Abstract
The cardiovascular effects of inhaled particle matter (PM) are responsible for a substantial morbidity and mortality attributed to air pollution. Ultrafine particles, like those in diesel exhaust emissions, are a major source of nanoparticles in urban environments, and it is these particles that have the capacity to induce the most significant health effects. Research has shown that diesel exhaust exposure can have many detrimental effects on the cardiovascular system both acutely and chronically. This review provides an overview of the cardiovascular effects on PM in air pollution, with an emphasis on ultrafine particles in vehicle exhaust. We consider the biological mechanisms underlying these cardiovascular effects of PM and postulate that cardiovascular dysfunction may be implicated in the effects of PM in other organ systems. The employment of multiple strategies to tackle air pollution, and especially ultrafine particles from vehicles, is likely to be accompanied by improvements in cardiovascular health.
1. Introduction
Public awareness of the health risks of air pollution has never been higher. Rarely is the subject out of the international media and its presence looms heavily over regulators and politicians. While we may have known for many centuries that air pollution is damaging to health, it is only in last two decades that the full magnitude of the problem has been recognized. The statistics are staggering. Air pollution is deemed to be responsible for several million premature deaths worldwide every single year.1,2 Recent estimates based on further analysis of a modelled airborne particle concentration–effect curve have suggested that there may up to 8.9 million excess deaths per year, specifically from outdoor air pollution.3 Lifelong exposure to pollution is accompanied by a drastic shortening of life that varies, on average, from 3 to 6 months in modestly polluted countries such as the UK and USA, to 1–2 years from the notorious high pollution found in many areas of Sub-Saharan Africa and Asia.4 Ambient (outdoor) air pollution is the 5th biggest risk factor for all-cause mortality, above more well-recognized risk factors such as low exercise and poor diet, and the number one environmental risk factor.5 Indeed, it has been estimated that reducing air pollution to WHO air quality guidelines globally would increase life expectancy by 0.6 years; a benefit similar in magnitude to eradicating lung and breast cancer.4 The morbidity accompanying air pollution is great (3.1% of global disability-adjusted life years), with phenomenal levels of associated economic cost ($1–3 trillion US dollars, per year worldwide).2,6 One of the reasons for these striking figures is that exposure to air pollution is near ubiquitous. It has been estimated that >90% of the world’s population lives in areas of above World Health Organization (WHO) guidelines.7 Levels of many air pollutants are on the rise in developing countries, but even in developed nations that have successfully lowered pollutants over the last 50 years, improvements have plateaued, and breeches of regulations in urban areas are common.
It has long been established that air pollution exacerbates respiratory conditions such as asthma and chronic pulmonary obstructive disease. Also, the once-surprising observation that air pollution is associated with cardiovascular disease is also now well-recognized in the 21st century. Nonetheless, the cardiovascular effects of pollution should not be dismissed. Cardiovascular conditions account for 40–60% of the premature mortality from air pollution.3,5,8 Additionally, the multifaceted cardiovascular effects represent a substantial burden in their own right as well as through exacerbation of comorbidities. This review discusses the cardiovascular effects of air pollution, focusing on airborne particles and vehicle emissions as key pollutants of interest. The review is intended as an overview of the area (we direct the reader to the following for further detail9–14) with discussion of the potential role of the cardiovascular system in the actions of air pollution in other areas of the body.
2. Constituents of air pollution
There is a plethora of substances in the air that our body is exposed to and may need to protect against. These include both natural (e.g. forest fires, volcanic eruptions, aerosolized soil and dusts, pollen, and moulds) and anthropogenic (e.g. industry, power plants, traffic, household heating, cooking, construction, mechanical wear, agriculture, etc.) sources. Urban ambient air pollution has received the most attention due to the high density of urban populations, greater levels of traffic-derived emissions, and general increasing urbanization of societies worldwide.
Urban pollution is a complex cocktail of chemicals that can also be broadly characterized into gases, semi-volatile liquids, and particles.15 Numerous gases are found in urban air, such as sulphur dioxide (SO2), carbon dioxide (CO2) and monoxide (CO), ozone (O3), and nitrogen dioxide (NO2). Several gases in air pollution have oxidative properties, as well as other means to affect biological systems. Gaseous pollutants have the potential to cause short- and long-term health effects, possibly in an additive manner to particulates.9 A diverse array of semi-volatile organic chemicals form the ‘liquid’ phase of air pollution, including methane, benzene, naphthalene, formaldehyde, polyaromatic hydrocarbons (PAHs), and alkanes. Semi-volatile chemicals associate or interact with gaseous and particulate phases of air pollutant. Consequently, distinguishing exposure to levels of individual pollutant constituents and isolating specific health effects can be challenging.
More consistent associations with health effects (and especially those of the cardiovascular system) tend to be found for particulate matter (PM) in the air. Environmental PM tends to be categorized, measured, and regulated in relation to particle size: coarse particles (PM10; particles with a diameter of 10 μm or less) and fine particles (PM2.5; diameter of 2.5 μm or less) (Figure 1). A third category, ultrafine particles (diameter of <100 nm, also termed ‘nanoparticles’), is believed to be especially important for health, although it is not possible to measure ultrafine PM using monitoring networks in the environment at present. In general, the smaller size fractions exert greater effects due to their large reactive surface area and their ability to penetrate deep into the alveoli of the lungs and potentially into the bloodstream.12
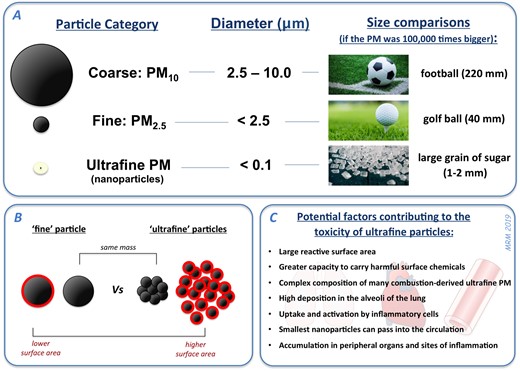
Figure 1
Different size categories of PM in air pollution. (A) PM10 and PM2.5 are routinely measured in the environment for monitoring and regulation of air pollution. Ultrafine PM (or nanoparticles: diameter <100 nm) comprise a proportion of urban PM10 and PM2.5, but due to the low mass of ultrafine PM it is not adequately accounted for by these larger PM metrics. (B) Ultrafine PM has a substantially higher surface area (red highlights) than an equivalent mass of fine PM. (C) The small size, and unique physicochemical properties, of ultrafine PM in urban environments/vehicle exhaust, engenders these particles with a higher potential to cause toxicity.
Urban PM has a complex and varied composition. Elemental and organic carbon form a major part of combustion-derived PM, but non-carbon constituents such as various mineral dusts, sea salt, ammonium, nitrates, and sulfates are also present. Additionally, there is a vast array of chemical species present in urban PM, including organic carbon species (PAHs, nitro-PAHs, alkanes, alkenes, alkyl-benzenes, quinones, etc.) and redox-active transition metals. The availability of these chemicals on the surface of PM determines a significant proportion of the biological response to these particles once they are inhaled. Additionally, particles may accumulate small amounts of biological material, such as endotoxin, that are likely to play a role in airway inflammation and other aspects of the pathophysiological response. Particles in vehicle exhaust have been the subject of substantial research interest due to high proportion of ultrafine particles in these emissions (especially so for diesel exhaust; DE). The composition of combustion-derived nanoparticles, the high surface area to mass ratio of these smaller particles and their ability to penetrate deep in the body (see below) all suggest that these particles may induce a greater relative toxicity of these particles compared to other sources of PM.
3. Particulate matter and cardiovascular disease
In 1993, a landmark study in America looked at levels of air pollution in six difference major US cities over a 14–16-year period.16 They found a clear relationship whereby higher levels of PM2.5 were associated with hospital admissions and deaths from cardiovascular disease. Subsequently, epidemiological studies have shown clear associations between air pollution and various cardiovascular diseases including coronary artery disease,17–19 cardiac arrhythmia and arrest,20–22 heart failure,23,24 cerebrovascular disease,17,25–29 peripheral arterial disease,30,31 and venous thromboembolism32 (see9 for further details).
There is evidence that acute exposure is linked to cardiovascular events. Peters et al.33 showed that individuals presenting with myocardial infarction were more likely to have been in traffic 1–2 h beforehand. Although accounting for confounding variables such as noise and stress is challenging, adjusting for level of exercise did not affect the association. Subsequent studies34 have confirmed this association, and that it is independent of the form of transport used.35
Epidemiological studies have associated air pollution with a number of endpoints underpinning cardiovascular conditions (Figure 2). Exposure to urban air pollution is associated with atherosclerosis in a range of arterial beds.36–38 While there is some inconsistency, PM exposure is also associated with a small (usually <5 mmHg for an interquartile increase in PM2.5), but significant elevation in blood pressure.39–42 Elevated blood pressure will be partially determined by constriction or reduced vasodilatation of resistance vessels, which is evident after exposure to PM.43 Exposure to PM2.5 and traffic (e.g. distance of residential address from a major road) is also linked to increased arterial stiffness,44–46 although not all studies have found significant associations.47,48
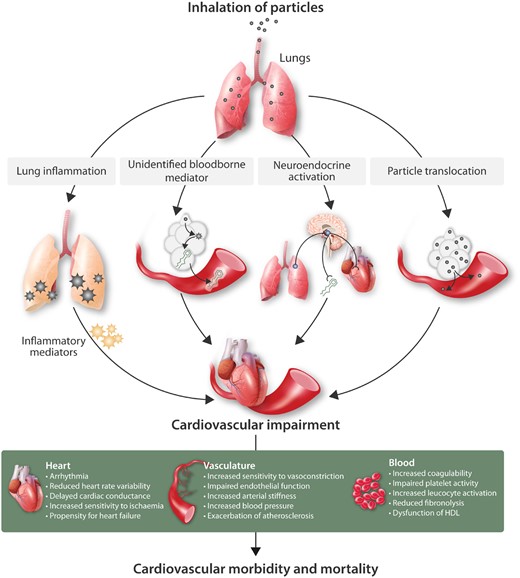
Figure 2
Biological mechanisms linking inhaled particles to cardiovascular morbidity and mortality. Several hypotheses have been proposed for the means by which inhaled particles have actions on the cardiovascular system. These include: the passage of biological mediators (e.g. not only inflammatory mediators but also unidentified mediators that are not traditional cytokines) from the lung into the circulation; activation of alveoli sensory receptors leading to triggering of neural afferents which can alter the activity of the autonomic nervous system or release of endocrine molecules; direct passage of particles (or chemicals eluting from particles) into the circulation to directly impair cardiovascular function. We emphasize that there is considerable interplay between the four pathways shown, with inflammation and oxidative stress, in particular, having the capacity to amply different stages of each pathways. The diagram also highlights that urban PM exerts many pathophysiological changes on different aspects of the cardiovascular system that ultimately increases cardiovascular morbidity and mortality.
A number of studies have made use of the non-invasive techniques to measure heart rate variability (HRV).11,49 There is an overall trend towards reduction in HRV parameters. Although there is a large degree of inconsistency between parameters and studies,49,50 the HRV effects associated with PM would be predictive of a worse prognosis at a population level. The relative ease of measuring HRV has been exploited in smaller panel studies where it is possible to measure personal exposure to pollutants (measured by portable devices, instead of estimates of pollution based on the nearest stationary monitor). In general, there is a trend towards detrimental HRV changes with PM exposure.51–55 Use of a facemask to lower exposure to particulate air pollution can attenuate these changes in HRV.56–58 The findings from epidemiological studies, results from animal studies (see below), and preventative effects of beta-blocker therapy,55,59 suggest that PM alters cardiac rhythm through imbalance of the autonomic nervous system to decrease the vagal tone and increased sympathetic tone.60 PM exposure also exacerbates cardiac ischaemia, as demonstrated by ST segment depression.9,61 Beyond HRV, PM2.5 has been linked with an increase in the incidence of arrhythmia.62 The nature of arrhythmia depends on the source of pollution, although anthropogenic sources were primarily responsible.
PM exposure is linked to prothrombotic pathways.63 While there are inconsistencies between studies looking at markers coagulation pathways,64–68 this may reflect differences in experimental protocol and variability in the extent of systemic inflammation occurring simultaneous (see below). Overall, there is as tendency for PM to increase blood fibrinogen, thrombin, von Willebrand factor and platelet activity, and decrease ex vivo coagulation times.63 Fibrinolysis is also blunted by PM, with decreases in t-PA release and up-regulation of PAI-1 pathways.63,69
Epidemiology studies have used blood and urine to look for mechanistic markers for the cardiovascular changes. PM or traffic exposure is associated with several biomarkers of oxidative stress, including oxidation of plasma proteins and lipids (e.g. malonaldehyde and protein 2-aminoadipic semialdehyde),70,71 urinary isoprostanes,72 and oxidative DNA adducts (e.g. 8-hydroxy-2′-deoxyguanosine).73–76 In many cases, indications of oxidative stress coincide with markers of inflammation [e.g. C-reactive protein (CRP), tumour necrosis factor alpha (TNF-α), various interleukins (IL)], although there is considerable variability in both.9,77 Furthermore, DE particles induce a greater inflammatory response in individuals with genetic deficiencies in various antioxidant systems.78,79 Recent work in healthy adults80 has also expanded on data from animal studies81,82 showing that PM2.5 exposure is linked to impaired high-density lipoprotein function, leading to greater levels of circulating oxidized low-density lipoprotein (LDL); a key mediator in the early stages of atherosclerosis.
4. Controlled exposure studies in human subjects
Controlled exposure studies in healthy volunteers provide a unique opportunity to study specific pollutants in isolation or together, and with the potential to avoid or control many of the confounding variables of epidemiological studies. These studies have been essential in determining the mechanisms for the acute biological effects of air pollution in humans (Figure 2). The majority of studies investigate diesel exhaust (DE) using a 1 or 2 h exposure period, and a dose of DE 100–300 μg PM/m3 to broadly model concentrations found from close proximity to exhaust emissions in heavy traffic, and representative of those reached in some international megacities.
Acute exposure to DE has prominent effects on the vasculature. Using forearm plethsymography, a 1 h exposure of DE impairs the ability of blood vessels to relax in response to infusions of vasodilator agents.83 The pattern of inhibition between vasodilators suggested an impairment of endothelial function and the nitric oxide (NO) pathway.83,84 DE also impairs endothelial responses in the skin microvasculature.85 DE attenuated vascular responses rapidly (within 2 h) and, concerningly, this impairment persisted for at least 24 h after the exposure.86 DE exhaust can also decrease brachial artery diameter, but not flow-mediated vasodilation, in healthy subjects and those with metabolic syndrome, possibly through increases in circulating endothelin-1.87 Others have found that, while DE increase vascular sensitivity to the endothelin pathway, these changes were more likely to be caused by decreased NO bioavailability rather that changes in ET-1 levels per se.88 DE can also increase arterial stiffness89 and raise systolic blood pressure,90 both of which occurred within 0.5–2 h from the beginning of the exposure.
Controlled exposure to DE alters cardiac function. A notably greater depression was seen in the ST segment of the ECG in patients with ischaemic heart disease on exercise, indicating that DE worsened the cardiac ischaemic stress.91 Acute exposures to DE tend not to be significantly associated with alterations in HRV parameters, suggesting that other constituents in urban air pollution are linked to effects on HRV that are observed in epidemiological studies.50 Indeed, Devlin et al.92 showed that controlled exposure to concentrated ambient particles (CAPs) caused changes in HRV and cardiac repolarization in elderly individuals. A later study from the group showed that ultrafine CAPs had similar effects in patients with metabolic syndrome who were null for GSTM1 allele (a prominent antioxidant gene), but not a comparative group from the general population.93 Likewise, Tong et al.94 showed that CAPs affected HRV in healthy volunteers, which could be prevented by taking omega-3 fatty acid supplements.
