-
PDF
- Split View
-
Views
-
Cite
Cite
Gabriel E Ferreira, John L Clark, Laura Clavijo, Alejandro Zuluaga, Alain Chautems, Michael J G Hopkins, Andrea O Araujo, Mathieu Perret, Phylogenetics, character evolution, and historical biogeography of the Neotropical genus Besleria (Gesneriaceae), Botanical Journal of the Linnean Society, Volume 206, Issue 1, September 2024, Pages 83–94, https://doi.org/10.1093/botlinnean/boae007
Close - Share Icon Share
Abstract
Besleria, a genus of perennial herbs, shrubs, or small trees growing in the understorey of rainforests, is one of the largest genera of neotropical Gesneriaceae, with over 165 species. Despite the ecological importance and ubiquity of Besleria in rainforest ecosystems, taxonomic and evolutionary studies of Besleria are limited. Here, we generated a phylogenetic analysis of Besleria using four nuclear and chloroplast DNA regions (ITS, matK, rps16, and trnL-trnF) covering more than 50% of the recognized species, along with two secondary calibration points to infer divergence times. Our results support the monophyly of Besleria and allowed us to revise the infrageneric classification and biogeographical history of the genus. We identified five major clades that do not correspond to sections or subsections in previous classifications. These clades are well circumscribed geographically but remain difficult to characterize using previously hypothesized morphological characters. Biogeographical reconstructions indicate an origin in the northern Andes during the Middle Miocene (ca. 15 Mya). The current distribution patterns of this plant group have been significantly shaped by geological and climatic events, particularly Andean uplift and the formation of the Panama Isthmus.
Introduction
Besleria Plum. ex L. is a neotropical genus of perennial herbs, shrubs, or small trees (Fig. 1) that grow in the understorey of rainforests from sea level to 3500 m in elevation. Besleria, with more than 165 species, is one of the most species-diverse and least studied genera of the neotropical Gesneriaceae (Möller and Clark 2013, Clark et al. 2020). It is the type genus of the tribe Beslerieae Bartl. (nine genera and about 260 species), which includes a broad range of morphological diversity in habits, corolla shapes, and fruit types that evolved over the last ca. 22–15 Myr (Roalson and Clark 2006, Clark et al. 2010, Weber et al. 2013, Roalson and Roberts 2016, Ogutcen et al. 2021). Besleria species occur in most neotropical rainforests, with centres of diversity in the tropical Andes (>100 species) and Central America (20 species). Other species are endemic to the Brazilian Atlantic Forest (11 spp.), Guiana Shield (8 spp.), and West Indies (7 spp.) (GRC 2023). Although most species are endemics, some are widely distributed, such as B. flavovirens Nees & Mart. and B. laxiflora Benth., which occur from Central America to the Brazilian Atlantic Rainforest. The most broadly distributed taxon in the genus is B. solanoides Kunth with a range from Nicaragua to Bolivia (Kvist et al. 2005, Clavijo et al. 2015, Araujo et al., 2020, GRC. 2023). Species of Besleria are usually recognized as unbranched terrestrial subshrubs with several pairs of large leaves, axillary ebracteate inflorescences, and fleshy globose berries (Wiehler 1975, Weber 2004). Most of the diagnostic characters for species identification rely on the presence or absence of a peduncle in the inflorescence, dimensions of calyx lobes, corolla colours (red–orange, yellow, or white), and corolla shapes ranging from narrow tubular to hypocyrtoid (i.e. with an apically inflated pouch on the lower surface below a constricted throat, cf. Fig. 1B). Flower characteristics of Besleria match with the definition of the hummingbird pollination syndrome, although few field observations have been recorded (Serrano-Serrano et al. 2017, Ogutcen et al. 2020). Fruit morphology has been largely overlooked in Besleria. However, it presents an interesting diversity in coloration and dehiscence ranging from indehiscent berries (Wiehler 1975) to a ‘rupturing berry’ that dehisces or bursts irregularly at maturity, displaying a red placental tissue embedded with red seeds (Berger et al. 2015). The conspicuously coloured fruit or rupturing display fruit is probably attractive to dispersing animals such as birds and/or small mammals (Berger et al. 2015). One species, Besleria formicaria Nowicke, is known to be myrmecophilous, hosting ants in domatia on both sides of the mid-vein of the leaves (Windsor and Jolivet 1996).
Broad morphological diversity in Besleria (Beslerieae, Gesneriaceae). A, flowers of B. iara. B, B. nitens. C, B. vestita. D, B. brevicalyx. E, axillary inflorescence in B. tambensis. F, pedunculate inflorescence in B. reticulata. G, a ‘rupturing berry’ of B. pauciflora. H, berry of B. solanoides. I, B. discreta, an understorey shrub. J, woody trunk of B. nitens. Photos: Gabriel E. Ferreira.
