-
PDF
- Split View
-
Views
-
Cite
Cite
Teresina Laragione, Carolyn Harris, Percio S Gulko, Combination therapy of a TRPV2 agonist with a TNF inhibitor achieves sustained suppression of disease severity and reduced joint damage, Clinical and Experimental Immunology, Volume 211, Issue 3, March 2023, Pages 233–238, https://doi.org/10.1093/cei/uxac124
Close - Share Icon Share
Abstract
We aimed to compare a transient receptor potential vanilloid 2 (TRPV2) agonist with a TNF inhibitor, and to test the potential of their combination in collagen-induced arthritis (CIA) as a potential future strategy for rheumatoid arthritis (RA). Following the onset of CIA DBA1/j mice were started on treatment with either vehicle, etanercept (8 mg/kg three times a week), the TRPV2 agonist O1821 (20–30 mg/kg/day), or a combination of both. Mice were scored over a 61-day period. Synovial tissues were obtained for RNA sequencing. Mice on monotherapy with either O1821 or etanercept developed milder clinical disease. The O1821 protection was observed at an earlier time-point than in the etanercept group. The combination therapy group achieved a more robust and sustained reduction in disease severity than either monotherapy group. All treatment groups had reduced scores for synovial inflammation, synovial hyperplasia, and erosive changes, compared with controls, with the combination group achieving the most significant protection. RNA sequencing and pathway analyses of synovial tissues identified pathways and processes regulated by the TRPV2 agonist, such as chemotaxis and cytokine receptor signaling, including IL6R. The combination therapy affected additional pathways not seen in the monotherapy groups. In conclusion, the TRPV2 agonist achieved an overall similar reduction in arthritis severity and histology scores as etanercept, but the combination therapy achieved a more sustained disease control and more pronounced reduction in joint damage, suggesting a potential future option for improving disease control in RA. RNA sequencing analyses identified new pathways regulated by TRPV2, and also by the combination treatment.
Introduction
Rheumatoid arthritis (RA) affects nearly 1% of the population and is associated with increased risk for disability, for reduced quality of living, as well as reduced survival [1]. New therapies developed over the past 20 years have significantly improved disease control. Yet, most patients only achieve mild to moderate improvements and disease remission remains uncommon and better treatments are needed. Current treatments for RA target the immune response, rendering patients increasingly susceptible to severe infections. Combination of different biologics targeting different key pro-inflammatory cytokines had no clear additional benefit and was associated with increased toxicity in patients with RA [2]. Yet, combination of therapies that target different aspects of disease pathogenesis have been successful in the treatment of cancers. Therefore, we consider that identifying the right targets for combination therapy might be the key to achieving better disease control and have focused our studies on the Fibroblast-like synoviocytes (FLS).
FLS have a central role in the RA pathogenesis [3]. The numbers of FLS are significantly increased in hyperplastic synovial tissues of RA patients and FLS have an aggressive behavior resembling that of cancer cells, invading and destroying cartilage and bone and releasing pro-inflammatory cytokines, chemokines, and proteases [3, 4]. The RA FLS in vitro invasive properties correlate with radiographic damage in RA [5] and with histologic changes in rodent models of arthritis [6]. Therefore, understanding the regulation of FLS invasiveness has the potential to generate new therapies aimed at reducing articular damage and improving disease control.
TRPV2 (transient receptor potential vanilloid subfamily, type 2 channel) is a non-selective cation channel belonging to the TRPV family, a multifunctional set of membrane proteins implicated in sensory functions between cells and their environment [7]. TRPV channels can be activated by different physical or chemical in vitro stimuli. However, human and mouse TRPV2 do not have any specific role in sensing thermal or painful stimuli [7, 8].
We have previously reported that TRPV2 is expressed by RA FLS [9] and its activation by synthetic agonists suppresses the invasiveness of RA FLS by preventing the activation of Rac1 and RhoA [10], and reducing the expression of MMP2 and MMP3 [9]. In vivo TRPV2 activation decreased disease severity in both collagen-induced arthritis (CIA) and KRN serum-induced arthritis in mice, and reduced numbers of synovial-infiltrating inflammatory cells [9].
