-
PDF
- Split View
-
Views
-
Cite
Cite
Panayiota Petrou, Ibrahim Kassis, Netta Levin, Friedemann Paul, Yael Backner, Tal Benoliel, Frederike Cosima Oertel, Michael Scheel, Michelle Hallimi, Nour Yaghmour, Tamir Ben Hur, Ariel Ginzberg, Yarden Levy, Oded Abramsky, Dimitrios Karussis, Beneficial effects of autologous mesenchymal stem cell transplantation in active progressive multiple sclerosis, Brain, Volume 143, Issue 12, December 2020, Pages 3574–3588, https://doi.org/10.1093/brain/awaa333
Close - Share Icon Share
Abstract
In this study (trial registration: NCT02166021), we aimed to evaluate the optimal way of administration, the safety and the clinical efficacy of mesenchymal stem cell (MSC) transplantation in patients with active and progressive multiple sclerosis. Forty-eight patients (28 males and 20 females) with progressive multiple sclerosis (Expanded Disability Status Scale: 3.0–6.5, mean : 5.6 ± 0.8, mean age: 47.5 ± 12.3) and evidence of either clinical worsening or activity during the previous year, were enrolled (between 2015 and 2018). Patients were randomized into three groups and treated intrathecally (IT) or intravenously (IV) with autologous MSCs (1 × 106/kg) or sham injections. After 6 months, half of the patients from the MSC-IT and MSC-IV groups were retreated with MSCs, and the other half with sham injections. Patients initially assigned to sham treatment were divided into two subgroups and treated with either MSC-IT or MSC-IV. The study duration was 14 months. No serious treatment-related safety issues were detected. Significantly fewer patients experienced treatment failure in the MSC-IT and MSC-IV groups compared with those in the sham-treated group (6.7%, 9.7%, and 41.9%, respectively, P = 0.0003 and P = 0.0008). During the 1-year follow-up, 58.6% and 40.6% of patients treated with MSC-IT and MSC-IV, respectively, exhibited no evidence of disease activity compared with 9.7% in the sham-treated group (P < 0.0001 and P < 0.0048, respectively). MSC-IT transplantation induced additional benefits on the relapse rate, on the monthly changes of the T2 lesion load on MRI, and on the timed 25-foot walking test, 9-hole peg test, optical coherence tomography, functional MRI and cognitive tests. Treatment with MSCs was well-tolerated in progressive multiple sclerosis and induced short-term beneficial effects regarding the primary end points, especially in the patients with active disease. The intrathecal administration was more efficacious than the intravenous in several parameters of the disease. A phase III trial is warranted to confirm these findings.
Introduction
Mesenchymal stem cells (MSCs) are non-haematopoietic stromal cells that reside mainly in the bone marrow compartment, but also in fat and other tissues. Their classical role is to support haematopoiesis and produce cells of the mesodermal lineage (Pittenger et al., 1999; Lennon and Caplan, 2006). Studies have described additional MSC properties, including immunomodulatory and neurotrophic effects (Caplan, 1991; Woodbury et al., 2000; Orlic et al., 2001; Blondheim et al., 2006; Caplan and Dennis, 2006; Kassis et al., 2006, 2008, 2011; Uccelli et al., 2007, 2008). In animal models, intravenous and intrathecal administration of MSCs has been shown to suppress experimental autoimmune encephalomyelitis (EAE) (Zappia et al., 2005; Kassis et al., 2008; Harris et al., 2012) and support remyelination following spinal trauma or induced demyelination (Cizkova et al., 2006; Zhang et al., 2010; Hedayatpour et al., 2013).
Few small, mostly open-label, clinical trials have reported indications of favourable effects of MSC treatment in stroke, multi-system atrophy, multiple sclerosis, and amyotrophic lateral sclerosis (Karussis et al., 2010; Yamout et al., 2010; Connick et al., 2012; Lee et al., 2012; Llufriu et al., 2014; Lublin et al., 2014a; Harris et al., 2016, 2018; Petrou et al., 2016; Cohen et al., 2018; ,Fernandez et al., 2018; Riordan et al., 2018). Whether the observed benefits were mediated by immunomodulatory mechanisms or by neurotrophic and neuroprotective effects remains controversial.
These earlier studies prompted us to perform a controlled clinical trial to examine the therapeutic efficacy of MSC transplantation in progressive multiple sclerosis, for which (at least for the subtype without activity) there is a critical unmet need for treatment. We also aimed to investigate the most favourable route of cell delivery.
We present here the results of this double-blind trial that compared the clinical effects of intravenous or intrathecal injections of autologous bone marrow-derived MSCs (1 × 106/kg) with those of sham injections, in 48 patients with active or worsening progressive multiple sclerosis.
Materials and methods
Study design
The study (NIH registration: NCT02166021) was initiated in February 2015 and completed in June 2018. It was approved by the local ethics committee and Ministry of Health, and monitored by an external contract research organization (CRO) (BRD, Israel) and an external safety committee. The inclusion criteria included: age ≤65 years; diagnosis of either active (with evidence of a relapse or MRI activity) or worsening [with deterioration in Expanded Disability Status Scale (EDSS)] progressive multiple sclerosis (according to the 2013 revised criteria by Lublin et al., 2014b); treatment failure with at least one line of multiple sclerosis therapy (as evidenced by either ≥2 relapses or by deterioration in EDSS and new MRI activity); and EDSS score between 3.0 and 6.5. The study included two phases (cycles of treatment): in the first phase, three groups of patients were formulated after randomization: one group (group 1) was assigned to receive an intrathecal injection of 1 × 106 MSCs (MSC-IT)/kg of body weight, and an intravenous sham injection of normal saline. The second group (group 2) was treated with an intrathecal sham injection (normal saline) and an intravenous injection of 1 × 106 MSCs (MSC-IV)/kg of body weight, and the third group (group 3: sham treatment) was treated only with normal saline (intravenously and intrathecally). In the second phase after 6 months, the treatment groups were crossed over and each one subdivided into two subgroups of eight patients (Fig. 1). The patients in group 3 who were in the placebo arm in the first phase, were treated after the 6 months with either MSC-IT (subgroup 3A, n = 8) or MSC-IV (subgroup 3B, n = 8). Half of the patients of groups 1 and 2, were retreated with the same treatment as in the first cycle (subgroup 1A with MSC-IT, n = 8 and subgroup 2A with MSC-IV, n = 8). The other halves of groups 1 and 2 (i.e. subgroups 1B and 2B, n = 8 each), were treated in this second cycle with placebo (Fig. 1).
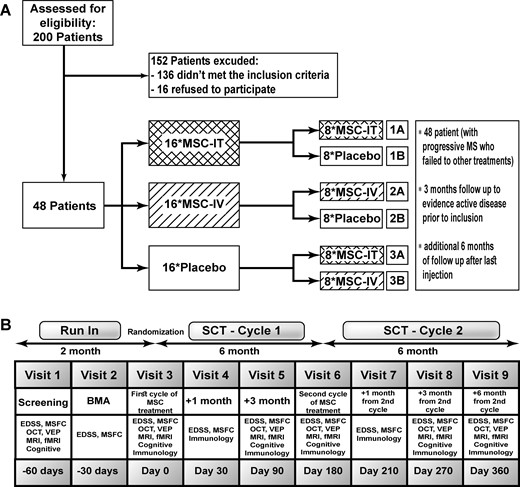
Study design and flow chart. fMRI = functional MRI; MS = multiple sclerosis; MSFC = Multiple Sclerosis Functional Composite; OCT = optical coherence tomography; VEP = visual evoked potentials.
The patients were followed-up in the outpatient multiple sclerosis clinic by the assigned treating physician (for adverse events) and by the examining physicians for the neurological testing. All physicians, clinic personnel, and patients were blinded to the treatment assignment. Patients underwent neurological examination (EDSS/Functional Systems scoring), the 9-hole peg test, timed 25-foot walking test, MRI (including resting functional MRI), visual evoked potentials (VEP), optical coherence tomography (OCT), and cognitive, immunological, and visual dynamic tests at predetermined time points, as displayed in the study flow chart (Fig. 1). In case of a relapse, the patient was treated with intravenous methylprednisolone (Solu-Medrol®) 1000 mg for 3–5 days and the scheduled neurological examination and scoring were postponed to 1 week after the end of steroid treatment. The MRI and OCT scans were transferred anonymously to a referral collaborating centre (Berlin University) and evaluated in a blinded way. The results were sent to the CRO.
MSC preparation and administration
MSCs were obtained from the bone marrow of each patient and prepared using a previously described protocol with slight modification (Karussis et al., 2010) (details of MSC preparation and culture are presented in the Supplementary material).
The cultured cells were diluted with normal saline and transferred to one of the two syringes that were prepared for each patient (according to the treatment group assignment by the CRO). One syringe contained MSCs (1 × 106/kg of body weight) resuspended in 3 ml of normal saline and one syringe contained only normal saline. To ensure blinding, the treating physician received from the Laboratory Unit (according to the randomization number), two sealed syringes covered with black adhesive, for every patient, at each treatment cycle, The full 3 ml of the content in the sealed syringe were injected to the cerebrospinal fluid via lumbar puncture at the level of L4–5, using a 20-gauge needle and three-way cannula. The 3 ml of the second syringe were injected into an aluminum-covered 500-ml sac with normal saline and infused into the patient over 30 min using a 20-gauge vein catheter. A volume of 3 ml of CSF was removed for future testing. During the two phases of the trial, each of the 48 patients received one intravenous and one intrathecal injection (of which, one or both was placebo), at each of the two treatment cycles.
Primary and secondary end points
The two predetermined primary end points of the trial were: (i) the safety of the MSC-IV and -IT treatments (incidence of adverse events versus those in the sham-treated group); and (ii) the differences among the three groups in EDSS score changes and the proportion of patients with treatment failure, as evidenced by an increase in EDSS score or deterioration in any of the functional systems, at 6 and 12 months. Secondary end points included the differences between the sham-treated and the MSC-IT or MSC-IV treated groups in: (i) the number of relapses and the relapse rate; (ii) the number of MRI gadolinium-enhancing lesions; (iii) the annualized rate of change in the T2 lesion load on MRI, total normalized brain volume (per cent brain volume change), and functional MRI-network connectivity strength versus the rates during the run-in period; (iv) the timed 25-foot walking and 9-hole peg tests; (v) cognitive functions; and (vi) the retinal nerve fibre layer thickness, and macular thickness and volume, as evaluated via OCT.
Lesion load on MRI and brain volumetric changes
For conventional 3 T MRI, raw data were sent to the NeuroCure Clinical Research Center, Charité – Universitätsmedizin Berlin, Germany and evaluated in a blinded manner. The methods of evaluation are described in the Supplementary material.
Functional MRI, visual evoked potential, optical coherence tomography and cognitive tests
Resting-state blood oxygenation level-dependent functional MRI, VEP, OCT, and a battery of cognitive tests that are sensitive to multiple sclerosis were performed [including the Paced Auditory Serial Addition Test (PASAT); Brief Visuospatial Memory Test-Revised (BVMT-R); Symbol Digit Modalities Test (SDMT); Owatonna Cognitive Behavioral Test (OWAT); KAVE-naming and fluency test (KAVE); Rey Auditory Verbal Learning Test (RAVLT); and Trail Making Test (TMT)], by standard techniques described in the Supplementary material.
Statistical analysis
The sizes of the experimental groups were calculated based on an assumption of efficacy of at least 50%, in reduction of the number of patients with treatment failure (evidenced by an EDSS step change) versus sham treatment to provide 80% power, assuming a standard deviation of differences of <75%, with a 0.050 two-sided significance level. We based our calculations both on published cohorts showing a mean annual change of 0.5 in EDSS and on our Centre cohort, in which there was a higher annual EDSS change of 0.7 (due to the selection criteria of only active or worsening patients who stopped all immunotherapies). The calculations were done for 6-month periods. Various models were evaluated that included the assumptions of the two cohorts and different scenarios of efficacy ranging from 50% to 72% and SDs of 50–75%. The locked database was transferred from the CRO (BRD, Israel) to an external company with expertise in medical statistical analysis (MediStat, Israel). All measured variables and derived parameters were assessed individually and tabulated using descriptive statistics. For categorical variables, summary tables were provided, which included the sample size and absolute and relative frequencies by study group. For continuous variables, summary tables presenting the sample size, arithmetic mean, standard deviation, median, minimum, and maximum by study group were formulated. Within-group changes from baseline or from the run-in period were analysed using paired t-tests (for the continuous values) and Wilcoxon signed-rank test (for EDSS).
For most of the statistical comparisons of the efficacy parameters, the two treatment cycles for each mode of treatment (i.e. sham, MSC-IV and MSC-IT) were pooled together: the 16 placebo patients from the first cycle were pooled with the two placebo subgroups of the second cycle (eight patients from group 1B and eight from the group 2B). Similarly, the 16 MSC-IT treated patients from the first cycle (group 1), were pooled with eight of each MSC-IT subgroup (1A and 3A) from the second cycle and the 16 MSC-IV treated patients from the first cycle (group 2), were pooled with eight of each MSC-IV subgroup (2A and 3B). In this way, three pooled groups of 6-months exposure to treatment with MSC-IT, MSC-IV or placebo were formulated (n = 32 each) for statistical comparisons. The two-sample non-parametric Wilcoxon-Mann-Whitney rank sum test was used to analyse the differences in quantitative parameters between the MSC-IT or MSC-IV and sham-treated groups. Noticeably, the ‘pooling’ of the two treatment periods, may have influenced somehow the statistical analysis of the treatment groups as independent ones, due to a possible ‘carry over effect’ in some of the patients from the first period to the second and therefore, the interpretation of this statistical analysis should be taken with caution. The chi-squared test was used to analyse the differences in binary parameters between the MSC-IT or MSC-IV and sham-treated groups. For functional MRI, a single functional z-score reflecting network connectivity was calculated per scan for each subject. For cognitive functions, values were expressed as z-scores, which were calculated using normative data from the literature, adjusted for age, sex, and educational level. All tests were two-tailed, and results with P-value ≤ 0.05 were considered statistically significant. The data were analysed using the SAS® version 9.3 software (SAS Institute, Cary, NC, USA).
Data availability
The authors confirm that the core of the data supporting the findings of this study are available within the article and its Supplementary material.
The full raw data are available on request from the corresponding author or the study coordinator (Ariel Ginzberg, [email protected]) or the Director of the external CRO, Dr Moshe Neuman, [email protected]). The data are not publicly available because they contain information that could compromise the privacy of research participants.