Species boundaries and infrageneric classification within Besleria remain poorly defined because the group lacks monographic and taxonomic studies, and a phylogenetic framework. The only recent revision of Besleria was published by Morton (1939) based on the study of herbarium collections at the Smithsonian Institution (USA). This treatment and later additional studies (Morton 1942, 1944, 1953, 1968) recognized 141 species that were placed into four sections, and 18 subsections based on morphological characters gathered from herbarium specimens related to flower morphology (calyx shape and nectary glands) and inflorescence structure (presence and absence of peduncle). Since Morton’s monograph, Besleria has been subject to few studies besides the segregation of Gasteranthus Benth. from Besleria (Wiehler, 1975) and regional floristic studies for Brazil (Lopes et al. 2008), Panama (Skog 1978), and the Guianas (Skog and Feuillet 2008). Therefore, Besleria needs a modern revision to improve the circumscription of currently recognized species and the classification of the growing number of newly described species in the genus (González et al. 2001, Feuillet 2008, Salinas 2008, Ferreira et al. 2016b, 2017, 2019, Cortés-Ceballos et al. 2017; Sánchez-Taborda et al. 2020.
Recent molecular phylogenetic studies in the tribe Beslerieae have confirmed the monophyly of the currently recognized genera and the sister relationship between Besleria and Cremosperma Benth. (Roalson and Clark 2006, Clark et al. 2010, Roalson and Roberts 2016). However, despite these advances, phylogenetic relationships within Besleria remain poorly defined. For example, Besleria species from the Brazilian Atlantic Rainforest have never been included in a phylogenetic study, leaving their systematic and biogeographical affinities unevaluated. To date, the most complete phylogenetic tree of Besleria has relied solely on the analysis of internal transcribed spacer (ITS) sequences and a limited taxonomic sampling mainly focused on Ecuadorian species (27 accessions; Roalson and Clark 2006). Although their study showed for the first time that Morton’s (1939) infrageneric classification is artificial and not supported, a more comprehensive and resolved phylogeny is necessary to delineate infrageneric lineages and revise diagnostic characters for supported clades. A species-level phylogeny of Besleria could also contribute to uncovering the biogeographical history of the group. Besleria has a widespread distribution throughout the Neotropics and is common in the understorey of tropical rainforests, making it a model to investigate the timing and the causes of plant diversification in these ecosystems (Antonelli et al. 2018, Cano et al. 2022). According to dating analyses available for Gesneriaceae (Perret et al. 2013, Roalson and Roberts 2016), Besleria diversified ca. 15 Mya, concomitantly with the beginning of a major geological period of uplift in the northern Andes (Hoorn et al. 2010). For example, the high species richness of Besleria found in the Andes of Colombia and Ecuador is congruent with the hypothesis that the Andean orogeny played a key role in the diversification of this plant group. However, it is unclear whether this area is the centre of origin of the genus and how and when the other regions, such as Central America, the Brazilian Atlantic Forest, and the West Indies, were colonized.
In this study, we estimated a time-calibrated phylogeny of Besleria based on one nuclear and three plastid regions. Our taxon sampling was designed to include representative species from all major neotropical regions. With this phylogenetic framework, we aimed to (i) evaluate the infrageneric classification of Besleria in the light of phylogenetic relationships, (ii) identify synapomorphies of major clades by reconstructing the evolutionary history of key morphological features, and (iii) infer the biogeographical processes that may have shaped the present-day distribution of Besleria species. The present study, which includes the most comprehensive species-level sampling assembled to date, will facilitate future revisions of this taxonomically challenging group and provide valuable insights into the timing and biogeographical context of understorey plant diversification across neotropical rainforests.
Materials and methods
Taxon sampling
Plant material was collected during fieldwork in Brazil and Colombia between 2014 and 2017 by G.E.F. Additional samples from the Andes (Colombia, Ecuador, and Peru), Central America (Panama), and the West Indies (Cuba) were provided by J.L.C., L.C., and M.P. We sampled a total of 155 accessions representing 117 species, among which 80 belong to the genus Besleria (~50% of all species of the genus). Sampling in Besleria was designed to include representative species of all subsections that Morton (1939) defined, and all biogeographical areas occupied by the genus (Weber et al. 2013). Several unidentified accessions (N = 12) from Colombia and Peru corresponding to potentially new species to science were also included to verify their phylogenetic position. Within other neotropical Gesneriaceae, we sampled representatives of all genera of the tribe Beslerieae, except Tylopsacas Leeuwenb., and representatives of all other genera recognized within the subfamilies Gesnerioideae and Sanangoideae (Weber et al. 2013): Columneinae [Columnea sanguinea (Pers.) Hanst. and Episcia fimbriata Fritsch], Gesneriinae (Gesneria humilis L.), Gloxiniinae [Gloxinia perennis (L.) Fritsch and Monopyle maxonii C.V.Morton], Ligeriinae [Sinningia brasiliensis (Regel & E.Schmidt) Wiehler & Chautems and Sinningia schiffneri Fritsch], Sphaerorrhizinae [Sphaerorrhiza sarmentiana (Gardner ex Hook.) Roalson & Boggan], and Sanango racemosum (Ruiz & Pav.) Barringer. Peltanthera floribunda Benth. was chosen as an outgroup based on previous phylogenetic results showing its sister relationship to the Gesneriaceae (APG IV, 2016; Luna et al. 2019, Ogutcen et al. 2021). All taxon and voucher information is listed in Supporting Information Table S1.