With goal of developing better and safer treatments for RA, in this study we compare the arthritis-suppressing and joint protective activity of a TRPV2 agonist with a standard of care biologic TNFi, etanercept, and examine the potential benefit of using them as a combination therapy.
Material and methods
Mice
Male DBA1/J mice were purchased from Jackson Laboratories (Farmington, CT). Mice were housed under specific pathogen-free conditions and all experiments conducted under a protocol approved by the Mount Sinai Institutional Animal Care and Use Committee.
CIA
Eight to 12 week-old male DBA1/J mice were injected subcutaneously in the tail on day 0 with a 50 µl of 1 mg/ml of chicken Type II collagen emulsion in Complete Freund’s Adjuvant (Hooke laboratories, Lawrence, MA), and received a booster containing 50 µl emulsion of 1 mg/ml of chicken Type II collagen emulsion in Incomplete Freund’s Adjuvant (Hooke laboratories) on day 21 [11]. Mice were followed for 61 days and scored three times a week.
Treatment
Starting on the first day of arthritis with at least one joint with a score ≥2 (day 34), mice (groups of 11–13 per treatment group) were randomized to the different groups (color coded for randomization without knowledge of the treatment group) and started on intraperitoneal injections of either control vehicle daily, O1821 20 mg/kg/day, O1821 30 mg/kg/day, etanercept 8 mg/kg three times a week [12], or O1821-30 mg/kg/day plus etanercept 8 mg/kg three times a week, all in the same volume.
Arthritis activity and severity scoring
The clinical arthritis score was determined in a blinded manner according to a scoring scale ranging from 0 to 16 per mouse per day as previously reported where 1 = swelling and erythema in a single joint, 2 = swelling and erythema in more than one joint, 3 = swelling of the entire paw, and 4 = swelling of paw and inability to bear weigh [9].
Histology and histological scoring
At the end of the arthritis observation period mice were euthanized, the paws fixed in 10% formaldehyde and then decalcified with a solution containing hydrochloric acid and 0.1 M EDTA (Cal-Ex; Fisher Scientific, Fairlawn, NJ). Tissues were embedded in paraffin, and slides prepared and stained with hematoxylin-eosin. The slides were evaluated in a blinded manner with a combined scoring system that included the following parameters: synovial inflammation, synovial hyperplasia, cartilage, and bone erosions (Supplementary Table 1) [13–15].
RNA sequencing
Total RNA isolated extracted from synovial tissues and quantified by Nanodrop and 400 ng per sample sent to Novogene (Beijing, China) for sequencing and analyses (for detailed methods see Supplementary Methods).
Statistics
Means were compared with the t-test and medians with the rank sum test as indicated, using GraphPad Prism 6 (San Diego, CA).
Results
Monotherapy with either the TRPV2 agonist O1821 or etanercept achieves similar disease suppressing activity in CIA
CIA was induced in DBA1/j male mice and on day 34, after the onset of disease, mice were started on one of the treatments. O1821 in two different dosing regimens (20 and 30 mg/kg) significantly reduced arthritis severity scores with a detectable effect as early as day 40, achieving significance from days 42 to 51 (Fig. 1A and B). Etanercept also reduced arthritis severity scores but achieved significance at a later stage in the course of CIA on days 58 and 61 (Fig. 1C).
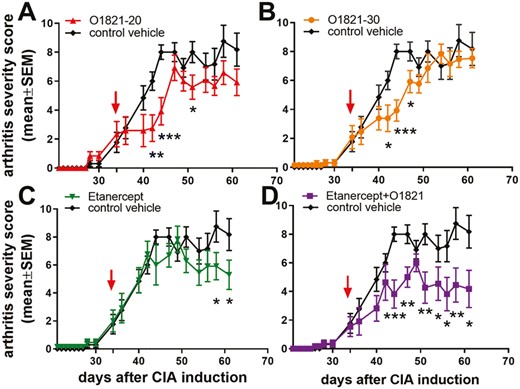
arthritis severity scores over time in mice with CIA. (A) O1821 at 20 mg/kg/day (n = 12) or (B) 30 mg/kg/day (n = 13) reduced arthritis severity scores. (C) Etanercept (n = 13) achieved a late onset protective effect. (D) Etanercept + O1821 (n = 11) induced an early and more sustained protective effect, compared with the monotherapy groups. (All treatments were started after the onset of arthritis on day 34, marked with an arrow; results shown as mean ± SEM; *P< 0.05, **P < 0.008, ***P < 0.0007, t-test; control group n = 13).