Using a Badimon system (an ex vivo model of thrombosis using human blood flowing over a damaged blood vessel), our group demonstrated that acute exposure to DE promoted blood clotting, the mechanisms of which included activation of platelets (e.g. increased numbers of platelet-monocyte aggregates)95 and reduced release of the fibrinolytic factor tissue-plasminogen activator (t-PA) from the vascular endothelium.83 DE alters the expression of several antioxidant pathways in peripheral blood monocytes,96 supporting a role for systemic oxidative stress in the cardiovascular actions of DE. While controlled exposure to DE does not appear to be associated with a consistent inflammatory response,97 controlled exposures to CAPs increase blood plasminogen and markers of acute phase response in individuals with genetic deficiencies in various antioxidant systems.93
Two studies indicate that particles drive the acute cardiovascular effects of DE exposure. A retrofit ‘particle trap’ on the engine exhaust efficiently reduces particle mass in the DE and completely prevented the thrombotic actions of DE.98 Filtering of particles from DE also prevented the vascular impairment observed with whole exhaust.99 This observation was supported by a study100 whereby volunteers were exposed to pure nitrogen dioxide at concentrations representative of whole exhaust; no acute cardiovascular effects were observed. Similarly, ozone exposure does not have acute cardiovascular effects,101 although co-exposure of PM and ozone can cause vasoconstriction.102
Particle composition is important to cardiovascular effects of inhaled PM. Pure carbon particles (i.e. without any surface chemicals present on diesel exhaust particles) were not associated with cardiovascular effects.99 Exposure to CAPs from rural environments can also increase blood pressure in comparison to that to that of filtered air,103 whereas CAPs that largely consisted of salt (e.g. PM largely arising from maritime winds) had no effect.104 Finally, both exhaust from idling engines and engines running in a ‘city cycle’ having detrimental cardiovascular effects.105
5. Mechanistic studies
There is a substantial body of work ranging from laboratory assays, cell cultures, isolated tissues, and in vivo studies in animals that build on findings in man to elucidate potential biological mechanisms for the cardiovascular effects of air pollution. This section provides a brief overview of this evidence and we refer readers to other reviews to broaden this narrative.9–13
Oxidative stress is a key biological mechanism by which diesel exhaust particles (DEP) exerts actions of the cardiovascular system.77 In the absence of biological tissue, DEP has the capacity to generate superoxide free radicals,106 and metals on the surface of DEP can assist in the production of hydroxyl free radicals via the Fenton reaction.107,108 Once in contact with cells, DEP can also trigger oxidative stress via a number of different cellular mechanisms including NADPH oxidase, xanthine oxidase, uncoupling of NO synthase, and mitochondrial dysfunction.77,109 The lung-lining fluid is rich in antioxidants that will presumably buffer the pro-oxidative actions of DEP, but particles can clearly overcome this defence to exert systemic oxidative actions.110 Whether inhaled particles can deplete antioxidant defences with high-dose or prolonged exposures, or if there are processes available by which particles can evade antioxidant defence, has yet to be fully established.
Inflammatory cells within the lung represent a defence mechanism for inhaled pollutants. Alveolar macrophages actively take up inhaled particles, as they would biological invaders. However, the physiochemical properties of combustion-derived PM promote activation of these cells111 and sufficient doses of PM can induce inflammatory responses that cause local, and potentially systemic, inflammation. Cell culture studies demonstrate that while DEP has only modest direct effects on many cells (unless at very high concentrations), prior interaction with macrophages leads to a release of inflammatory mediators that can then induce marked inflammatory changes in other cells types including endothelial cells.112 Oxidative stress and inflammation are likely to act synergistically to amplify each other’s effects. For example, DEP can oxidatively modify lipids,82 and that DEP and oxidized lipids together have synergistic actions of the expression profile of pathways linked to vascular inflammation.113 Similarly, the ox-LDL receptor mediates a number of cardiovascular effects of vehicle exhaust emissions in atherosclerotic mice, including infiltration of monocytes and macrophages in the vessel wall.114 While there is potential for inflammation to exacerbate the cardiovascular effects of inhaled particles in many different respects,115 there is some doubt as to whether inflammation alone is the underlying cause of these effects.77
Experiments with isolated tissues have shown that DEP can directly induce endothelial dysfunction in the absence of inflammatory cells.43,106,116 Pulmonary exposure to DEP in rodent models in vivo can induce vascular dysfunction with a similar profile of impairment to that seen with controlled exposure to DE in man, albeit depending on the model used and vascular bed studied.43 As well as effects on the pulmonary and peripheral vasculature, animal models have shown that PM has detrimental effects on the coronary circulation.117–120 PM2.5 can also affect vascular smooth muscle phenotypes, and in vivo exposure increases the incidence of abdominal aortic aneurysm in angiotensin-II-infused atherosclerotic mice.121
Mouse models of atherosclerosis have been valuable for addressing the vascular effects of chronic exposure to PM.43 Inhalation of PM promotes early events in atherogenesis, e.g. oxidation of LDL122 and the adherence of leucocytes to the vascular wall.123 Particles such as DEP increase the burden of atherosclerosis by a range of mechanisms including oxidative stress,81,82,113,124–129 changes in arachidonic acid metabolites,82,114,130 endothelin-1 pathways,131 dysfunctional high-density lipoprotein (HDL),81,132 endothelial nitric oxide synthase (eNOS) uncoupling,133 and signalling through lectin-like oxidized LDL receptors.114,134 Markers of plaque vulnerability are also increased by PM,126,128 suggesting that urban PM and DEP exposure could trigger plaque rupture. Such observations would support those of epidemiological studies linking exposure to traffic with hospital admissions for acute myocardial infarction.
In vivo models have been used to look at the actions of PM on other facets of the cardiovascular system. Pulmonary exposure of DEP to rats potentiated the thrombotic occlusion of the carotid artery following arterial injury.135 The response was more notable in response to DEP exposure compared to pure carbon nanoparticles or quartz particles, again emphasizing the importance of particle composition. Platelet activation and impaired fibrinolysis were important mechanisms,135 complementing the findings of the clinical exposures to DE.83,95 A range of thrombotic pathways could contribute to the prothrombotic effects of PM, including inflammation and oxidative stress,136–138 tissue factor,139 fibrinogen binding,140 impaired fibrinolysis,134,141 and platelets142 (reviewed by Robertson and Miller63).
Farraj et al. have performed detailed preclinical characterization of the cardiac effects of combustion-derived particles (see143 for a review). As well as effects in healthy mice, the group have used isoproterenol-induced models of cardiomyopathy to show that inhalation of various types of PM promote arrhythmias, alterations in HRV and delays cardiac conductance.144–146 Long-term exposure to PM promotes myocardial hypertrophy and loss of cardiac function.147 Pulmonary exposure to DEP also induced arrhythmia and a greater degree of myocardial infarction in a rat model of myocardial infarction after coronary ligation.148 Pharmacological inhibition of pulmonary sensory receptors or neural pathways diminished these effects, demonstrating a role for the neural systems in the cardiac effects of this air pollutant.148,149 The renin–angiotensin system and oxidative stress have also been implicated in the cardiac effects of PM.150 Direct exposure of cardiac myocytes to DEP can alter myocardial contractility and calcium handling,151 suggesting that particle translocation (see below) may be a feasible mechanism accounting for the cardiac effects of inhaled PM.
6. From the lung to the cardiovascular system
The weight of mechanistic evidence provides confidence in the biological causality for epidemiological associations between PM and CVD. Both the initial pulmonary response to inhaled PM and the multifaceted nature of cardiovascular impairment have been well characterized. However, uncertainty in the biological processes that link inhalation of particles to that of the cardiovascular system still casts a shadow over the field. Three main theories have been proposed for the potential linking pathways152,153 (Figure 2). The classical hypothesis is that inhaled pollutants activate inflammatory cells in the lung, leading to the release of inflammatory mediators that pass into the circulation to influence cardiovascular function.154 There is a convincing case to link together lung inflammation and cardiovascular disease in general.155 Also, markers of a systemic inflammation and oxidative stress are found in the blood after exposure to PM in blood humans and animal studies.9,156–158 However, there is considerable inconsistency across different biomarkers and between studies, especially in human subjects, and the time-course of the inflammatory response often does not match other systemic effects. Nonetheless, there is a clear role for both inflammation and oxidative stress in multiple stages of the mode of action of inhaled PM77,152 and these pathways represent a key means to amplify the signal from PM112 even if they are not the critical underlying cause. A convincing argument has also been postulated for acute phase proteins (e.g. CRP or serum amyloid A) in mediating certain cardiovascular effects.159 Finally, while conventional cytokines such as TNF-α, IL-6, and CRP do not seem to fully fit the bill, there is convincing evidence for an, as-yet, unidentified blood-borne mediator that could bring together the different stages of this pathway.160–162
The second theory is that inhaled pollutants activate alveolar receptors, stimulating sensory afferents that alter cardiovascular function via changes in autonomic balance or neuroendocrine regulation.143,163,164 The pattern of HRV changes in response to PM would clearly indicate a shift in the balance of the autonomic nervous system towards reduced parasympathetic activity and increased sympathetic activity. There is strong evidence from animal studies showing that pharmacological inhibition of either alveolar sensory receptors or the beta-adrenergic receptors can attenuate the cardiac effects of DEP.148,149 Chemicals species on the surface of DEP such as PAHs appear to be key in activating sensory afferents.165 The role of this pathway on non-cardiac aspects of the cardiovascular system, such as the vasculature and blood, are less certain. However, the implication of the central nervous system (e.g. the hypothalamus–pituitary–adrenal axis) with subsequent endocrine release into the blood could fill this gap.164,166,167
The third hypothesis, termed ‘particle translocation’, is that the nanoparticle fraction of PM is small enough to cross over the lung epithelial barrier and enter the pulmonary circulation, whereby particles can then be carried in the blood directly to harm other areas of the cardiovascular system.168 Proving this hypothesis is challenging given the technical limitations of visualizing these minute particles and detecting the very low levels of particles once they enter the systemic circulation. Studies in animals supported this possibility,169–172 but evidence in man was limited. Recently our group used gold nanoparticles as a model to investigate this pathway.173,174 Following 2 h inhalation of gold particles (5 nm primary size, median ∼20 nm particles aggregates in aerosol) in healthy volunteers, gold could be detected in the blood within 15 min after the exposure, and by 24 h, all volunteers had measurable levels of gold in both their blood and urine. Gold was still present in blood and urine on recall of the volunteers 3 months later, indicating that these particles persist in the body and blood for very long periods of time. Subsequent studies in animals used a range of sizes of gold nanoparticles and showed that particles with primary size of <30 nm, but not larger particles, gained entry to the blood. Importantly, translocated gold in mice preferentially accumulated in atherosclerotic arteries compared to arteries without disease. Furthermore, inhaled gold reached areas of carotid vascular disease in patients with a history of stroke.173 Recent studies by the laboratories of Calderon-Garciduenas and Maher175,176 have used advanced electron microscopy techniques to identify iron-based particles in the brain and heart177 of cadavers from heavily polluted Mexico City. The crystal structure and smooth-rounded surface suggests that these particles derive from combustion processes. While there are still some uncertainties as to whether these are inhaled particles, or that carbon-based particles also behave in the same way, these meticulous studies provide compelling evidence that exogenous particles can penetrate several organs of the body. Additionally, it is notable that the particles identified were associated with cellular damage, reinforcing the concept that particle translocation directly contributes to pathophysiological injury. Whether or not sufficient particle numbers translocate to induce cardiovascular effects is open for debate. However, the ability of DEP to generate free radicals, activate inflammatory cells, and directly impair vascular and cardiac function, suggests that should PM such as DEP also translocate in a similar manner to the gold nanoparticles, then these particles can promote cardiovascular disease.
7. Role of the cardiovascular system in the systemic effects of air pollution
In light of the many biological mechanisms by which PM can promote disease, it is logical that exposure will be associated with a range of extrapulmonary effects.178 Nonetheless, the diversity of these effects is somewhat alarming (Figure 3). These include exacerbation of metabolic syndrome and diabetes,42,179–181 chronic kidney disease,182,183 various cancers,184–187 inflammatory bowel disease,188 osteoporosis,189 and liver disease.190 PM exposure alters circulating stem cell populations191,192 and may promote rejection of organ transplants.193 Air pollution has also been linked to skin diseases,194 autoimmune diseases,195 and infertility.196

Figure 3
Emerging evidence showing that air pollution has effects throughout the body. Over the last few decades it has become apparent that air pollution can effects beyond the pulmonary and cardiovascular system. This schematic highlights a number of examples of extrapulmonary effects of air pollution in general, although in most cases there is strong evidence for a role of particulates specifically. COPD, chronic obstructive pulmonary disease; HDL, high-density lipoprotein.
Research into air pollution on the central nervous system has discovered links between air pollution and impaired cognition,197–199 dementia200–202 and even antisocial behaviour,203 and teenage psychosis.204 There is substantial attention on the early life effects of air pollution where maternal exposure has been linked to poor birth outcomes (e.g. preterm birth, low birth weight, stillbirth, or spontaneous abortion),205–207 congenital defects,208 and ill health of the children later in life.209 Furthermore, early childhood exposure has been linked to effects on asthma,210 lung development,211 childhood leukaemia,212 obesity,213 attention disorders,214 and autism.215
While the range of possible harmful effects of air pollution across the body is disturbing, our growing understanding of the mechanisms of air pollutions goes some way to explaining these observations. Oxidative stress and inflammation are ubiquitous pathways in most diseases, and characteristically amplify each other’s molecular pathophysiology. While, the nature of the mechanism that ‘carries’ this ‘signal’ from the lung to other organs still requires elucidation, nanoparticle translocation, and neuroendocrine pathways could also bridge this gap. Additionally, several systemic effects of air pollution may be indirectly caused, or at least exacerbated, by the impairments in cardiovascular function caused by inhaled PM (Figure 4).
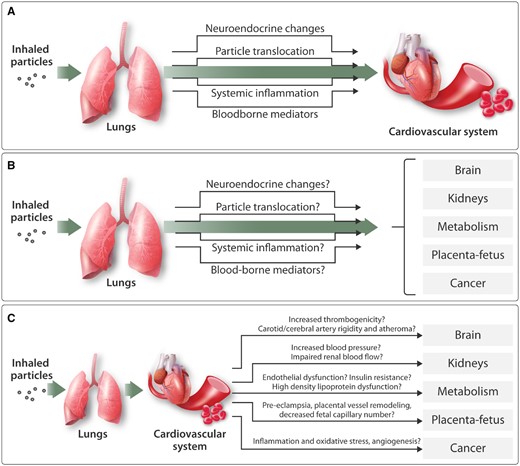
Figure 4
Biological mechanisms by which inhaled particles can impair the cardiovascular system and affect other organs in the body. (A) Several mechanisms have the potential to account for the ability of inhaled particles to induce cardiovascular actions: Inhaled particles stimulate sensory receptors in the lung, leading to changes in neural activity and endocrine release; small nanoparticles may pass (‘translocate’) into the circulation and directly act on the cardiovascular system; Induction of lung inflammation may result in a ‘spill-over’ of inflammatory mediators into the systemic circulation; other, as-yet unidentified, blood-borne mediators may drive the cardiovascular effects of inhaled particles. (B) The mechanisms proposed to account for the cardiovascular actions of inhaled particles could also be responsible for a range of effects in other organs of the body. (C) Various impairments in cardiovascular function could also indirectly contribute to the action of inhaled particles in other organs of the body.
Widespread vascular dysfunction would be expected to be accompanied by raised blood pressure; a recognized risk factor for multiple morbidities beyond the cardiovascular system. Similarly, impaired circulatory control could result in inadequate perfusion of organs and potentially ischaemic damage when combined with an increased propensity for thromboembolism. In particular, the risk factors for diabetes are intricately linked to those of cardiovascular disease, with pathophysiology of the multiple vascular beds commonly developing with progression of Type 2 diabetes mellitus. Endothelial dysfunction usual precedes insulin resistance, and the dual vascular and proinflammatory effect of PM on both glucose homoeostasis179 and impairment of cardiovascular function will contribute to the co-morbidity. Indeed, animal studies show synergism between air pollution and hypercholesterolaemia, promoting insulin resistance, enlargement of fat beds, and inflammation of visceral adipose tissue, in addition to endothelial dysfunction180,181 and development of atherosclerosis.216
Research into the effect of air pollution on the kidney is in its infancy. However, PM2.5 has been linked to membranous nephropathy, decline in renal function and increased incidence of end-stage renal disease.183 Many of the cardiovascular actions of PM could exacerbate the pathophysiology of kidney disease through elevated blood pressure, impaired renal perfusion, or inflammation. Indeed, administration of DEP to a rat model of adenine-induced chronic kidney disease decreased renal blood flow, in addition to increasing systemic blood pressure.182 Work with different sized gold nanoparticles suggests that some particles be small enough to pass from the lung to the blood, but not be small enough to pass through the filtering mechanisms of the kidney.173 Theoretically, accumulation of particles in the kidney would be expected to promote inflammation and oxidative stress and predispose other disease processes in the kidney.