Results
Patients
Over 200 patients from the Hadassah MS Centre and Unit of Neuroimmunology were prescreened for inclusion in the trial. Of these, 136 patients did not meet the inclusion criteria and 16 refused to participate. In total, 48 patients (21 female, 27 male), were included with a mean EDSS score at inclusion of 5.60 ± 0.88, mean age of 47.63 ± 9.72 years, and mean disease duration of 12.70 ± 7.5 years (Table 1 and Fig. 1). The higher proportion of male versus female patients in our sample (which is not in line with the gender distribution of multiple sclerosis) may be explained by the ‘biased’ inclusion of only patients with progressive and active multiple sclerosis, who were non-responders to conventional multiple sclerosis treatments.
| . | All patients (n = 48) . | MSC-IT (n = 16) . | MSC-IV (n = 16) . | Sham treatment (n = 16) . | P-value (Kruskal-Wallis test) . |
|---|---|---|---|---|---|
| Gender | 28M, 20F | 10M, 6F | 6M, 10F | 12M, 4F | 0.312 |
| Age at inclusion, years | 47.63 ± 9.72 | 49.05 ± 7.2 | 47.42 ± 10.4 | 45.89 ± 10.9 | 0.566 |
| Disease coursea | 20 activea: 18 SPMS, 2 PPMS 28 non-active: 23 SPMS, 5 PPMS | 6 activea: 5 SPM, 1 PPMS 10 non-active: 8 SPMS, 2 PPMS | 7 activea: 7 SPMS 9 non-active: 8 SPMS, 1 PPMS | 7 activea: 6 SPMS, 1 PPMS 9 non-active: 7 SPMS, 2 PPMS | 0.552 |
| Disease onset (y) | 12.70 ± 7.51 | 10.28 ± 4.48 | 12.90 ± 8.74 | 14.94 ± 8.27 | 0.361 |
| EDSS increase at last year | 0.73 ± 0.51 | 0.72 ± 0.51 | 0.78 ± 0.75 | 0.69 ± 0.57 | 0.641 |
| EDSS at inclusion | 0.819 | ||||
| Mean ± SD | 5.60 ± 0.88 | 5.75 ± 0.77 | 5.63 ± 0.83 | 5.44 ± 1.05 | |
| Median (IQR) | 5.75 (5.5–6.5) | 5.75 (5.5–6.5) | 5.75 (5.5–6.5) | 5.75 (4.87–6.12) | |
| EDSS at baseline | 0.583 | ||||
| Mean ± SD | 5.88 ± 0.80 | 6.19 ± 0.31 | 5.81 ± 0.77 | 5.66 ± 1.08 | |
| Median (IQR) | 6.0 (5.87–6.5) | 6.0 (6.0–6.5) | 6.0 (6.0–6.5) | (4.87–6.5) | |
| Number of previous DMT | 2.58 ± 1.18 | 2.62 ± 1.02 | 2.87 ± 1.36 | 2.25 ± 1.24 | 0.403 |
| . | All patients (n = 48) . | MSC-IT (n = 16) . | MSC-IV (n = 16) . | Sham treatment (n = 16) . | P-value (Kruskal-Wallis test) . |
|---|---|---|---|---|---|
| Gender | 28M, 20F | 10M, 6F | 6M, 10F | 12M, 4F | 0.312 |
| Age at inclusion, years | 47.63 ± 9.72 | 49.05 ± 7.2 | 47.42 ± 10.4 | 45.89 ± 10.9 | 0.566 |
| Disease coursea | 20 activea: 18 SPMS, 2 PPMS 28 non-active: 23 SPMS, 5 PPMS | 6 activea: 5 SPM, 1 PPMS 10 non-active: 8 SPMS, 2 PPMS | 7 activea: 7 SPMS 9 non-active: 8 SPMS, 1 PPMS | 7 activea: 6 SPMS, 1 PPMS 9 non-active: 7 SPMS, 2 PPMS | 0.552 |
| Disease onset (y) | 12.70 ± 7.51 | 10.28 ± 4.48 | 12.90 ± 8.74 | 14.94 ± 8.27 | 0.361 |
| EDSS increase at last year | 0.73 ± 0.51 | 0.72 ± 0.51 | 0.78 ± 0.75 | 0.69 ± 0.57 | 0.641 |
| EDSS at inclusion | 0.819 | ||||
| Mean ± SD | 5.60 ± 0.88 | 5.75 ± 0.77 | 5.63 ± 0.83 | 5.44 ± 1.05 | |
| Median (IQR) | 5.75 (5.5–6.5) | 5.75 (5.5–6.5) | 5.75 (5.5–6.5) | 5.75 (4.87–6.12) | |
| EDSS at baseline | 0.583 | ||||
| Mean ± SD | 5.88 ± 0.80 | 6.19 ± 0.31 | 5.81 ± 0.77 | 5.66 ± 1.08 | |
| Median (IQR) | 6.0 (5.87–6.5) | 6.0 (6.0–6.5) | 6.0 (6.0–6.5) | (4.87–6.5) | |
| Number of previous DMT | 2.58 ± 1.18 | 2.62 ± 1.02 | 2.87 ± 1.36 | 2.25 ± 1.24 | 0.403 |
Active patients: those with relapse or MRI activity during the year prior to inclusion.
DMT = disease-modifying therapy; F = female; IQR = interquartile range; M = male; PPMS = primary progressive multiple sclerosis; SD = standard deviation; SPMS = secondary progressive multiple sclerosis.
| . | All patients (n = 48) . | MSC-IT (n = 16) . | MSC-IV (n = 16) . | Sham treatment (n = 16) . | P-value (Kruskal-Wallis test) . |
|---|---|---|---|---|---|
| Gender | 28M, 20F | 10M, 6F | 6M, 10F | 12M, 4F | 0.312 |
| Age at inclusion, years | 47.63 ± 9.72 | 49.05 ± 7.2 | 47.42 ± 10.4 | 45.89 ± 10.9 | 0.566 |
| Disease coursea | 20 activea: 18 SPMS, 2 PPMS 28 non-active: 23 SPMS, 5 PPMS | 6 activea: 5 SPM, 1 PPMS 10 non-active: 8 SPMS, 2 PPMS | 7 activea: 7 SPMS 9 non-active: 8 SPMS, 1 PPMS | 7 activea: 6 SPMS, 1 PPMS 9 non-active: 7 SPMS, 2 PPMS | 0.552 |
| Disease onset (y) | 12.70 ± 7.51 | 10.28 ± 4.48 | 12.90 ± 8.74 | 14.94 ± 8.27 | 0.361 |
| EDSS increase at last year | 0.73 ± 0.51 | 0.72 ± 0.51 | 0.78 ± 0.75 | 0.69 ± 0.57 | 0.641 |
| EDSS at inclusion | 0.819 | ||||
| Mean ± SD | 5.60 ± 0.88 | 5.75 ± 0.77 | 5.63 ± 0.83 | 5.44 ± 1.05 | |
| Median (IQR) | 5.75 (5.5–6.5) | 5.75 (5.5–6.5) | 5.75 (5.5–6.5) | 5.75 (4.87–6.12) | |
| EDSS at baseline | 0.583 | ||||
| Mean ± SD | 5.88 ± 0.80 | 6.19 ± 0.31 | 5.81 ± 0.77 | 5.66 ± 1.08 | |
| Median (IQR) | 6.0 (5.87–6.5) | 6.0 (6.0–6.5) | 6.0 (6.0–6.5) | (4.87–6.5) | |
| Number of previous DMT | 2.58 ± 1.18 | 2.62 ± 1.02 | 2.87 ± 1.36 | 2.25 ± 1.24 | 0.403 |
| . | All patients (n = 48) . | MSC-IT (n = 16) . | MSC-IV (n = 16) . | Sham treatment (n = 16) . | P-value (Kruskal-Wallis test) . |
|---|---|---|---|---|---|
| Gender | 28M, 20F | 10M, 6F | 6M, 10F | 12M, 4F | 0.312 |
| Age at inclusion, years | 47.63 ± 9.72 | 49.05 ± 7.2 | 47.42 ± 10.4 | 45.89 ± 10.9 | 0.566 |
| Disease coursea | 20 activea: 18 SPMS, 2 PPMS 28 non-active: 23 SPMS, 5 PPMS | 6 activea: 5 SPM, 1 PPMS 10 non-active: 8 SPMS, 2 PPMS | 7 activea: 7 SPMS 9 non-active: 8 SPMS, 1 PPMS | 7 activea: 6 SPMS, 1 PPMS 9 non-active: 7 SPMS, 2 PPMS | 0.552 |
| Disease onset (y) | 12.70 ± 7.51 | 10.28 ± 4.48 | 12.90 ± 8.74 | 14.94 ± 8.27 | 0.361 |
| EDSS increase at last year | 0.73 ± 0.51 | 0.72 ± 0.51 | 0.78 ± 0.75 | 0.69 ± 0.57 | 0.641 |
| EDSS at inclusion | 0.819 | ||||
| Mean ± SD | 5.60 ± 0.88 | 5.75 ± 0.77 | 5.63 ± 0.83 | 5.44 ± 1.05 | |
| Median (IQR) | 5.75 (5.5–6.5) | 5.75 (5.5–6.5) | 5.75 (5.5–6.5) | 5.75 (4.87–6.12) | |
| EDSS at baseline | 0.583 | ||||
| Mean ± SD | 5.88 ± 0.80 | 6.19 ± 0.31 | 5.81 ± 0.77 | 5.66 ± 1.08 | |
| Median (IQR) | 6.0 (5.87–6.5) | 6.0 (6.0–6.5) | 6.0 (6.0–6.5) | (4.87–6.5) | |
| Number of previous DMT | 2.58 ± 1.18 | 2.62 ± 1.02 | 2.87 ± 1.36 | 2.25 ± 1.24 | 0.403 |
Active patients: those with relapse or MRI activity during the year prior to inclusion.
DMT = disease-modifying therapy; F = female; IQR = interquartile range; M = male; PPMS = primary progressive multiple sclerosis; SD = standard deviation; SPMS = secondary progressive multiple sclerosis.
Forty-one patients had secondary progressive multiple sclerosis and seven had primary progressive multiple sclerosis. Twenty of the patients had either superimposed relapses or MRI activity (progressive multiple sclerosis with activity). Most patients (77%) had been previously treated with two or more of the accepted immunotherapeutic drugs for multiple sclerosis. All immunomodulatory treatments were stopped at least 3–6 months before the screening visit. Detailed demographic data (including previous treatments) of each patient are presented in Supplementary Tables 1 and 2. At baseline, the three treatment-groups showed no significant differences in EDSS score, gender, disease course and duration, proportion of active patients and previous treatments (Table 1 and Supplementary Table 1).
One patient (Patient 005) withdrew his consent 3 weeks after the first treatment. Compliance with the trial was excellent, and only nine of the scheduled 528 visits were missed.
The ‘blindness’ of the study was assessed by a questionnaire filled by the patients and the involved physicians at the end of the trial. According to this, 17 of 48 patients guessed correctly at least one of the treatment assignments. The correct answers from the treating and evaluating physicians were 17% and 22%, respectively.
Primary end points
Safety
Three serious adverse events occurred during the study resulting in patient hospitalization. Two were related to relapses of multiple sclerosis, and one was due to an upper respiratory infection (not related to the treatment) that was resolved after a course of antibiotics. No other serious adverse events were observed during the 14 months of the trial. The full list of adverse events is presented in Table 2. All of them were transient/short-lasting and there was no carry-over adverse event between the two cycles of treatment (see also the detailed description of adverse events in Supplementary Table 3).
| Adverse event, n . | Run-in n = 48 . | MSC-IT n = 16 + 16 . | MSC-IV n = 16 + 16 . | Sham treatment n = 16 + 16 . | Related to procedure . | Related to treatment . |
|---|---|---|---|---|---|---|
| No adverse events | – | 5 | 4 | 3 | ||
| Headache | 0 | 9 | 10 | 8 | Yes (all) | No |
| Back pain | 1 | 2 | 2 | 3 | Yes (all except one) | No |
| Viral infection | 3 | 0 | 2 | 1 | No | No |
| Upper respiratory infection | 1 | 1 | 0 | 0 | No | No |
| Fever | 1 | 1 | 1 | 2 | No | No |
| Urinary tract infection | 2 | 1 | 0 | 0 | No | No |
| Sinusitis | 0 | 2 | 0 | 0 | No | No |
| Fall | 1 | 1 | 2 | 1 | No | No |
| Fracture (leg/hand) | 2 | 1 | 0 | 0 | No | No |
| Dizziness | 0 | 2 | 1 | 0 | No | No |
| Haematoma | 1 | 1 | 0 | 0 | No | No |
| Nausea | 0 | 0 | 2 | 0 | No | No |
| Melanoma (in situ) | 1 | 0 | 0 | 0 | No | No |
| Scabies infection | 0 | 1 | 0 | 0 | No | No |
| Peripheral facial nerve palsy | 0 | 1 | 0 | 0 | No | No |
| Toothache | 0 | 0 | 1 | 0 | No | No |
| Anorexia | 0 | 0 | 1 | 0 | No | No |
| Gout | 1 | 0 | 0 | 0 | No | No |
| Facial rash | 0 | 1 | 0 | 0 | No | No |
| Cervical pain | 0 | 0 | 1 | 0 | Possible | No |
| Infection distal arm | 0 | 0 | 0 | 1 | No | No |
| Adverse event, n . | Run-in n = 48 . | MSC-IT n = 16 + 16 . | MSC-IV n = 16 + 16 . | Sham treatment n = 16 + 16 . | Related to procedure . | Related to treatment . |
|---|---|---|---|---|---|---|
| No adverse events | – | 5 | 4 | 3 | ||
| Headache | 0 | 9 | 10 | 8 | Yes (all) | No |
| Back pain | 1 | 2 | 2 | 3 | Yes (all except one) | No |
| Viral infection | 3 | 0 | 2 | 1 | No | No |
| Upper respiratory infection | 1 | 1 | 0 | 0 | No | No |
| Fever | 1 | 1 | 1 | 2 | No | No |
| Urinary tract infection | 2 | 1 | 0 | 0 | No | No |
| Sinusitis | 0 | 2 | 0 | 0 | No | No |
| Fall | 1 | 1 | 2 | 1 | No | No |
| Fracture (leg/hand) | 2 | 1 | 0 | 0 | No | No |
| Dizziness | 0 | 2 | 1 | 0 | No | No |
| Haematoma | 1 | 1 | 0 | 0 | No | No |
| Nausea | 0 | 0 | 2 | 0 | No | No |
| Melanoma (in situ) | 1 | 0 | 0 | 0 | No | No |
| Scabies infection | 0 | 1 | 0 | 0 | No | No |
| Peripheral facial nerve palsy | 0 | 1 | 0 | 0 | No | No |
| Toothache | 0 | 0 | 1 | 0 | No | No |
| Anorexia | 0 | 0 | 1 | 0 | No | No |
| Gout | 1 | 0 | 0 | 0 | No | No |
| Facial rash | 0 | 1 | 0 | 0 | No | No |
| Cervical pain | 0 | 0 | 1 | 0 | Possible | No |
| Infection distal arm | 0 | 0 | 0 | 1 | No | No |
Three serious adverse events occurred during the experiment resulting in hospitalization of the patient. Two events were related to relapses of multiple sclerosis (Patient 018, Group 3B, during the placebo cycle and Patient 038, Group 1A, during the MSC-IT cycle), and one was due to upper respiratory infection, which resolved after treatment with antibiotics (Patient 011, Group 1A, during the MSC-IT cycle).