Histological analyses showed marked synovial inflammation, synovial hyperplasia, cartilage erosions, and bone erosions in the vehicle group, and significant reduction in all these parameters in the three treatment groups (Fig. 2A and B). There was a dose-response effect of O1821, with more pronounced reduction of synovial inflammation, synovial hyperplasia, as well as in cartilage and bone erosions in the 30 mg/kg group (P < 0.05; Fig. 2A and B). The etanercept and O1821 (30 mg/kg) groups had overall similar reduction in the synovial histology parameters (Fig. 2A and B).
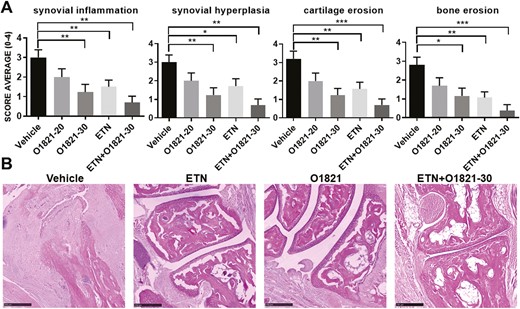
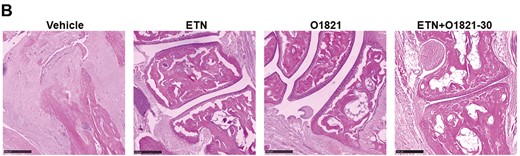
histology images and scoring. (A) O1821 30 mg/kg/day (n = 13), etanercept (ETN) (n = 13) and the combination treatment (n = 11) significantly reduced the histology scores for synovial inflammation, hyperplasia, cartilage erosions, and bone erosions, compared with controls (n = 13). The most significant reduction in cartilage and bone erosion was achieved in the combination therapy group (results shown as mean ± SEM; *P < 0.05, **P ≤ 0.0083, ***P ≤ 0.0008, rank-sum test). (B) Representative images of each treatment group. Black bar = 250 µM.
Combination therapy of the TRPV2 agonist O1821 with etanercept achieved sustained reduction in arthritis severity
The combination therapy of O1821 and etanercept significantly reduced arthritis severity scores by as much as 60%, by day 44, and achieved a more sustained suppression of disease severity scores than the monotherapy groups (Fig. 1D). The combination therapy group had the most significant reduction in histology scores for inflammation, hyperplasia, and cartilage and bone erosions of all treatment of groups, compared with the control group (Fig. 2A and B).
RNA sequencing analyses of the synovial tissues
At the end of the CIA study (day 61), synovial tissues were obtained and RNA used for sequencing and analysis. There were 619 genes differentially expressed between control vehicle and O1821 (30 mg/kg)-treated mice (P ≤ 0.01), with 499 increased and 120 decreased in the treatment group (Fig. 3A and B). Pathway analyses revealed significant differences (adjusted P-value) in the Gene Ontology (GO) and KEGG database pathway groups, including genes implicated on both leukocyte, neutrophil, and myeloid cell migration and chemotaxis, receptor ligand activity, receptor regulator activity, cytokine binding, cytokine signaling and receptor activity, interleukin-1 and interleukin-6 production and responses, acute inflammatory response, and adaptive immune response, among others (Fig. 4 and Supplementary Tables 2 and 3).
