-
PDF
- Split View
-
Views
-
Cite
Cite
Rowan J Schley, Alex D Twyford, R Toby Pennington, Hybridization: a ‘double-edged sword’ for Neotropical plant diversity, Botanical Journal of the Linnean Society, Volume 199, Issue 1, May 2022, Pages 331–356, https://doi.org/10.1093/botlinnean/boab070
Close - Share Icon Share
Abstract
Hybridization can facilitate both evolutionary diversification and extinction and has had a critical role in plant evolution, with c. 25% of species known to hybridize in some temperate floras. However, in the species-rich Neotropical flora, the role of hybridization in the evolution of diversity remains unclear. Our review examines studies of hybridization in seed plants from across the Neotropics and explores its outcomes on Neotropical plant evolution. We review studies on a per-biome basis and a spectrum of evolutionary outcomes from hybridization are evident across Neotropical biomes and taxa. These range from short-term impacts, such as the broadening of ecological amplitude in hybrid progeny with transgressive phenotypes and genetic swamping, through to long term impacts, such as the generation of new lineages. Among these studies certain themes emerge, such as the pervasive hybridization among species-rich plant radiations from the Andean páramos, suggesting a role for hybridization in rapid diversification events. Finally, we highlight that hybridization is relatively understudied in the Neotropical flora, despite its remarkable species richness. The advent of genomic techniques can facilitate the study of hybridization and its effects in understudied biomes and plant groups. The increasing availability of genomic resources will eventually allow comparisons between tropical and temperate floras and therefore shed light on the evolutionary impacts of hybridization across the latitudinal biodiversity gradient.
INTRODUCTION
Hybridization (i.e. reproduction between species) is a common occurrence among certain lineages and is known to generate evolutionary novelty, particularly in plants (Rieseberg et al., 2007). For example, hybridization occurs in an estimated 25% of species in the British flora (Mallet, 2005) and in up to 40% of species in some genera of this flora (e.g. Euphrasia L.) (Preston & Pearman, 2015). Hybridization shows a strong phylogenetic signal, occurring far more frequently in certain plant families than others, and tends to be most common in a few genera [e.g. occurring across 40% of plant families, but restricted to 16% of genera, in several North American, European and Australian floras (Whitney et al., 2010; Marques et al., 2018)}. However, hybridization is a relatively unexplored area of evolutionary biology in speciose tropical floras, such as that of the Neotropics, and to date its impact has not been systematically reviewed. Having been historically thought of as a rare occurrence, at least in tropical rainforests (Ashton, 1969; Ehrendorfer, 1970; Gentry, 1982), empirical studies are emerging that provide evidence of hybridization as a modulator, and perhaps a catalyst, of speciation in the Neotropical flora. Here, we review these studies and aim to explore the influence of hybridization on the evolution of Neotropical plant diversity.
Evolutionary outcomes of hybridization
For the evolution of diversity, hybridization is a ‘double-edged sword’ leading to a variety of evolutionary outcomes, ranging from the passage of adaptive loci precipitating extremely rapid radiations (Meier et al., 2017) through to the homogenization of divergence and extinction of lineages (reviewed by Todesco et al., 2016). The outcomes of hybridization are shaped by a range of processes, including divergence between parental species (Comeault & Matute, 2018), recombination (Martin et al., 2019) and selection (Suarez-Gonzalez, Lexer & Cronk, 2018).
The boundaries between plant species are often semi-permeable (Mallet, 2007), and the degree of this permeability generally correlates with the level of divergence between species (Comeault & Matute, 2018). In closely related species or subspecies which interbreed frequently, these boundaries (i.e. ‘barrier’ loci, regions of the genome that remain differentiated in the presence of gene flow; Harrison & Larson, 2014), are usually loci that confer adaptation to different environments and are under divergent selection (Payseur, 2010). In more distantly related species, further barriers to gene flow crystalize around these adaptive loci as species diverge (Feder, Egan & Nosil, 2012). In many cases, these genetic barriers preclude hybridization between species, but in some cases, these may be broken down by recombination over subsequent generations of backcrossing. Indeed, the rate of recombination can greatly impact the porosity of divergent genomes to introgression (Martin et al., 2019).