DNA sequencing and alignment
DNA was isolated from silica-gel-dried leaf tissue using the NucleoSpin Plant II (Macherey-Nagel, Düren, Germany) following the manufacturer’s protocol. Sequences of ITS, matK, rps16 intron, and trnL intron and trnL-trnF spacer were newly acquired for this study or obtained from published data (Perret et al. 2013, Serrano-Serrano et al. 2017). The selected loci have been extensively sequenced across the Gesneriaceae and their use has largely contributed to improve Gesneriaceae taxonomy, although we recognize that sequencing a larger number of nuclear genes using the sequence capture kit recently developed for the Gesneriaceae might have been a more robust strategy to resolve phylogenetic relationship within this group (Ogutcen et al. 2021). Both polymerase chain reaction (PCR) amplifications and sequencing reactions followed the procedures described in Perret et al. (2013). The primers used to amplify the matK, rps16 intron, and trnL intron and intergenic spacer between trnL and trnF (here designated as trnL-trnF) are given in Perret et al. (2003, 2013). ITS was amplified and sequenced with primers ITS 5P and ITS 8P (Möller and Cronk 1997). The program Sequencher 4.7 (Gene Code Corp., Ann Arbor, MI, USA) was used to edit and assemble complementary strands. Newly acquired sequences have been deposited in GenBank (Supporting Information Table S1). All sequences were first aligned using MAFFT (Katoh and Toh 2010) and then imported to Mesquite 3.03 (Maddison and Maddison 2017) for verification and manual adjustment.
Phylogenetic analysis and divergence times
Phylogenetic relationships between species were reconstructed by maximum likelihood (ML) using the software RAxML (Stamatakis 2014). Analyses were performed on the CIPRES portal (www.phylo.org; Miller et al. 2010). Each DNA region was treated as a separate data partition, allowing parameters of each region to be unlinked. The most suitable substitution model for each DNA region was determined using jModelTest2 (Darriba et al. 2012) based on the Akaike information criterion (AIC). The analysis identified HKY as the best-fitting model for trnL-trnF, while GTR+Γ was chosen for ITS, matK, and rps16. To ensure robust results, we conducted 100 runs using the preferred models for the specified data partitions. The robustness of the tree was calculated with nonparametric standard bootstrap resampling and 1000 pseudoreplicates (Felsenstein 1985). To examine potential incongruences between datasets, we inspected whether the topology resulting from the combined analyses of all the regions conflicted with the topologies obtained through separate analyses of the plastidial and nuclear sequences. Only nonconflicting nodes with ≥60% bootstrap support (BS) were considered.
Divergence time analyses were conducted in BEAST 1.8.4 (Drummond et al. 2012, Heled and Drummond 2012), applying the same partitions and substitution models as described above. Substitution and clock models were set as unlinked, whereas tree models were linked among partitions. We performed two runs of 100 million generations each, sampling every 20 000th generation, assuming an uncorrelated lognormal clock and a Yule speciation model (Yule 1925). Convergence of individual runs was assessed by inspecting the highest posterior density (HPD) of the Markov chain Monte Carlo (MCMC) parameters in Tracer v.1.6 (Drummond and Rambaut 2007). In addition, we checked that effective sample sizes for all relevant parameters were above the recommended minimum of 200 (Drummond and Rambaut 2007). After removing 10% as burn-in, tree files from the independent chains were combined using TreeAnnotator v.1.8.3 (Drummond and Rambaut 2007, Drummond et al. 2012). Because of the lack of known fossils in Gesneriaceae, we used secondary calibration points derived from the time-calibrated phylogenetic tree of Gesneriaceae obtained by Roalson and Roberts (2016). We chose to use the divergence between Peltanthera and the rest of Gesneriaceae, estimated at 81.9 Mya, to constrain the age of the root (star 1 in Supporting Information Fig. S1), and time constrained the divergence between Gesnerioideae and the remaining Gesneriaceae (star 2 in Fig. S1), estimated at 69.66 Mya. For each calibration point, we used a normal distribution prior with a high degree of flexibility (SD 5.0).