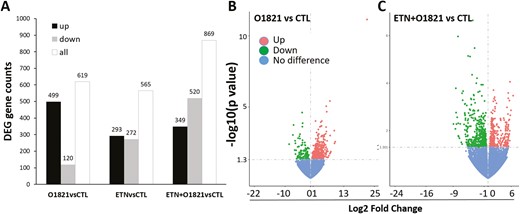
differentially expressed gene numbers and volcano plots. (A) Total (all) number of genes differentially expressed, and numbers of gene expressed in increased (up) or decreased levels in the treatment groups compared with control (CTL). (B) Volcano plots of the analyses of O1821 vs. control, and of (C) etanercept + O1821 vs. control (ETN = etanercept).
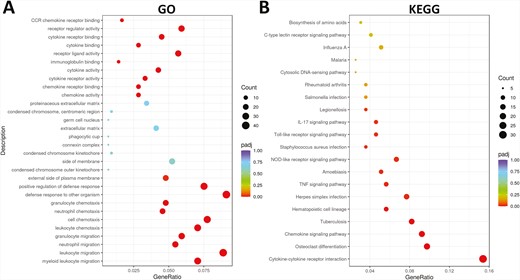
dot plot of pathways differentially represented in the RNAseq analyses between control vehicle and O1821 (30 mg/kg/day). (A) GO pathways and (B) KEG processes differentially expressed.
In the etanercept versus control analyses, there were 565 genes differentially expressed genes (P ≤ 0.01), 293 with increased and 272 with decreased expression in synovial tissues of etanercept group. GO and KEGG pathways most significantly affected by etanercept included antigen presentation and processing, protein digestion and absorption, hematopoietic cell lineage, autoimmune, and inflammatory diseases including RA, systemic lupus, Type I diabetes, allograft rejection, graft-versus-host, asthma, autoimmune thyroid disease, viral myocarditis, and inflammatory bowel disease (Supplementary Fig. 1A and B, and Supplementary Tables 4 and 5).
We next analyzed the effect of the combination therapy on the synovial transcriptome, compared with the control group. In this analysis there were 869 differentially expressed genes, with 349 expressed in increased levels and 520 in decreased levels in the combination group, compared with control (Fig. 3A and C). The combination treatment group signature and pathways included not only part of those identified in the transcriptomes of the monotherapy groups compared with control, but also unique pathways including nonsense-mediated decay (NMD), extracellular matrix organization and proteoglycans, ribosome, and transmission across chemical synapses. There were also additional pathways of interest such as presynaptic and postsynaptic nicotinic acetylcholine receptors, acethylcholine binding and receptors, fatty acid metabolism, regulation of lipolysis in adipocytes, mTOR signaling, and BCR signaling, but their significance disappears after adjustments for multiple comparisons (Supplementary Tables 6–8). These results raise the possibility that the combination of etanercept and O1821 can potentially modify the activity of additional pathways also important in disease pathogenesis, with a potential additionally benefit.
Discussion
There is continued need for more effective treatments for RA, similarly effective but less risky treatments, or more effective drug combinations. Currently available biologics and small molecule JAK inhibitors achieve similar rates of responses in RA patients, and are associated with increased risk for severe infections [16]. Combination therapies of biologic agents targeting different pathways has been limited by toxicity [2].
We have previously identified TRPV2 as a new suppressor of the invasive properties of FLS and then showed that stimulation of TRPV2 with agonists reduced disease severity in two different mouse models of RA [17]. TNFi are typically the first line of biologic treatment in RA, and therefore we conducted a study comparing the TRPV2 agonist O1821 with etanercept, and also the effect of their combination. O1821 was similarly effective as etanercept in reducing disease severity and histologic joint inflammation, synovial hyperplasia, and damage. However, the O1821 protective effect was noticeable at an earlier stage in the CIA disease course, while the etanercept’s reached statistical significance at a later stage.
We next examined the synovial transcriptomes of these mice. Tissues were collected at the end of the experiment, which is typically an advanced stage in CIA. Yet, while both O1821 and etanercept affected important processes in arthritis pathogenesis and joint damage, both had clearly distinct transcriptomes and GO and KEGG pathways, underlying their different mechanisms of action. O1821 affected chemotaxis, cytokine receptor signaling, and receptor activity, including interleukin-1 and interleukin-6 production and responses, acute inflammatory response, and adaptive immune response, while etanercept affected antigen presentation and processing, hematopoietic cell lineage, collagen fibril organization, and degradation of extracellular matrix, and several genes and pathways implicated in autoimmune and inflammatory diseases including RA and systemic lupus.