Whereas recombination can act to break down barriers to gene flow between species and promote introgression, selection can both promote introgression (e.g. Whitney, Randell & Rieseberg, 2010) and reinforce barriers to gene flow (Hopkins, 2013). In addition to its effect on pre-zygotic barriers, selection impacts the outcomes of hybridization largely through its action on hybrid progeny, and the factors driving selection can either be intrinsic or extrinsic. Intrinsic selection is often mediated by negative epistatic interactions between alleles with different evolutionary histories [e.g. Bateson–Dobzhansky–Muller incompatibilities (Bateson, 1909; Dobzhansky, 1936; Muller, 1942)] that drive hybrid sterility. Extrinsic processes driving selection are often ecological in nature, caused by adaptation to contrasting environments in parental species, rendering hybrid progeny maladapted to either environment and facilitating selection against hybrids. However, selection may also favour hybrid genotypes when loci of adaptive benefit are passed between species (Rundle & Nosil, 2005).
The outcomes of hybridization are further mediated by the geographical context of the hybridization taking place (Harrison & Larson, 2014) and dispersal (Kovach et al., 2015). For example, where the ranges of two species overlap a stable hybrid zone may form, within which hybrids between highly divergent species may be unfit and the hybrid zone is maintained by dispersal and recurrent hybridization (i.e. ‘tension zones; Barton & Hewitt, 1985’). Alternatively, where hybrid offspring experience higher fitness than either parental species in intermediate environments, but lower fitness in either parental environment, this is known as a ‘bounded superiority hybrid zone’ (Moore, 1977; Abbott, 2017). Such zones tend to form at ecotones, and so may occur across large areas of the Neotropics where biomes interdigitate (e.g. between Amazonian rainforest and cerrado savanna). Finally, in a patchwork of different environments where parental species occupy contrasting habitats and hybrids occur where these parental species overlap, a ‘mosaic’ hybrid zone may form (Harrison & Rand, 1989). This type of hybrid zone is characterized by hybrids with either lower, higher or variable fitness relative to the parental species and where both parents and hybrids have a patchy distribution across the site. This type of hybrid zone may occur in the Neotropics where there is a high diversity of different habitats at the landscape scale (e.g. among Andean valleys that host dry forest, montane forest and montane grassland in relatively close proximity).
Within these hybrid zones, hybrids between less divergent species may persist for generations in sympatry through backcrossing, or hybrid individuals may locally outcompete parental forms resulting in a zone of ‘orphan’ hybrids disjunct from progenitor species (e.g. Groh et al., 2019). Similarly, in many cases hybrid offspring are geographically or ecologically displaced from their parental species, with the occurrence of gene flow prevented by geographical distance or ecologically mediated selection. This displacement often occurs after periods of climatic change that cause the ranges of parental species to shift, bringing them into secondary contact where they hybridize. Following this, further climatic change may drive the parental species back into habitat refugia, and the evolutionary novelty generated in hybrid lineages allows them to persist in the original area of secondary contact (Kadereit, 2015). This often occurs in hybrid speciation, which is further discussed next and in the following sections.
In terms of evolutionary outcomes, hybridization may first be deleterious through maladaptive gene flow (hereafter termed ‘negative’ outcomes). Indeed, in many lineages hybridization simply results in the formation of maladapted hybrids which are quickly outcompeted by parental species in sympatry, driving disruptive selection (Tavares et al., 2018). Where selection against hybrids is relaxed, adaptive alleles may be homogenized between populations through ‘genetic swamping’ (Buggs & Pannell, 2006), ultimately leading to the extinction of the rarer species (Balao et al., 2015) or possibly ‘speciation reversal’, resulting in a single lineage with an admixed mosaic genome (Hegde et al., 2006; Vonlanthen et al., 2012; Kearns et al., 2018).