Historical biogeography
The distribution of each species of Beslerieae was categorized into eight eco-geographical regions, defined to reflect floristic and vegetation patterns as well as the geological history of each region (Cabrera and Willink 1973, Antonelli et al. 2009, Hoorn et al. 2010, Ribas et al. 2012, Perret et al. 2013, Morrone 2014). These regions are defined as: (C) Central America; (N) North Andes, from Venezuela to Ecuador, limited by the Western Andean Portal (Antonelli et al. 2009); (S) South Andes, including Bolivia and Peru; (A) Amazon basin (Amazonia lowlands); (B) Brazilian Atlantic Rainforest; (G) Guiana Shield and northeast Venezuela; and (W) West Indies. Taxa ranging outside the American continents were assigned to (O) Old World. The occurrence of each species was obtained through the Global Biodiversity Information Facility (GBIF doi.org/10.15468/dl.raohzu, http://www.gbif.org) and Gesneriaceae Resource Centre (GRC 2023).
Ancestral area reconstruction (AAR) was performed using the DEC (dispersal–extinction–cladogenesis; Ree and Smith 2008) model implemented in the R package BioGeoBEARS (Matzke 2014, Van Dam and Matzke 2016). The AAR analyses were done on the pruned BEAST-calibrated tree to keep only one accession for each monophyletic taxon. Accessions representing nonmonophyletic or unidentified species were maintained in the analyses since they could represent undescribed species. The DEC model was implemented with and without the parameter ‘j’ accounting for the probability of founder-event speciation (Matzke et al. 2014, 2022). Since the impossibility of statistically comparing the DEC and DEC+J models based on log-likelihood differences and the potential flaw of DEC+J to correctly model the probability of range inheritance events with respect to time (Ree and Sanmartín 2018), we decided to only consider the biogeographical events supported by both DEC and DEC+J reconstructions.
Morphological traits and ancestral character reconstructions
Herbarium specimens were examined or obtained from loans from the following herbaria: ALCB, B, BAH, BHCB, CAY, CEN, CEPEC, CESJ, COL, ESA, FCAB, FLOR, F, G, GZU, HAS, HERBAM, HPL, HRCB, HUEFS, HUFU, IAC, INPA, IPA, LE, M, MBM, MBML, MG, MO, NY, P, RB, RON, S, SP, SPSF, UB, UEC, UPCB, US, VIES, and W (acronyms according to Thiers 2023). Whenever possible, floral and fruit features were examined in the field on living material, on parts kept in alcohol (pickled collection), or from pictures using the extensive images library made available by J.L.C. The traits we considered and recorded for each species include inflorescence type (pedunculate vs epedunculate), corolla shape (tubular, ventricose, hypocyrtoid), corolla colour (orange, yellow, white), and fruit dehiscence type (indehiscent berry, rupturing berry, dehiscent capsule). These traits have been selected because they were used by Morton (1939) in his treatment of Besleria or because of their potential as synapomorphies for defining clades (e.g. berry type, Berger et al. 2015).
Reconstructions of ancestral character states were performed using ML as implemented in RASP v.4 (Yu et al. 2020), using 100 randomly chosen trees derived from the BEAST analysis. We conducted model selection for trait evolution, evaluating three distinct models: the Equal Rates (ER) model (where a single parameter governs all transition rates equally), the Symmetrical (SYM) rate transition model (in which forward and reverse transitions share the same rate), and the All Rates Different (ARD) model (where each rate is treated as a unique parameter). The best-fitting model for each character was selected based on comparison of AIC scores (Paradis et al. 2004).
Results
Phylogenetic analyses
The combined matrix of all DNA regions comprised 4433 characters with 2055 variable sites. Of these, 3631 sites (1582 variable) derived from plastid sequences (trnL-trnF region, matK, and rps16) and 802 sites (473 variable) from nuclear sequences (ITS). The comparison of phylogenetic analyses derived from separate analyses of nuclear and plastid datasets did not support topological incongruences [BS > 60%, posterior probability (PP) > 0.95], and therefore the data were combined in a total evidence analysis. The ML and BEAST analyses resulted in congruent topologies (Figs 2, 3). In both analyses, the tribe Beslerieae is monophyletic and sister to Titanotrichum, although this relationship is poorly supported. Besleria (BS 100%, PP 1) is monophyletic and sister to Cremosperma (BS 95%, PP 1) (Fig. 2). Within Besleria, species represented by multiple samples were retrieved as monophyletic except for B. aggregata, B. barbata, B. quadrangulata, B. solanoides, and B. tambensis. These nonmonophyletic species are probably due to misidentifications or overly broad circumscriptions. Further investigations will be necessary to accurately circumscribe these taxa. Our analyses resolved six major clades that are characterized by specific geographical distributions (Fig. 2). Clade A is sister to all other Besleria (BS 99%, PP 1), and comprises endemic species with a restricted distribution in the Guiana highlands or endemic to Caribbean islands, but it also includes species in the North Andes and the most widely distributed species in Besleria such as B. flavovirens and B. laxiflora that both range from eastern Brazil to Mexico and B. pauciflora distributed from Nicaragua to Bolivia (Fig. 2). Clade B (BS 100%, PP 1) comprised two samples of Besleria sprucei Britton ex Rusby distributed in the foothills along the eastern side of the Andes and in the Amazon lowlands (North and South Andes and Amazon Basin; Fig. 2). Clade C (BS 62%, PP 1) mainly comprises Andean species except one, Besleria aggregata (Mart.) Hanst., the range of which extends into the Amazon basin. Clades D (BS 93%, PP 1) and E (BS 72%, PP 1) are sister clades but present a distinct distribution: Clade D is an endemic lineage from the Atlantic Forest in southeastern Brazil, whereas Clade E is centred in the Southern Andes between Bolivia, Ecuador, Venezuela, Colombia, and Peru (Fig. 2). Finally, Clade F (BS 42%, PP 1) encompasses species that are distributed across diverse regions, including the North Andes, Central America, Guiana Shield, and Amazon basin (Fig. 2).