It is reasonable to assume that the vascular effects of air pollution seen in many other areas of the body will be relevant to the brain. Given that approximately a third of stroke survivors would be expected to develop dementia within 5 years,217 the established link between air pollution (notably PM) and stroke26,29,218 would also be expected to extend to cognitive impairment and dementias linked to circulatory causes. Atherosclerosis (both in the carotid and cerebral arteries), increased thrombogenicity and loss of vascular flexibility are plausible cardiovascular mechanisms by which PM could induce ischaemic and haemorrhagic stroke, respectively. In this regard, PM is associated with increases in carotid-intima thickness9,37,219 and extensive carotid atheroma can have clinical manifestations linked to cerebral ischaemia or embolism. Increased endothelin-1220 and angiotensin-II221 concentrations are observed in the cerebral cortex in Alzheimer’s disease, and build-up of these vasoconstrictors may contribute to the impairments in blood flow within the brain. Addressing the effects of PM on cerebral vasculature in man is more challenging, although Wellenius et al.222 used transcranial Doppler ultrasound to look at non-structural changes in blood flow in the middle cerebral artery in elderly human subjects. An 8% increase in vascular resistance was observed per interquartile (3 μg/m3) increase in PM2.5.222 Polymorphisms in apolipoprotein E alleles are the most prevalent genetic risk factor for Alzheimer’s disease, with carriers of the E4 allele experiencing accelerated cognitive decline and neurodegeneration.223 However, apoE4 is also intrinsically linked to insulin resistance, microvascular integrity (including the blood–brain barrier) and atherosclerosis. Indeed ApoE4 is important in both the control of LDL-cholesterol in the blood and for clearance of amyloid-beta from the brain, the accumulation of which is a hallmark of several forms of dementia. Thus it is interesting that carriers of the APOE4 allele also have greater susceptibility to the effects of air pollution than non-carriers, in terms of cognition224–226 and indicators of metabolic syndrome.223,227
The effects of air pollution during pregnancy are a highly active area of research, and again there is a case for the cardiovascular effects of PM to play a role. Pre-eclampsia and other hypertensive disorders during pregnancy occur in ∼10% of pregnancies. These conditions have a substantial health burden, potentially leading to seizures, long-term disabilities, and maternal or perinatal mortality.228 The effects of exposure to air pollution on systemic blood pressure are relatively small and it is unclear whether such effect would have a significant contribution to pre-eclampsia. Nonetheless, research has suggested that in the region of 8% of hypertensive disorders during pregnancy we attributable to exposure to PM2.5 (based on >9 μg/m3 PM2.5 exposure throughout the entire pregnancy).229 While a systemic endothelial dysfunction would be a likely mechanism by which PM could contribute to pre-eclampsia, PM exerts specific effects on the foetal-placental circulation. Exposure to ambient PM (close to roadside in Sao Paulo, Brazil) during pregnancy changes the structural integrity of the umbilical cord in mice.230 Gestational exposure to diesel exhaust decreases placental blood flow and foetal capillary numbers in rabbits.231 DE can also induce haemorrhage and compaction of the labyrinth vascular spaces in mice.232 Furthermore, an early report makes claims to have found suspect PM in the placenta of non-smoking mothers living in polluted areas233 and there are some indications from rodent studies that blood-borne (non-environmental model) nanoparticles may reach the foetus.234,235 It is also plausible that translocated particles could induce a localized inflammation and oxidative stress within the placenta, that induces chronic vascular remodelling and potentially increases in vascular permeability that could aid the passage of particles to the unborn child.
Finally, the vascular effects of certain pollutants may also play a role in tumour growth. The International Agency for Research on Cancer (IARC) classified diesel exhaust as a Class 1 carcinogen in 2012 based on the combined evidence from studies in miners, animal data, and the potential for DEP to cause genotoxicity in vitro.236 However, while there is a clear case for setting occupational exposure limits to diesel exhaust, extrapolation to risk for the general public is challenging.237 Nonetheless, given the prevalence of diesel particles at roadsides (measured as elemental carbon) and lifetime exposure, researchers have postulated that this pollutant will contribute to the incidence of cancer in the general public.238 Interestingly, a study by Xu et al.239 investigated the influence of DEP on angiogenesis, with regard to the role that blood vessel formation plays in tumour formation. The investigators showed that inhalation of diesel exhaust led to increased growth of new blood vessels in hypoxic tissues in mice, while decreasing eNOS expression and increasing inflammatory cell penetration. It should be noted that the concentrations of DE used in the study were high (1 mg PM/m3) for a prolonged period to time (2–8 weeks). Furthermore, artificial in vivo models of ischaemia were employed as a trigger for angiogenesis, whereas in other scenarios new blood vessel formation could be viewed as a beneficial response to improve blood supply to ischaemic regions. Therefore, while it remains to be established whether this process would contribute to the growth and development of tumours, the observations are intriguing nonetheless, and expand further on the growing list of cardiovascular effects of vehicle exhaust.
8. Conclusions and implications
The cardiovascular effects of ambient PM make an extensive contribution to the substantial burden of air pollution on health. Epidemiological studies have found sizeable (and largely consistent) associations between exposure to urban PM and cardiovascular mortality and morbidities, including myocardial infarction and stroke. Furthermore, exposure to PM has both acute (e.g. alterations in heart rate and increased blood pressure) and chronic (e.g. exacerbation of atherosclerosis) actions on the cardiovascular system. Pollutants such as diesel exhaust particles can exert a multitude of effects on different facets of the cardiovascular system. These include vascular dysfunction, increased susceptibility of the heart to ischaemic damage, and an increased propensity for thrombosis. An array of different biological pathways appears to underlie the cardiovascular actions of inhaled PM. Oxidative stress and inflammation remain key pathways by which inhaled PM may cause harm to the body, although the ability of these nanoparticle fractions of PM to translocate into the circulation could also account for the widespread effects of PM around the body. Translocation appears to be largely restricted to the smallest nanoparticles, and it is feasible that this could include a proportion of the particulates found in vehicle emissions such as diesel exhaust. Given the large reactive surface area of these particles, their recognized toxicity, and the potential harm from co-pollutants such as nitrogen dioxide, it is clear that reducing emissions from vehicles should be a priority for air quality strategies.
There are clear socioeconomic inequalities of air pollution exposure, both in terms of exposure to pollutants (low-cost housing is often in the areas of highest pollution) and associations with other risk factors for disease (deprivation is linked to poor diet, inactivity, and, often, greater levels of smoking, alcohol, and drug use). As well as the overlap and interactions between these risk factors, the impaired cardiovascular function associated with air pollution will further compound health. Raised blood pressure, propensity for thromboembolism, and cardiac inefficiency will, in themselves, be risk factors for co-morbidities. In addition to this, from a pathophysiological perspective, there are a number of means by which cardiovascular dysfunction linked to PM exposure may indirect contribute to effect in other organ systems. These include links to cardiovascular complications of metabolic disease and diabetes, stroke and vascular dementia, changes to circulatory system of the placenta in pregnancy, and potentially vascularization of tumours. To what extent the cardiovascular system contributes to these conditions remains to be determined. However, these possibilities further emphasize the widespread effects of air pollution throughout the body. Practically, they also suggest that interventions that reduce air pollution could lead to beneficial effects on multiple organ systems.
While significant improvements in air quality have been made in many countries, air pollution is increasing beyond the already extreme levels frequently found in many developing nations. Furthermore, there are clear differences in guidelines for different limit levels of pollutants in different nations (e.g. WHO recommended annual PM2.5: <10 μg/m3; USA: <12 μg/m3; Europe: <25 μg/m3; and China: <35 μg/m3). Furthermore, based on the current data available, there is no recognized level of air pollution that is considered ‘safe’. Certainly, robust studies have shown that levels of PM and air pollution below stringent guidelines (e.g. WHO, PM2.5 <10 μg/m3) are still associated with significant health effects.129,240 It is clear that air pollution is global concern that should not be met with complacency.
A combination of strategies should be used to reduce air pollution effectively. Indeed, a recent examination of the potential interventions in the UK has identified a number of strategies by which different sectors can tackle air pollution.241 However, the report emphasizes that a concerted implementation of measures is required to realize gains in air quality that are unlikely to be possible from isolated interventions. In terms of pollutants, from a cardiovascular perspective alone, reducing PM should be a priority. Epidemiological assessments of cities that have successfully decreased particulate air pollution levels have reduced cardiovascular mortality.42 Indeed, it is encouraging that a number of studies have shown that reduction of personal exposure to urban PM through use of facemasks has been accompanied by improvements in cardiovascular parameters.42,56–58,242 Indoor air purifiers also appear to have beneficial effects in this regard, especially in cities with high levels of pollution.42,243 The long-term consequences of these short-term benefits need to be established.
The mass metrics of PM10 and PM2.5 are not ideal for quantifying ultrafine particles from vehicle exhaust.244 Given the strong mechanistic evidence for the scale of harm that combustion-derived particles exert on the cardiovascular system, targeting PM from vehicle emissions may well be accompanied by improvements in cardiovascular health beyond that which would be estimated from reductions in PM10 and PM2.5. Experimental studies in man have already shown that removal of particulates from diesel exhaust can prevent the acute cardiovascular parameters associated with this pollutant.98,99 Rodent studies have also shown the potential for fuel additives that reduce particle numbers in tailpipe emissions to prevent the pro-atherosclerotic effects of DEP.245 Modern combustion engines are substantially more efficient, with Euro 6 diesel engine emitting, on average, 10× less PM than a Euro 3 engine (albeit these are based on mass metrics from Euro emission standards for regulated diesel vehicles—emissions vary considerably with the type of vehicle and the situation is considerably more complicated and variable under real-world driving conditions).246 Furthermore, the rapid rise in popularity of electric vehicle (and improvements in charging infrastructure, battery efficiency, and the electrification of commercial vehicles) suggests that the eradication of combustion-derived particles from vehicles may be achievable. And if this aspiration can be realized, significant improvements in cardiovascular health will likely follow.
Conflict of interest: none declared.
Funding
This work was supported by the British Heart Foundation [SP/15/8/31575, CH/09/002 to M.R.M.; CH/09/002, RG/10/9/28286; RE/18/5/34216 to D.E.N.] and is the recipient of a Wellcome Trust Senior Investigator Award [WT103782AIA].
References
2Lim
SS
, Vos
T
, Flaxman
AD
, Danaei
G
, Shibuya
K
, Adair-Rohani
H
, Amann
M
, Anderson
HR
, Andrews
KG
, Aryee
M
, Atkinson
C
, Bacchus
LJ
, Bahalim
AN
, Balakrishnan
K
, Balmes
J
, Barker-Collo
S
, Baxter
A
, Bell
ML
, Blore
JD
, Blyth
F
, Bonner
C
, Borges
G
, Bourne
R
, Boussinesq
M
, Brauer
M
, Brooks
P
, Bruce
NG
, Brunekreef
B
, Bryan-Hancock
C
, Bucello
C
, Buchbinder
R
, Bull
F
, Burnett
RT
, Byers
TE
, Calabria
B
, Carapetis
J
, Carnahan
E
, Chafe
Z
, Charlson
F
, Chen
H
, Chen
JS
, Cheng
AT
, Child
JC
, Cohen
A
, Colson
KE
, Cowie
BC
, Darby
S
, Darling
S
, Davis
A
, Degenhardt
L
, Dentener
F
, Des Jarlais
DC
, Devries
K
, Dherani
M
, Ding
EL
, Dorsey
ER
, Driscoll
T
, Edmond
K
, Ali
SE
, Engell
RE
, Erwin
PJ
, Fahimi
S
, Falder
G
, Farzadfar
F
, Ferrari
A
, Finucane
MM
, Flaxman
S
, Fowkes
FG
, Freedman
G
, Freeman
MK
, Gakidou
E
, Ghosh
S
, Giovannucci
E
, Gmel
G
, Graham
K
, Grainger
R
, Grant
B
, Gunnell
D
, Gutierrez
HR
, Hall
W
, Hoek
HW
, Hogan
A
, Hosgood
HD
3rd, Hoy
D
, Hu
H
, Hubbell
BJ
, Hutchings
SJ
, Ibeanusi
SE
, Jacklyn
GL
, Jasrasaria
R
, Jonas
JB
, Kan
H
, Kanis
JA
, Kassebaum
N
, Kawakami
N
, Khang
YH
, Khatibzadeh
S
, Khoo
JP
, Kok
C
, Laden
F
, Lalloo
R
, Lan
Q
, Lathlean
T
, Leasher
JL
, Leigh
J
, Li
Y
, Lin
JK
, Lipshultz
SE
, London
S
, Lozano
R
, Lu
Y
, Mak
J
, Malekzadeh
R
, Mallinger
L
, Marcenes
W
, March
L
, Marks
R
, Martin
R
, McGale
P
, McGrath
J
, Mehta
S
, Mensah
GA
, Merriman
TR
, Micha
R
, Michaud
C
, Mishra
V
, Mohd Hanafiah
K
, Mokdad
AA
, Morawska
L
, Mozaffarian
D
, Murphy
T
, Naghavi
M
, Neal
B
, Nelson
PK
, Nolla
JM
, Norman
R
, Olives
C
, Omer
SB
, Orchard
J
, Osborne
R
, Ostro
B
, Page
A
, Pandey
KD
, Parry
CD
, Passmore
E
, Patra
J
, Pearce
N
, Pelizzari
PM
, Petzold
M
, Phillips
MR
, Pope
D
, Pope
CA
3rd, Powles
J
, Rao
M
, Razavi
H
, Rehfuess
EA
, Rehm
JT
, Ritz
B
, Rivara
FP
, Roberts
T
, Robinson
C
, Rodriguez-Portales
JA
, Romieu
I
, Room
R
, Rosenfeld
LC
, Roy
A
, Rushton
L
, Salomon
JA
, Sampson
U
, Sanchez-Riera
L
, Sanman
E
, Sapkota
A
, Seedat
S
, Shi
P
, Shield
K
, Shivakoti
R
, Singh
GM
, Sleet
DA
, Smith
E
, Smith
KR
, Stapelberg
NJ
, Steenland
K
, Stockl
H
, Stovner
LJ
, Straif
K
, Straney
L
, Thurston
GD
, Tran
JH
, Van Dingenen
R
, van Donkelaar
A
, Veerman
JL
, Vijayakumar
L
, Weintraub
R
, Weissman
MM
, White
RA
, Whiteford
H
, Wiersma
ST
, Wilkinson
JD
, Williams
HC
, Williams
W
, Wilson
N
, Woolf
AD
, Yip
P
, Zielinski
JM
, Lopez
AD
, Murray
CJ
, Ezzati
M
, AlMazroa
MA
, Memish
ZA.
A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010
.
Lancet
2012
;
380
:
2224
–
2260
.
3Burnett
R
, Chen
H
, Szyszkowicz
M
, Fann
N
, Hubbell
B
, Pope
CA
3rd, Apte
JS
, Brauer
M
, Cohen
A
, Weichenthal
S
, Coggins
J
, Di
Q
, Brunekreef
B
, Frostad
J
, Lim
SS
, Kan
H
, Walker
KD
, Thurston
GD
, Hayes
RB
, Lim
CC
, Turner
MC
, Jerrett
M
, Krewski
D
, Gapstur
SM
, Diver
WR
, Ostro
B
, Goldberg
D
, Crouse
DL
, Martin
RV
, Peters
P
, Pinault
L
, Tjepkema
M
, van Donkelaar
A
, Villeneuve
PJ
, Miller
AB
, Yin
P
, Zhou
M
, Wang
L
, Janssen
NAH
, Marra
M
, Atkinson
RW
, Tsang
H
, Quoc Thach
T
, Cannon
JB
, Allen
RT
, Hart
JE
, Laden
F
, Cesaroni
G
, Forastiere
F
, Weinmayr
G
, Jaensch
A
, Nagel
G
, Concin
H
, Spadaro
JV.
Global estimates of mortality associated with long-term exposure to outdoor fine particulate matter
.
Proc Natl Acad Sci U S A
2018
;
115
:
9592
–
9597
.
4Apte
JS
, Brauer
M
, Cohen
A
, Ezzati
M
, Pope
CA
3rd.
Ambient PM2.5 reduces global and regional life expectancy
.
Environ Sci Technol Lett
2018
;
5
:
546
–
551
.
5Cohen
AJ
, Brauer
M
, Burnett
R
, Anderson
HR
, Frostad
J
, Estep
K
, Balakrishnan
K
, Brunekreef
B
, Dandona
L
, Dandona
R
, Feigin
V
, Freedman
G
, Hubbell
B
, Jobling
A
, Kan
H
, Knibbs
L
, Liu
Y
, Martin
R
, Morawska
L
, Pope
CA
3rd, Shin
H
, Straif
K
, Shaddick
G
, Thomas
M
, van Dingenen
R
, van Donkelaar
A
, Vos
T
, Murray
CJL
, Forouzanfar
MH.
Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the Global Burden of Diseases Study 2015
.
Lancet
2017
;
389
:
1907
–
1918
.
8Lelieveld
J
, Klingmuller
K
, Pozzer
A
, Poschl
U
, Fnais
M
, Daiber
A
, Munzel
T.
Cardiovascular disease burden from ambient air pollution in Europe reassessed using novel hazard ratio functions
.
Eur Heart J
2019
;
40
:
1590
–
1596
.
9Brook
RD
, Rajagopalan
S
, Pope
CA
3rd, Brook
JR
, Bhatnagar
A
, Diez-Roux
AV
, Holguin
F
, Hong
Y
, Luepker
RV
, Mittleman
MA
, Peters
A
, Siscovick
D
, Smith
SC
Jr, Whitsel
L
, Kaufman
JD.
Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association
.
Circulation
2010
;
121
:
2331
–
2378
.
10Bourdrel
T
, Bind
MA
, Bejot
Y
, Morel
O
, Argacha
JF.
Cardiovascular effects of air pollution
.
Arch Cardiovasc Dis
2017
;
110
:
634
–
642
.