Second, hybridization may be maintained over many generations while having a minimal effect on evolutionary dynamics (hereafter termed ‘neutral’ outcomes). Hybridization and differential selection across different genomic regions can result in variable rates of introgression, where loci under divergent selection remain distinct between species due to negative fitness effects for hybrid genotypes at those loci. Conversely, loci that are subject to little selection and are physically distant from loci under selection are homogenized between species through gene flow (Gompert, Parchman & Buerkle, 2012). Accordingly, divergent lineages may remain distinct in the face of gene flow, with divergence between them being maintained only at a few loci (Wu, 2001) known as ‘islands of divergence’ in the genome (Turner, Hahn & Nuzhdin, 2005; shown for flower colour loci by Stankowski, Sobel & Streisfeld, 2017; Tavares et al., 2018).
Third, there are multiple selectively beneficial impacts of hybridization (hereafter termed ‘positive’ outcomes). One adaptive outcome of persistent hybridization is the provision of increased genetic diversity, which can act as a mechanism by which ‘syngameons’ of interfertile species with low population densities negate Allee effects and inbreeding depression (Cannon & Lerdau, 2015; Suarez-Gonzalez et al., 2018). Similarly, where hybrid individuals are favoured by selection due to the increased fitness imparted by introgressed loci, this is known as ‘adaptive introgression’ (Whitney, Randell & Rieseberg, 2010; Taylor & Larson, 2019). Adaptive introgression is particularly common in novel or disturbed environments, especially where introgressed offspring experience higher fitness than parental species in the novel environment (Rieseberg et al., 2007).
Finally, hybridization can drive the evolution of new species and even lead to rapid, phenotypically diverse radiations (Meier et al., 2017; Meier et al., 2019). The most direct way in which hybridization can generate diversity is through hybrid speciation (Mallet et al., 2007), which occurs when the hybrid offspring of two lineages are reproductively isolated from their parental lineages and may only reproduce with other hybrids of similar composition (Abbott, Barton & Good, 2016). Hybrid speciation can either be ‘polyploid’ (i.e. where hybrid offspring have a different ploidy to parental species, often entailing instantaneous reproductive isolation between hybrids and parents), or ‘homoploid’ (i.e. between species with the same ploidy, where reproductive isolation takes longer to evolve) (Coyne & Orr, 2004; Schumer, Rosenthal & Andolfatto, 2014).
A related mechanism by which hybridization may generate diversity is through ‘combinatorial speciation’ (Marques, Meier & Seehausen, 2019). The combinatorial model posits that hybridization between two or more species can lead to a re-assembly of existing genetic variation that has accrued between the lineages and has already been subject to ‘assessment’ by selection. This leads to novel combinations of genetic variants that generate a range of phenotypic variation beyond that of the progenitor lineages, many of which confer adaptation to highly contrasting environments and can cause reproductive isolation (Rieseberg et al., 2003; Kagawa & Takimoto, 2018; Wang et al., 2021). This variation accumulates at a rate much higher than if adaptive variants were accumulated by mutation de novo (Grant & Grant, 1994), and so leads to rapid adaptive radiations.
Studying hybridization in the genomic era
The advent of genomic data has provided new insights into the dynamics of hybridization and associated evolutionary processes. The shift from sparse genetic markers (e.g. microsatellites) to genomic data that allow us to examine variation across the entire genome with much higher resolution (e.g. RADseq) has provided deeper insights into hybridization than was previously possible (e.g. Eaton & Ree, 2013; Royer, Streisfeld & Smith, 2016; MacGuigan & Near, 2019). In temperate floras in particular, novel genomic data and associated bioinformatic approaches have allowed the detection of gene flow, which was previously undetectable using morphological methods (Mitchell & Holsinger, 2018). These approaches have also shed light on the impacts of selection in hybrid zones (Hamilton & Aitken, 2013; Ma et al., 2019), allowed the exploration of the role of hybridization in generating diversity (White et al., 2020) and, more generally, facilitated the detection of introgression in taxonomically complex groups (Buck et al., 2020).