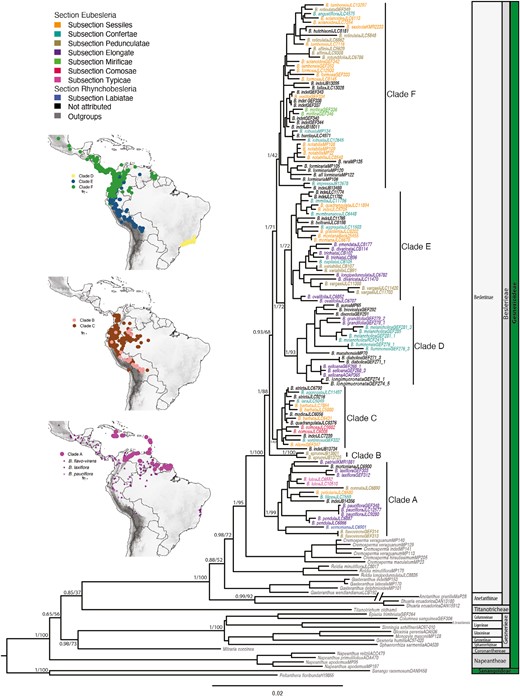
Maximum-likelihood tree for 155 accessions representing 80 species of Besleria and outgroups obtained from the combined analysis of plastid loci matK, rps16, and trnL-trnF, and the nuclear ITS region. Numbers on branches are Bayesian posterior probabilities and maximum-likelihood bootstrap support values. Taxon names are coloured according to Morton’s subsections. Main clades of Besleria are labelled A, B, C, D, E, and F for text discussion and major clades of Gesneriaceae are indicated on the right-hand side. The inset maps show the geographical distribution of the main clades of Besleria. Distributions of the three widely distributed species of Clade A (B. flavovirens, B. laxiflora, and B. pauciflora) are displayed. Scale bar represents the number of subsitutions per site.
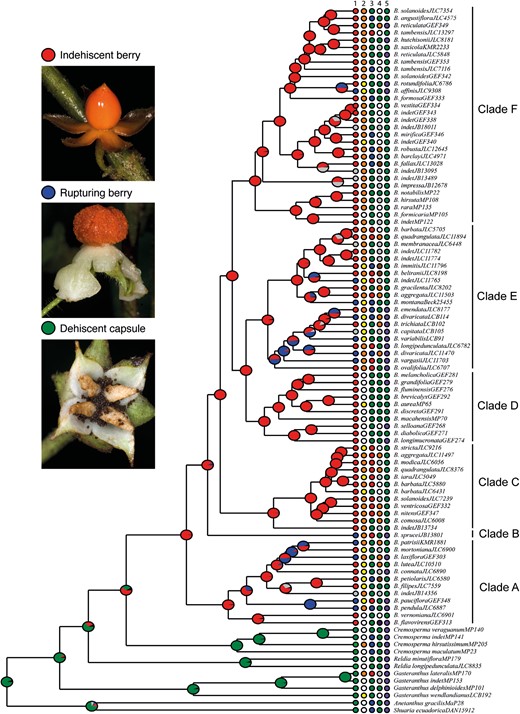
Morphological diversity and evolution of fruit dehiscence type in Beslerieae plotted on the BEAST consensus tree pruned to include only one accession per monophyletic species. Pie charts represent the probabilities of different ancestral character states at each node estimated from maximum-likelihood inference. Coloured boxes at the tip of each species show the character states for (1) fruit dehiscence type being indehiscent berry (red), rupturing berry (blue), or dehiscent capsule (green); (2) corolla colour being orange (orange), yellow (yellow), or white (white); (3) corolla shape being tubular (blue), ventricose (green), or hypocyrtoid (red); (4) fruit colour being green (green), white (white), orange (orange), brown (brown), or purple (purple); and (5) inflorescence type being pedunculate (purple) or epedunculate (green). Reconstructions of ancestral character states for traits 2–5 are provided in the Supporting Information. Photos: John L. Clark.