| Adverse event, n . | Run-in n = 48 . | MSC-IT n = 16 + 16 . | MSC-IV n = 16 + 16 . | Sham treatment n = 16 + 16 . | Related to procedure . | Related to treatment . |
|---|---|---|---|---|---|---|
| No adverse events | – | 5 | 4 | 3 | ||
| Headache | 0 | 9 | 10 | 8 | Yes (all) | No |
| Back pain | 1 | 2 | 2 | 3 | Yes (all except one) | No |
| Viral infection | 3 | 0 | 2 | 1 | No | No |
| Upper respiratory infection | 1 | 1 | 0 | 0 | No | No |
| Fever | 1 | 1 | 1 | 2 | No | No |
| Urinary tract infection | 2 | 1 | 0 | 0 | No | No |
| Sinusitis | 0 | 2 | 0 | 0 | No | No |
| Fall | 1 | 1 | 2 | 1 | No | No |
| Fracture (leg/hand) | 2 | 1 | 0 | 0 | No | No |
| Dizziness | 0 | 2 | 1 | 0 | No | No |
| Haematoma | 1 | 1 | 0 | 0 | No | No |
| Nausea | 0 | 0 | 2 | 0 | No | No |
| Melanoma (in situ) | 1 | 0 | 0 | 0 | No | No |
| Scabies infection | 0 | 1 | 0 | 0 | No | No |
| Peripheral facial nerve palsy | 0 | 1 | 0 | 0 | No | No |
| Toothache | 0 | 0 | 1 | 0 | No | No |
| Anorexia | 0 | 0 | 1 | 0 | No | No |
| Gout | 1 | 0 | 0 | 0 | No | No |
| Facial rash | 0 | 1 | 0 | 0 | No | No |
| Cervical pain | 0 | 0 | 1 | 0 | Possible | No |
| Infection distal arm | 0 | 0 | 0 | 1 | No | No |
| Adverse event, n . | Run-in n = 48 . | MSC-IT n = 16 + 16 . | MSC-IV n = 16 + 16 . | Sham treatment n = 16 + 16 . | Related to procedure . | Related to treatment . |
|---|---|---|---|---|---|---|
| No adverse events | – | 5 | 4 | 3 | ||
| Headache | 0 | 9 | 10 | 8 | Yes (all) | No |
| Back pain | 1 | 2 | 2 | 3 | Yes (all except one) | No |
| Viral infection | 3 | 0 | 2 | 1 | No | No |
| Upper respiratory infection | 1 | 1 | 0 | 0 | No | No |
| Fever | 1 | 1 | 1 | 2 | No | No |
| Urinary tract infection | 2 | 1 | 0 | 0 | No | No |
| Sinusitis | 0 | 2 | 0 | 0 | No | No |
| Fall | 1 | 1 | 2 | 1 | No | No |
| Fracture (leg/hand) | 2 | 1 | 0 | 0 | No | No |
| Dizziness | 0 | 2 | 1 | 0 | No | No |
| Haematoma | 1 | 1 | 0 | 0 | No | No |
| Nausea | 0 | 0 | 2 | 0 | No | No |
| Melanoma (in situ) | 1 | 0 | 0 | 0 | No | No |
| Scabies infection | 0 | 1 | 0 | 0 | No | No |
| Peripheral facial nerve palsy | 0 | 1 | 0 | 0 | No | No |
| Toothache | 0 | 0 | 1 | 0 | No | No |
| Anorexia | 0 | 0 | 1 | 0 | No | No |
| Gout | 1 | 0 | 0 | 0 | No | No |
| Facial rash | 0 | 1 | 0 | 0 | No | No |
| Cervical pain | 0 | 0 | 1 | 0 | Possible | No |
| Infection distal arm | 0 | 0 | 0 | 1 | No | No |
Three serious adverse events occurred during the experiment resulting in hospitalization of the patient. Two events were related to relapses of multiple sclerosis (Patient 018, Group 3B, during the placebo cycle and Patient 038, Group 1A, during the MSC-IT cycle), and one was due to upper respiratory infection, which resolved after treatment with antibiotics (Patient 011, Group 1A, during the MSC-IT cycle).
Clinical efficacy
The intention to treat analysis of the statistical analysis plan predetermined primary efficacy end point, showed that the percentage of patients with treatment failure (increase in EDSS score of 1 point for patients with baseline EDSS values of ≤5.0 and of 0.5 degree for baseline EDSS > 5.0), confirmed by two consecutive evaluations at the end of each treatment cycle (i.e. at 6 months or 12 months), was significantly lower in the MSC-IT and MSC-IV groups (6.7% and 9.7%, respectively) compared with the sham-treated patients (41.9%) (Table 3 and Fig. 2) (P = 0.0003 between MSC-IT and sham treatment, P = 0.0008 between MSC-IV and sham treatment, chi-squared test). Among the sham-treated patients, 76.7% experienced a deterioration in at least one Functional Systems score, and only 31% and 27.6% experienced similar deterioration in the MSC-IT and MSC-IV groups, respectively (P = 0.0002 and P = 0.0004, chi-squared test) (Table 3).
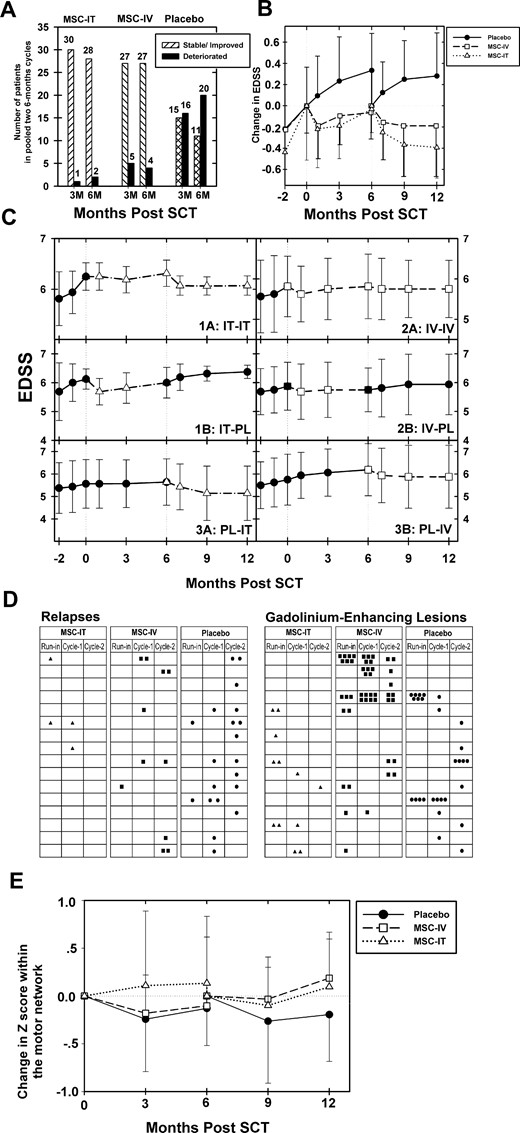
Clinical and radiological effects of MSC-IT and MSC-IV transplantation versus sham treatment in progressive multiple sclerosis. (A) Beneficial effect of MSC treatment on the progression of multiple sclerosis as evidenced by the changes in EDSS. Number of patients in each treatment subgroup (MSC-IT, MSC-IC, and sham treatment) who deteriorated in EDSS score or were stable or improved in EDSS, during each 3- or 6-month period (pooled data from the two cycles of treatment). P < 0.0001 (3 months) and P = 0.0003 (6 months) for MSC-IT versus sham treatment (chi-squared test); P = 0.0085 (3 months), P = 0.0008 (6 months) for MSC-IV versus sham treatment (chi-squared test). (B) Treatment with MSC induces beneficial effects on the progression of multiple sclerosis, as evidenced by the changes in EDSS score. Longitudinal follow-up of the mean changes in EDSS scores in each of the major treatment group (MSC-IT, MSC-IV, and placebo/sham treatment), during the run-in pretreatment period, and at 3 and 6 months following each cycle of treatment. (P < 0.0001 for MSC-IT versus sham at 3 months and P < 0.0001 at 6 months; the corresponding P-values for MSC-IV versus sham, were 0.001 at 3 months and 0.0002 at 6 months, Wilcoxon signed-rank Test.) (C) Differential clinical effects in each of the six treatment subgroups during the two phases of the trial (longitudinal changes in the EDSS score). Longitudinal follow-up of the mean EDSS scores in each of the six treatment subgroups (1A: MSC-IT/MSC-IT, 1B: MSC-IT/Placebo, 2A: MSC-IV/MSC-IV, 2B: MSC-IV/Placebo, 3A: Placebo/MSC-IT and 3B: Placebo/MSC-IV), during the run-in pretreatment period, and the two cycles of the study. Comparison between the 12 months of treatment versus the run-in period: P < 0.001 (for the repeated MSC-IT treatment), (Wilcoxon signed-rank test) P = not significant (for the single MSC-IT treatment), (Wilcoxon signed-rank test) P < 0.001 (for the repeated MSC-IV treatment), (Wilcoxon signed-rank test) P = not significant (for the single MSC-IV treatment), (Wilcoxon signed-rank test) P = 0.023 (between repeated versus single MSC-IT treatment), (Mann-Whitney test) P = 0.50 (between repeated versus single MSC-IV treatment), (Mann-Whitney test). (D) Incidence of clinical relapses and gadolinium-enhancing lesions in MRI. Number of relapses and gadolinium-enhancing lesions (presented as black dots for sham treatment, black squares for MSC-IV, and triangles for MSC-IT) in each patient of the three treatment subgroups (MSC-IT, MSC-IV, and sham treatment) during both cycles (two 6-month periods). (Pooled data from the two cycles of treatment.) Cyc-1 = first cycle; Cyc-2 = second cycle. P < 0.0005 for relapses in the MSC-IT group versus sham treatment. P = 0.052 for relapses in the MSC-IV group versus sham treatment. There was a good correlation between the incidence of relapses and the presence of gadolinium-enhancing lesions in each of the treatment groups. Mean number of gadolinium-enhancing lesions on MRI in the two cycles of treatment pooled together: 0.17 ± 0.47 in the MSC-IT group, 0.97 ± 1.93 in the MSC-IV group, and 0.55 ± 1.03 in the sham-treated group (P = 0.062 for MSC-IT versus sham treatment, P = 0.90 for MSC-IV versus sham treatment, P = 0.077 for MSC-IT versus MSC-IV, Wilcoxon test). (E) MSC treatment induces beneficial effects on the motor network on functional MRI. Changes in mean z-values of the motor network on functional MRI in the group of patients treated with MSC-IT or MSC-IV versus the sham-treated group, during both cycles of the study. An increase was observed in the mean z-score in the MSC-IT group (annualized change of 0.108 ± 1.06 and 0.156 ± 0.68 at 3 and 6 months, respectively; pooled data), and a deterioration in the sham-treated group (−0.504 ± 1.06 and −0.288 ± 0.61 at 3 and 6 months, respectively). The changes in the MSC-IV group were +0.036 ± 0.88 at 3 months, and −0.06 ± 0.816 at 6 months (P = 0.0675 and P = 0.042 for MSC-IT versus sham treatment at 3 and 6 months, respectively; P = 0.031 and P = 0.077 for MSC-IV versus sham treatment at 3 and 6 months, respectively).