Such novel analytical approaches vary in their scope, depending on the temporal depth of hybridization they are trying to detect. For detecting ancestral hybridization deep in a phylogenetic tree, most methods examine patterns of phylogenetic incongruence between gene trees, under the assumption that hybridization results in one incongruent topology being more common than all others (e.g. ABBA-BABA tests: Green et al., 2010; Durand et al., 2011; PhyloNetworks: Solís-Lemus, Bastide & Ané, 2017). This contrasts with the expectation that all incongruent topologies will be equally common under incomplete lineage sorting (ILS), caused by stochastic inheritance of alleles (Doyle, 1992). For detecting more recent hybridization, most methods estimate the degree of shared genetic variation between adjacent or co-occurring species (e.g. STRUCTURE: Pritchard, Stephens & Donnelly, 2000; Raj, Stephens & Pritchard, 2014; NEWHYBRIDS: Anderson & Thompson, 2002), including the calculation of ‘hybrid indices’ to assess the proportion of ancestry inherited from each parental progenitor (e.g. hindex: Buerkle, 2005) and often use genetic ‘clines’ within hybrid zones to understand the interplay between hybridization and selection (e.g. bgc: Gompert & Buerkle, 2012). The broad approaches for examining hybridization at deep and shallow evolutionary timescales are highlighted in Figure 1. Novel analytical approaches to studying hybridization are now also facilitating the exploration of the evolutionary impact of hybridization in diverse tropical floras such as those of the tropical Americas, which are often understudied.
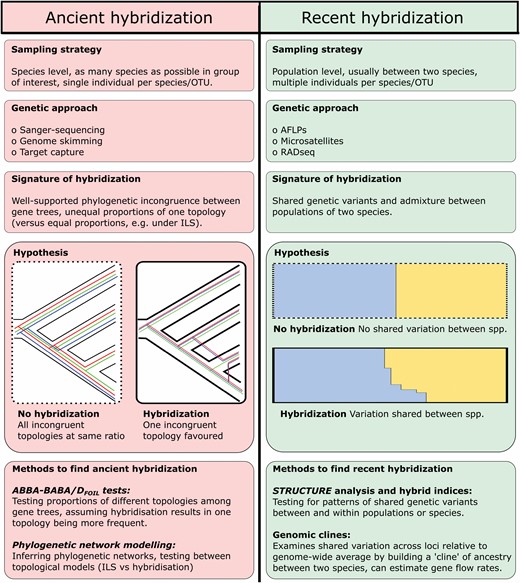
Summary of some methods and hypotheses underlying detection of deep (usually ancient) and shallow, recent hybridization using genetic and genomic data.
Neotropical plant diversity
The tropical Americas are among the most species-rich areas on Earth for vascular plants, with c.125 000 species (Antonelli & Sanmartín, 2011; Ulloa Ulloa et al., 2017). Although a large portion of this diversity is held in the rainforests of the region, the largest being the Amazon, which covers c. 6 000 000 km2 (ter Steege et al., 2013), recent estimates for other major biomes, or portions of them, are also suggestive of outstanding species diversity. For example, whereas the Brazilian Amazon contains 11 349 angiosperm species, the Brazilian cerrado, the largest area of savanna in the Neotropics, contains 11 384 (35 more; Forzza et al., 2010). Furthermore, floristic inventories of just 1610 sites in seasonally dry tropical forest (SDTF) across the Neotropics recorded 7338 species of free-standing woody plants alone (DRYFLOR: Banda et al., 2016), indicative of a species diversity that rivals that in rainforests and savannas. Another major portion of the biological diversity of the Neotropics is found in the Andes, which harbour different biomes, distinct elevational zones and isolated valleys, making the wider cordillera an ideal location for speciation and adaptive radiation (Hughes & Eastwood, 2006; Elias et al., 2009; Madriñán, Cortés & Richardson, 2013). Notable among these Andean biomes for its diversity and the rapid accumulation of species is the high-elevation páramo, which hosts c. 3400 species of vascular plants (Luteyn et al., 1999).