12Ohlwein
S
, Kappeler
R
, Kutlar Joss
M
, Kunzli
N
, Hoffmann
B.
Health effects of ultrafine particles: a systematic literature review update of epidemiological evidence
.
Int J Public Health
2019
;
64
:
547.
13Rajagopalan
S
, Al-Kindi
SG
, Brook
RD.
Air pollution and cardiovascular disease: JACC state-of-the-art review
.
J Am Coll Cardiol
2018
;
72
:
2054
–
2070
.
14Franchini
M
, Guida
A
, Tufano
A
, Coppola
A.
Air pollution, vascular disease and thrombosis: linking clinical data and pathogenic mechanisms
.
J Thromb Haemost
2012
;
10
:
2438
–
2451
.
15Kelly
FJ
, Fussell
JC.
Air pollution and public health: emerging hazards and improved understanding of risk
.
Environ Geochem Health
2015
;
37
:
631
–
649
.
16Dockery
DW
, Pope
CA
3rd, Xu
X
, Spengler
JD
, Ware
JH
, Fay
ME
, Ferris
BG
Jr, Speizer
FE.
An association between air pollution and mortality in six U.S. cities
.
N Engl J Med
1993
;
329
:
1753
–
1759
.
17Miller
KA
, Siscovick
DS
, Sheppard
L
, Shepherd
K
, Sullivan
JH
, Anderson
GL
, Kaufman
JD.
Long-term exposure to air pollution and incidence of cardiovascular events in women
.
N Engl J Med
2007
;
356
:
447
–
458
.
18Chen
H
, Goldberg
MS
, Burnett
RT
, Jerrett
M
, Wheeler
AJ
, Villeneuve
PJ.
Long-term exposure to traffic-related air pollution and cardiovascular mortality
.
Epidemiology
2013
;
24
:
35
–
43
.
19Cesaroni
G
, Forastiere
F
, Stafoggia
M
, Andersen
ZJ
, Badaloni
C
, Beelen
R
, Caracciolo
B
, de Faire
U
, Erbel
R
, Eriksen
KT
, Fratiglioni
L
, Galassi
C
, Hampel
R
, Heier
M
, Hennig
F
, Hilding
A
, Hoffmann
B
, Houthuijs
D
, Jöckel
K-H
, Korek
M
, Lanki
T
, Leander
K
, Magnusson
PKE
, Migliore
E
, Ostenson
C-G
, Overvad
K
, Pedersen
NL
, J
JP
, Penell
J
, Pershagen
G
, Pyko
A
, Raaschou-Nielsen
O
, Ranzi
A
, Ricceri
F
, Sacerdote
C
, Salomaa
V
, Swart
W
, Turunen
AW
, Vineis
P
, Weinmayr
G
, Wolf
K
, de Hoogh
K
, Hoek
G
, Brunekreef
B
, Peters
A.
Long term exposure to ambient air pollution and incidence of acute coronary events: prospective cohort study and meta-analysis in 11 European cohorts from the ESCAPE Project
.
BMJ
2014
;
348
:
f7412.
20Pope
CA
3rd, Muhlestein
JB
, May
HT
, Renlund
DG
, Anderson
JL
, Horne
BD.
Ischemic heart disease events triggered by short-term exposure to fine particulate air pollution
.
Circulation
2006
;
114
:
2443
–
2448
.
21Raza
A
, Bellander
T
, Bero-Bedada
G
, Dahlquist
M
, Hollenberg
J
, Jonsson
M
, Lind
T
, Rosenqvist
M
, Svensson
L
, Ljungman
PL.
Short-term effects of air pollution on out-of-hospital cardiac arrest in Stockholm
.
Eur Heart J
2014
;
35
:
861
–
868
.
22Watkins
A
, Danilewitz
M
, Kusha
M
, Masse
S
, Urch
B
, Quadros
K
, Spears
D
, Farid
T
, Nanthakumar
K.
Air pollution and arrhythmic risk: the smog is yet to clear
.
Can J Cardiol
2013
;
29
:
734
–
741
.
23Atkinson
RW
, Carey
IM
, Kent
AJ
, van Staa
TP
, Anderson
HR
, Cook
DG.
Long-term exposure to outdoor air pollution and incidence of cardiovascular diseases
.
Epidemiology
2013
;
24
:
44
–
53
.
24Shah
AS
, Langrish
JP
, Nair
H
, McAllister
DA
, Hunter
AL
, Donaldson
K
, Newby
DE
, Mills
NL.
Global association of air pollution and heart failure: a systematic review and meta-analysis
.
Lancet
2013
;
382
:
1039
–
1048
.
25Low
RB
, Bielory
L
, Qureshi
AI
, Dunn
V
, Stuhlmiller
DF
, Dickey
DA.
The relation of stroke admissions to recent weather, airborne allergens, air pollution, seasons, upper respiratory infections, and asthma incidence, September 11, 2001, and day of the week
.
Stroke
2006
;
37
:
951
–
957
.
26Shah
AS
, Lee
KK
, McAllister
DA
, Hunter
A
, Nair
H
, Whiteley
W
, Langrish
JP
, Newby
DE
, Mills
NL.
Short term exposure to air pollution and stroke: systematic review and meta-analysis
.
BMJ
2015
;
350
:
h1295.
27Stafoggia
M
, Cesaroni
G
, Peters
A
, Andersen
ZJ
, Badaloni
C
, Beelen
R
, Caracciolo
B
, Cyrys
J
, de Faire
U
, de Hoogh
K
, Eriksen
KT
, Fratiglioni
L
, Galassi
C
, Gigante
B
, Havulinna
AS
, Hennig
F
, Hilding
A
, Hoek
G
, Hoffmann
B
, Houthuijs
D
, Korek
M
, Lanki
T
, Leander
K
, Magnusson
PK
, Meisinger
C
, Migliore
E
, Overvad
K
, Ostenson
CG
, Pedersen
NL
, Pekkanen
J
, Penell
J
, Pershagen
G
, Pundt
N
, Pyko
A
, Raaschou-Nielsen
O
, Ranzi
A
, Ricceri
F
, Sacerdote
C
, Swart
WJ
, Turunen
AW
, Vineis
P
, Weimar
C
, Weinmayr
G
, Wolf
K
, Brunekreef
B
, Forastiere
F.
Long-term exposure to ambient air pollution and incidence of cerebrovascular events: results from 11 European cohorts within the ESCAPE project
.
Environ Health Perspect
2014
;
122
:
919
–
925
.
28Miller
MR
, Shah
A.
Ambient particles and cerebrovascular disease. In Bondy
SC
, Campbell
A
(eds).
Inflammation, Aging and Oxidative Stress
.
Switzerland
:
Springer International
;
2016
. p
133
–
160
.
29Lee
KK
, Miller
MR
, Shah
A.
Air pollution and stroke
.
J Stroke
2018
;
20
:
2
–
11
.
30Hoffmann
B
, Moebus
S
, Kroger
K
, Stang
A
, Mohlenkamp
S
, Dragano
N
, Schmermund
A
, Memmesheimer
M
, Erbel
R
, Jockel
KH.
Residential exposure to urban air pollution, ankle–brachial index, and peripheral arterial disease
.
Epidemiology
2009
;
20
:
280
–
288
.
31Peng
RD
, Chang
HH
, Bell
ML
, McDermott
A
, Zeger
SL
, Samet
JM
, Dominici
F.
Coarse particulate matter air pollution and hospital admissions for cardiovascular and respiratory diseases among Medicare patients
.
JAMA
2008
;
299
:
2172
–
2179
.
32Baccarelli
A
, Martinelli
I
, Pegoraro
V
, Melly
S
, Grillo
P
, Zanobetti
A
, Hou
L
, Bertazzi
PA
, Mannucci
PM
, Schwartz
J.
Living near major traffic roads and risk of deep vein thrombosis
.
Circulation
2009
;
119
:
3118
–
3124
.
33Peters
A
, Dockery
DW
, Muller
JE
, Mittleman
MA.
Increased particulate air pollution and the triggering of myocardial infarction
.
Circulation
2001
;
103
:
2810
–
2815
.
34Bhaskaran
K
, Hajat
S
, Armstrong
B
, Haines
A
, Herrett
E
, Wilkinson
P
, Smeeth
L.
The effects of hourly differences in air pollution on the risk of myocardial infarction: case crossover analysis of the MINAP database
.
BMJ
2011
;
343
:
d5531.
35Peters
A
, von Klot
S
, Mittleman
MA
, Meisinger
C
, Hormann
A
, Kuch
B
, Wichmann
HE.
Triggering of acute myocardial infarction by different means of transportation
.
Eur J Prev Cardiol
2013
;
20
:
750
–
758
.
36Hoffmann
B
, Moebus
S
, Mohlenkamp
S
, Stang
A
, Lehmann
N
, Dragano
N
, Schmermund
A
, Memmesheimer
M
, Mann
K
, Erbel
R
, Jockel
KH.
Residential exposure to traffic is associated with coronary atherosclerosis
.
Circulation
2007
;
116
:
489
–
496
.
37Kunzli
N
, Jerrett
M
, Mack
WJ
, Beckerman
B
, LaBree
L
, Gilliland
F
, Thomas
D
, Peters
J
, Hodis
HN.
Ambient air pollution and atherosclerosis in Los Angeles
.
Environ Health Perspect
2005
;
113
:
201
–
206
.
38Provost
EB
, Madhloum
N
, Int Panis
L
, De Boever
P
, Nawrot
TS.
Carotid intima-media thickness, a marker of subclinical atherosclerosis, and particulate air pollution exposure: the meta-analytical evidence
.
PLoS One
2015
;
10
:
e0127014.
39Brook
RD
, Rajagopalan
S.
Particulate matter, air pollution, and blood pressure
.
J Am Soc Hypertens
2009
;
3
:
332
–
350
.
40Liang
R
, Zhang
B
, Zhao
X
, Ruan
Y
, Lian
H
, Fan
Z.
Effect of exposure to PM2.5 on blood pressure: a systematic review and meta-analysis
.
J Hypertens
2014
;
32
:
2130
–
2140
.
41Yang
BY
, Qian
Z
, Howard
SW
, Vaughn
MG
, Fan
SJ
, Liu
KK
, Dong
GH.
Global association between ambient air pollution and blood pressure: a systematic review and meta-analysis
.
Environ Pollut
2018
;
235
:
576
–
588
.
42Munzel
T
, Sorensen
M
, Gori
T
, Schmidt
FP
, Rao
X
, Brook
J
, Chen
LC
, Brook
RD
, Rajagopalan
S.
Environmental stressors and cardio-metabolic disease: part I—epidemiologic evidence supporting a role for noise and air pollution and effects of mitigation strategies
.
Eur Heart J
2017
;
38
:
550
–
556
.
43Moller
P
, Mikkelsen
L
, Vesterdal
LK
, Folkmann
JK
, Forchhammer
L
, Roursgaard
M
, Danielsen
PH
, Loft
S.
Hazard identification of particulate matter on vasomotor dysfunction and progression of atherosclerosis
.
Crit Rev Toxicol
2011
;
41
:
339
–
368
.
44Scheers
H
, Nawrot
TS
, Nemery
B
, Casas
L.
Changing places to study short-term effects of air pollution on cardiovascular health: a panel study
.
Environ Health
2018
;
17
:
80.
45Mehta
AJ
, Zanobetti
A
, Koutrakis
P
, Mittleman
MA
, Sparrow
D
, Vokonas
P
, Schwartz
J.
Associations between short-term changes in air pollution and correlates of arterial stiffness: the Veterans Affairs Normative Aging Study, 2007–2011
.
Am J Epidemiol
2014
;
179
:
192
–
199
.
46Zhang
S
, Wolf
K
, Breitner
S
, Kronenberg
F
, Stafoggia
M
, Peters
A
, Schneider
A.
Long-term effects of air pollution on ankle–brachial index
.
Environ Int
2018
;
118
:
17
–
25
.
47Ljungman
PLS
, Li
W
, Rice
MB
, Wilker
EH
, Schwartz
J
, Gold
DR
, Koutrakis
P
, Benjamin
EJ
, Vasan
RS
, Mitchell
GF
, Hamburg
NM
, Mittleman
MA.
Long- and short-term air pollution exposure and measures of arterial stiffness in the Framingham Heart Study
.
Environ Int
2018
;
121
:
139
–
147
.
48Zanoli
L
, Lentini
P
, Granata
A
, Gaudio
A
, Fatuzzo
P
, Serafino
L
, Rastelli
S
, Fiore
V
, D'Anca
A
, Signorelli
SS
, Castellino
P.
A systematic review of arterial stiffness, wave reflection and air pollution
.
Mol Med Rep
2017
;
15
:
3425
–
3429
.
49Buteau
S
, Goldberg
MS.
A structured review of panel studies used to investigate associations between ambient air pollution and heart rate variability
.
Environ Res
2016
;
148
:
207
–
247
.
50Mills
NL
, Finlayson
AE
, Gonzalez
MC
, Tornqvist
H
, Barath
S
, Vink
E
, Goudie
C
, Langrish
JP
, Soderberg
S
, Boon
NA
, Fox
KA
, Donaldson
K
, Sandstrom
T
, Blomberg
A
, Newby
DE.
Diesel exhaust inhalation does not affect heart rhythm or heart rate variability
.
Heart
2011
;
97
:
544
–
550
.
51Chan
CC
, Chuang
KJ
, Shiao
GM
, Lin
LY.
Personal exposure to submicrometer particles and heart rate variability in human subjects
.
Environ Health Perspect
2004
;
112
:
1063
–
1067
.
52Wu
S
, Deng
F
, Niu
J
, Huang
Q
, Liu
Y
, Guo
X.
Association of heart rate variability in taxi drivers with marked changes in particulate air pollution in Beijing in 2008
.
Environ Health Perspect
2010
;
118
:
87
–
91
.
53Feng
Y
, Huang
X
, Sun
H
, Liu
C
, Zhang
B
, Zhang
Z
, Sharma Tengur
V
, Chen
W
, Wu
T
, Yuan
J
, Zhang
X.
Framingham risk score modifies the effect of PM10 on heart rate variability
.
Sci Total Environ
2015
;
523
:
146
–
151
.
54Lee
MS
, Eum
KD
, Fang
SC
, Rodrigues
EG
, Modest
GA
, Christiani
DC.
Oxidative stress and systemic inflammation as modifiers of cardiac autonomic responses to particulate air pollution
.
Int J Cardiol
2014
;
176
:
166
–
170
.
55Folino
AF
, Scapellato
ML
, Canova
C
, Maestrelli
P
, Bertorelli
G
, Simonato
L
, Iliceto
S
, Lotti
M.
Individual exposure to particulate matter and the short-term arrhythmic and autonomic profiles in patients with myocardial infarction
.
Eur Heart J
2009
;
30
:
1614
–
1620
.
56Langrish
JP
, Li
X
, Wang
S
, Lee
MMY
, Barnes
GD
, Miller
MR
, Cassee
FR
, Boon
NA
, Donaldson
K
, Li
J
, Li
L
, Mills
NL
, Newby
DE
, Jiang
L.
Reducing particulate air pollution exposure in patients with coronary heart disease improved cardiovascular health
.
Environ Health Perspect
2012
;
120
:
367
–
372
.
57Langrish
JP
, Mills
NL
, Chan
JK
, Leseman
DL
, Aitken
RJ
, Fokkens
PH
, Cassee
FR
, Li
J
, Donaldson
K
, Newby
DE
, Jiang
L.
Beneficial cardiovascular effects of reducing exposure to particulate air pollution with a simple facemask
.
Part Fibre Toxicol
2009
;
6
:
8.
58Yang
X
, Jia
X
, Dong
W
, Wu
S
, Miller
MR
, Hu
D
, Li
H
, Pan
L
, Deng
F
, Guo
X.
Cardiovascular benefits of reducing personal exposure to traffic-related noise and particulate air pollution: a randomized crossover study in the Beijing subway system
.
Indoor Air
2018
;
28
:
777.
59de Hartog
JJ
, Lanki
T
, Timonen
KL
, Hoek
G
, Janssen
NA
, Ibald-Mulli
A
, Peters
A
, Heinrich
J
, Tarkiainen
TH
, van Grieken
R
, van Wijnen
JH
, Brunekreef
B
, Pekkanen
J.
Associations between PM2.5 and heart rate variability are modified by particle composition and beta-blocker use in patients with coronary heart disease
.
Environ Health Perspect
2009
;
117
:
105
–
111
.
60Suh
HH
, Zanobetti
A.
Exposure error masks the relationship between traffic-related air pollution and heart rate variability
.
J Occup Environ Med
2010
;
52
:
685
–
692
.
61Pekkanen
J
, Peters
A
, Hoek
G
, Tiittanen
P
, Brunekreef
B
, de Hartog
J
, Heinrich
J
, Ibald-Mulli
A
, Kreyling
WG
, Lanki
T
, Timonen
KL
, Vanninen
E.
Particulate air pollution and risk of ST-segment depression during repeated submaximal exercise tests among subjects with coronary heart disease: the exposure and risk assessment for fine and ultrafine particles in ambient air (ULTRA) study
.