| . | IT-MSC . | IV-MSC . | Sham treatment . | MSC-IT versus sham, P . | MSC-IV versus sham, P . | MSC-IT versus MSC-IV, P . |
|---|---|---|---|---|---|---|
| Primary end point | ||||||
| Treatment failurea at 6 mob | 6.7% (2/30) | 9.7% (3/31) | 41.9% (13/31) | 0.0003* | 0.0008* | NS |
| Treatment failurec at 6 mob | 31.0% (9/29) | 27.6% (8/29) | 76.7% (23/30) | 0.0004 | 0.0002 | NS |
| Secondary end points | ||||||
| Change in EDSS | ||||||
At 3 mo Median (IQR) | −0.29 ± 0.3 −0.5 (−0.5, 0) | −0.12 ± 0.4 0 (−0.5, 0) | +0.24 ± 0.4 0 (0, +0.5) | <0.0001 | 0.0010 | 0.0624 |
At 6 mo Median (IQR) | −0.20 ± 0.3 0 (−0.5, 0) | −0.13 ± 0.4 0 (−0.5, 0) | +0.30 ± 0.4 0 (0, +0.5) | <0.0001 | 0.0002 | 0.3280 |
| Treatment failurec at 3 mo | 16.1% (5/31) | 24.1% (7/29) | 73.3% (22/30) | <0.0001* | 0.0002* | NS |
| Change in ambulation score | ||||||
At 3 mo Median (IQR) | −0.93 ± 1.1 −1 (−2, 0) | −0.28 ± 1.2 0 (−1, 0) | +1.1 ± 1.2 +1 (0, +2)] | <0.0001 | 0.1938 | 0.0375 |
At 6 mo Median (IQR) | −0.97 ± 1.7 −1 (−1.75, 0) | −0.35 ± 1.1 0 (−1, 0) | +1.3 ± 1.3 +1 (0, +2) | 0.0009 | 0.0938 | 0.1239 |
| Change in sum of functional scores | ||||||
At 3 mo Median (IQR) | −2.81 ± 2.4 −3 (−4, −2) | −1.50 ± 2.1 −1 (−2.25, 0) | +0.48 ± 1.7 +1 (−0.5, +1) | <0.0001 | 0.0009 | 0.0177 |
At 6 mo Median (IQR) | −2.93 ± 2.4 −3 (−4, −2) | −1.29 ± 2.3 −1 (−2, 0) | +0.84 ± 2.1 +1 (−0.5, +2)] | <0.0001 | 0.0006 | 0.0127 |
| Relapses per patient, n, mean ± SD | 0.06 ± 0.25 | 0.28 ± 0.58 | 0.56 ± 0.67 | 0.0005 | 0.052 | 0.074 |
| Proportion/patients relapse-free (n) | 93.8% (30/32) | 78.1% (25/32) | 53.1% (17/32) | 0.001 | 0.1 | |
| T25FW % changes over 6 mo | −5.3 ± 16.3 | −6.4 ± 17.7 | +14.0 ± 25.4 | 0.0017 | 0.0009 | 0.81 |
| 9-HPT % changes over 6 mo | ||||||
| Dominant hand | −3.0 ± 10.1 | −2.1 ± 5.1 | +0.5 ± 8.9 | 0.129 | 0.300 | 0.43 |
| Non-dominant hand | −5.5 ± 7.9 | −1.6 ± 7.5 | +1.3 ± 11.2 | 0.0136 | 0.391 | 0.043 |
| MRI: % monthly changes in FLAIR T2 lesion volume at 6 mo (median) | −0.024 ± 0.053 (−0.004) | −0.016 ± 0.036 (−0.004) | +0.003 ± 0.029 (−0.0) | 0.029 | 0.123 | 0.50 |
| MRI: mean number of Gd-enhancing lesionsb (total lesion number) | 0.17 ± 0.47 (9) | 0.97 ± 1.93 (49) | 0.55 ± 1.03 (30) | 0.0636 | 0.9086 | 0.0776 |
| MRI: % total brain volume change versus baselineb, mean ± SD | ||||||
| At 3 mo | +0.45 ± 1.36 | −0.29 ± 1.16 | −3.58 ± 17.2 | 0.09 | 0.93 | 0.03 |
| At 6 mo | +0.28 ± 1.18 | −0.13 ± 1.48 | +0.29 ± 1.67 | 0.74 | 0.29 | 0.30 |
| PASAT cognitive test % change | ||||||
| At 3 mo | +69.9 ± 204.4 | +37.8 ± 320.0 | −36.1 ± 143.7 | 0.0007 | 0.240 | 0.014 |
| At 6 mo | +11.9 ± 166.2 | +129.5 ± 545.0 | −6.5 ± 203.2 | 0.327 | 0.939 | 0.334 |
| Cognitive test score change at 3 mo (z-scores) | ||||||
| OWAT (KAVE) | +0.474 ± 0.83 | +0.122 ± 0.80 | −0.282 ± 0.60 | 0.013 | 0.12 | |
| SDMT | +0.1 ± 0.7 | −0.3 ± 0.9 | −0.1 ± 0.6 | 0.18 | 0.20 | 0.02 |
| OCT-RNFL (G) % changes over 6 mo (median) | ||||||
| Right eye | −0.2 ± 3.2 (0) | +0.1 ± 2.4 (0) | −0.3 ± 2.7 (−0.4) | 0.844 | 0.429 | 0.57 |
| Left eye | +1.0 ± 2.6 | +0.1 ± 3.2 | −0.7 ± 3.1 | 0.038 | 0.417 | 0.273 |
| % VEP latency changes at 6 mo | ||||||
| Left eye | 0.4 ± 6.6 | 0.7 ± 6.3 | 3.6 ± 15.9 | 0.36 | 0.26 | |
| Right eye | 0.8 ± 5.2 | −1.8 ± 6.9 | 1.8 ± 5.8 | 0.63 | 0.034 | |
| Functional MRI annual changes in motor network, z-scores | ||||||
| Over 3 mo | +0.108 ± 1.06 | +0.036 ± 0.88 | −0.504 ± 1.06 | 0.0675 | 0.0312 | 0.74 |
| Over 6 mo | +0.156 ± 0.684 | −0.06 ± 0.816 | −0.288 ± 0.612 | 0.0425 | 0.0774 | 0.80 |
| Immunology: CD4+/CD25+high T cells % change at 6 mo versus baseline | 307.2 ± 487.5 | 249.5 ± 382.8 | 123.6 ± 281.9 | 0.05 | 0.11 | 0.62 |
| NEDA over 6 mo | 58.6% (17/29) | 40.6% (13/32) | 9.7% (3/31) | <0.0001 | 0.0048 | 0.160 |
| NEDA-4 (including <−0.4% annual change in total brain volume) | 44.8% (13/29) | 28.1% (9/32) | 9.7% (3/31) | 0.005 | 0.12 | 0.17 |
| . | IT-MSC . | IV-MSC . | Sham treatment . | MSC-IT versus sham, P . | MSC-IV versus sham, P . | MSC-IT versus MSC-IV, P . |
|---|---|---|---|---|---|---|
| Primary end point | ||||||
| Treatment failurea at 6 mob | 6.7% (2/30) | 9.7% (3/31) | 41.9% (13/31) | 0.0003* | 0.0008* | NS |
| Treatment failurec at 6 mob | 31.0% (9/29) | 27.6% (8/29) | 76.7% (23/30) | 0.0004 | 0.0002 | NS |
| Secondary end points | ||||||
| Change in EDSS | ||||||
At 3 mo Median (IQR) | −0.29 ± 0.3 −0.5 (−0.5, 0) | −0.12 ± 0.4 0 (−0.5, 0) | +0.24 ± 0.4 0 (0, +0.5) | <0.0001 | 0.0010 | 0.0624 |
At 6 mo Median (IQR) | −0.20 ± 0.3 0 (−0.5, 0) | −0.13 ± 0.4 0 (−0.5, 0) | +0.30 ± 0.4 0 (0, +0.5) | <0.0001 | 0.0002 | 0.3280 |
| Treatment failurec at 3 mo | 16.1% (5/31) | 24.1% (7/29) | 73.3% (22/30) | <0.0001* | 0.0002* | NS |
| Change in ambulation score | ||||||
At 3 mo Median (IQR) | −0.93 ± 1.1 −1 (−2, 0) | −0.28 ± 1.2 0 (−1, 0) | +1.1 ± 1.2 +1 (0, +2)] | <0.0001 | 0.1938 | 0.0375 |
At 6 mo Median (IQR) | −0.97 ± 1.7 −1 (−1.75, 0) | −0.35 ± 1.1 0 (−1, 0) | +1.3 ± 1.3 +1 (0, +2) | 0.0009 | 0.0938 | 0.1239 |
| Change in sum of functional scores | ||||||
At 3 mo Median (IQR) | −2.81 ± 2.4 −3 (−4, −2) | −1.50 ± 2.1 −1 (−2.25, 0) | +0.48 ± 1.7 +1 (−0.5, +1) | <0.0001 | 0.0009 | 0.0177 |
At 6 mo Median (IQR) | −2.93 ± 2.4 −3 (−4, −2) | −1.29 ± 2.3 −1 (−2, 0) | +0.84 ± 2.1 +1 (−0.5, +2)] | <0.0001 | 0.0006 | 0.0127 |
| Relapses per patient, n, mean ± SD | 0.06 ± 0.25 | 0.28 ± 0.58 | 0.56 ± 0.67 | 0.0005 | 0.052 | 0.074 |
| Proportion/patients relapse-free (n) | 93.8% (30/32) | 78.1% (25/32) | 53.1% (17/32) | 0.001 | 0.1 | |
| T25FW % changes over 6 mo | −5.3 ± 16.3 | −6.4 ± 17.7 | +14.0 ± 25.4 | 0.0017 | 0.0009 | 0.81 |
| 9-HPT % changes over 6 mo | ||||||
| Dominant hand | −3.0 ± 10.1 | −2.1 ± 5.1 | +0.5 ± 8.9 | 0.129 | 0.300 | 0.43 |
| Non-dominant hand | −5.5 ± 7.9 | −1.6 ± 7.5 | +1.3 ± 11.2 | 0.0136 | 0.391 | 0.043 |
| MRI: % monthly changes in FLAIR T2 lesion volume at 6 mo (median) | −0.024 ± 0.053 (−0.004) | −0.016 ± 0.036 (−0.004) | +0.003 ± 0.029 (−0.0) | 0.029 | 0.123 | 0.50 |
| MRI: mean number of Gd-enhancing lesionsb (total lesion number) | 0.17 ± 0.47 (9) | 0.97 ± 1.93 (49) | 0.55 ± 1.03 (30) | 0.0636 | 0.9086 | 0.0776 |
| MRI: % total brain volume change versus baselineb, mean ± SD | ||||||
| At 3 mo | +0.45 ± 1.36 | −0.29 ± 1.16 | −3.58 ± 17.2 | 0.09 | 0.93 | 0.03 |
| At 6 mo | +0.28 ± 1.18 | −0.13 ± 1.48 | +0.29 ± 1.67 | 0.74 | 0.29 | 0.30 |
| PASAT cognitive test % change | ||||||
| At 3 mo | +69.9 ± 204.4 | +37.8 ± 320.0 | −36.1 ± 143.7 | 0.0007 | 0.240 | 0.014 |
| At 6 mo | +11.9 ± 166.2 | +129.5 ± 545.0 | −6.5 ± 203.2 | 0.327 | 0.939 | 0.334 |
| Cognitive test score change at 3 mo (z-scores) | ||||||
| OWAT (KAVE) | +0.474 ± 0.83 | +0.122 ± 0.80 | −0.282 ± 0.60 | 0.013 | 0.12 | |
| SDMT | +0.1 ± 0.7 | −0.3 ± 0.9 | −0.1 ± 0.6 | 0.18 | 0.20 | 0.02 |
| OCT-RNFL (G) % changes over 6 mo (median) | ||||||
| Right eye | −0.2 ± 3.2 (0) | +0.1 ± 2.4 (0) | −0.3 ± 2.7 (−0.4) | 0.844 | 0.429 | 0.57 |
| Left eye | +1.0 ± 2.6 | +0.1 ± 3.2 | −0.7 ± 3.1 | 0.038 | 0.417 | 0.273 |
| % VEP latency changes at 6 mo | ||||||
| Left eye | 0.4 ± 6.6 | 0.7 ± 6.3 | 3.6 ± 15.9 | 0.36 | 0.26 | |
| Right eye | 0.8 ± 5.2 | −1.8 ± 6.9 | 1.8 ± 5.8 | 0.63 | 0.034 | |
| Functional MRI annual changes in motor network, z-scores | ||||||
| Over 3 mo | +0.108 ± 1.06 | +0.036 ± 0.88 | −0.504 ± 1.06 | 0.0675 | 0.0312 | 0.74 |
| Over 6 mo | +0.156 ± 0.684 | −0.06 ± 0.816 | −0.288 ± 0.612 | 0.0425 | 0.0774 | 0.80 |
| Immunology: CD4+/CD25+high T cells % change at 6 mo versus baseline | 307.2 ± 487.5 | 249.5 ± 382.8 | 123.6 ± 281.9 | 0.05 | 0.11 | 0.62 |
| NEDA over 6 mo | 58.6% (17/29) | 40.6% (13/32) | 9.7% (3/31) | <0.0001 | 0.0048 | 0.160 |
| NEDA-4 (including <−0.4% annual change in total brain volume) | 44.8% (13/29) | 28.1% (9/32) | 9.7% (3/31) | 0.005 | 0.12 | 0.17 |
Paired t-test, Wilcoxon signed-rank test and Mann-Whitney test, were used as described in the ‘Materials and methods’ section. 9-HPT = 9-hole peg test; mo = months; NEDA = no evidence of disease activity; NS = not significant; OWAT = Owatonna Cognitive Behavioral Test; RNFL = retinal nerve fibre layer; SDMT = Symbol Digit Modalities Test; T25FW - timed 25-foot walking test; VEP = visual evoked potential. *χ2. Values in bold indicate statistically significant difference versus placebo (i.e. P < 0.05).
Confirmed increase in EDSS/1 point in EDSS for patients with baseline scores ≤5.0 and/0.5 degree for baseline EDSS > 5.0.
Pooled analysis/both cycles/treatment.
Increase in at least one Functional System score.