Many authors have proposed mechanisms with which to explain the disproportionate number of plant species found in the Neotropics. Suggested mechanisms for the evolution of Neotropical plant diversity range from the stability of ecosystems and long timespans over which species have accumulated (Stebbins, 1974) to geological processes such as the Andean orogeny (Hoorn et al., 2010). Moreover, ecological processes such as dispersal, speciation processes across biome boundaries (e.g. Pennington & Dick, 2004; Erkens et al., 2007; Dexter et al., 2017; Antonelli et al., 2018), species interactions (Kay et al., 2005; Kay & Schemske, 2008; Kursar et al., 2009) and edaphic specialization (Fine, Mesones & Coley, 2004; Fine et al., 2005) have also been suggested to help explain Neotropical plant diversity.
These factors have precipitated both slow accumulation of plant lineages (Couvreur et al., 2011; Schley et al., 2018) and rapid, ‘explosive’ radiation of hundreds of species in a few million years (Richardson et al., 2001; Hughes & Eastwood, 2006; Cortés et al., 2018; Vasconcelos et al., 2020) in the Neotropical flora. In particular, it is becoming increasingly clear that rapid evolutionary radiations are likely to explain a significant portion of the exceptional species diversity found in the Neotropics (e.g. Richardson et al., 2001; Koenen et al., 2015), with a compelling new model of ‘episodic evolutionary turnover’ suggesting that such radiations have occurred constantly through geological time (Koenen et al., 2015; Pennington, Hughes & Moonlight, 2015).
Given that many rapid plant radiations elsewhere also present significant evidence of hybridization (e.g. Hawaiian silverswords: Barrier et al., 1999), our goal in this review is to evaluate the role that interspecific gene flow has played in generating Neotropical plant diversity. Historically, there have been differing views on the occurrence of hybridization and its role as an evolutionary stimulus in the Tropics. In rainforest trees, for example, the prevailing view was that hybridization is an exceptionally rare event (Ashton, 1969; Ehrendorfer, 1970; Gentry, 1982). It was suggested that hybrids between rainforest tree species are poor competitors, and that fertile hybrid populations are almost non-existent (Ashton, 1969). In contrast, hybridization was previously theorized to play more of a role in speciation in herbaceous taxa (Gentry, 1982), especially those from the Andes (e.g. Fuchsia L.: Berry, 1982).
Given these contrasting historical perspectives and the increasing evidence for hybridization underlying radiation events (Marques et al., 2019), the goal of this review is therefore to investigate the role of hybridization in seed plant diversification in the tropical Americas as a whole. In particular, we aim to summarize the impact of natural hybridization on the evolution of Neotropical plant diversity from the population level through to macroevolutionary scales. Here, we distinguish natural hybridization from other processes such as recent hybridization between species that are shifting their ranges as a result of anthropogenic disturbance (e.g. van Hengstum et al., 2012) or following the introduction of alien invasive species (e.g. Ayres, Zaremba & Strong, 2004).
We structure our discussion of natural hybridization in Neotropical seed plants by major biome, including rainforest, páramo, savanna, deserts and wetlands, among others. Our justification for this is that although biomes are most usually defined by their physiognomy and ecological processes, it has been shown that in South America they can be defined by distinct species composition (Silva de Miranda et al., 2018). Such biotic distinctiveness has led some to view biomes as distinct evolutionary arenas (e.g. Pennington, Lavin & Oliveira-Filho, 2009; Hughes, Pennington & Antonelli, 2013) because evolutionary and biogeographic processes within them may differ significantly.