Divergence times
The divergence time estimates suggest that Beslerieae split from the other Gesneriaceae during the Palaeocene (60 Mya, 59.95–74.87 Mya; mean, 95% HPD) and that current diversity in Besleria originated in the Early Miocene (19 Mya, 15.13–25.17 Ma) (Supporting Information Fig. S1). All major crown groups recognized within Besleria emerged between 11 and 8 Mya and diversified intensively during the Late Miocene.
Biogeographical analyses
The biogeographical history reconstructed with the DEC+J (Fig. 4) and DEC (Supporting Information Fig. S2) models were similar, and only patterns common to both reconstructions were taken into account. These results indicate that Besleria probably originated in the Northern Andes around the Middle Miocene. Subsequent colonization of the other neotropical regions occupied by Besleria followed different clade-specific trajectories. The most recent common ancestor (MRCA) of Clades A and C were probably distributed in the North Andes (combined with Amazon lowlands for Clade C). Diversification of Clade C occurred mainly within the North Andes, whereas Clade A species reached the Guiana Shield and the West Indies ~7 Mya. Range expansion of a few species (e.g. B. laxiflora and B. falvovirens) into the lowland rainforests of Eastern Brazil (Bahia State), and Central America account for the widespread distribution of Clade A along the eastern portion of the Neotropics up to Mexico. The remaining Besleria species belong to sister lineages showing a north–south disjunction: one distributed in the South Andes and southeastern Brazil (Clade D/E), the other distributed in the North Andes and Central America (Clade F). The dispersal event to the South Andes, followed by a founder event to southeastern Brazil, or a vicariant event between the South Andes and the Brazilian Atlantic Forest, ~10 Mya, gave rise to the endemic radiations in the South Andes (Clade E) and in the Brazilian Atlantic Forest (Clade D). Range expansion into Central America was reconstructed at the root of Clade F ~8–10 Mya. This was followed by vicariance, which gave rise to a Central American endemic lineage (Besleria notabilis C.V.Morton, Besleria hirsuta Hanst., Besleria rara L.E.Skog, and Besleria formicaria Nowicke). At the same time, the remaining Clade F in the North Andes continued to diversify and expanded later into Central America.
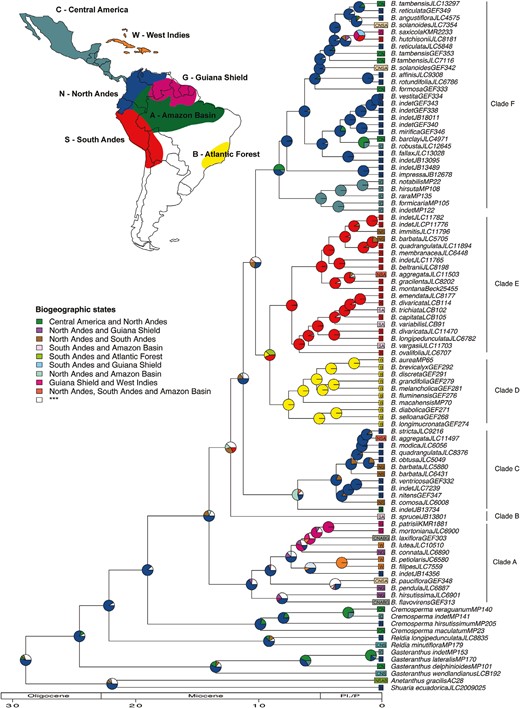
Biogeographical reconstruction plotted on the BEAST consensus tree of Beslerieae using the DEC+J model in BioGeoBEARS. Pie charts represent the relative probability of the ancestral area reconstructed for each node, with colours corresponding to the areas highlighted on the map. Geological time scale in millions of years is shown at the bottom, and major clades of Besleria are indicated on the right-hand side.
Morphological traits and ancestral character reconstructions
We inferred the ancestral states of five diagnostic characters widely used to define Besleria species, including features such as pedunculate or epedunculate inflorescences, corolla shape or colour, fruit colour, and fruit dehiscence type (Morton 1939, Berger et al. 2015). The best-fit model for each character was found to be the ER model, suggesting that a single parameter governs all transition rates equally (see Supporting Information Table S3 for a comparison of the performance of the three models). We found that these traits exhibit a high degree of convergence, and identified few synapomorphies for the main clades (Fig. 3 and Figs S3, S4, S5, S6). According to our reconstruction (Fig. 3), rupturing berries evolved seven times independently from indehiscent berries, the ancestral condition in Besleria. Fruits pigmented in orange, brown or purple, as well as white, co-occur in most clades and could have derived from greenish fruits (Fig. S3). Hypocyrtoid corollas evolved mainly within Clades C and E from ventricose corollas (Fig. S4). Pedunculate inflorescences evolved from epdunculate inflorescences in Clades A, D, E, and F (Fig. S5). Finally, shifts from orange to yellow corollas were frequent in Besleria, whereas loss of corolla pigmentation occurred in Clade D, which exhibits predominantly white flowers, with only a single reversal to yellow flowers (Fig. S6). This loss of pigmentation in the corolla can be considered a synapomorphy for Clade D. However, it is important to note that white flowers also evolved in Clade D (Fig. 3) in B. flavovirens and B. hirsutissima.