| . | IT-MSC . | IV-MSC . | Sham treatment . | MSC-IT versus sham, P . | MSC-IV versus sham, P . | MSC-IT versus MSC-IV, P . |
|---|---|---|---|---|---|---|
| Primary end point | ||||||
| Treatment failurea at 6 mob | 6.7% (2/30) | 9.7% (3/31) | 41.9% (13/31) | 0.0003* | 0.0008* | NS |
| Treatment failurec at 6 mob | 31.0% (9/29) | 27.6% (8/29) | 76.7% (23/30) | 0.0004 | 0.0002 | NS |
| Secondary end points | ||||||
| Change in EDSS | ||||||
At 3 mo Median (IQR) | −0.29 ± 0.3 −0.5 (−0.5, 0) | −0.12 ± 0.4 0 (−0.5, 0) | +0.24 ± 0.4 0 (0, +0.5) | <0.0001 | 0.0010 | 0.0624 |
At 6 mo Median (IQR) | −0.20 ± 0.3 0 (−0.5, 0) | −0.13 ± 0.4 0 (−0.5, 0) | +0.30 ± 0.4 0 (0, +0.5) | <0.0001 | 0.0002 | 0.3280 |
| Treatment failurec at 3 mo | 16.1% (5/31) | 24.1% (7/29) | 73.3% (22/30) | <0.0001* | 0.0002* | NS |
| Change in ambulation score | ||||||
At 3 mo Median (IQR) | −0.93 ± 1.1 −1 (−2, 0) | −0.28 ± 1.2 0 (−1, 0) | +1.1 ± 1.2 +1 (0, +2)] | <0.0001 | 0.1938 | 0.0375 |
At 6 mo Median (IQR) | −0.97 ± 1.7 −1 (−1.75, 0) | −0.35 ± 1.1 0 (−1, 0) | +1.3 ± 1.3 +1 (0, +2) | 0.0009 | 0.0938 | 0.1239 |
| Change in sum of functional scores | ||||||
At 3 mo Median (IQR) | −2.81 ± 2.4 −3 (−4, −2) | −1.50 ± 2.1 −1 (−2.25, 0) | +0.48 ± 1.7 +1 (−0.5, +1) | <0.0001 | 0.0009 | 0.0177 |
At 6 mo Median (IQR) | −2.93 ± 2.4 −3 (−4, −2) | −1.29 ± 2.3 −1 (−2, 0) | +0.84 ± 2.1 +1 (−0.5, +2)] | <0.0001 | 0.0006 | 0.0127 |
| Relapses per patient, n, mean ± SD | 0.06 ± 0.25 | 0.28 ± 0.58 | 0.56 ± 0.67 | 0.0005 | 0.052 | 0.074 |
| Proportion/patients relapse-free (n) | 93.8% (30/32) | 78.1% (25/32) | 53.1% (17/32) | 0.001 | 0.1 | |
| T25FW % changes over 6 mo | −5.3 ± 16.3 | −6.4 ± 17.7 | +14.0 ± 25.4 | 0.0017 | 0.0009 | 0.81 |
| 9-HPT % changes over 6 mo | ||||||
| Dominant hand | −3.0 ± 10.1 | −2.1 ± 5.1 | +0.5 ± 8.9 | 0.129 | 0.300 | 0.43 |
| Non-dominant hand | −5.5 ± 7.9 | −1.6 ± 7.5 | +1.3 ± 11.2 | 0.0136 | 0.391 | 0.043 |
| MRI: % monthly changes in FLAIR T2 lesion volume at 6 mo (median) | −0.024 ± 0.053 (−0.004) | −0.016 ± 0.036 (−0.004) | +0.003 ± 0.029 (−0.0) | 0.029 | 0.123 | 0.50 |
| MRI: mean number of Gd-enhancing lesionsb (total lesion number) | 0.17 ± 0.47 (9) | 0.97 ± 1.93 (49) | 0.55 ± 1.03 (30) | 0.0636 | 0.9086 | 0.0776 |
| MRI: % total brain volume change versus baselineb, mean ± SD | ||||||
| At 3 mo | +0.45 ± 1.36 | −0.29 ± 1.16 | −3.58 ± 17.2 | 0.09 | 0.93 | 0.03 |
| At 6 mo | +0.28 ± 1.18 | −0.13 ± 1.48 | +0.29 ± 1.67 | 0.74 | 0.29 | 0.30 |
| PASAT cognitive test % change | ||||||
| At 3 mo | +69.9 ± 204.4 | +37.8 ± 320.0 | −36.1 ± 143.7 | 0.0007 | 0.240 | 0.014 |
| At 6 mo | +11.9 ± 166.2 | +129.5 ± 545.0 | −6.5 ± 203.2 | 0.327 | 0.939 | 0.334 |
| Cognitive test score change at 3 mo (z-scores) | ||||||
| OWAT (KAVE) | +0.474 ± 0.83 | +0.122 ± 0.80 | −0.282 ± 0.60 | 0.013 | 0.12 | |
| SDMT | +0.1 ± 0.7 | −0.3 ± 0.9 | −0.1 ± 0.6 | 0.18 | 0.20 | 0.02 |
| OCT-RNFL (G) % changes over 6 mo (median) | ||||||
| Right eye | −0.2 ± 3.2 (0) | +0.1 ± 2.4 (0) | −0.3 ± 2.7 (−0.4) | 0.844 | 0.429 | 0.57 |
| Left eye | +1.0 ± 2.6 | +0.1 ± 3.2 | −0.7 ± 3.1 | 0.038 | 0.417 | 0.273 |
| % VEP latency changes at 6 mo | ||||||
| Left eye | 0.4 ± 6.6 | 0.7 ± 6.3 | 3.6 ± 15.9 | 0.36 | 0.26 | |
| Right eye | 0.8 ± 5.2 | −1.8 ± 6.9 | 1.8 ± 5.8 | 0.63 | 0.034 | |
| Functional MRI annual changes in motor network, z-scores | ||||||
| Over 3 mo | +0.108 ± 1.06 | +0.036 ± 0.88 | −0.504 ± 1.06 | 0.0675 | 0.0312 | 0.74 |
| Over 6 mo | +0.156 ± 0.684 | −0.06 ± 0.816 | −0.288 ± 0.612 | 0.0425 | 0.0774 | 0.80 |
| Immunology: CD4+/CD25+high T cells % change at 6 mo versus baseline | 307.2 ± 487.5 | 249.5 ± 382.8 | 123.6 ± 281.9 | 0.05 | 0.11 | 0.62 |
| NEDA over 6 mo | 58.6% (17/29) | 40.6% (13/32) | 9.7% (3/31) | <0.0001 | 0.0048 | 0.160 |
| NEDA-4 (including <−0.4% annual change in total brain volume) | 44.8% (13/29) | 28.1% (9/32) | 9.7% (3/31) | 0.005 | 0.12 | 0.17 |
| . | IT-MSC . | IV-MSC . | Sham treatment . | MSC-IT versus sham, P . | MSC-IV versus sham, P . | MSC-IT versus MSC-IV, P . |
|---|---|---|---|---|---|---|
| Primary end point | ||||||
| Treatment failurea at 6 mob | 6.7% (2/30) | 9.7% (3/31) | 41.9% (13/31) | 0.0003* | 0.0008* | NS |
| Treatment failurec at 6 mob | 31.0% (9/29) | 27.6% (8/29) | 76.7% (23/30) | 0.0004 | 0.0002 | NS |
| Secondary end points | ||||||
| Change in EDSS | ||||||
At 3 mo Median (IQR) | −0.29 ± 0.3 −0.5 (−0.5, 0) | −0.12 ± 0.4 0 (−0.5, 0) | +0.24 ± 0.4 0 (0, +0.5) | <0.0001 | 0.0010 | 0.0624 |
At 6 mo Median (IQR) | −0.20 ± 0.3 0 (−0.5, 0) | −0.13 ± 0.4 0 (−0.5, 0) | +0.30 ± 0.4 0 (0, +0.5) | <0.0001 | 0.0002 | 0.3280 |
| Treatment failurec at 3 mo | 16.1% (5/31) | 24.1% (7/29) | 73.3% (22/30) | <0.0001* | 0.0002* | NS |
| Change in ambulation score | ||||||
At 3 mo Median (IQR) | −0.93 ± 1.1 −1 (−2, 0) | −0.28 ± 1.2 0 (−1, 0) | +1.1 ± 1.2 +1 (0, +2)] | <0.0001 | 0.1938 | 0.0375 |
At 6 mo Median (IQR) | −0.97 ± 1.7 −1 (−1.75, 0) | −0.35 ± 1.1 0 (−1, 0) | +1.3 ± 1.3 +1 (0, +2) | 0.0009 | 0.0938 | 0.1239 |
| Change in sum of functional scores | ||||||
At 3 mo Median (IQR) | −2.81 ± 2.4 −3 (−4, −2) | −1.50 ± 2.1 −1 (−2.25, 0) | +0.48 ± 1.7 +1 (−0.5, +1) | <0.0001 | 0.0009 | 0.0177 |
At 6 mo Median (IQR) | −2.93 ± 2.4 −3 (−4, −2) | −1.29 ± 2.3 −1 (−2, 0) | +0.84 ± 2.1 +1 (−0.5, +2)] | <0.0001 | 0.0006 | 0.0127 |
| Relapses per patient, n, mean ± SD | 0.06 ± 0.25 | 0.28 ± 0.58 | 0.56 ± 0.67 | 0.0005 | 0.052 | 0.074 |
| Proportion/patients relapse-free (n) | 93.8% (30/32) | 78.1% (25/32) | 53.1% (17/32) | 0.001 | 0.1 | |
| T25FW % changes over 6 mo | −5.3 ± 16.3 | −6.4 ± 17.7 | +14.0 ± 25.4 | 0.0017 | 0.0009 | 0.81 |
| 9-HPT % changes over 6 mo | ||||||
| Dominant hand | −3.0 ± 10.1 | −2.1 ± 5.1 | +0.5 ± 8.9 | 0.129 | 0.300 | 0.43 |
| Non-dominant hand | −5.5 ± 7.9 | −1.6 ± 7.5 | +1.3 ± 11.2 | 0.0136 | 0.391 | 0.043 |
| MRI: % monthly changes in FLAIR T2 lesion volume at 6 mo (median) | −0.024 ± 0.053 (−0.004) | −0.016 ± 0.036 (−0.004) | +0.003 ± 0.029 (−0.0) | 0.029 | 0.123 | 0.50 |
| MRI: mean number of Gd-enhancing lesionsb (total lesion number) | 0.17 ± 0.47 (9) | 0.97 ± 1.93 (49) | 0.55 ± 1.03 (30) | 0.0636 | 0.9086 | 0.0776 |
| MRI: % total brain volume change versus baselineb, mean ± SD | ||||||
| At 3 mo | +0.45 ± 1.36 | −0.29 ± 1.16 | −3.58 ± 17.2 | 0.09 | 0.93 | 0.03 |
| At 6 mo | +0.28 ± 1.18 | −0.13 ± 1.48 | +0.29 ± 1.67 | 0.74 | 0.29 | 0.30 |
| PASAT cognitive test % change | ||||||
| At 3 mo | +69.9 ± 204.4 | +37.8 ± 320.0 | −36.1 ± 143.7 | 0.0007 | 0.240 | 0.014 |
| At 6 mo | +11.9 ± 166.2 | +129.5 ± 545.0 | −6.5 ± 203.2 | 0.327 | 0.939 | 0.334 |
| Cognitive test score change at 3 mo (z-scores) | ||||||
| OWAT (KAVE) | +0.474 ± 0.83 | +0.122 ± 0.80 | −0.282 ± 0.60 | 0.013 | 0.12 | |
| SDMT | +0.1 ± 0.7 | −0.3 ± 0.9 | −0.1 ± 0.6 | 0.18 | 0.20 | 0.02 |
| OCT-RNFL (G) % changes over 6 mo (median) | ||||||
| Right eye | −0.2 ± 3.2 (0) | +0.1 ± 2.4 (0) | −0.3 ± 2.7 (−0.4) | 0.844 | 0.429 | 0.57 |
| Left eye | +1.0 ± 2.6 | +0.1 ± 3.2 | −0.7 ± 3.1 | 0.038 | 0.417 | 0.273 |
| % VEP latency changes at 6 mo | ||||||
| Left eye | 0.4 ± 6.6 | 0.7 ± 6.3 | 3.6 ± 15.9 | 0.36 | 0.26 | |
| Right eye | 0.8 ± 5.2 | −1.8 ± 6.9 | 1.8 ± 5.8 | 0.63 | 0.034 | |
| Functional MRI annual changes in motor network, z-scores | ||||||
| Over 3 mo | +0.108 ± 1.06 | +0.036 ± 0.88 | −0.504 ± 1.06 | 0.0675 | 0.0312 | 0.74 |
| Over 6 mo | +0.156 ± 0.684 | −0.06 ± 0.816 | −0.288 ± 0.612 | 0.0425 | 0.0774 | 0.80 |
| Immunology: CD4+/CD25+high T cells % change at 6 mo versus baseline | 307.2 ± 487.5 | 249.5 ± 382.8 | 123.6 ± 281.9 | 0.05 | 0.11 | 0.62 |
| NEDA over 6 mo | 58.6% (17/29) | 40.6% (13/32) | 9.7% (3/31) | <0.0001 | 0.0048 | 0.160 |
| NEDA-4 (including <−0.4% annual change in total brain volume) | 44.8% (13/29) | 28.1% (9/32) | 9.7% (3/31) | 0.005 | 0.12 | 0.17 |
Paired t-test, Wilcoxon signed-rank test and Mann-Whitney test, were used as described in the ‘Materials and methods’ section. 9-HPT = 9-hole peg test; mo = months; NEDA = no evidence of disease activity; NS = not significant; OWAT = Owatonna Cognitive Behavioral Test; RNFL = retinal nerve fibre layer; SDMT = Symbol Digit Modalities Test; T25FW - timed 25-foot walking test; VEP = visual evoked potential. *χ2. Values in bold indicate statistically significant difference versus placebo (i.e. P < 0.05).
Confirmed increase in EDSS/1 point in EDSS for patients with baseline scores ≤5.0 and/0.5 degree for baseline EDSS > 5.0.
Pooled analysis/both cycles/treatment.
Increase in at least one Functional System score.
The mean EDSS score deteriorated in the sham-treated group and was improved in the MSC-IT and MSC-IV groups during both treatment cycles (P = 0.0002 and P = 0.007, respectively, versus sham treatment; Mann-Whitney test) (Fig. 2 and Table 3). Two patients showed improvement in EDSS during the first cycle of treatment with MSC-IT and 11 during the second cycle (ranging from 0.5 to 1.0 degrees). The respective numbers of patients with improvement in the MSC-IV group, were three and six in the two cycles; one patient showed improvement in the sham treatment group (Table 4). There was no clear association between the treatment-induced benefit with the status of activity of the disease (presence of relapse or MRI activity) during the year prior to inclusion and the run-in period (Table 4). At inclusion, seven patients had active disease in the placebo group, six in the MSC-IT group and seven in the MSC-IV group. During the second cycle of the treatment, only one patient in the MSC-IT group showed activity compared with 12 patients in the sham-treated group (Table 4and Supplementary Table 4). During only the first treatment cycle (comparison of parallel groups before the crossover, n = 16 in each group), as seen in Supplementary Table 4, the annualized EDSS change during the year prior to the inclusion and the run-in period (total 14 months) was similar in the subgroup of the patients with activity and those without, in all three groups, and was significantly reduced after MSC-IV and MSC-IT treatment, similar to the active and the inactive patients (e.g. in the MSC-IT group the annualized ΔEDSS was reduced from +0.88 to −0.11 in the active patients and from +1.01 to +0.14 in the inactive subgroup). However, since the majority of the included patients had some activity of the disease, either in the year prior to the inclusion or in the run-in period (in total, before starting MSC transplantation 27 patients were active and 21 non-active and three more became active during the first placebo cycle, further increasing the number of patients with active disease to 30 of 48), it seems that MSC transplantation benefits predominantly active progressive multiple sclerosis.