HYBRIDIZATION IN THE NEOTROPICAL FLORA
We reviewed studies of Neotropical plant hybridization by using the search terms ‘Neotropical’, ‘hybridization’, ‘reticulation’ and ‘introgression’ in Google Scholar (scholar.google.com, accessed between 10 January 2021 and 8 July 2021), adding the names of several important Neotropical countries and biomes in combination with the search terms above (including ‘Belize’, ‘Costa Rica’, ‘El Salvador’, ‘Guatemala’, ‘Honduras’, ‘Mexico’, ‘Nicaragua’, ‘Panama’, ‘Bolivia’, ‘Brazil’, ‘Colombia’, ‘Ecuador’, ‘French Guiana’, ‘Guyana’, ‘Peru’, ‘Suriname’, ‘Venezuela’, ‘Amazonia’, ‘Llanos’, ‘Páramo’, ‘cerrado’, ‘Andes’ and ‘Caatinga’). We included all studies from any year examining natural hybridization. Key studies discussed in the text are shown in Figure 2 and a table of all studies explored in this review, including details of the species examined, data type used, outcome of hybridization and biome in which they are found is provided in the Supporting Information (1).
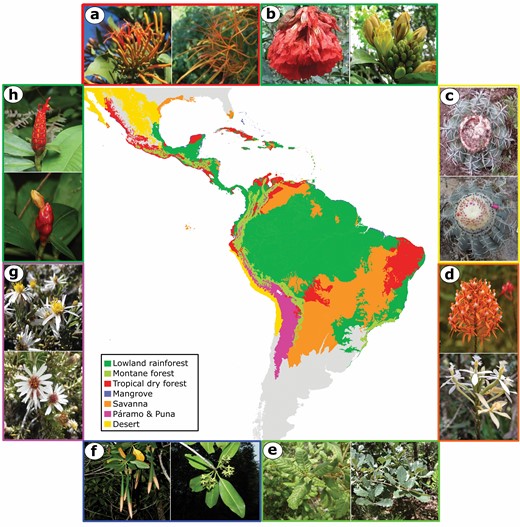
Schematic biome map of the Neotropics based on ecoregions from Olson et al. (2001), adapted by Dick & Pennington (2019) and further modified here. Images around the periphery show hybridising species pairs from a selection of studies examining hybridization in Neotropical plant species, with border colours corresponding to biomes, as explained by the map legend. Species shown are: A, Psittacanthus calyculatus and P. schiedeanus (Baena-Diaz et al., 2018; photographs modified from study); B, Brownea grandiceps and B. jaramilloi (Schley et al., 2018; photographs modified from study); C, Melocactus concinnus and M. glaucescens (Khan et al., 2020; photographs modified from study); D, Epidendrum cochlidium (photograph © Ecuador Megadiverso) and E. schistochilum (photograph © Nicolás Ramírez) as in Vega et al., 2013; E, Quercus rugosa (photograph © anthony_mendoza) and Quercus glabrescens (photograph © Dr. Alexey Yakovlev) as in Castillo-Mendoza et al., 2019; F, Rhizophora racemosa (photograph © Reinaldo Aguilar) and R. mangle (photograph © copepodo), as in Ceron-Souza et al., 2010); G, Diplostephium cinereum and D. meyenii (Vargas et al., 2017, photographs modified from study); H, Costus scaber (photograph © berniedup) and C. pulverulentus (photograph © Hans Hillewaert) as in Surget-Groba & Kay, 2013). Photographs taken from studies for A and B under CC BY 4.0 license (http://creativecommons.org/licenses/by/4.0/), license obtained for reprinting photographs from studies C and G.