Circulation
2002
;
106
:
933
–
938
.
62Feng
B
, Song
X
, Dan
M
, Yu
J
, Wang
Q
, Shu
M
, Xu
H
, Wang
T
, Chen
J
, Zhang
Y
, Zhao
Q
, Wu
R
, Liu
S
, Yu
JZ
, Wang
T
, Huang
W.
High level of source-specific particulate matter air pollution associated with cardiac arrhythmias
.
Sci Total Environ
2019
;
657
:
1285
–
1293
.
63Robertson
S
, Miller
MR.
Ambient air pollution and thrombosis
.
Part Fibre Toxicol
2018
;
15
:
1.
64Zuurbier
M
, Hoek
G
, Oldenwening
M
, Meliefste
K
, Krop
E
, van den Hazel
P
, Brunekreef
B.
In-traffic air pollution exposure and CC16, blood coagulation, and inflammation markers in healthy adults
.
Environ Health Perspect
2011
;
119
:
1384
–
1389
.
65Emmerechts
J
, Hoylaerts
MF.
The effect of air pollution on haemostasis
.
Hamostaseologie
2012
;
32
:
5
–
13
.
66Franchini
M
, Mannucci
PM.
Thrombogenicity and cardiovascular effects of ambient air pollution
.
Blood
2011
;
118
:
2405
–
2412
.
67Routledge
HC
, Manney
S
, Harrison
RM
, Ayres
JG
, Townend
JN.
Effect of inhaled sulphur dioxide and carbon particles on heart rate variability and markers of inflammation and coagulation in human subjects
.
Heart
2006
;
92
:
220
–
227
.
68Strak
M
, Hoek
G
, Godri
KJ
, Gosens
I
, Mudway
IS
, van Oerle
R
, Spronk
HM
, Cassee
FR
, Lebret
E
, Kelly
FJ
, Harrison
RM
, Brunekreef
B
, Steenhof
M
, Janssen
NA.
Composition of PM affects acute vascular inflammatory and coagulative markers—the RAPTES project
.
PLoS One
2013
;
8
:
e58944.
69Langrish
JP
, Bosson
J
, Unosson
J
, Muala
A
, Newby
DE
, Mills
NL
, Blomberg
A
, Sandstrom
T.
Cardiovascular effects of particulate air pollution exposure: time course and underlying mechanisms
.
J Intern Med
2012
;
272
:
224
–
239
.
70Brucker
N
, Moro
AM
, Charao
MF
, Durgante
J
, Freitas
F
, Baierle
M
, Nascimento
S
, Gauer
B
, Bulcao
RP
, Bubols
GB
, Ferrari
PD
, Thiesen
FV
, Gioda
A
, Duarte
MM
, de Castro
I
, Saldiva
PH
, Garcia
SC.
Biomarkers of occupational exposure to air pollution, inflammation and oxidative damage in taxi drivers
.
Sci Total Environ
2013
;
463–464
:
884
–
893
.
71Sorensen
M
, Daneshvar
B
, Hansen
M
, Dragsted
LO
, Hertel
O
, Knudsen
L
, Loft
S.
Personal PM2.5 exposure and markers of oxidative stress in blood
.
Environ Health Perspect
2003
;
111
:
161
–
166
.
72Li
W
, Wilker
EH
, Dorans
KS
, Rice
MB
, Schwartz
J
, Coull
BA
, Koutrakis
P
, Gold
DR
, Keaney
JF
Jr, Lin
H
, Vasan
RS
, Benjamin
EJ
, Mittleman
MA.
Short-term exposure to air pollution and biomarkers of oxidative stress: the Framingham Heart Study
.
J Am Heart Assoc
2016
;
5
. pii: e002742. doi: 10.1161/JAHA.115.002742.
73Bagryantseva
Y
, Novotna
B
, Rossner
P
Jr, Chvatalova
I
, Milcova
A
, Svecova
V
, Lnenickova
Z
, Solansky
I
, Sram
RJ.
Oxidative damage to biological macromolecules in Prague bus drivers and garagemen: impact of air pollution and genetic polymorphisms
.
Toxicol Lett
2010
;
199
:
60
–
68
.
74Chuang
KJ
, Chan
CC
, Su
TC
, Lee
CT
, Tang
CS.
The effect of urban air pollution on inflammation, oxidative stress, coagulation, and autonomic dysfunction in young adults
.
Am J Respir Crit Care Med
2007
;
176
:
370
–
376
.
75Ren
C
, Fang
S
, Wright
RO
, Suh
H
, Schwartz
J.
Urinary 8-hydroxy-2′-deoxyguanosine as a biomarker of oxidative DNA damage induced by ambient pollution in the Normative Aging Study
.
Occup Environ Med
2011
;
68
:
562
–
569
.
76Rossner
P
Jr, Rossnerova
A
, Sram
RJ.
Oxidative stress and chromosomal aberrations in an environmentally exposed population
.
Mutat Res
2011
;
707
:
34
–
41
.
77Miller
MR
, Shaw
CA
, Langrish
JP.
From particles to patients: oxidative stress and the cardiovascular effects of air pollution
.
Future Cardiol
2012
;
8
:
577
–
602
.
78Weichenthal
SA
, Godri-Pollitt
K
, Villeneuve
PJ.
PM2.5, oxidant defence and cardiorespiratory health: a review
.
Environ Health
2013
;
12
:
40.
79Zanobetti
A
, Baccarelli
A
, Schwartz
J.
Gene-air pollution interaction and cardiovascular disease: a review
.
Prog Cardiovasc Dis
2011
;
53
:
344
–
352
.
80Li
J
, Zhou
C
, Xu
H
, Brook
RD
, Liu
S
, Yi
T
, Wang
Y
, Feng
B
, Zhao
M
, Wang
X
, Zhao
Q
, Chen
J
, Song
X
, Wang
T
, Liu
S
, Zhang
Y
, Wu
R
, Gao
J
, Pan
B
, Pennathur
S
, Rajagopalan
S
, Huo
Y
, Zheng
L
, Huang
W.
Ambient air pollution is associated with HDL (high-density lipoprotein) dysfunction in healthy adults
.
Arterioscler Thromb Vasc Biol
2019
;
39
:
513
–
522
.
81Araujo
JA
, Barajas
B
, Kleinman
M
, Wang
X
, Bennett
BJ
, Gong
KW
, Navab
M
, Harkema
J
, Sioutas
C
, Lusis
AJ
, Nel
AE.
Ambient particulate pollutants in the ultrafine range promote early atherosclerosis and systemic oxidative stress
.
Circ Res
2008
;
102
:
589
–
596
.
82Yin
F
, Lawal
A
, Ricks
J
, Fox
JR
, Larson
T
, Navab
M
, Fogelman
AM
, Rosenfeld
ME
, Araujo
JA.
Diesel exhaust induces systemic lipid peroxidation and development of dysfunctional pro-oxidant and proinflammatory high-density lipoprotein
.
Arterioscler Thromb Vasc Biol
2013
;
33
:
1153
–
1161
.
83Mills
NL
, Tornqvist
H
, Robinson
SD
, Gonzalez
M
, Darnley
K
, MacNee
W
, Boon
NA
, Donaldson
K
, Blomberg
A
, Sandstrom
T
, Newby
DE.
Diesel exhaust inhalation causes vascular dysfunction and impaired endogenous fibrinolysis
.
Circulation
2005
;
112
:
3930
–
3936
.
84Langrish
JP
, Unosson
J
, Bosson
J
, Barath
S
, Muala
A
, Blackwell
S
, Soderberg
S
, Pourazar
J
, Megson
IL
, Treweeke
A
, Sandstrom
T
, Newby
DE
, Blomberg
A
, Mills
NL.
Altered nitric oxide bioavailability contributes to diesel exhaust inhalation-induced cardiovascular dysfunction in man
.
J Am Heart Assoc
2013
;
2
:
e004309.
85Wauters
A
, Dreyfuss
C
, Pochet
S
, Hendrick
P
, Berkenboom
G
, van de Borne
P
, Argacha
JF.
Acute exposure to diesel exhaust impairs nitric oxide-mediated endothelial vasomotor function by increasing endothelial oxidative stress
.
Hypertension
2013
;
62
:
352
–
358
.
86Tornqvist
H
, Mills
NL
, Gonzalez
M
, Miller
MR
, Robinson
SD
, Megson
IL
, Macnee
W
, Donaldson
K
, Soderberg
S
, Newby
DE
, Sandstrom
T
, Blomberg
A.
Persistent endothelial dysfunction in humans after diesel exhaust inhalation
.
Am J Respir Crit Care Med
2007
;
176
:
395
–
400
.
87Peretz
A
, Sullivan
JH
, Leotta
DF
, Trenga
CA
, Sands
FN
, Allen
J
, Carlsten
C
, Wilkinson
CW
, Gill
EA
, Kaufman
JD.
Diesel exhaust inhalation elicits acute vasoconstriction in vivo
.
Environ Health Perspect
2008
;
116
:
937
–
942
.
88Langrish
JP
, Lundback
M
, Mills
NL
, Johnston
NR
, Webb
DJ
, Sandstrom
T
, Blomberg
A
, Newby
DE.
Contribution of endothelin 1 to the vascular effects of diesel exhaust inhalation in humans
.
Hypertension
2009
;
54
:
910
–
915
.
89Lundback
M
, Mills
NL
, Lucking
A
, Barath
S
, Donaldson
K
, Newby
DE
, Sandstrom
T
, Blomberg
A.
Experimental exposure to diesel exhaust increases arterial stiffness in man
.
Part Fibre Toxicol
2009
;
6
:
7.
90Cosselman
KE
, Krishnan
RM
, Oron
AP
, Jansen
K
, Peretz
A
, Sullivan
JH
, Larson
TV
, Kaufman
JD.
Blood pressure response to controlled diesel exhaust exposure in human subjects
.
Hypertension
2012
;
59
:
943
–
948
.
91Mills
NL
, Tornqvist
H
, Gonzalez
MC
, Vink
E
, Robinson
SD
, Soderberg
S
, Boon
NA
, Donaldson
K
, Sandstrom
T
, Blomberg
A
, Newby
DE.
Ischemic and thrombotic effects of dilute diesel-exhaust inhalation in men with coronary heart disease
.
N Engl J Med
2007
;
357
:
1075
–
1082
.
92Devlin
RB
, Ghio
AJ
, Kehrl
H
, Sanders
G
, Cascio
W.
Elderly humans exposed to concentrated air pollution particles have decreased heart rate variability
.
Eur Respir J Suppl
2003
;
40
:
76s
–
80s
.
93Devlin
RB
, Smith
CB
, Schmitt
MT
, Rappold
AG
, Hinderliter
A
, Graff
D
, Carraway
MS.
Controlled exposure of humans with metabolic syndrome to concentrated ultrafine ambient particulate matter causes cardiovascular effects
.
Toxicol Sci
2014
;
140
:
61
–
72
.
94Tong
H
, Rappold
AG
, Diaz-Sanchez
D
, Steck
SE
, Berntsen
J
, Cascio
WE
, Devlin
RB
, Samet
JM.
Omega-3 fatty acid supplementation appears to attenuate particulate air pollution-induced cardiac effects and lipid changes in healthy middle-aged adults
.
Environ Health Perspect
2012
;
120
:
952
–
957
.
95Lucking
AJ
, Lundback
M
, Mills
NL
, Faratian
D
, Barath
SL
, Pourazar
J
, Cassee
FR
, Donaldson
K
, Boon
NA
, Badimon
JJ
, Sandstrom
T
, Blomberg
A
, Newby
DE.
Diesel exhaust inhalation increases thrombus formation in man
.
Eur Heart J
2008
;
29
:
3043
–
3051
.
96Peretz
A
, Peck
EC
, Bammler
TK
, Beyer
RP
, Sullivan
JH
, Trenga
CA
, Srinouanprachnah
S
, Farin
FM
, Kaufman
JD.
Diesel exhaust inhalation and assessment of peripheral blood mononuclear cell gene transcription effects: an exploratory study of healthy human volunteers
.
Inhal Toxicol
2007
;
19
:
1107
–
1119
.
97Ghio
AJ
, Sobus
JR
, Pleil
JD
, Madden
MC.
Controlled human exposures to diesel exhaust
.
Swiss Med Wkly
2012
;
142
:
w13597.
98Lucking
AJ
, Lundback
M
, Barath
SL
, Mills
NL
, Sidhu
MK
, Langrish
JP
, Boon
NA
, Pourazar
J
, Badimon
JJ
, Gerlofs-Nijland
ME
, Cassee
FR
, Boman
C
, Donaldson
K
, Sandstrom
T
, Newby
DE
, Blomberg
A.
Particle traps prevent adverse vascular and prothrombotic effects of diesel engine exhaust inhalation in men
.
Circulation
2011
;
123
:
1721
–
1728
.
99Mills
NL
, Miller
MR
, Lucking
AJ
, Beveridge
J
, Flint
L
, Boere
AJ
, Fokkens
PH
, Boon
NA
, Sandstrom
T
, Blomberg
A
, Duffin
R
, Donaldson
K
, Hadoke
PW
, Cassee
FR
, Newby
DE.
Combustion-derived nanoparticulate induces the adverse vascular effects of diesel exhaust inhalation
.
Eur Heart J
2011
;
32
:
2660
–
2671
.
100Langrish
JP
, Lundback
M
, Barath
S
, Soderberg
S
, Mills
NL
, Newby
DE
, Sandstrom
T
, Blomberg
A.
Exposure to nitrogen dioxide is not associated with vascular dysfunction in man
.
Inhal Toxicol
2010
;
22
:
192
–
198
.
101Barath
S
, Langrish
JP
, Lundback
M
, Bosson
JA
, Goudie
C
, Newby
DE
, Sandstrom
T
, Mills
NL
, Blomberg
A.
Short-term exposure to ozone does not impair vascular function or affect heart rate variability in healthy young men
.
Toxicol Sci
2013
;
135
:
292
–
299
.
102Brook
RD
, Brook
JR
, Urch
B
, Vincent
R
, Rajagopalan
S
, Silverman
F.
Inhalation of fine particulate air pollution and ozone causes acute arterial vasoconstriction in healthy adults
.
Circulation
2002
;
105
:
1534
–
1536
.
103Brook
RD
, Bard
RL
, Morishita
M
, Dvonch
JT
, Wang
L
, Yang
HY
, Spino
C
, Mukherjee
B
, Kaplan
MJ
, Yalavarthi
S
, Oral
EA
, Ajluni
N
, Sun
Q
, Brook
JR
, Harkema
J
, Rajagopalan
S.
Hemodynamic, autonomic, and vascular effects of exposure to coarse particulate matter air pollution from a rural location
.
Environ Health Perspect
2014
;
122
:
624
–
630
.
104Mills
NL
, Robinson
SD
, Fokkens
PH
, Leseman
DL
, Miller
MR
, Anderson
D
, Freney
EJ
, Heal
MR
, Donovan
RJ
, Blomberg
A
, Sandstrom
T
, MacNee
W
, Boon
NA
, Donaldson
K
, Newby
DE
, Cassee
FR.
Exposure to concentrated ambient particles does not affect vascular function in patients with coronary heart disease
.
Environ Health Perspect
2008
;
116
:
709
–
715
.
105Barath
S
, Mills
NL
, Lundback
M
, Tornqvist
H
, Lucking
AJ
, Langrish
JP
, Soderberg
S
, Boman
C
, Westerholm
R
, Londahl
J
, Donaldson
K
, Mudway
IS
, Sandstrom
T
, Newby
DE
, Blomberg
A.
Impaired vascular function after exposure to diesel exhaust generated at urban transient running conditions
.
Part Fibre Toxicol
2010
;
7
:
19.
106Miller
MR
, Borthwick
SJ
, Shaw
CA
, McLean
SG
, McClure
D
, Mills
NL
, Duffin
R
, Donaldson
K
, Megson
IL
, Hadoke
PW
, Newby
DE.
Direct impairment of vascular function by diesel exhaust particulate through reduced bioavailability of endothelium-derived nitric oxide induced by superoxide free radicals
.
Environ Health Perspect
2009
;
117
:
611
–
616
.
107Donaldson
K
, Brown
DM
, Mitchell
C
, Dineva
M
, Beswick
PH
, Gilmour
P
, MacNee
W.
Free radical activity of PM10: iron-mediated generation of hydroxyl radicals
.
Environ Health Perspect
1997
;
105
(
Suppl 5
):
1285
–
1289
.
108DiStefano
E
, Eiguren-Fernandez
A
, Delfino
RJ
, Sioutas
C
, Froines
JR
, Cho
AK.
Determination of metal-based hydroxyl radical generating capacity of ambient and diesel exhaust particles
.
Inhal Toxicol
2009
;
21
:
731
–
738
.
109Risom
L
, Moller
P
, Loft
S.
Oxidative stress-induced DNA damage by particulate air pollution
.
Mutat Res
2005
;
592
:
119
–
137
.