Detailed data about EDSS step changes in each patient/group and the activity status (relapse or MRI activity) in each patient
| . | Pre-study year + run in period . | First cycle of treatment . | Second cycle of treatment . |
|---|---|---|---|
| Patients with activitya | |||
| Placebo (n = 16) | 9 | 8 | 12 |
| MSC-IV (n = 16) | 10 | 6 | 9 |
| MSC-IT (n = 16) | 9 | 5 | 1 |
| Patients with worsening | |||
| Placebo (n = 16) | 14 | 7 (5 who were active before the start of treatment and 2 who were non-active) | 8 (6 who were active before the start of treatment and 2 who were non-active) |
| ΔEDSS | +0.5; +0.5; +0.5; +0.5; +0.5; +0.5; +1.0; +1.0; +1.0; +1.0; +1.5; +1.5; +1.5; +2.0 | +0.5; +0.5; +0.5; +0.5; +0.5; +1.0; +1.5 | +0.5; +0.5; +0.5; +0.5; +0.5; +0.5; +1.0; +1.0 |
| MSC-IV (n = 16) | 15 | 1 | 3 |
| ΔEDSS | +0.5; +0.5; +0.5; +0.5; +1.0; +1.0; +1.0; +1.0; +1.0; +1.0; +1.0; +1.5; +1.5; +1.5; +2.5 | +0.5 | +0.5; +0.5; +0.5 |
| MSC-IT (n = 16) | 15 | 2 | 0 |
| ΔEDSS | +0.5; +0.5; +0.5; +0.5; +1.0; +1.0; +1.0; +1.0; +1.5; +1.5; +1.5; +1.5; +2.0; +2.0; +2.0 | +0.5; +0.5 | − |
| Patients with improvement | |||
| Placebo (n = 16) | 0 | 0 | 1 −0.5 |
| MSC-IV (n = 16) | 0 | 3 | 6 (4 who were active before the start of treatment and 2 who were non-active) |
| ΔEDSS | − | −0.5; −0.5; −0.5 | −0.5; −0.5; −0.5; −1.0; −1.0 |
| MSC-IT (n = 16) | 0 | 2 | 11 (6 who were active before the start of treatment and 5 who were non-active) |
| ΔEDSS | − | −0.5; −0.5 | −0.5; −0.5; −0.5; −0.5; −0.5; −0.5; −0.5 − 0.5; −0.5; −0.5; −1.0 |
| . | Pre-study year + run in period . | First cycle of treatment . | Second cycle of treatment . |
|---|---|---|---|
| Patients with activitya | |||
| Placebo (n = 16) | 9 | 8 | 12 |
| MSC-IV (n = 16) | 10 | 6 | 9 |
| MSC-IT (n = 16) | 9 | 5 | 1 |
| Patients with worsening | |||
| Placebo (n = 16) | 14 | 7 (5 who were active before the start of treatment and 2 who were non-active) | 8 (6 who were active before the start of treatment and 2 who were non-active) |
| ΔEDSS | +0.5; +0.5; +0.5; +0.5; +0.5; +0.5; +1.0; +1.0; +1.0; +1.0; +1.5; +1.5; +1.5; +2.0 | +0.5; +0.5; +0.5; +0.5; +0.5; +1.0; +1.5 | +0.5; +0.5; +0.5; +0.5; +0.5; +0.5; +1.0; +1.0 |
| MSC-IV (n = 16) | 15 | 1 | 3 |
| ΔEDSS | +0.5; +0.5; +0.5; +0.5; +1.0; +1.0; +1.0; +1.0; +1.0; +1.0; +1.0; +1.5; +1.5; +1.5; +2.5 | +0.5 | +0.5; +0.5; +0.5 |
| MSC-IT (n = 16) | 15 | 2 | 0 |
| ΔEDSS | +0.5; +0.5; +0.5; +0.5; +1.0; +1.0; +1.0; +1.0; +1.5; +1.5; +1.5; +1.5; +2.0; +2.0; +2.0 | +0.5; +0.5 | − |
| Patients with improvement | |||
| Placebo (n = 16) | 0 | 0 | 1 −0.5 |
| MSC-IV (n = 16) | 0 | 3 | 6 (4 who were active before the start of treatment and 2 who were non-active) |
| ΔEDSS | − | −0.5; −0.5; −0.5 | −0.5; −0.5; −0.5; −1.0; −1.0 |
| MSC-IT (n = 16) | 0 | 2 | 11 (6 who were active before the start of treatment and 5 who were non-active) |
| ΔEDSS | − | −0.5; −0.5 | −0.5; −0.5; −0.5; −0.5; −0.5; −0.5; −0.5 − 0.5; −0.5; −0.5; −1.0 |
aActive patients: those with relapse or MRI activity during the year prior to inclusion.
Detailed data about EDSS step changes in each patient/group and the activity status (relapse or MRI activity) in each patient
| . | Pre-study year + run in period . | First cycle of treatment . | Second cycle of treatment . |
|---|---|---|---|
| Patients with activitya | |||
| Placebo (n = 16) | 9 | 8 | 12 |
| MSC-IV (n = 16) | 10 | 6 | 9 |
| MSC-IT (n = 16) | 9 | 5 | 1 |
| Patients with worsening | |||
| Placebo (n = 16) | 14 | 7 (5 who were active before the start of treatment and 2 who were non-active) | 8 (6 who were active before the start of treatment and 2 who were non-active) |
| ΔEDSS | +0.5; +0.5; +0.5; +0.5; +0.5; +0.5; +1.0; +1.0; +1.0; +1.0; +1.5; +1.5; +1.5; +2.0 | +0.5; +0.5; +0.5; +0.5; +0.5; +1.0; +1.5 | +0.5; +0.5; +0.5; +0.5; +0.5; +0.5; +1.0; +1.0 |
| MSC-IV (n = 16) | 15 | 1 | 3 |
| ΔEDSS | +0.5; +0.5; +0.5; +0.5; +1.0; +1.0; +1.0; +1.0; +1.0; +1.0; +1.0; +1.5; +1.5; +1.5; +2.5 | +0.5 | +0.5; +0.5; +0.5 |
| MSC-IT (n = 16) | 15 | 2 | 0 |
| ΔEDSS | +0.5; +0.5; +0.5; +0.5; +1.0; +1.0; +1.0; +1.0; +1.5; +1.5; +1.5; +1.5; +2.0; +2.0; +2.0 | +0.5; +0.5 | − |
| Patients with improvement | |||
| Placebo (n = 16) | 0 | 0 | 1 −0.5 |
| MSC-IV (n = 16) | 0 | 3 | 6 (4 who were active before the start of treatment and 2 who were non-active) |
| ΔEDSS | − | −0.5; −0.5; −0.5 | −0.5; −0.5; −0.5; −1.0; −1.0 |
| MSC-IT (n = 16) | 0 | 2 | 11 (6 who were active before the start of treatment and 5 who were non-active) |
| ΔEDSS | − | −0.5; −0.5 | −0.5; −0.5; −0.5; −0.5; −0.5; −0.5; −0.5 − 0.5; −0.5; −0.5; −1.0 |
| . | Pre-study year + run in period . | First cycle of treatment . | Second cycle of treatment . |
|---|---|---|---|
| Patients with activitya | |||
| Placebo (n = 16) | 9 | 8 | 12 |
| MSC-IV (n = 16) | 10 | 6 | 9 |
| MSC-IT (n = 16) | 9 | 5 | 1 |
| Patients with worsening | |||
| Placebo (n = 16) | 14 | 7 (5 who were active before the start of treatment and 2 who were non-active) | 8 (6 who were active before the start of treatment and 2 who were non-active) |
| ΔEDSS | +0.5; +0.5; +0.5; +0.5; +0.5; +0.5; +1.0; +1.0; +1.0; +1.0; +1.5; +1.5; +1.5; +2.0 | +0.5; +0.5; +0.5; +0.5; +0.5; +1.0; +1.5 | +0.5; +0.5; +0.5; +0.5; +0.5; +0.5; +1.0; +1.0 |
| MSC-IV (n = 16) | 15 | 1 | 3 |
| ΔEDSS | +0.5; +0.5; +0.5; +0.5; +1.0; +1.0; +1.0; +1.0; +1.0; +1.0; +1.0; +1.5; +1.5; +1.5; +2.5 | +0.5 | +0.5; +0.5; +0.5 |
| MSC-IT (n = 16) | 15 | 2 | 0 |
| ΔEDSS | +0.5; +0.5; +0.5; +0.5; +1.0; +1.0; +1.0; +1.0; +1.5; +1.5; +1.5; +1.5; +2.0; +2.0; +2.0 | +0.5; +0.5 | − |
| Patients with improvement | |||
| Placebo (n = 16) | 0 | 0 | 1 −0.5 |
| MSC-IV (n = 16) | 0 | 3 | 6 (4 who were active before the start of treatment and 2 who were non-active) |
| ΔEDSS | − | −0.5; −0.5; −0.5 | −0.5; −0.5; −0.5; −1.0; −1.0 |
| MSC-IT (n = 16) | 0 | 2 | 11 (6 who were active before the start of treatment and 5 who were non-active) |
| ΔEDSS | − | −0.5; −0.5 | −0.5; −0.5; −0.5; −0.5; −0.5; −0.5; −0.5 − 0.5; −0.5; −0.5; −1.0 |
aActive patients: those with relapse or MRI activity during the year prior to inclusion.
The changes in ambulation scores and in the sum of all Functional System scores, followed the same trend, strongly favouring MSC-IT and MSC-IV treatments over sham treatment (Tables 3 and 5) (the detailed EDSS, Functional System and ambulation scores of each individual patient at each visit, are provided in Supplementary Tables 4–9). These clinical effects do not seem to have been influenced by steroidal treatment (which was administered due to relapse, deterioration or MRI activity). Actually more patients in the sham-treated group received steroids (11:4 in the first cycle and 7 in the second, versus 6 in the MSC-IV group and 4 in the MSC-IT group, during the two cycles of the study) (detailed data on steroid treatment of each patient in Supplementary Table 4).
Changes in clinical disability parameters during the run-in period and from baseline to 12 months visit (end of study)
| Change from baseline to 12 months in: . | MSC-IT x2 n = 8 . | MSC-IT x1 n = 8 . | MSC-IV x2 n = 8 . | MSC-IV x1 n = 8 . | Any treatment n = 48 . | |
|---|---|---|---|---|---|---|
| EDSS | ||||||
| Mean ± SD | −0.188 ± 0.26 | 0.250 ± 0.27 | −0.063 ± 0.18 | 0.063 ± 0.32 | 0.016 ± 0.30 | |
| Median | 0.0 | 0.25 | 0.0 | 0.0 | 0.0 | |
| IQR | −0.5, 0 | 0, 0.5 | 0, 0 | 0, 0.37 | 0, 0 | |
Change during run-in period, P-value* | 0.438 ± 0.32 P < 0.001 | 0.438 ± 0.68 NS | 0.250 ± 0.27 P < 0.001 | 0.188 ± 0.26 NS | 0.188 ± 0.26 P < 0.001 | |
| Comparison between single versus repeated treatment** | MSC-IT: P = 0.0238 | MSC-IV: P = 0.4965 | ||||
| Ambulation score | ||||||
| Mean ± SD | −1.25 ± 1.28 | 1.25 ± 1.16 | −0.25 ± 0.89 | 1.00 ± 1.31 | 0.02 ± 1.84 | |
| Median | −1.0 | 1.5 | 0.0 | 0.5 | 0.0 | |
| IQR | −2.5, −0.5 | 0, +2 | −0.25, 0 | 0, +1.5 | −1, +1 | |
Change during run-in period, P-value* | 1.75 ± 1.28 P = 0.00738 | 0.87 ± 1.13 P = 0.351 | 0.37 ± 1.30 P = 0.217 | 0.50 ± 0.76 P = 0.227 | 0.89 ± 1.36 P = 0.0355 | |
| Comparison between single versus repeated treatment** | MSC-IT: P = 0.0045 | MSC-IV: P = 0.0836 | ||||
| Sum of functional systems | ||||||
| Mean ± SD | −4.86 ± 3.72 | −0.25 ± 2.71 | −1.50 ± 2.83 | −0.75 ± 1.83 | −2.20 ± 3.61 | |
| Median | −4.0 | 0.0 | −0.5 | −0.5 | −2.0 | |
| IQR | −6.0, −2.5 | −2.25, +1.25 | −2.5, 0 | −2.25, +0.25 | −4, 0 | |
Change during run-in period, P-value* | 1.12 ± 2.03 P = 0.0334 | −0.12 ± 0.64 P = 0.909 | 0.12 ± 1.13 P = 0.183 | 0.37 ± 0.92 P = 0.229 | 0.46 ± 1.37 P = 0.00017 | |
| Comparison between single versus repeated treatment** | MSC-IT: P = 0.0183 | MSC-IV: P = 0.2263 | ||||
| 25-Foot timed walking | ||||||
| Mean ± SD | −5.80 ± 7.23 | 5.28 ± 7.72 | −1.17 ± 8.12 | 4.52 ± 5.69 | 0.45 ± 7.03 | |
| Median | −3.45 | 5.85 | −0.45 | 2.4 | −0.15 | |
| IQR | −9.7, −1.7 | 1.5, +10.85 | −1.16, +1.14 | 1.21, +5.09 | −2.26, +2.52 | |
Change during run-in period, P-value* | −0.12 ± 1.97 P = 0.105 | 1.26 ± 3.87 P = 0.316 | 2.71 ± 5.77 P = 0.166 | 0.03 ± 0.87 P = 0.082 | 1.86 ± 5.55 P = 0.252 | |
| Comparison between single versus repeated treatment** | MSC-IT: P = 0.0168 | MSC-IV: P = 0.0455 | ||||
| Change from baseline to 12 months in: . | MSC-IT x2 n = 8 . | MSC-IT x1 n = 8 . | MSC-IV x2 n = 8 . | MSC-IV x1 n = 8 . | Any treatment n = 48 . | |
|---|---|---|---|---|---|---|
| EDSS | ||||||
| Mean ± SD | −0.188 ± 0.26 | 0.250 ± 0.27 | −0.063 ± 0.18 | 0.063 ± 0.32 | 0.016 ± 0.30 | |
| Median | 0.0 | 0.25 | 0.0 | 0.0 | 0.0 | |
| IQR | −0.5, 0 | 0, 0.5 | 0, 0 | 0, 0.37 | 0, 0 | |
Change during run-in period, P-value* | 0.438 ± 0.32 P < 0.001 | 0.438 ± 0.68 NS | 0.250 ± 0.27 P < 0.001 | 0.188 ± 0.26 NS | 0.188 ± 0.26 P < 0.001 | |
| Comparison between single versus repeated treatment** | MSC-IT: P = 0.0238 | MSC-IV: P = 0.4965 | ||||
| Ambulation score | ||||||
| Mean ± SD | −1.25 ± 1.28 | 1.25 ± 1.16 | −0.25 ± 0.89 | 1.00 ± 1.31 | 0.02 ± 1.84 | |
| Median | −1.0 | 1.5 | 0.0 | 0.5 | 0.0 | |
| IQR | −2.5, −0.5 | 0, +2 | −0.25, 0 | 0, +1.5 | −1, +1 | |
Change during run-in period, P-value* | 1.75 ± 1.28 P = 0.00738 | 0.87 ± 1.13 P = 0.351 | 0.37 ± 1.30 P = 0.217 | 0.50 ± 0.76 P = 0.227 | 0.89 ± 1.36 P = 0.0355 | |
| Comparison between single versus repeated treatment** | MSC-IT: P = 0.0045 | MSC-IV: P = 0.0836 | ||||
| Sum of functional systems | ||||||
| Mean ± SD | −4.86 ± 3.72 | −0.25 ± 2.71 | −1.50 ± 2.83 | −0.75 ± 1.83 | −2.20 ± 3.61 | |
| Median | −4.0 | 0.0 | −0.5 | −0.5 | −2.0 | |
| IQR | −6.0, −2.5 | −2.25, +1.25 | −2.5, 0 | −2.25, +0.25 | −4, 0 | |
Change during run-in period, P-value* | 1.12 ± 2.03 P = 0.0334 | −0.12 ± 0.64 P = 0.909 | 0.12 ± 1.13 P = 0.183 | 0.37 ± 0.92 P = 0.229 | 0.46 ± 1.37 P = 0.00017 | |
| Comparison between single versus repeated treatment** | MSC-IT: P = 0.0183 | MSC-IV: P = 0.2263 | ||||
| 25-Foot timed walking | ||||||
| Mean ± SD | −5.80 ± 7.23 | 5.28 ± 7.72 | −1.17 ± 8.12 | 4.52 ± 5.69 | 0.45 ± 7.03 | |
| Median | −3.45 | 5.85 | −0.45 | 2.4 | −0.15 | |
| IQR | −9.7, −1.7 | 1.5, +10.85 | −1.16, +1.14 | 1.21, +5.09 | −2.26, +2.52 | |
Change during run-in period, P-value* | −0.12 ± 1.97 P = 0.105 | 1.26 ± 3.87 P = 0.316 | 2.71 ± 5.77 P = 0.166 | 0.03 ± 0.87 P = 0.082 | 1.86 ± 5.55 P = 0.252 | |
| Comparison between single versus repeated treatment** | MSC-IT: P = 0.0168 | MSC-IV: P = 0.0455 | ||||
Comparison between the 12 months post treatment change versus the change in the run-in period; paired t-test and Wilcoxon signed rank test for EDSS; **Mann-Whitney test. Values in bold indicate statistically significant difference versus placebo (i.e. P < 0.05).