Discussion
Phylogenetic relationships and systematic implications
The relationships recovered within the tribe Beslerieae are consistent with the phylogenetic analyses by Clark et al. (2010) including the segregation of Gasteranthus from Besleria as proposed by Wiehler (1975), and the division of Beslerieae into two subtribes: the Besleriinae comprising Besleria, Cremosperma, Reldia, and Gasteranthus, and the Anetanthinae, represented by Anetanthus and Shuaria (Weber et al. 2013). The sister relationship found between Besleria and Cremosperma agrees with previous studies based on ITS or ITS + trnL-trnF (Roalson and Clark 2006, Clark et al. 2010), but differs from a recent phylogenomic analysis of Gesneriaceae based on 418 nuclear genes that places Besleria sister to a clade including Cremosperma and Gasteranthus (Ogutcen et al. 2021). On the other hand, the phylogenetic position of Titanotrichum as sister to all Beslerieae (BS 37%, PP 0.85) is in agreement with Ogutcen et al. (2021).
The current phylogenetic tree corroborates the monophyly of Besleria and provides a far more resolved topology than previous results derived from the analysis of 28 accessions of Besleria using ITS only (Roalson and Clark 2006) or from a family-scale analysis of Gesneriaceae (Roalson and Roberts 2016). Based on our analysis, we found that none of the sections and subsections proposed by Morton (1939) and revised by Wiehler (1975) in Besleria are monophyletic (Fig. 2). For example, subsection Sessiles defined by short calyx lobes and inflorescences without peduncles and subsection Pedunculatae characterized in contrast by a well-developed peduncle (Morton 1939) are both segregated across four different clades (Fig. 2).
Our reconstructions of the evolution of morphological traits have identified few synapomorphies that could be used to characterize the main clades in Besleria. The loss of pigmentation in the corolla was the only clear synapomorphy defining the Brazilian endemic Clade D (Supporting Information Fig. S6). This preliminary survey of the morphological diversity of Besleria stresses the need to explore additional morphological traits beyond those considered by Morton (1939), such as pollen morphology that proved to be useful to discriminate closely related Besleria species from Colombia (Cortés-Ceballos et al. 2021). However, the limited phylogenetic signal of morphological traits observed in Besleria is consistent with the pattern of morphological convergence that has been identified across diverse lineages of Gesneriaceae, underlining the challenges of defining taxa and resolving evolutionary relationships in this plant family based solely on morphological data (Clark et al. 2011, 2012, 2015, Serrano-Serrano et al. 2015, Ferreira et al. 2016a).
Historical biogeography
Our ancestral area estimation supports the origins of Besleria in the North Andes around 19 Mya, coinciding with the onset of the northern Andean uplift (Garzione et al. 2008). The fact that Besleria diversity peaked at mid-elevation (500–2000 m; Morton 1939) suggests that diversification of this genus was probably promoted by Andean uplifts that increased the availability of suitable habitats and their connectivity with the surrounding areas (Antonelli et al. 2009, Luebert and Weigend 2014). Around 10 Mya, Besleria dispersed southward into the South Andes and southeastern Brazil and northward into Central America, resulting in the radiation of three main lineages with little overlap in their distribution (Clades D, E, and F; Fig. 4). The first unambiguous colonization of the South Andes was reconstructed at the root of Clade D/E. This dispersal event coincides with closure of the Western Andean Portal (WAP), which served as a corridor for marine incursions into the Amazon basin until the Middle Miocene (13–11 Mya; Jaramillo et al. 2017b). This region, also named Amotape–Huancabamba Zone, is located between southern Ecuador and northern Peru and has frequently been referred to as a barrier for the dispersal of Andean plant groups (Antonelli et al. 2009, Struwe et al. 2009, Weigend et al. 2010, Mutke et al. 2014). A similar timing for the colonization of the South Andes from the north has been recorded in other plant groups such as Rubiaceae and Chlorantaceae, suggesting that the WAP acted as an effective dispersal barrier for mid-elevation plants in the Andes (Antonelli et al. 2009, Antonelli and Sanmartin 2011, Luebert and Weigend 2014, Pérez-Escobar et al. 2022). The mainly in situ diversification of Clade E within the South Andes and the limited subsequent recolonization of the North Andes indicate that the Amotape–Huancabamba zone acted as a dispersal barrier for Besleria even after closure of the WAP due to Andean uplift. Colonization of Besleria in southeastern Brazil probably involved a single founder event from the South Andes 9–8 Mya, although vicariance between these areas cannot be excluded. Our results indicate that Besleria dispersed into the Atlantic Forest before the assemblage of the Cerrado biome 10–5 Mya (Simon et al. 2011) when neotropical rainforests were probably more interconnected than today (Morley 2000, Sobral-Souza et al. 2015). The strict biogeographical disjunction between the Andean and the Brazilian clades and their endemic radiations within these areas are consistent with previous studies that suggest the formation of a SW–NE diagonal of dry vegetation across South America, which strongly limited biotic exchanges between the Atlantic Forest and other surrounding biomes (Costa 2003, Fouquet et al. 2012, Werneck et al. 2012, Ferreira et al. 2016a).