110Kelly
FJ
, Dunster
C
, Mudway
I.
Air pollution and the elderly: oxidant/antioxidant issues worth consideration
.
Eur Respir J Suppl
2003
;
40
:
70s
–
75s
.
111Shaw
CA
, Mortimer
GM
, Deng
ZJ
, Carter
ES
, Connell
SP
, Miller
MR
, Duffin
R
, Newby
DE
, Hadoke
PW
, Minchin
RF.
Protein corona formation in bronchoalveolar fluid enhances diesel exhaust nanoparticle uptake and pro-inflammatory responses in macrophages
.
Nanotoxicology
2016
;
10
:
981
–
991
.
112Shaw
CA
, Robertson
S
, Miller
MR
, Duffin
R
, Tabor
CM
, Donaldson
K
, Newby
DE
, Hadoke
PW.
Diesel exhaust particulate—exposed macrophages cause marked endothelial cell activation
.
Am J Respir Cell Mol Biol
2011
;
44
:
840
–
851
.
113Gong
KW
, Zhao
W
, Li
N
, Barajas
B
, Kleinman
M
, Sioutas
C
, Horvath
S
, Lusis
AJ
, Nel
A
, Araujo
JA.
Air-pollutant chemicals and oxidized lipids exhibit genome-wide synergistic effects on endothelial cells
.
Genome Biol
2007
;
8
:
R149.
114Lund
AK
, Lucero
J
, Harman
M
, Madden
MC
, McDonald
JD
, Seagrave
JC
, Campen
MJ.
The oxidized low-density lipoprotein receptor mediates vascular effects of inhaled vehicle emissions
.
Am J Respir Crit Care Med
2011
;
184
:
82
–
91
.
115Donaldson
K
, Mills
N
, MacNee
W
, Robinson
S
, Newby
D.
Role of inflammation in cardiopulmonary health effects of PM
.
Toxicol Appl Pharmacol
2005
;
207
:
483
–
488
.
116Ikeda
M
, Suzuki
M
, Watarai
K
, Sagai
M
, Tomita
T.
Impairment of endothelium-dependent relaxation by diesel exhaust particles in rat thoracic aorta
.
Jpn J Pharmacol
1995
;
68
:
183
–
189
.
117Bartoli
CR
, Wellenius
GA
, Coull
BA
, Akiyama
I
, Diaz
EA
, Lawrence
J
, Okabe
K
, Verrier
RL
, Godleski
JJ.
Concentrated ambient particles alter myocardial blood flow during acute ischemia in conscious canines
.
Environ Health Perspect
2009
;
117
:
333
–
337
.
118Campen
MJ
, Babu
NS
, Helms
GA
, Pett
S
, Wernly
J
, Mehran
R
, McDonald
JD.
Nonparticulate components of diesel exhaust promote constriction in coronary arteries from ApoE−/− mice
.
Toxicol Sci
2005
;
88
:
95
–
102
.
119Cherng
TW
, Campen
MJ
, Knuckles
TL
, Gonzalez Bosc
LV
, Kanagy
NL.
Impairment of coronary endothelial cell ETB receptor function following short-term inhalation exposure to whole diesel emissions
.
Am J Physiol Regul Integr Comp Physiol
2009
;
297
:
R640
–
R647
.
120Lemos
M
, Mohallen
SV
, Macchione
M
, Dolhnikoff
M
, Assuncao
JV
, Godleski
JJ
, Saldiva
PH.
Chronic exposure to urban air pollution induces structural alterations in murine pulmonary and coronary arteries
.
Inhal Toxicol
2006
;
18
:
247
–
253
.
121Jun
X
, Jin
G
, Fu
C
, Jinxuan
Z
, Xuelin
L
, Jiaxin
H
, Shuaihua
Q
, Anqi
S
, Jianzhou
C
, Lian
Z
, Xiwen
Z
, Baoli
Z
, Biao
X.
PM2.5 promotes abdominal aortic aneurysm formation in angiotensin-infused ApoE−/− mice
.
Biomed Pharmacother
2018
;
104
:
550
–
557
.
122Ikeda
M
, Shitashige
M
, Yamasaki
H
, Sagai
M
, Tomita
T.
Oxidative modification of low density lipoprotein by diesel exhaust particles
.
Biol Pharm Bull
1995
;
18
:
866
–
871
.
123Yatera
K
, Hsieh
J
, Hogg
JC
, Tranfield
E
, Suzuki
H
, Shih
CH
, Behzad
AR
, Vincent
R
, van Eeden
SF.
Particulate matter air pollution exposure promotes recruitment of monocytes into atherosclerotic plaques
.
Am J Physiol Heart Circ Physiol
2008
;
294
:
H944
–
H953
.
124Bai
N
, Kido
T
, Kavanagh
TJ
, Kaufman
JD
, Rosenfeld
ME
, van Breemen
C
, van Eeden
SF.
Exposure to diesel exhaust up-regulates iNOS expression in ApoE knockout mice
.
Toxicol Appl Pharmacol
2011
;
255
:
184
–
192
.
125Lund
AK
, Knuckles
TL
, Obot Akata
C
, Shohet
R
, McDonald
JD
, Gigliotti
A
, Seagrave
JC
, Campen
MJ.
Gasoline exhaust emissions induce vascular remodeling pathways involved in atherosclerosis
.
Toxicol Sci
2007
;
95
:
485
–
494
.
126Miller
MR
, McLean
SG
, Duffin
R
, Lawal
AO
, Araujo
JA
, Shaw
CA
, Mills
NL
, Donaldson
K
, Newby
DE
, Hadoke
PW.
Diesel exhaust particulate increases the size and complexity of lesions in atherosclerotic mice
.
Part Fibre Toxicol
2013
;
10
:
61.
127Kampfrath
T
, Maiseyeu
A
, Ying
Z
, Shah
Z
, Deiuliis
JA
, Xu
X
, Kherada
N
, Brook
RD
, Reddy
KM
, Padture
NP
, Parthasarathy
S
, Chen
LC
, Moffatt-Bruce
S
, Sun
Q
, Morawietz
H
, Rajagopalan
S.
Chronic fine particulate matter exposure induces systemic vascular dysfunction via NADPH oxidase and TLR4 pathways
.
Circ Res
2011
;
108
:
716
–
726
.
128Sun
Q
, Wang
A
, Jin
X
, Natanzon
A
, Duquaine
D
, Brook
RD
, Aguinaldo
JG
, Fayad
ZA
, Fuster
V
, Lippmann
M
, Chen
LC
, Rajagopalan
S.
Long-term air pollution exposure and acceleration of atherosclerosis and vascular inflammation in an animal model
.
JAMA
2005
;
294
:
3003
–
3010
.
129Wan
G
, Rajagopalan
S
, Sun
Q
, Zhang
K.
Real-world exposure of airborne particulate matter triggers oxidative stress in an animal model
.
Int J Physiol Pathophysiol Pharmacol
2010
;
2
:
64
–
68
.
130Soares
SR
, Carvalho-Oliveira
R
, Ramos-Sanchez
E
, Catanozi
S
, da Silva
LF
, Mauad
T
, Gidlund
M
, Goto
H
, Garcia
ML.
Air pollution and antibodies against modified lipoproteins are associated with atherosclerosis and vascular remodeling in hyperlipemic mice
.
Atherosclerosis
2009
;
207
:
368
–
373
.
131Lund
AK
, Lucero
J
, Lucas
S
, Madden
MC
, McDonald
JD
, Seagrave
JC
, Knuckles
TL
, Campen
MJ.
Vehicular emissions induce vascular MMP-9 expression and activity associated with endothelin-1-mediated pathways
.
Arterioscler Thromb Vasc Biol
2009
;
29
:
511
–
517
.
132Li
R
, Navab
M
, Pakbin
P
, Ning
Z
, Navab
K
, Hough
G
, Morgan
TE
, Finch
CE
, Araujo
JA
, Fogelman
AM
, Sioutas
C
, Hsiai
T.
Ambient ultrafine particles alter lipid metabolism and HDL anti-oxidant capacity in LDLR-null mice
.
J Lipid Res
2013
;
54
:
1608
–
1615
.
133Cherng
TW
, Paffett
ML
, Jackson-Weaver
O
, Campen
MJ
, Walker
BR
, Kanagy
NL.
Mechanisms of diesel-induced endothelial nitric oxide synthase dysfunction in coronary arterioles
.
Environ Health Perspect
2011
;
119
:
98
–
103
.
134Kodavanti
UP
, Thomas
R
, Ledbetter
AD
, Schladweiler
MC
, Shannahan
JH
, Wallenborn
JG
, Lund
AK
, Campen
MJ
, Butler
EO
, Gottipolu
RR
, Nyska
A
, Richards
JE
, Andrews
D
, Jaskot
RH
, McKee
J
, Kotha
SR
, Patel
RB
, Parinandi
NL.
Vascular and cardiac impairments in rats inhaling ozone and diesel exhaust particles
.
Environ Health Perspect
2011
;
119
:
312
–
318
.
135Tabor
CM
, Shaw
CA
, Robertson
S
, Miller
MR
, Duffin
R
, Donaldson
K
, Newby
DE
, Hadoke
PW.
Platelet activation independent of pulmonary inflammation contributes to diesel exhaust particulate-induced promotion of arterial thrombosis
.
Part Fibre Toxicol
2015
;
13
:
6.
136Mutlu
GM
, Green
D
, Bellmeyer
A
, Baker
CM
, Burgess
Z
, Rajamannan
N
, Christman
JW
, Foiles
N
, Kamp
DW
, Ghio
AJ
, Chandel
NS
, Dean
DA
, Sznajder
JI
, Budinger
GR.
Ambient particulate matter accelerates coagulation via an IL-6-dependent pathway
.
J Clin Invest
2007
;
117
:
2952
–
2961
.
137Nemmar
A
, Subramaniyan
D
, Ali
BH.
Protective effect of curcumin on pulmonary and cardiovascular effects induced by repeated exposure to diesel exhaust particles in mice
.
PLoS One
2012
;
7
:
e39554.
138Ali
BH
, Al Za'abi
M
, Shalaby
A
, Manoj
P
, Waly
MI
, Yasin
J
, Fahim
M
, Nemmar
A.
The effect of thymoquinone treatment on the combined renal and pulmonary toxicity of cisplatin and diesel exhaust particles
.
Exp Biol Med (Maywood)
2015
;
240
:
1698
–
1707
.
139Kilinc
E
, Van Oerle
R
, Borissoff
JI
, Oschatz
C
, Gerlofs-Nijland
ME
, Janssen
NA
, Cassee
FR
, Sandstrom
T
, Renne
T
, Ten Cate
H
, Spronk
HM.
Factor XII activation is essential to sustain the procoagulant effects of particulate matter
.
J Thromb Haemost
2011
;
9
:
1359
–
1367
.
140Wilson
DW
, Aung
HH
, Lame
MW
, Plummer
L
, Pinkerton
KE
, Ham
W
, Kleeman
M
, Norris
JW
, Tablin
F.
Exposure of mice to concentrated ambient particulate matter results in platelet and systemic cytokine activation
.
Inhal Toxicol
2010
;
22
:
267
–
276
.
141Budinger
GR
, McKell
JL
, Urich
D
, Foiles
N
, Weiss
I
, Chiarella
SE
, Gonzalez
A
, Soberanes
S
, Ghio
AJ
, Nigdelioglu
R
, Mutlu
EA
, Radigan
KA
, Green
D
, Kwaan
HC
, Mutlu
GM.
Particulate matter-induced lung inflammation increases systemic levels of PAI-1 and activates coagulation through distinct mechanisms
.
PLoS One
2011
;
6
:
e18525.
142Emmerechts
J
, Jacobs
L
, Van Kerckhoven
S
, Loyen
S
, Mathieu
C
, Fierens
F
, Nemery
B
, Nawrot
TS
, Hoylaerts
MF.
Air pollution-associated procoagulant changes: the role of circulating microvesicles
.
J Thromb Haemost
2012
;
10
:
96
–
106
.
143Perez
CM
, Hazari
MS
, Farraj
AK.
Role of autonomic reflex arcs in cardiovascular responses to air pollution exposure
.
Cardiovasc Toxicol
2015
;
15
:
69
–
78
.
144Carll
AP
, Haykal-Coates
N
, Winsett
DW
, Hazari
MS
, Ledbetter
AD
, Richards
JH
, Cascio
WE
, Costa
DL
, Farraj
AK.
Cardiomyopathy confers susceptibility to particulate matter-induced oxidative stress, vagal dominance, arrhythmia and pulmonary inflammation in heart failure-prone rats
.
Inhal Toxicol
2015
;
27
:
100
–
112
.
145Carll
AP
, Haykal-Coates
N
, Winsett
DW
, Rowan
WH
3rd, Hazari
MS
, Ledbetter
AD
, Nyska
A
, Cascio
WE
, Watkinson
WP
, Costa
DL
, Farraj
AK.
Particulate matter inhalation exacerbates cardiopulmonary injury in a rat model of isoproterenol-induced cardiomyopathy
.
Inhal Toxicol
2010
;
22
:
355
–
368
.
146Carll
AP
, Lust
RM
, Hazari
MS
, Perez
CM
, Krantz
QT
, King
CJ
, Winsett
DW
, Cascio
WE
, Costa
DL
, Farraj
AK.
Diesel exhaust inhalation increases cardiac output, bradyarrhythmias, and parasympathetic tone in aged heart failure-prone rats
.
Toxicol Sci
2013
;
131
:
583
–
595
.
147Wold
LE
, Ying
Z
, Hutchinson
KR
, Velten
M
, Gorr
MW
, Velten
C
, Youtz
DJ
, Wang
A
, Lucchesi
PA
, Sun
Q
, Rajagopalan
S.
Cardiovascular remodeling in response to long-term exposure to fine particulate matter air pollution
.
Circ Heart Fail
2012
;
5
:
452
–
461
.
148Robertson
S
, Thomson
AL
, Carter
R
, Stott
HR
, Shaw
CA
, Hadoke
PW
, Newby
DE
, Miller
MR
, Gray
GA.
Pulmonary diesel particulate increases susceptibility to myocardial ischemia/reperfusion injury via activation of sensory TRPV1 and beta1 adrenoreceptors
.
Part Fibre Toxicol
2014
;
11
:
12.
149Hazari
MS
, Haykal-Coates
N
, Winsett
DW
, Krantz
QT
, King
C
, Costa
DL
, Farraj
AK.
TRPA1 and sympathetic activation contribute to increased risk of triggered cardiac arrhythmias in hypertensive rats exposed to diesel exhaust
.
Environ Health Perspect
2011
;
119
:
951
–
957
.
150Ghelfi
E
, Wellenius
GA
, Lawrence
J
, Millet
E
, Gonzalez-Flecha
B.
Cardiac oxidative stress and dysfunction by fine concentrated ambient particles (CAPs) are mediated by angiotensin-II
.
Inhal Toxicol
2010
;
22
:
963
–
972
.
151Gorr
MW
, Youtz
DJ
, Eichenseer
CM
, Smith
KE
, Nelin
TD
, Cormet-Boyaka
E
, Wold
LE.
In vitro particulate matter exposure causes direct and lung-mediated indirect effects on cardiomyocyte function
.
Am J Physiol Heart Circ Physiol
2015
;
309
:
H53
–
H62
.
152Miller
MR.
The role of oxidative stress in the cardiovascular actions of particulate air pollution
.
Biochem Soc Trans
2014
;
42
:
1006
–
1011
.
153Fiordelisi
A
, Piscitelli
P
, Trimarco
B
, Coscioni
E
, Iaccarino
G
, Sorriento
D.
The mechanisms of air pollution and particulate matter in cardiovascular diseases
.
Heart Fail Rev
2017
;
22
:
337
–
347
.
154Seaton
A
, MacNee
W
, Donaldson
K
, Godden
D.
Particulate air pollution and acute health effects
.
Lancet
1995
;
345
:
176
–
178
.
155Van Eeden
S
, Leipsic
J
, Paul Man
SF
, Sin
DD.
The relationship between lung inflammation and cardiovascular disease
.
Am J Respir Crit Care Med
2012
;
186
:
11
–
16
.
156Forbes
LJ
, Patel
MD
, Rudnicka
AR
, Cook
DG
, Bush
T
, Stedman
JR
, Whincup
PH
, Strachan
DP
, Anderson
RH.
Chronic exposure to outdoor air pollution and markers of systemic inflammation
.
Epidemiology
2009
;
20
:
245
–
253
.
157Goodman
JE
, Prueitt
RL
, Sax
SN
, Pizzurro
DM
, Lynch
HN
, Zu
K
, Venditti
FJ.
Ozone exposure and systemic biomarkers: evaluation of evidence for adverse cardiovascular health impacts
.
Crit Rev Toxicol
2015
;
45
:
412
–
452
.
158Robertson
S
, Gray
GA
, Duffin
R
, McLean
SG
, Shaw
CA
, Hadoke
PW
, Newby
DE
, Miller
MR.