We reviewed 60 studies of hybridization in the Neotropical flora, of which 5% showed ‘negative’ evolutionary outcomes of hybridization, 50% showed ‘neutral’ outcomes and 45% showed ‘positive’ outcomes of hybridization, as discussed in the previous section. Studies of hybridization were most common in rainforests and high-elevation environments (e.g. Andean páramos and montane forest), with most studies focusing on the Orchidaceae, Fabaceae, Fagaceae and Bromeliaceae. This is congruent with the fact that these are among the largest flowering plant families and that the occurrence of hybridization is largely governed by plant family, being more common in some families than others (Whitney et al., 2010). The number of hybridization studies by biome, growth form, plant family and genus are summarized and plotted in Supporting Information (1).
Rainforest
Rainforests cover much of lowland South America and significant portions of Central America, and include the largest expanse of rainforest on Earth in the Amazon basin (ter Steege et al., 2013). The expansive lowland forests of Amazonia provide relatively few geographical barriers to gene flow, especially among tree species (Pennington & Dick, 2010), and many Amazonian tree communities contain multiple closely related species in sympatry (Dexter et al., 2017). Indeed, in Amazonia, one factor that could promote hybridization widely among tree species is the remarkable level of sympatry caused by high levels of dispersal into local communities. For example, up to 19 species of Inga Mill. can coexist in a single hectare, and such high local diversity is also typical of many other species-rich Amazonian tree genera such as Protium Burm.f., Pouteria Aubl. and Eschweilera Mart. ex DC. (e.g. Valencia, Balslev & Miño, 1994).
Recent work has provided new evidence of hybridization in Neotropical rainforest trees, the most current of which uncovered signals of hybridization at both deep and shallow evolutionary scales in Brownea Jacq. (Fabaceae, a family that dominates Amazonian rainforest) (Schley et al., 2020). Older hybridization events gave rise to phylogenetic discordance among hundreds of nuclear genes among the 27 accepted Brownea spp. and was not caused purely by ILS. This reticulation deep in the phylogenetic tree, coupled with population genomic evidence of recent asymmetric introgression, suggested that hybridization and introgression within syngameons of interfertile species was a prominent feature of Brownea spp. This investigation is further explored as a case study for examining hybridization in Neotropical seed plants in Figure 3.
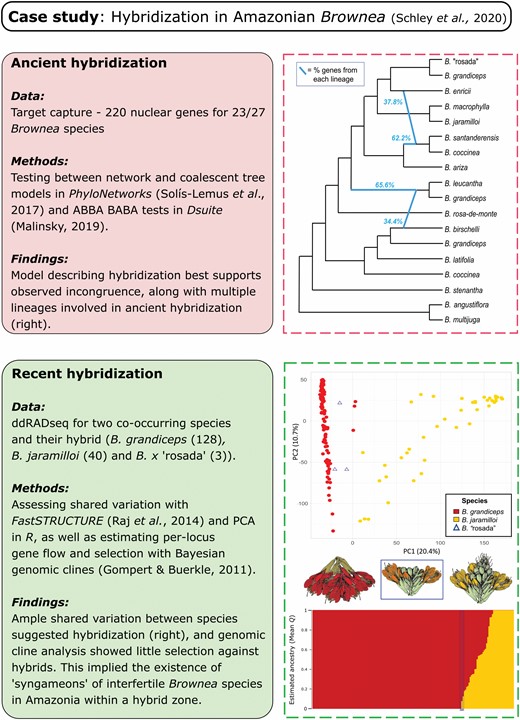
Case study of hybridization in the Neotropical flora, taken from Schley et al. (2020). Boxes show methods and results from investigation of hybridization at both deep (‘ancient’) and shallow (‘recent’) evolutionary timescales using phylogenomic and population genomic approaches, respectively. Inset figures modified from Schley et al. (2020).