The differences in pathways affected by each agent raised the possibility that the combination therapy of O1821 and etanercept might provide an advantage by interfering with additional pathogenic processes. The combination treatment group disease severity curve was a combination of both the O1821 and the etanercept curves, with an early onset and a more sustained disease severity reduction effect compared with the monotherapy groups. The combination group achieved the most significant reduction in total arthritis severity scores, and the most significant protection in histologic scores compared with control vehicle.
At the transcriptomic level, the combination group had several of the changes observed in the monotherapy treatment groups. However, the combination group also had unique pathways of interest nearly reaching a significant adjusted P-value such as NMD, Ribosome, extracellular proteoglycans. NMD is involved in RNA quality control and gene regulatory mechanisms, including inflammation and immune response genes [18, 19], and to our knowledge has not yet been implicated in chronic autoimmune or inflammatory diseases making these findings of potential interest. The extracellular proteoglycans pathway effect may be one of the reasons for the reduced erosive changes seen on histology.
Others pathways of potential interest identified only in the combination treatment group included presynaptic and postsynaptic nicotinic acetylcholine receptors, acethylcholine binding, fatty acyl-CoA biosynthesis, BCR signaling, and others. Some of these, such as BCR signaling are implicated in RA pathogenesis. Others, such as acetylcholine receptors are of interest given the anti-inflammatory properties associated with the alpha7 nicotinic receptors [20]. However, the nicotinic receptors alpha1, alpha2, gamma, and delta and not the alpha7 were among those affected by the combination therapy, and will be interesting to explore their potential role in disease pathogenesis.
Fatty acyl-CoA biosynthesis was another interesting pathway in the combination treatment group. Fat metabolism has been shown to regulate macrophage function and the M1 versus M2 phenotypes [21], and fatty acids and their metabolites have been recently implicated in the pathogenesis of RA, and perhaps in response to treatment [22, 23].
This is the first time that a TRPV2 agonist is directly compared with a standard of care biologic agent such as etanercept, and our results in CIA suggest comparable efficacy but with an earlier onset of disease suppression with O1821. We have previously shown that TRPV2 activation reduces receptor tyrosine kinase-induced activation of Rac1 and RhoA [10]. There is also evidence that signaling through IL6R [24, 25] and IL1R [26] involve Rac1 and RhoA activation. The present transcriptome data suggest that by stimulating TRPV2, O1821 is also interfering with the IL6R and IL1R signaling processes, which are central to arthritis pathogenesis and a targets of FDA-approved biologics.
In conclusion, we describe for the first time a side-by-side comparison of a TRPV2 agonist and a TNFi, and show earlier onset of disease suppression in the TRPV2 group, but similar histologic protection on both. Transcriptomic analyses revealed known and previously unknown pathways regulated by TRPV2. Furthermore, the combination therapy identified pathways that were beyond the purely additive effect of the two treatments, suggesting that together they may modulate additional and important process with the potential to achieve better disease control.
Abbreviations
- CIA
collagen-induced arthritis
- FLS
Fibroblast-like synoviocytes
- RA
rheumatoid arthritis
- TNFi
TNF inhibitor
- TRPV2
transient receptor potential vanilloid 2
Ethical approval
The experiments were conducted in accordance to the ARRIVE guidelines.
Conflict of interests
P.S.G. is an inventor in patent of TRPV2 agonists (none used in this study). T.L. and C.H. have no conflicts to disclose.
Funding
Icahn School of Medicine at Mount Sinai.
Data availability
All data is made available via published and supplementary figures and tables.
Author contributions
Conceptualization, analyses, and supervision carried out by T.L. and P.S.G.; methodology and data acquisition were done by T.L. and C.H.; funding acquisition, project administration, and writing – original draft did byP.S.G.; writing – review & editing were done by T.L., C.H. and P.S.G.
Clinical trial registration
This is not a clinical trial.