Changes in clinical disability parameters during the run-in period and from baseline to 12 months visit (end of study)
| Change from baseline to 12 months in: . | MSC-IT x2 n = 8 . | MSC-IT x1 n = 8 . | MSC-IV x2 n = 8 . | MSC-IV x1 n = 8 . | Any treatment n = 48 . | |
|---|---|---|---|---|---|---|
| EDSS | ||||||
| Mean ± SD | −0.188 ± 0.26 | 0.250 ± 0.27 | −0.063 ± 0.18 | 0.063 ± 0.32 | 0.016 ± 0.30 | |
| Median | 0.0 | 0.25 | 0.0 | 0.0 | 0.0 | |
| IQR | −0.5, 0 | 0, 0.5 | 0, 0 | 0, 0.37 | 0, 0 | |
Change during run-in period, P-value* | 0.438 ± 0.32 P < 0.001 | 0.438 ± 0.68 NS | 0.250 ± 0.27 P < 0.001 | 0.188 ± 0.26 NS | 0.188 ± 0.26 P < 0.001 | |
| Comparison between single versus repeated treatment** | MSC-IT: P = 0.0238 | MSC-IV: P = 0.4965 | ||||
| Ambulation score | ||||||
| Mean ± SD | −1.25 ± 1.28 | 1.25 ± 1.16 | −0.25 ± 0.89 | 1.00 ± 1.31 | 0.02 ± 1.84 | |
| Median | −1.0 | 1.5 | 0.0 | 0.5 | 0.0 | |
| IQR | −2.5, −0.5 | 0, +2 | −0.25, 0 | 0, +1.5 | −1, +1 | |
Change during run-in period, P-value* | 1.75 ± 1.28 P = 0.00738 | 0.87 ± 1.13 P = 0.351 | 0.37 ± 1.30 P = 0.217 | 0.50 ± 0.76 P = 0.227 | 0.89 ± 1.36 P = 0.0355 | |
| Comparison between single versus repeated treatment** | MSC-IT: P = 0.0045 | MSC-IV: P = 0.0836 | ||||
| Sum of functional systems | ||||||
| Mean ± SD | −4.86 ± 3.72 | −0.25 ± 2.71 | −1.50 ± 2.83 | −0.75 ± 1.83 | −2.20 ± 3.61 | |
| Median | −4.0 | 0.0 | −0.5 | −0.5 | −2.0 | |
| IQR | −6.0, −2.5 | −2.25, +1.25 | −2.5, 0 | −2.25, +0.25 | −4, 0 | |
Change during run-in period, P-value* | 1.12 ± 2.03 P = 0.0334 | −0.12 ± 0.64 P = 0.909 | 0.12 ± 1.13 P = 0.183 | 0.37 ± 0.92 P = 0.229 | 0.46 ± 1.37 P = 0.00017 | |
| Comparison between single versus repeated treatment** | MSC-IT: P = 0.0183 | MSC-IV: P = 0.2263 | ||||
| 25-Foot timed walking | ||||||
| Mean ± SD | −5.80 ± 7.23 | 5.28 ± 7.72 | −1.17 ± 8.12 | 4.52 ± 5.69 | 0.45 ± 7.03 | |
| Median | −3.45 | 5.85 | −0.45 | 2.4 | −0.15 | |
| IQR | −9.7, −1.7 | 1.5, +10.85 | −1.16, +1.14 | 1.21, +5.09 | −2.26, +2.52 | |
Change during run-in period, P-value* | −0.12 ± 1.97 P = 0.105 | 1.26 ± 3.87 P = 0.316 | 2.71 ± 5.77 P = 0.166 | 0.03 ± 0.87 P = 0.082 | 1.86 ± 5.55 P = 0.252 | |
| Comparison between single versus repeated treatment** | MSC-IT: P = 0.0168 | MSC-IV: P = 0.0455 | ||||
| Change from baseline to 12 months in: . | MSC-IT x2 n = 8 . | MSC-IT x1 n = 8 . | MSC-IV x2 n = 8 . | MSC-IV x1 n = 8 . | Any treatment n = 48 . | |
|---|---|---|---|---|---|---|
| EDSS | ||||||
| Mean ± SD | −0.188 ± 0.26 | 0.250 ± 0.27 | −0.063 ± 0.18 | 0.063 ± 0.32 | 0.016 ± 0.30 | |
| Median | 0.0 | 0.25 | 0.0 | 0.0 | 0.0 | |
| IQR | −0.5, 0 | 0, 0.5 | 0, 0 | 0, 0.37 | 0, 0 | |
Change during run-in period, P-value* | 0.438 ± 0.32 P < 0.001 | 0.438 ± 0.68 NS | 0.250 ± 0.27 P < 0.001 | 0.188 ± 0.26 NS | 0.188 ± 0.26 P < 0.001 | |
| Comparison between single versus repeated treatment** | MSC-IT: P = 0.0238 | MSC-IV: P = 0.4965 | ||||
| Ambulation score | ||||||
| Mean ± SD | −1.25 ± 1.28 | 1.25 ± 1.16 | −0.25 ± 0.89 | 1.00 ± 1.31 | 0.02 ± 1.84 | |
| Median | −1.0 | 1.5 | 0.0 | 0.5 | 0.0 | |
| IQR | −2.5, −0.5 | 0, +2 | −0.25, 0 | 0, +1.5 | −1, +1 | |
Change during run-in period, P-value* | 1.75 ± 1.28 P = 0.00738 | 0.87 ± 1.13 P = 0.351 | 0.37 ± 1.30 P = 0.217 | 0.50 ± 0.76 P = 0.227 | 0.89 ± 1.36 P = 0.0355 | |
| Comparison between single versus repeated treatment** | MSC-IT: P = 0.0045 | MSC-IV: P = 0.0836 | ||||
| Sum of functional systems | ||||||
| Mean ± SD | −4.86 ± 3.72 | −0.25 ± 2.71 | −1.50 ± 2.83 | −0.75 ± 1.83 | −2.20 ± 3.61 | |
| Median | −4.0 | 0.0 | −0.5 | −0.5 | −2.0 | |
| IQR | −6.0, −2.5 | −2.25, +1.25 | −2.5, 0 | −2.25, +0.25 | −4, 0 | |
Change during run-in period, P-value* | 1.12 ± 2.03 P = 0.0334 | −0.12 ± 0.64 P = 0.909 | 0.12 ± 1.13 P = 0.183 | 0.37 ± 0.92 P = 0.229 | 0.46 ± 1.37 P = 0.00017 | |
| Comparison between single versus repeated treatment** | MSC-IT: P = 0.0183 | MSC-IV: P = 0.2263 | ||||
| 25-Foot timed walking | ||||||
| Mean ± SD | −5.80 ± 7.23 | 5.28 ± 7.72 | −1.17 ± 8.12 | 4.52 ± 5.69 | 0.45 ± 7.03 | |
| Median | −3.45 | 5.85 | −0.45 | 2.4 | −0.15 | |
| IQR | −9.7, −1.7 | 1.5, +10.85 | −1.16, +1.14 | 1.21, +5.09 | −2.26, +2.52 | |
Change during run-in period, P-value* | −0.12 ± 1.97 P = 0.105 | 1.26 ± 3.87 P = 0.316 | 2.71 ± 5.77 P = 0.166 | 0.03 ± 0.87 P = 0.082 | 1.86 ± 5.55 P = 0.252 | |
| Comparison between single versus repeated treatment** | MSC-IT: P = 0.0168 | MSC-IV: P = 0.0455 | ||||
Comparison between the 12 months post treatment change versus the change in the run-in period; paired t-test and Wilcoxon signed rank test for EDSS; **Mann-Whitney test. Values in bold indicate statistically significant difference versus placebo (i.e. P < 0.05).
For the changes in EDSS and Functional System scores, the MSC-IT was superior to the MSC-IV treatment (Tables 3 and 5). Notably, half of the patients treated twice with MSC-IT, had a confirmed disability improvement (CDI) at the end of the whole trial (12 months), and none of them exhibited confirmed disability progression.
An additional analysis using the ‘any treatment scenario’ during the whole 12-month duration of the study (i.e. combining all treatment groups together; n = 48), the EDSS score, showed a significant deterioration during the pretreatment run-in period (from 5.58 ± 0.88 at screening to 5.88 ± 0.80 at baseline visit, P < 0.001, Wilcoxon signed-rank test), and a stabilization during the whole 12 months of the two treatment-cycles (5.88 ± 0.80 at baseline versus 5.87 ± 0.9, on final visit at 12 months). The difference between the change in EDSS in the run-in period versus the change from baseline to the 12-month value, for all 48 patients, was statistically significant (P < 0.001, Wilcoxon signed-rank test). The patients treated twice intrathecally with MSC had the best outcome at 12 months and the difference between single versus double intrathecal treatment, was statistically significant in all the major efficacy parameters (EDSS, sum of functional systems, ambulation score) (Table 5).
Fifteen of 32 patients in the sham-treated groups experienced at least one relapse during both 6-month periods of the study, compared with only seven in the MSC-IV group and 2 of 32 in the MSC-IT group (46.9%, 21.9%, and 6.3%, respectively, P = 0.0002 for MSC-IT, and P = 0.035 for MSC-IV versus the sham treatment) (Table 3 and Fig. 2). The mean annual relapse rates in both cycles of treatment were 0.06 ± 0.25, 0.28 ± 0.57, and 0.56 ± 0.67 in the MSC-IT, MSC-IV, and sham-treated groups, respectively (P = 0.0005, for MSC-IT versus sham treatment; P = 0.052, for MSC-IV versus sham treatment, Wilcoxon test).
Secondary end points
MRI
The mean number of gadolinium-enhancing lesions per patient during the two cycles of treatment were: 0.55 ± 1.03 in the sham-treated group, 0.17 ± 0.47 in the MSC-IT group, and 0.97 ± 1.93 in the MSC-IV group (P = 0.062 for MSC-IT versus sham treatment; P = 0.90 for MSC-IV versus sham treatment; P = 0.077 for MSC-IT versus MSC-IV, Wilcoxon test) (Fig. 2 and Table 3). The mean monthly rate of the T2-FLAIR lesion volume change compared to the rate during the run-in period was −0.024 ± 0.053 in the pooled group of patients treated with MSC-IT; −0.016 ± 0.036 in the MSC-IV group; and 0.003 ± 0.029 in the sham-treated group (P = 0.029 for MSC-IT versus sham treatment; P = 0.123 for MSC-IV versus sham treatment) (Table 3). The changes in total brain volume, although showing a trend in favour of the MSC-IT treatment (Table 3), were not statistically significant. This may be related to possible effects on brain volume of the intrathecal injection itself, and also to diurnal and water intake fluctuations. Notably, there was an increase of brain volume in all three groups (+0.21% for MSC-IT, +0.15% for MSC-IV and +0.41% for placebo) during the second cycle of the trial, potentially reflecting carryover effects of the first treatment and therefore, difficult to interpret.
Functional MRI
Testing of the motor networks revealed a significant annual increase in the mean z-score of the MSC-IT group (0.108 ± 1.06 and 0.156 ± 0.68 at 3 and 6 months, respectively; pooled data), and a decrease/deterioration in the sham-treated group (−0.504 ± 1.06 and −0.288 ± 0.61 at 3 and 6 months, respectively). The z-scores changes in the MSC-IV group were 0.036 ± 0.88 at 3 months and −0.06 ± 0.816 at 6 months (P = 0.0675 and P = 0.042 for MSC-IT versus sham treatment at 3 and 6 months, respectively; P = 0.031 and P = 0.077 for MSC-IV versus sham treatment at 3 and 6 months, respectively) (Fig. 2).
No evidence of disease activity
Seventeen of 29 (58.6%) MSC-IT-treated patients [for whom there were data for all parameters of no evidence of disease activity (NEDA)] had no evidence of disease activity (NEDA-3) during the two pooled 6-month treatment periods, as compared with 40.6% (13/32) in the MSC-IV group and 9.7% (3/31) in the sham-treated group, (P < 0.0001 for MSC-IT versus sham treatment and 0.0048 for MSC-IV versus sham treatment). The percentages of patients with NEDA-4 (Kappos et al., 2016)status (i.e. including annual brain volume loss of <0.4% according to MRI) were 44.8% in the MSC-IT, 28.1% in the MSC-IV, and 9.7% in the sham-treated groups (P = 0.005 for MSC-IT versus sham treatment and P = 0.12 for MSC-IV versus sham treatment) (Table 3).
Other parameters
Statistically significant benefits were observed in the MSC-IT group in the 25-foot timed walking test, 9-hole peg test, OCT of the retinal nerve fibre layer, PASAT, and KAVE/OWAT cognitive tests. Trends of beneficial effects were also observed in VEP, the SDMT, and the proportion of CD4+CD25+highcells (increased) (Table 3).