Range extension of Besleria into Central America occurred for the first time 7–10 Mya, coinciding with the formation of the Panama Isthmus during the Middle Miocene and a period of intense biotic exchange between South and North America (Bacon et al. 2015, Jaramillo et al. 2017a). Early vicariance in Clade F gave rise to a subclade endemic to Panama and Costa Rica (B. formicaria, B. hirsuta, B. notabilis, and B. rara), whereas remaining Besleria diversity in Central America is explained by recent range expansions of species distributed in both the Andes and Central America (Fig. 4). All Besleria species occurring in the Guiana Shield, the West Indies and northeastern Brazil belong to the same Clade A (Fig. 4). Our results suggest that dispersals from the North Andes to the Guiana Shield and West Indies probably started 6–8 Mya, after drying of the Pebas system (Hoorn et al. 2010, 2022) and marine incursions (Jaramillo et al. 2017b) that probably limited biotic interchange between the western and eastern portions of the Neotropics (Antonelli et al. 2009). Finally, our analysis suggests that the widespread distribution of B. laxiflora and B. flavovirens in the lowland rainforests of Central America, NE South America, and eastern Brazil (Fig. 4) is probably the result of recent range expansions that occurred around 5–7 Mya. Although the origin of this disjunct distribution may involve long-distance dispersal, a more probable hypothesis is that range expansions of these species have followed the global expansion of neotropical rainforests that led to the connection of the Amazon and Atlantic Forest during the Last Glacial Maximum (Sobral-Souza et al. 2015).
Conclusions
Our study provides the most comprehensive taxon sampling and resolved phylogenetic and biogeographical hypothesis of Besleria to date. Most of the six major clades identified in this study have not been recovered or recognized in previous phylogenetic or taxonomic studies. The reconstruction of the ancestral characters of morphological traits shows that the subsections of Morton (1939) are artificially defined, and many of the characters used in Morton’s classification are convergent. This finding underlines the need for further revisions in the infrageneric classification and morphological characterization of Besleria. In this context, the use of new methods based on the sequence capture of a larger number of nuclear genes might offer a promising way to better define phylogenetic relationships within the Beslerieae (Ogutcen et al. 2021).
Our divergence time analyses suggest that Besleria started diversifying in the Early Miocene probably in the North Andes (Fig. 4). The biogeographical history of Besleria, including its expansion in South and Central America, was impacted mainly by major geological and palaeogeographical events that occurred in the Neotropics during the Miocene. These events encompass the closure of the Panama Isthmus (Montes et al. 2015) that coincided with the first colonization of Besleria in Central America (Fig. 4) and the emergence of a dry diagonal that maintained the taxa of the Brazilian Atlantic Forest isolated from the other rainforest systems. The marked concordance between phylogenetic and biogeographical signals indicates that clades of Besleria mainly diversified locally within different regions where similar morphological characters evolved independently (e.g. rupturing berries).
Supplementary data
Supplementary data are available at Botanical Journal of the Linnean Society online.
Acknowledgments
We thank Régine Niba for her contribution to the laboratory work; and Àngela Cano, Charles Zartman, Helder Vechi, Fernanda Cabral, João Paulo Zorzanelli, Lukas Daneu, Marta Pereira, Mauro Peixoto, and Susana Costa for their help during field expeditions. G.E.F. acknowledges funding support from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) of Brazil for supporting his doctoral dissertation research, a Cuatrecasas Fellowship Award, an Elvin McDonald Research Endowment Fund grant, a Nellie D. Sleeth Scholarship Endowment Fund offered by The Gesneriad Society, a Research Grant from the American Society for Plant Taxonomy (ASPT), and a Research Grant-Program in Plant Systematics of the International Association for Plant Taxonomy (IAPT); M.P. acknowledges funding from the Swiss National Science foundation (grant no. 31003A_175655/1). Laboratory work was performed at the Conservatory and Botanical Garden of Geneva (Switzerland). All samples were collected by researchers following current regulations. Collecting permits were granted by ICMBio SISBIO licence number43226-4 in Brazil, the ANAM (SC/P-43–10) in Panama and the Autoridad National de Licencias Ambientales - ANLA, resolucion 1070 and 01004 in Colombia.



