-
PDF
- Split View
-
Views
-
Cite
Cite
Ziv Gan-Or, Jennifer A. Ruskey, Dan Spiegelman, Isabelle Arnulf, Yves Dauvilliers, Birgit Högl, Christelle Monaca-Charley, Ronald B. Postuma, Jacques Y. Montplaisir, Guy A. Rouleau, Heterozygous PINK1 p.G411S in rapid eye movement sleep behaviour disorder, Brain, Volume 140, Issue 6, June 2017, Page e32, https://doi.org/10.1093/brain/awx076
Close - Share Icon Share
Sir,
In a recent study published in Brain, Puschmann et al. (2016) strengthened the suggested association between the heterozygous PINK1 mutation p.G411S and Parkinson’s disease. They performed a meta-analysis showing that patients with Parkinson’s disease had a pooled odds ratio (OR) of 2.89 for carrying the p.G411S mutation. Furthermore, the authors demonstrated that the p.G411S mutation led to reduced kinase activity and interfered with ubiquitin phosphorylation by PINK1 (Puschmann et al., 2016). Biallelic mutations in PINK1 are well-established causes of autosomal-recessive early onset Parkinson’s disease, and account for 3.7% of patients with early onset Parkinson’s disease (Kilarski et al., 2012). However, the role of heterozygous PINK1 mutations in Parkinson’s disease is still not clear. In a study of rare PINK1 variants, the carrier frequencies in Parkinson’s disease patients and controls were 1.8% and 1.5%, respectively, and a meta-analysis with previous studies resulted in non-significant OR of 1.62 (Marongiu et al., 2008). Other studies, however, suggested that heterozygous PINK1 variants may indeed increase the risk of developing Parkinson’s disease (Abou-Sleiman et al., 2006; Toft et al., 2007).
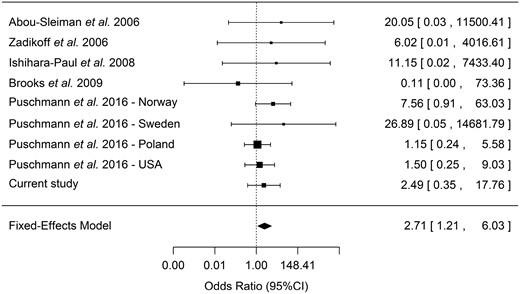
Forest plot of the effect of the PINK1 p.G411S mutation in Parkinson’s disease and RBD. A forest plot including data from eight previously published populations, which included data on carriers of PINK p.G411S in other patients and controls. As this mutation was shown to have a functional effect, which is not random and should be similar across all carriers, a fixed-effect model was used. When usinga random-effect model, a marginal P-value of 0.06 was achieved. The heterogeneity was not significant across the different populations (Tarone’s test for heterogeneity P = 0.62), further suggesting the fit of a fixed-effect model.
The average coverage of the probe covering the PINK1 p.G411S across all samples was >300×. Four carriers were identified: two RBD patients (0.6%) and two controls (0.2%, P > 0.05, Fisher’s exact test). Although not statistically significant, these frequencies have similar effect size and directionality as those reported by Puschmann et al. (2016). The age at diagnosis of RBD of the two patients was 65 and 69 years, and while the first patient had not yet converted to an overt synucleinopathy, the second patient was diagnosed with Parkinson’s disease at the age of 70. As RBD can be considered as a synucleinopathy in progress (Postuma et al., 2015), the current data could be added to the meta-analysis from Puschmann et al., which used studies that identified carriers of the PINK1 p.G411S mutation in patients or controls (Abou-Sleiman et al., 2006; Zadikoff et al., 2006; Ishihara-Paul et al., 2008; Brooks et al., 2009; Puschmann et al., 2016). Adding our data, the OR for having the PINK1 p.G411S mutation was 2.71 (95% confidence interval 1.21–6.03, P = 0.016, Tarone’s test for heterogeneity P = 0.62, Fig. 1).
Our results provide some additional support for the association between the PINK1 p.G411S mutation and Parkinson’s disease. There are conflicting reports on synucleinopathy and Lewy bodies in PINK1-associated Parkinson’s disease, as some post-mortem studies identified Lewy bodies in PINK1-associated Parkinson’s disease while others did not (Samaranch et al., 2010; Takanashi et al., 2016). The identification of two carriers of the PINK1 p.G411S mutation among the RBD cohort, who have de facto early-stage synucleinopathy, may suggest that PINK1, at least in some cases, may be associated with α-synuclein pathology. Of note, although the ORs calculated here and in previous studies are higher than those typically seen in genome-wide association studies of Parkinson’s disease (Nalls et al., 2014), the overall effect of the p.G411S mutation is still very small. If the life-time risk for Parkinson’s disease is 1–3%, and if the OR represents the risk for Parkinson’s disease, carriers of the PINK1 p.G411S mutation have more than a 90% chance of never developing Parkinson’s disease. Considering this reduced penetrance with the low frequency of this variant (allele frequency of 0.002 in the ExAC database, http://exac.broadinstitute.org/), it seems that the role of this variant in Parkinson’s disease is minor. However, it is important to note that other PINK1 mutations may also contribute to Parkinson’s disease in the heterozygous form. A previous meta-analysis of various rare PINK1 mutations suggested that they do not confer increased risk for Parkinson’s disease (Marongiu et al., 2008); however, collapsing all rare PINK1 variants together into a single meta-analysis is based on the hidden assumption that different rare PINK1 variants carry the same effect on risk for Parkinson’s disease. It is more likely that different variants have different effects on Parkinson’s disease risk, as occur in other Parkinson’s disease-related genes, such as GBA (Gan-Or et al., 2015a) and LRRK2 (Gan-Or et al., 2015a). While a mutation such as p.G411S may indeed be a risk variant, other PINK1 mutations may have no effect on Parkinson’s disease risk, or the opposite effect (i.e. they may be protective), and pooling them together may hide the effects of specific variants. Studies of individual mutations and more advanced analysis methods that take into account different effect directions of individual variants should be performed to better study the role of heterozygous PINK1 mutations in Parkinson’s disease.
Acknowledgements
We would like to thank the individuals who participated in this study. We thank Stephanie Strong, Simon C. Warby, Claire S. Leblond, Ambra Stefani, Patrick A. Dion, Alex Desautels, Jean-François Gagnon, Cynthia Bourassa, Jay P. Ross, Sandra Laurent, Helene Catoire, Pascale Hince and Vessela Zaharieva for their assistance.
Funding
This study was funded by a grant from the Michael J. Fox Foundation.


