate immunity, showcasing considerable application potential. Future in-depth studies should focus on the specific transformation conditions and mechanisms for better application of NK-like CD8 T cells.
Supplementary data
Supplementary data is available at Clinical and Experimental Immunology online.
Abbreviations:
- AICD
activation-induced cell death
- CARs
chimeric antigen receptors
- COVID-19
corona virus disease 2019
- CRC
colorectal cancer
- HAV
hepatitis virus A
- HCMV
human cytomegalovirus
- HER2
epidermal growth factor receptor 2
- HLA
human leukocyte antigen
- IgSF
immunoglobulin superfamily
- iIELs
intestinal intraepithelial lymphocytes
- IL-
interleukin-
- iNKT
invariant natural killer T cells
- ITAM
immunoreceptor tyrosine activation motif
- ITIMs
immunoreceptor tyrosine-based inhibitory motifs
- ITNKs
induced T-to-NK cells
- KLRG1
killer cell lectin-like receptor G1
- MAIT cells
mucosal-associated invariant T cells
- MAPK
mitogen-activated protein kinase
- MDSCs
myeloid-derived suppressor cells
- MICA
MHC class I chain-related protein A
- MPEC
memory precursors effector cells
- MS
multiple sclerosis
- NCRs
natural cytotoxicity receptors
- NKRs
NK cell-associated receptors
- PD-1
programmed death 1
- PI3K
phosphoinositide 3-kinase
- SLECs
short-lived effector cells
- TCRs
T cell receptors
- TEMRA
effector memory RA T cells
- TGF-β
transforming growth factor beta
- TVM
virtual memory T cells
- ULBP
UL16-binding proteins
Funding
These studies were supported by 81871234 and 82172763 (S.X.), 82272824 (M.Z.), and 82172725 (Z.G.) from National Natural Science Foundation of China.
Data availability
Data availability is not applicable to this article as no new data were created or analyzed in this study.
Author contributions
M.Z. and S.X. designed the project. Q.W. wrote the manuscript and S.C. prepared the figures. S.X. and Z.G. revised the manuscript.
Conflict of interests
The authors declared no financial and non-financial competing interests.