Diesel exhaust particulate induces pulmonary and systemic inflammation in rats without impairing endothelial function ex vivo or in vivo
.
Part Fibre Toxicol
2012
;
9
:
9.
159Saber
AT
, Jacobsen
NR
, Jackson
P
, Poulsen
SS
, Kyjovska
ZO
, Halappanavar
S
, Yauk
CL
, Wallin
H
, Vogel
U.
Particle-induced pulmonary acute phase response may be the causal link between particle inhalation and cardiovascular disease
.
Wiley Interdiscip Rev Nanomed Nanobiotechnol
2014
;
6
:
517
–
531
.
160Channell
MM
, Paffett
ML
, Devlin
RB
, Madden
MC
, Campen
MJ.
Circulating factors induce coronary endothelial cell activation following exposure to inhaled diesel exhaust and nitrogen dioxide in humans: evidence from a novel translational in vitro model
.
Toxicol Sci
2012
;
127
:
179
–
186
.
161Schisler
JC
, Ronnebaum
SM
, Madden
M
, Channell
M
, Campen
M
, Willis
MS.
Endothelial inflammatory transcriptional responses to an altered plasma exposome following inhalation of diesel emissions
.
Inhal Toxicol
2015
;
27
:
272
–
280
.
162Brower
JB
, Doyle-Eisele
M
, Moeller
B
, Stirdivant
S
, McDonald
JD
, Campen
MJ.
Metabolomic changes in murine serum following inhalation exposure to gasoline and diesel engine emissions
.
Inhal Toxicol
2016
;
28
:
241
–
250
.
163Pope
CA
3rd, Verrier
RL
, Lovett
EG
, Larson
AC
, Raizenne
ME
, Kanner
RE
, Schwartz
J
, Villegas
GM
, Gold
DR
, Dockery
DW.
Heart rate variability associated with particulate air pollution
.
Am Heart J
1999
;
138
:
890
–
899
.
164Kodavanti
UP.
Stretching the stress boundary: linking air pollution health effects to a neurohormonal stress response
.
Biochim Biophys Acta
2016
;
1860
:
2880
–
2890
.
165Robinson
RK
, Birrell
MA
, Adcock
JJ
, Wortley
MA
, Dubuis
ED
, Chen
S
, McGilvery
CM
, Hu
S
, Shaffer
MSP
, Bonvini
SJ
, Maher
SA
, Mudway
IS
, Porter
AE
, Carlsten
C
, Tetley
TD
, Belvisi
MG.
Mechanistic link between diesel exhaust particles and respiratory reflexes
.
J Allergy Clin Immunol
2018
;
141
:
1074
–
1084.e1079
.
166Niu
Y
, Chen
R
, Xia
Y
, Cai
J
, Ying
Z
, Lin
Z
, Liu
C
, Chen
C
, Peng
L
, Zhao
Z
, Zhou
W
, Chen
J
, Wang
D
, Huo
J
, Wang
X
, Fu
Q
, Kan
H.
Fine particulate matter constituents and stress hormones in the hypothalamus–pituitary–adrenal axis
.
Environ Int
2018
;
119
:
186
–
192
.
167Li
H
, Cai
J
, Chen
R
, Zhao
Z
, Ying
Z
, Wang
L
, Chen
J
, Hao
K
, Kinney
PL
, Chen
H
, Kan
H.
Particulate matter exposure and stress hormone levels: a randomized, double-blind, crossover trial of air purification
.
Circulation
2017
;
136
:
618
–
627
.
168Oberdorster
G
, Sharp
Z
, Atudorei
V
, Elder
A
, Gelein
R
, Lunts
A
, Kreyling
W
, Cox
C.
Extrapulmonary translocation of ultrafine carbon particles following whole-body inhalation exposure of rats
.
J Toxicol Environ Health A
2002
;
65
:
1531
–
1543
.
169Husain
M
, Wu
D
, Saber
AT
, Decan
N
, Jacobsen
NR
, Williams
A
, Yauk
CL
, Wallin
H
, Vogel
U
, Halappanavar
S.
Intratracheally instilled titanium dioxide nanoparticles translocate to heart and liver and activate complement cascade in the heart of C57BL/6 mice
.
Nanotoxicology
2015
;
9
:
1013
–
1022
.
170Kreyling
WG
, Semmler
M
, Erbe
F
, Mayer
P
, Takenaka
S
, Schulz
H
, Oberdorster
G
, Ziesenis
A.
Translocation of ultrafine insoluble iridium particles from lung epithelium to extrapulmonary organs is size dependent but very low
.
J Toxicol Environ Health A
2002
;
65
:
1513
–
1530
.
171Choi
HS
, Ashitate
Y
, Lee
JH
, Kim
SH
, Matsui
A
, Insin
N
, Bawendi
MG
, Semmler-Behnke
M
, Frangioni
JV
, Tsuda
A.
Rapid translocation of nanoparticles from the lung airspaces to the body
.
Nat Biotechnol
2010
;
28
:
1300
–
1303
.
172Furuyama
A
, Kanno
S
, Kobayashi
T
, Hirano
S.
Extrapulmonary translocation of intratracheally instilled fine and ultrafine particles via direct and alveolar macrophage-associated routes
.
Arch Toxicol
2009
;
83
:
429
–
437
.
173Miller
MR
, Raftis
JB
, Langrish
JP
, McLean
SG
, Samutrtai
P
, Connell
SP
, Wilson
S
, Vesey
AT
, Fokkens
PHB
, Boere
AJF
, Krystek
P
, Campbell
CJ
, Hadoke
PWF
, Donaldson
K
, Cassee
FR
, Newby
DE
, Duffin
R
, Mills
NL.
Inhaled nanoparticles accumulate at sites of vascular disease
.
ACS Nano
2017
;
11
:
4542
–
4552
.
174Miller
MR
, Raftis
JB
, Langrish
JP
, McLean
SG
, Samutrtai
P
, Connell
SP
, Wilson
S
, Vesey
AT
, Fokkens
PHB
, Boere
AJF
, Krystek
P
, Campbell
CJ
, Hadoke
PWF
, Donaldson
K
, Cassee
FR
, Newby
DE
, Duffin
R
, Mills
NL.
Correction to “inhaled nanoparticles accumulate at sites of vascular disease”
.
ACS Nano
2017
;
11
:
10623
–
10624
.
175Maher
BA
, Ahmed
IAM
, Karloukovski
V
, MacLaren
DA
, Foulds
PG
, Allsop
D
, Mann
DMA
, Torres-Jardón
R
, Calderon-Garciduenas
L.
Magnetite pollution nanoparticles in the human brain
.
Proc Natl Acad Sci U S A
2016
;
113
:
10797
–
10801
.
176Calderon-Garciduenas
L
, Gonzalez-Maciel
A
, Reynoso-Robles
R
, Delgado-Chavez
R
, Mukherjee
PS
, Kulesza
RJ
, Torres-Jardon
R
, Avila-Ramirez
J
, Villarreal-Rios
R.
Hallmarks of Alzheimer disease are evolving relentlessly in Metropolitan Mexico City infants, children and young adults. APOE4 carriers have higher suicide risk and higher odds of reaching NFT stage V at </=40 years of age
.
Environ Res
2018
;
164
:
475
–
487
.
177Calderon-Garciduenas
L
, Gonzalez-Maciel
A
, Mukherjee
PS
, Reynoso-Robles
R
, Perez-Guille
B
, Gayosso-Chavez
C
, Torres-Jardon
R
, Cross
JV
, Ahmed
IAM
, Karloukovski
VV
, Maher
BA.
Combustion- and friction-derived magnetic air pollution nanoparticles in human hearts
.
Environ Res
2019
;
176
:
108567.
178Schraufnagel
DE
, Balmes
JR
, Cowl
CT
, De Matteis
S
, Jung
SH
, Mortimer
K
, Perez-Padilla
R
, Rice
MB
, Riojas-Rodriguez
H
, Sood
A
, Thurston
GD
, To
T
, Vanker
A
, Wuebbles
DJ.
Air pollution and noncommunicable diseases: a review by the Forum of International Respiratory Societies' Environmental Committee, part 1: the damaging effects of air pollution
.
Chest
2019
;
155
:
409
–
416
.
179Rajagopalan
S
, Brook
RD.
Air pollution and type 2 diabetes: mechanistic insights
.
Diabetes
2012
;
61
:
3037
–
3045
.
180Sun
Q
, Yue
P
, Deiuliis
JA
, Lumeng
CN
, Kampfrath
T
, Mikolaj
MB
, Cai
Y
, Ostrowski
MC
, Lu
B
, Parthasarathy
S
, Brook
RD
, Moffatt-Bruce
SD
, Chen
LC
, Rajagopalan
S.
Ambient air pollution exaggerates adipose inflammation and insulin resistance in a mouse model of diet-induced obesity
.
Circulation
2009
;
119
:
538
–
546
.
181Xu
X
, Yavar
Z
, Verdin
M
, Ying
Z
, Mihai
G
, Kampfrath
T
, Wang
A
, Zhong
M
, Lippmann
M
, Chen
LC
, Rajagopalan
S
, Sun
Q.
Effect of early particulate air pollution exposure on obesity in mice: role of p47phox
.
Arterioscler Thromb Vasc Biol
2010
;
30
:
2518
–
2527
.
182Al Suleimani
YM
, Al Mahruqi
AS
, Al Za'abi
M
, Shalaby
A
, Ashique
M
, Nemmar
A
, Ali
BH.
Effect of diesel exhaust particles on renal vascular responses in rats with chronic kidney disease
.
Environ Toxicol
2016
;
32
:
541
–
549
.
183Xu
X
, Nie
S
, Ding
H
, Hou
FF.
Environmental pollution and kidney diseases
.
Nat Rev Nephrol
2018
;
14
:
313
–
324
.
184Nagel
G
, Stafoggia
M
, Pedersen
M
, Andersen
ZJ
, Galassi
C
, Munkenast
J
, Jaensch
A
, Sommar
J
, Forsberg
B
, Olsson
D
, Oftedal
B
, Krog
NH
, Aamodt
G
, Pyko
A
, Pershagen
G
, Korek
M
, De Faire
U
, Pedersen
NL
, Ostenson
CG
, Fratiglioni
L
, Sorensen
M
, Tjonneland
A
, Peeters
PH
, Bueno-de-Mesquita
B
, Vermeulen
R
, Eeftens
M
, Plusquin
M
, Key
TJ
, Concin
H
, Lang
A
, Wang
M
, Tsai
MY
, Grioni
S
, Marcon
A
, Krogh
V
, Ricceri
F
, Sacerdote
C
, Ranzi
A
, Cesaroni
G
, Forastiere
F
, Tamayo-Uria
I
, Amiano
P
, Dorronsoro
M
, de Hoogh
K
, Beelen
R
, Vineis
P
, Brunekreef
B
, Hoek
G
, Raaschou-Nielsen
O
, Weinmayr
G.
Air pollution and incidence of cancers of the stomach and the upper aerodigestive tract in the European Study of Cohorts for Air Pollution Effects (ESCAPE)
.
Int J Cancer
2018
;
17
:
86
.
185Andersen
ZJ
, Stafoggia
M
, Weinmayr
G
, Pedersen
M
, Galassi
C
, Jorgensen
JT
, Oudin
A
, Forsberg
B
, Olsson
D
, Oftedal
B
, Aasvang
GM
, Aamodt
G
, Pyko
A
, Pershagen
G
, Korek
M
, De Faire
U
, Pedersen
NL
, Ostenson
CG
, Fratiglioni
L
, Eriksen
KT
, Tjonneland
A
, Peeters
PH
, Bueno-de-Mesquita
B
, Plusquin
M
, Key
TJ
, Jaensch
A
, Nagel
G
, Lang
A
, Wang
M
, Tsai
MY
, Fournier
A
, Boutron-Ruault
MC
, Baglietto
L
, Grioni
S
, Marcon
A
, Krogh
V
, Ricceri
F
, Sacerdote
C
, Migliore
E
, Tamayo-Uria
I
, Amiano
P
, Dorronsoro
M
, Vermeulen
R
, Sokhi
R
, Keuken
M
, de Hoogh
K
, Beelen
R
, Vineis
P
, Cesaroni
G
, Brunekreef
B
, Hoek
G
, Raaschou-Nielsen
O.
Long-term exposure to ambient air pollution and incidence of postmenopausal breast cancer in 15 European cohorts within the ESCAPE project
.
Environ Health Perspect
2017
;
125
:
107005.
186Janitz
AE
, Campbell
JE
, Magzamen
S
, Pate
A
, Stoner
JA
, Peck
JD.
Traffic-related air pollution and childhood acute leukemia in Oklahoma
.
Environ Res
2016
;
148
:
102
–
111
.
187Pope
CA
3rd, Burnett
RT
, Thun
MJ
, Calle
EE
, Krewski
D
, Ito
K
, Thurston
GD.
Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution
.
JAMA
2002
;
287
:
1132
–
1141
.
188Abegunde
AT
, Muhammad
BH
, Bhatti
O
, Ali
T.
Environmental risk factors for inflammatory bowel diseases: evidence based literature review
.
World J Gastroenterol
2016
;
22
:
6296
–
6317
.
189Prada
D
, Zhong
J
, Colicino
E
, Zanobetti
A
, Schwartz
J
, Dagincourt
N
, Fang
SC
, Kloog
I
, Zmuda
JM
, Holick
M
, Herrera
LA
, Hou
L
, Dominici
F
, Bartali
B
, Baccarelli
AA.
Association of air particulate pollution with bone loss over time and bone fracture risk: analysis of data from two independent studies
.
Lancet Planet Health
2017
;
1
:
e337
–
e347
.
190Kim
JW
, Park
S
, Lim
CW
, Lee
K
, Kim
B.
The role of air pollutants in initiating liver disease
.
Toxicol Res
2014
;
30
:
65
–
70
.
191DeJarnett
N
, Yeager
R
, Conklin
DJ
, Lee
J
, O’Toole
TE
, McCracken
J
, Abplanalp
W
, Srivastava
S
, Riggs
DW
, Hamzeh
I
, Wagner
S
, Chugh
A
, DeFilippis
A
, Ciszewski
T
, Wyatt
B
, Becher
C
, Higdon
D
, Ramos
KS
, Tollerud
DJ
, Myers
JA
, Rai
SN
, Shah
J
, Zafar
N
, Krishnasamy
SS
, Prabhu
SD
, Bhatnagar
A.
Residential proximity to major roadways is associated with increased levels of AC133+ circulating angiogenic cells
.
Arterioscler Thromb Vasc Biol
2015
;
35
:
2468
–
2477
.
192Cui
Y
, Sun
Q
, Liu
Z.
Ambient particulate matter exposure and cardiovascular diseases: a focus on progenitor and stem cells
.
J Cell Mol Med
2016
;
20
:
782
–
793
.
193Ruttens
D
, Verleden
SE
, Bijnens
EM
, Winckelmans
E
, Gottlieb
J
, Warnecke
G
, Meloni
F
, Morosini
M
, Van Der Bij
W
, Verschuuren
EA
, Sommerwerck
U
, Weinreich
G
, Kamler
M
, Roman
A
, Gomez-Olles
S
, Berastegui
C
, Benden
C
, Holm
AM
, Iversen
M
, Schultz
HH
, Luijk
B
, Oudijk
EJ
, Kwakkel-van Erp
JM
, Jaksch
P
, Klepetko
W
, Kneidinger
N
, Neurohr
C
, Corris
P
, Fisher
AJ
, Lordan
J
, Meachery
G
, Piloni
D
, Vandermeulen
E
, Bellon
H
, Hoffmann
B
, Vienneau
D
, Hoek
G
, de Hoogh
K
, Nemery
B
, Verleden
GM
, Vos
R
, Nawrot
TS
, Vanaudenaerde
BM.
An association of particulate air pollution and traffic exposure with mortality after lung transplantation in Europe
.
Eur Respir J
2017
;
49
:
1600484.
194Kim
KE
, Cho
D
, Park
HJ.
Air pollution and skin diseases: adverse effects of airborne particulate matter on various skin diseases
.
Life Sci
2016
;
152
:
126
–
134
.
195Zhao
CN
, Xu
Z
, Wu
GC
, Mao
YM
, Liu
LN
, Qian
W
, Dan
YL
, Tao
SS
, Zhang
Q
, Sam
NB
, Fan
YG
, Zou
YF
, Ye
DQ
, Pan
HF.
Emerging role of air pollution in autoimmune diseases
.
Autoimmun Rev
2019
;
18
:
607
–
614
.
196Carre
J
, Gatimel
N
, Moreau
J
, Parinaud
J
, Leandri
R.
Does air pollution play a role in infertility? A systematic review
.
Environ Health
2017
;
16
:
82.
197Kilian
J
, Kitazawa
M.
The emerging risk of exposure to air pollution on cognitive decline and Alzheimer's disease—evidence from epidemiological and animal studies
.
Biomed J
2018
;
41
:
141
–
162
.
198Allen
JL
, Klocke
C
, Morris-Schaffer
K
, Conrad
K
, Sobolewski
M
, Cory-Slechta
DA.