Discussion
With an aim to evaluate the safety and clinical efficacy of autologous MSC transplantation in progressive multiple sclerosis, our study revealed positive results in all predefined primary end points. No serious, treatment-related adverse effects were observed and significantly fewer patients in the MSC-IT and MSC-IV groups experienced treatment failure (based on the EDSS changes), as compared with the sham-treated group (Table 4). Significant changes favouring MSC-IT treatment over sham treatment were also observed in the ambulation index, sum of functional scores, 25-foot timed walking test, 9-hole peg test, PASAT, and OWAT/KAVE cognitive tests, and the rate of change in T2 lesion load on MRI, as well as newer biomarkers, such as OCT (retinal nerve fibre layer) and functional MRI (motor network). Repeated intrathecal injection of MSCs at Month 6 significantly boosted the effects observed during the first cycle of treatment. Beneficial (but less significant) effects were also observed in the MSC-IV group. Overall, the robust effects of MSC transplantation on various parameters that reflect neurological dysfunction and especially on multiple sclerosis activity, may indicate the involvement of (central and peripheral) immunomodulatory and possibly also neuroprotective mechanisms.
These benefits seem to be of particular clinical significance, as they were observed in patients with progressive multiple sclerosis unresponsive to conventional immunotherapies, and for which limited treatment options exist. To have a crude estimation of the magnitude of our findings versus previously published randomized trials in progressive multiple sclerosis (although it is not scientifically sound to compare different trials with different designs), the difference in the EDSS change between the placebo arm and the MSC-IT group in our study was 0.5 (in favour of the MSC-IT treatment) as compared with an EDSS difference between placebo and active treatment of 0.36 in a mitoxantrone trial and 0.16 in a biotin trial (Hartung et al., 2002; Tourbah et al., 2016; Montalban et al., 2017).
Previous trials using various types of MSC in multiple sclerosis have been small (mostly open-label) and mainly showed the clinical safety of MSC-administration, providing in parallel some preliminary indications of potential clinical benefits (Karussis et al., 2010; Yamout et al., 2010; Connick et al., 2012; Cohen et al., 2018; Harris et al., 2018; Riordan et al., 2018). A triple blinded study, in which 30 patients were infused (11 placebo, 10 low-dose and 9 high-dose) intravenously with adipose-MSC showed an inconclusive trend of efficacy (Fernandez et al., 2018). Another small double blind, placebo-controlled trial (Llufriu et al., 2014) in nine multiple sclerosis patients treated intravenously with MSC, showed a positive trend in the primary end point of MRI gadolinium-enhancing lesions, but no significant differences in the secondary end points of EDSS and multiple sclerosis functional scores. In a phase Ib study (Lublin et al., 2014a), human placental MSCs (PDA-001) were injected intravenously in 16 patients in a double blind dose-ranging trial. Most patients were stable or improved after the treatment.
Despite the development of highly efficient and more targeted immunotherapies, two major unmet needs in the treatment of multiple sclerosis still exist. First, the need for treatment to suppress compartmentalized and meningeal inflammation in the CNS, which seems to drive tissue injury and progression of disability (Magliozzi et al., 2007; Lucchinetti et al., 2011; Ruggieri et al., 2015; Eshaghi et al., 2018; Reich et al., 2018). These compartmentalized inflammatory and degenerative activities seem to be less responsive to the majority of immunomodulatory drugs, accounting for the relatively poor efficacy of the majority of registered multiple sclerosis therapies in progressive multiple sclerosis, especially in patients with no prominent inflammatory activity, with the exception of very few (Hartung et al., 2002; Shirani et al., 2016; Tourbah et al., 2016; Montalban et al., 2017).
Second, the need for a treatment that may substantially promote regeneration–remyelination. Generally, the CNS loses its capacity for efficient regeneration and remyelination over time. This is especially pronounced in chronic neuroinflammatory and neurodegenerative diseases such as multiple sclerosis, possibly due to an insufficiency of growth factors or defective mobilization of intrinsic CNS stem cells/oligodendrocyte progenitors (Ben-Hur et al., 2005; Karussis and Kassis, 2007; Einstein and Ben-Hur, 2008; Karussis et al., 2013).
Based on their well-described properties (Ben-Hur et al., 2005; Freedman et al., 2010; Freedman and Uccelli, 2012; Karussis et al., 2013; Scolding et al., 2017), stem cells may represent a ‘logical’ treatment approach to achieve those unmet needs and induce neuroprotection and enhance endogenous remyelination. Moreover, stem cells are strong immunomodulators that may potentially downregulate the localized and compartmentalized inflammation upon their migration to the CNS (Magliozzi et al., 2007; Lucchinetti et al., 2011; Reich et al., 2018). Several studies have shown that embryonic, neuronal, and other adult stem cells can induce beneficial clinicopathological effects in animal models of neurological diseases, including multiple sclerosis (Pluchino et al., 2003, 2009; Ben-Hur et al., 2004; Zappia et al., 2005; Aharonowiz et al., 2008; Karussis and Kassis, 2008; Kassis et al., 2008; Harris et al., 2012). MSCs are commonly used for such therapies, as they have several practical advantages for clinical use over other types of stem cells: (i) they can be easily cultured and expanded in large quantities; (ii) they can be obtained from the patient, thus eliminating the need for a donor, the risk of rejection, or the need for chemotherapy; and (iii) they seem to be safe and carry low risks of malignant transformation. During the past decade, MSC treatments have been applied to various neurological diseases in small or pilot open-label trials (Karussis et al., 2010; Yamout et al., 2010; Connick et al., 2012; Lee et al., 2012; Llufriu et al., 2014; Lublin et al., 2014a; Petrou et al., 2016; Steinberg et al., 2016; Cohen et al., 2018; ,Fernandez et al., 2018), with promising indications.
The putative mechanism of action of MSCs in neurological diseases is controversial. Some investigators claim that the most prominent effects are mediated through peripheral immunomodulation (Karussis and Kassis, 2007, 2008; Uccelli et al., 2007, 2008, 2011; Karussis et al., 2008; Kassis et al., 2011). We have long advocated that neuroprotective and neurotrophic mechanisms also play a crucial role, as supported by our findings in animal models and pilot trials of multiple sclerosis and amyotrophic lateral sclerosis (Karussis et al., 2010; Petrou et al., 2016). We speculate that intrathecal injection, which brings a higher proportion of the injected cells into close proximity with damaged areas of the CNS, may induce more robust effects than intravenous injection. In the current study, intrathecal transplantation of MSCs was indeed shown to be superior to intravenous administration in several efficacy parameters (Table 3). If peripheral immunomodulation was the dominant mechanism, we would expect the opposite to be true. Moreover, the clinical beneficial effects were observed more prominently in patients with active disease (i.e. with relapses or MRI activity) but also in those without activity (Table 4). The improvements observed in OCT and motor networks on functional MRI, and the trend towards increased brain volume may indicate the involvement of neurotrophic or even neuroregenerative mechanisms, but to demonstrate whether autologous MSCs may facilitate remyelination or regeneration, a larger trial with appropriate biomarkers in progressive patients with disability worsening without disease actvity, is warranted. On the other hand, the less pronounced effects of the treatment on gadolinium-enhancing lesions on MRI, advocate against a major contribution of peripheral immunomodulatory mechanisms.
The strengths of our trial include: (i) the inclusion of patients with active progressive multiple sclerosis, for which existing immunotherapies are usually ineffective; (ii) the double-blind design, making this the first randomized controlled trial comparing intrathecal versus intravenous methods of MSC administration and single versus repeated treatment; and (iii) the robust, though short-term, clinical benefits observed in several disease activity parameters, including newer biomarkers, such as functional MRI-network connectivity, OCT, and cognitive testing. The limitations of our study include the small number of patients in each group, the short duration, and the crossover design (in the second cycle), which may have introduced a ‘carry-over’ effect from the first cycle of treatment.
An additional possible limitation of our findings could be related to the severe progression of half of the patients during the run-in period before the treatment. This may be related to the patients’ population (highly active patients non-responders to a mean of 2.58 previous treatments) and the discontinuation of the immunotherapies before the study. A rebound effect could partially explain this deterioration and the higher than in other cohorts progression rate of the placebo group, especially after treatment with fingolimod, which was the last treatment in 21 of 48 patients (with a rather equal distribution in the three treatment groups). Some of the beneficial effects therefore could be theoreticaly related to a ‘regression to the mean’ phenomenon. However, such regression, although may have affected the clinical changes at some degree (especially in the first months of the study), cannot—to our view—explain the finding of more robust benefits during the second cycle of treatment and of the improvements in several clinical and paraclinical parameters at various time-points, during both phases of the trial.
In summary, our results provide clear signals of short-term clinical efficacy and possible indications of neuroprotection, induced by the administration of autologous MSCs in patients with progressive multiple sclerosis. Our findings suggest a superiority of intrathecal over intravenous administration, and indicate a possible boost of the beneficial effects by a repeated injection of the cells. These data may contribute to the design of future trials with cell therapies and the use of objective biomarkers for the evaluation of neurodegeneration and neuronal regeneration. A larger, phase III study is warranted to confirm these observations and further evaluate the therapeutic potential of cellular therapy in neuroinflammatory and neurodegenerative diseases such as multiple sclerosis.
Research in context and evidence before this study
We searched PubMed up to 1 April 2019 for English language articles using the search terms: ‘mesenchymal stem cells’, ‘multiple sclerosis’ and ‘clinical trial’.
We found six published clinical trials using treatment with MSC in multiple sclerosis during the past 10 years. Almost all of them were open pilot studies and only one small trial was randomized and controlled by a placebo group. These phase I and IIa studies mainly investigated the safety of intravenous or intrathecal MSC. One was performed in our centre (Karussis et al., 2010) and was an open safety phase I/II trial including 15 multiple sclerosis and 19 amyotrophic lateral sclerosis patients; MSCs were injected both intravenously and intrathecally. No serious side effects were reported and there were indications of clinical efficacy in terms of stabilization or improvement of EDSS and amyotrophic lateral sclerosis functional rating scale (ALSFRS) scores.
In the second, an open label trial by Yamout et al. (2010), MSCs were injected into 10 patients with multiple sclerosis intrathecally and the results showed hints of clinical, but no radiological, efficacy. The third was an open label study, by Connick et al. (2012), MSCs were injected intravenously into 10 progressive multiple sclerosis patients, with primary objective being safety, and the secondary objective being the effects on visual pathways. No serious side effects were observed and there was some evidence of structural, functional and physiological improvement in few of the visual end points. In a larger open trial by Cohen et al. (2018), 25 patients and eight controls were injected intravenously with MSC. The authors reported only data for the feasibility and safety of this treatment but no clinical data. In a small double blind, placebo-controlled trial by Llufriu et al. (2014), nine patients with multiple sclerosis were injected with MSC intravenously. The study used as primary end point the number of gadolinium-enhancing lesions and showed a positive trend in this primary end point, but no statistically significant differences in the secondary end points of EDSS and multiple sclerosis functional scores.
Further to these trials in which autologous unmodified bone marrow MSCs were used, there are four additional trials using different types of modified or enhanced MSCs. In the first, a phase Ib study by Lublin et al. (2014a), human placental MSCs (PDA-001) were injected intravenously into 16 patients in a double-blind dose-ranging trial. The primary end point was to rule out any negative clinical effect of the procedure. Most patients were stable or improved after the treatment. Fernandez et al. (2018) used adipose-derived MSCs and injected different doses of the cells into 19 multiple sclerosis patients and 11 controls. The authors showed the safety of the procedure and indicated possible beneficial clinical and radiological effects. Riordan et al. (2018), in an open study, injected umbilical cord tissue-derived MSCs into 20 multiple sclerosis patients intravenously using a protocol of multiple infusions. The procedure was shown as safe and induced some clinical improvements. Finally, Harris et al. (2018), in an open trial (n = 20), injected MSC-derived neural progenitors intrathecally, in three doses spaced 3 months apart. No serious adverse events were observed and there were indications of clinical benefits.
Added value of this study
Our current study was a phase II double-blind trial, randomized and controlled by placebo and included a significantly higher number of patients (n = 48) than in previous trials. It compared for the first time the efficacy of two methods of administration of the MSCs (intrathecal and intravenous) using a parallel-group and crossover design. In addition, we compared the effects of repeated (two) MSC infusions with those of a single injection.
This is the first trial—to our knowledge—that used several objective and novel biomarkers to evaluate clinical efficacy and indications of neuro-regeneration (timed walking, hand dexterity tests, cognitive tests, quantitative neuroradiological measurements, functional MRI, OCT, VEP and dynamic visual tests).
Implications of all the available evidence
Various types of MSCs were used in small (mostly open label) clinical trials in multiple sclerosis during the last decade. These studies showed the clinical safety of MSC-administration and provided some preliminary indications of potential clinical benefits. The protocols of preparation and administration of MSC and the patients’ populations were highly variable. In our trial, which was randomized and placebo-controlled, we recruited a significant larger and well-defined group of patients with precisely defined active progressive multiple sclerosis.
Acknowledgements
The authors wish to thank Dr Moshe Neuman for his monitoring of the trial and summary of all the data and formulation of the final study report, Dr Gil Harari (Medistat) for the statistical analysis of the data, Prof. Reuven Or and Prof. Polina Stepensky for their collaboration and contribution related to bone marrow harvesting, Prof. Moshe Gomori for his comments and help in analysis of the gadolinium-enhancing lesions in the MRI, Dr Haya Glick-Shames, Ms Devora Marks, and the MRI Unit at Shaare Tzedeq Hospital, for their help and contribution to the accomplishment of this trial. Special thanks to the members of the external steering-safety committee, Prof. Yehuda Shoenfeld (Sheba Medical Center and Tel Aviv University), Prof. Ariel Miller (Carmel Medical Center, Haifa and Technion University) and Dr Ron Milo (Barzilai Hospital, Ashkelon). The study was supported by personal research grants of the PI and donations to the department (before the initiation of the trial). We especially thank Shwartz family foundation for their research grant to the department of Neurology. The PI (D.K.) had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All information and materials in the manuscript are original. The authors would like to thank Prof. Hans-Peter Hartung for his constructive critique, comments and corrections on our manuscript.
Funding
This study was solely funded by the principal investigator’s personal research grants, an award of the Judy and Sidney Swartz Foundation to T.B.H., and other donations to the Hospital for this project. Donations to the hospital for this project were not required for a candidate to be evaluated or entered on the trial. According to our Institute rules, no donations were allowed if the donor had any relation to a candidate patient. No commercial company was involved at any stage.
Competing interests
The authors report no competing interests.
Supplementary material
Supplementary material is available at Brain online.
References
- EDSS =
Expanded Disability Status Scale;
- MSC =
mesenchymal stem cell;
- OCT =
optical coherence tomography