Cognitive effects of air pollution exposures and potential mechanistic underpinnings
.
Curr Environ Health Rep
2017
;
4
:
180
–
191
.
199Power
MC
, Adar
SD
, Yanosky
JD
, Weuve
J.
Exposure to air pollution as a potential contributor to cognitive function, cognitive decline, brain imaging, and dementia: a systematic review of epidemiologic research
.
Neurotoxicology
2016
;
56
:
235
–
253
.
200The Lancet N
. Air pollution and brain health: an emerging issue
.
Lancet Neurol
2018
;
17
:
103
.
201Sram
RJ
, Veleminsky
M
Jr, Veleminsky
M
Sr, Stejskalova
J.
The impact of air pollution to central nervous system in children and adults
.
Neuro Endocrinol Lett
2017
;
38
:
389
–
396
.
202Peters
R
, Ee
N
, Peters
J
, Booth
A
, Mudway
I
, Anstey
KJ.
Air pollution and dementia: a systematic review
.
J Alzheimers Dis
2019
;
70
:
S145
–
S163
.
203Haynes
EN
, Chen
A
, Ryan
P
, Succop
P
, Wright
J
, Dietrich
KN.
Exposure to airborne metals and particulate matter and risk for youth adjudicated for criminal activity
.
Environ Res
2011
;
111
:
1243
–
1248
.
204Newbury
JB
, Arseneault
L
, Beevers
S
, Kitwiroon
N
, Roberts
S
, Pariante
CM
, Kelly
FJ
, Fisher
HL.
Association of air pollution exposure with psychotic experiences during adolescence
.
Environ Pollut
2017
;
76
:
614
–
623
.
205Li
X
, Huang
S
, Jiao
A
, Yang
X
, Yun
J
, Wang
Y
, Xue
X
, Chu
Y
, Liu
F
, Liu
Y
, Ren
M
, Chen
X
, Li
N
, Lu
Y
, Mao
Z
, Tian
L
, Xiang
H.
Association between ambient fine particulate matter and preterm birth or term low birth weight: an updated systematic review and meta-analysis
.
Environ Pollut
2017
;
227
:
596
–
605
.
206Grippo
A
, Zhang
J
, Chu
L
, Guo
Y
, Qiao
L
, Zhang
J
, Myneni
AA
, Mu
L.
Air pollution exposure during pregnancy and spontaneous abortion and stillbirth
.
Rev Environ Health
2018
;
33
:
247
–
264
.
207Klepac
P
, Locatelli
I
, Korosec
S
, Kunzli
N
, Kukec
A.
Ambient air pollution and pregnancy outcomes: a comprehensive review and identification of environmental public health challenges
.
Environ Res
2018
;
167
:
144
–
159
.
208Baldacci
S
, Gorini
F
, Santoro
M
, Pierini
A
, Minichilli
F
, Bianchi
F.
Environmental and individual exposure and the risk of congenital anomalies: a review of recent epidemiological evidence
.
Epidemiol Prev
2018
;
42
:
1
–
34
.
209Wang
L
, Pinkerton
KE.
Air pollutant effects on fetal and early postnatal development
.
Birth Defects Res C Embryo Today
2007
;
81
:
144
–
154
.
210Jung
CR
, Chen
WT
, Tang
YH
, Hwang
BF.
Fine particulate matter exposure during pregnancy and infancy periods and incident asthma
.
J Allergy Clin Immunol
2019
;
143
:
2254
–
2262.e5
.
211Veras
MM
, de Oliveira Alves
N
, Fajersztajn
L
, Saldiva
P.
Before the first breath: prenatal exposures to air pollution and lung development
.
Cell Tissue Res
2017
;
367
:
445
–
455
.
212Filippini
T
, Heck
JE
, Malagoli
C
, Del Giovane
C
, Vinceti
M.
A review and meta-analysis of outdoor air pollution and risk of childhood leukemia
.
J Environ Sci Health C Environ Carcinog Ecotoxicol Rev
2015
;
33
:
36
–
66
.
213Kim
JS
, Alderete
TL
, Chen
Z
, Lurmann
F
, Rappaport
E
, Habre
R
, Berhane
K
, Gilliland
FD.
Longitudinal associations of in utero and early life near-roadway air pollution with trajectories of childhood body mass index
.
Environ Health
2018
;
17
:
64.
214Myhre
O
, Lag
M
, Villanger
GD
, Oftedal
B
, Ovrevik
J
, Holme
JA
, Aase
H
, Paulsen
RE
, Bal-Price
A
, Dirven
H.
Early life exposure to air pollution particulate matter (PM) as risk factor for attention deficit/hyperactivity disorder (ADHD): need for novel strategies for mechanisms and causalities
.
Toxicol Appl Pharmacol
2018
;
354
:
196
–
214
.
215Flores-Pajot
MC
, Ofner
M
, Do
MT
, Lavigne
E
, Villeneuve
PJ.
Childhood autism spectrum disorders and exposure to nitrogen dioxide, and particulate matter air pollution: a review and meta-analysis
.
Environ Res
2016
;
151
:
763
–
776
.
216Maresh
JG
, Campen
MJ
, Reed
MD
, Darrow
AL
, Shohet
RV.
Hypercholesterolemia potentiates aortic endothelial response to inhaled diesel exhaust
.
Inhal Toxicol
2011
;
23
:
1
–
10
.
217Vijayan
M
, Reddy
PH.
Stroke, vascular dementia, and Alzheimer's disease: molecular links
.
J Alzheimers Dis
2016
;
54
:
427
–
443
.
218Babadjouni
RM
, Hodis
DM
, Radwanski
R
, Durazo
R
, Patel
A
, Liu
Q
, Mack
WJ.
Clinical effects of air pollution on the central nervous system; a review
.
J Clin Neurosci
2017
;
43
:
16
–
24
.
219Liu
X
, Lian
H
, Ruan
Y
, Liang
R
, Zhao
X
, Routledge
M
, Fan
Z.
Association of exposure to particular matter and carotid intima-media thickness: a systematic review and meta-analysis
.
Int J Environ Res Public Health
2015
;
12
:
12924
–
12940
.
220Palmer
JC
, Tayler
HM
, Love
S.
Endothelin-converting enzyme-1 activity, endothelin-1 production, and free radical-dependent vasoconstriction in Alzheimer's disease
.
J Alzheimers Dis
2013
;
36
:
577
–
587
.
221Miners
JS
, van Helmond
Z
, Raiker
M
, Love
S
, Kehoe
PG.
ACE variants and association with brain Abeta levels in Alzheimer's disease
.
Am J Transl Res
2010
;
3
:
73
–
80
.
222Wellenius
GA
, Boyle
LD
, Wilker
EH
, Sorond
FA
, Coull
BA
, Koutrakis
P
, Mittleman
MA
, Lipsitz
LA.
Ambient fine particulate matter alters cerebral hemodynamics in the elderly
.
Stroke
2013
;
44
:
1532
–
1536
.
223Calderon-Garciduenas
L
, de la Monte
SM.
Apolipoprotein E4, gender, body mass index, inflammation, insulin resistance, and air pollution interactions: recipe for Alzheimer's disease development in Mexico City young females
.
J Alzheimers Dis
2017
;
58
:
613
–
630
.
224Alemany
S
, Vilor-Tejedor
N
, Garcia-Esteban
R
, Bustamante
M
, Dadvand
P
, Esnaola
M
, Mortamais
M
, Forns
J
, van Drooge
BL
, Alvarez-Pedrerol
M
, Grimalt
JO
, Rivas
I
, Querol
X
, Pujol
J
, Sunyer
J.
Traffic-related air pollution, APOEepsilon4 status, and neurodevelopmental outcomes among school children enrolled in the BREATHE project (Catalonia, Spain)
.
Environ Health Perspect
2018
;
126
:
087001
.
225Schikowski
T
, Vossoughi
M
, Vierkötter
A
, Schulte
T
, Teichert
T
, Sugiri
D
, Fehsel
K
, Tzivian
L
, Bae
I-S
, Ranft
U
, Hoffmann
B
, Probst-Hensch
N
, Herder
C
, Krämer
U
, Luckhaus
C.
Association of air pollution with cognitive functions and its modification by APOE gene variants in elderly women
.
Environ Res
2015
;
142
:
10
–
16
.
226Calderón-Garcidueñas
L
, Mora-Tiscareño
A
, Franco-Lira
M
, Zhu
H
, Lu
Z
, Solorio
E
, Torres-Jardón
R
, D'Angiulli
A.
Decreases in short term memory, IQ, and altered brain metabolic ratios in urban apolipoprotein epsilon4 children exposed to air pollution
.
J Alzheimers Dis
2015
;
45
:
757
–
770
.
227Calderon-Garciduenas
L
, Jewells
V
, Galaz-Montoya
C
, van Zundert
B
, Perez-Calatayud
A
, Ascencio-Ferrel
E
, Valencia-Salazar
G
, Sandoval-Cano
M
, Carlos
E
, Solorio
E
, Acuna-Ayala
H
, Torres-Jardon
R
, D'Angiulli
A.
Interactive and additive influences of gender, BMI and apolipoprotein 4 on cognition in children chronically exposed to high concentrations of PM2.5 and ozone. APOE 4 females are at highest risk in Mexico City
.
Environ Res
2016
;
150
:
411
–
422
.
229Xue
T
, Zhu
T
, Lin
W
, Talbott
EO.
Association between hypertensive disorders in pregnancy and particulate matter in the contiguous United States, 1999–2004
.
Hypertension
2018
;
72
:
77
–
84
.
230Veras
MM
, Guimaraes-Silva
RM
, Caldini
EG
, Saldiva
PH
, Dolhnikoff
M
, Mayhew
TM.
The effects of particulate ambient air pollution on the murine umbilical cord and its vessels: a quantitative morphological and immunohistochemical study
.
Reprod Toxicol
2012
;
34
:
598
–
606
.
231Valentino
SA
, Tarrade
A
, Aioun
J
, Mourier
E
, Richard
C
, Dahirel
M
, Rousseau-Ralliard
D
, Fournier
N
, Aubriere
MC
, Lallemand
MS
, Camous
S
, Guinot
M
, Charlier
M
, Aujean
E
, Al Adhami
H
, Fokkens
PH
, Agier
L
, Boere
JA
, Cassee
FR
, Slama
R
, Chavatte-Palmer
P.
Maternal exposure to diluted diesel engine exhaust alters placental function and induces intergenerational effects in rabbits
.
Part Fibre Toxicol
2015
;
13
:
39.
232Weldy
CS
, Liu
Y
, Liggitt
HD
, Chin
MT.
In utero exposure to diesel exhaust air pollution promotes adverse intrauterine conditions, resulting in weight gain, altered blood pressure, and increased susceptibility to heart failure in adult mice
.
PLoS One
2014
;
9
:
e88582.
233Liu
N.
Abstract no: PA360, “Late Breaking Abstract—do inhaled carbonaceous particles translocate from the lung to the placenta?”, Norrice Liu; Occupational and environmental lung diseases: asthma and the airways, 08:30 hrs CEST, Sunday 16 September, Paris Expo Porte de Versailles. European_Respiratory_Congress,
2019
.
234Semmler-Behnke
M
, Lipka
J
, Wenk
A
, Hirn
S
, Schäffler
M
, Tian
F
, Schmid
G
, Oberdörster
G
, Kreyling
WG.
Size dependent translocation and fetal accumulation of gold nanoparticles from maternal blood in the rat
.
Part Fibre Toxicol
2014
;
11
:
33.
235Hougaard
KS
, Campagnolo
L
, Chavatte-Palmer
P
, Tarrade
A
, Rousseau-Ralliard
D
, Valentino
S
, Park
MV
, de Jong
WH
, Wolterink
G
, Piersma
AH
, Ross
BL
, Hutchison
GR
, Hansen
JS
, Vogel
U
, Jackson
P
, Slama
R
, Pietroiusti
A
, Cassee
FR.
A perspective on the developmental toxicity of inhaled nanoparticles
.
Reprod Toxicol
2015
;
56
:
118
–
140
.
236Benbrahim-Tallaa
L
, Baan
RA
, Grosse
Y
, Lauby-Secretan
B
, El Ghissassi
F
, Bouvard
V
, Guha
N
, Loomis
D
, Straif
K.
International agency for research on cancer monograph working G. Carcinogenicity of diesel-engine and gasoline-engine exhausts and some nitroarenes
.
Lancet Oncol
2012
;
13
:
663
–
664
.
237Taxell
P
, Santonen
T.
Diesel engine exhaust: basis for occupational exposure limit value
.
Toxicol Sci
2017
;
158
:
243
–
251
.
238Vermeulen
R
, Silverman
DT
, Garshick
E
, Vlaanderen
J
, Portengen
L
, Steenland
K.
Exposure–response estimates for diesel engine exhaust and lung cancer mortality based on data from three occupational cohorts
.
Environ Health Perspect
2014
;
122
:
172
–
177
.
239Xu
X
, Kherada
N
, Hong
X
, Quan
C
, Zheng
L
, Wang
A
, Wold
LE
, Lippmann
M
, Chen
LC
, Rajagopalan
S
, Sun
Q.
Diesel exhaust exposure induces angiogenesis
.
Toxicol Lett
2009
;
191
:
57
–
68
.
240Beelen
R
, Raaschou-Nielsen
O
, Stafoggia
M
, Andersen
ZJ
, Weinmayr
G
, Hoffmann
B
, Wolf
K
, Samoli
E
, Fischer
P
, Nieuwenhuijsen
M
, Vineis
P
, Xun
WW
, Katsouyanni
K
, Dimakopoulou
K
, Oudin
A
, Forsberg
B
, Modig
L
, Havulinna
AS
, Lanki
T
, Turunen
A
, Oftedal
B
, Nystad
W
, Nafstad
P
, De Faire
U
, Pedersen
NL
, Ostenson
CG
, Fratiglioni
L
, Penell
J
, Korek
M
, Pershagen
G
, Eriksen
KT
, Overvad
K
, Ellermann
T
, Eeftens
M
, Peeters
PH
, Meliefste
K
, Wang
M
, Bueno-de-Mesquita
B
, Sugiri
D
, Kramer
U
, Heinrich
J
, de Hoogh
K
, Key
T
, Peters
A
, Hampel
R
, Concin
H
, Nagel
G
, Ineichen
A
, Schaffner
E
, Probst-Hensch
N
, Kunzli
N
, Schindler
C
, Schikowski
T
, Adam
M
, Phuleria
H
, Vilier
A
, Clavel-Chapelon
F
, Declercq
C
, Grioni
S
, Krogh
V
, Tsai
MY
, Ricceri
F
, Sacerdote
C
, Galassi
C
, Migliore
E
, Ranzi
A
, Cesaroni
G
, Badaloni
C
, Forastiere
F
, Tamayo
I
, Amiano
P
, Dorronsoro
M
, Katsoulis
M
, Trichopoulou
A
, Brunekreef
B
, Hoek
G.
Effects of long-term exposure to air pollution on natural-cause mortality: an analysis of 22 European cohorts within the multicentre ESCAPE project
.
Lancet
2014
;
383
:
785
–
795
.
242Guan
T
, Hu
S
, Han
Y
, Wang
R
, Zhu
Q
, Hu
Y
, Fan
H
, Zhu
T.
The effects of facemasks on airway inflammation and endothelial dysfunction in healthy young adults: a double-blind, randomized, controlled crossover study
.
Part Fibre Toxicol
2018
;
15
:
30.
243Chen
R
, Zhao
A
, Chen
H
, Zhao
Z
, Cai
J
, Wang
C
, Yang
C
, Li
H
, Xu
X
, Ha
S
, Li
T
, Kan
H.
Cardiopulmonary benefits of reducing indoor particles of outdoor origin: a randomized, double-blind crossover trial of air purifiers
.
J Am Coll Cardiol
2015
;
65
:
2279
–
2287
.
244Delfino
RJ
, Sioutas
C
, Malik
S.
Potential role of ultrafine particles in associations between airborne particle mass and cardiovascular health
.
Environ Health Perspect
2005
;
113
:
934
–
946
.
245Cassee
FR
, Campbell
A
, Boere
AJ
, McLean
SG
, Duffin
R
, Krystek
P
, Gosens
I
, Miller
MR.
The biological effects of subacute inhalation of diesel exhaust following addition of cerium oxide nanoparticles in atherosclerosis-prone mice
.
Environ Res
2012
;
115
:
1
–
10
.
246Hooftman
N
, Oliveira
L
, Messagie
M
, Coosemans
T
, van Mierlo
J.
Environmental analysis of petrol, diesel and electric passenger cars in a Belgian urban setting
.
Energies
2016
;
9
:
84
.
© The Author(s) 2019. Published by Oxford University Press on behalf of the European Society of Cardiology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (
http://creativecommons.org/licenses/by-nc/4.0/), which permits non-commercial re-use, distribution, and reproduction in any medium, provided the original work is properly cited. For commercial re-use, please contact
[email protected]
PDF