Similar patterns of reticulation-driven discordance are evident in other lineages of Neotropical rainforest trees, likely resulting from overlapping ranges within the past few million years. Such a pattern was demonstrated between two members of the species-rich genus Ficus L. (Moraceae) (Costa et al., 2017) using three Sanger-sequenced loci and between two species of Carapa Aubl. (Meliaceae) using microsatellite data (Scotti-Saintagne et al., 2013a), which also uncovered evidence of more recent hybridization. Both of these studies also uncovered a history of either asymmetric introgression or discordance between nuclear and plastid loci, possibly through plastid capture, suggesting multiple generations of persistent backcrossing. In Amazonian Brownea, Carapa and Ficus, this asymmetry of introgression may suggest invasion of peripatric ranges of daughter species by more populous parental species (Pennington et al., 2015; Dexter et al., 2017). Some evidence of hybridization was also found between two ecologically divergent species of the diverse genus Inga (Fabaceae) using microsatellite loci (Rollo et al., 2016), probably due to a low degree of genetic divergence and outcrossing between species with overlapping ranges.
In non-woody plants, multiple studies indicate extensive hybridization occurs among rainforest orchids, especially in Epidendrum L. This genus comprises > 1600 species endemic to the Americas that display a variety of growth forms (e.g. epiphytic, lithophytic and rupicolous) (Hágsater & Soto-Arenas, 2005; WCVP, 2020). Many studies have used AFLPs and morphological data to examine hybrid zones between species pairs in montane Andean rainforests and Amazonia (e.g. Vega et al., 2013; Marques et al., 2014). One such study showed that the two focal Epidendrum spp. exhibited diverse morphology, with 72–78% of sampled individuals being of hybrid origin (Vega et al., 2013). A range of evolutionary outcomes from recurrent hybridization are evident in these studies, from the erosion of divergence between parental species to the formation of novel lineages with diverse morphologies (Marques et al., 2014). In these studies, persistent hybrid lineages occur in all hybrid zones, suggesting hybrids are fit and persist over time. More broadly, these studies indicate that hybridization is generating novel combinations of traits with broad ecological amplitude in Epidendrum spp.
Hybridization in Epidendrum has also been studied in other Neotropical forests using microsatellite data, revealing subtly different outcomes. For example, E. fulgens Brongn. and E. puniceoluteum F.Pinheiro & F.Barros from coastal Brazil hybridize extensively, despite different ploidies and habitat preferences in the parental species (Pinheiro et al., 2010). Moreover, backcrossing events are common between parents and hybrid offspring, which does not occur frequently between other lineages with different ploidies due to both extrinsic and intrinsic barriers (Coyne & Orr, 2004). Although there is also evidence of habitat-mediated selection shaping introgression across different habitats, the authors concluded that hybridization probably plays a significant evolutionary role and may increase the ecological amplitude of resultant offspring, as also shown in Marques et al. (2014) and Sujii, Cozzolino & Pinheiro (2019). Recent transcriptomic work has also highlighted that hybrid individuals resulting from crossing of these two species preferentially express genes which code for flooding tolerance, as possessed by one of the parental species, and that hybridization underlies the generation of some of their phenotypic diversity (Leal et al., 2020), as suggested by morphological work on other Epidendrum spp. (Pansarin & Amaral, 2008).
However, starkly different outcomes of hybridization are evident in epiphytes in Brazilian coastal rainforests. In Vriesea Lindl. (Bromeliaceae) from the Atlantic Forest region of Brazil, Neri, Wendt & Palma-Silva (2018) used microsatellites to show that minimal introgression occurred among lineages despite recurrent hybridization between species in sympatry. They observed that species boundaries in this group were maintained despite gene flow, probably due to a mix of pre- and post-zygotic barriers. These barriers included divergent reproductive systems between species (one of which is a selfer and the other an outcrosser), divergent floral traits and low hybrid viability. Moreover, whereas microsatellite data and plastid loci identified potential bidirectional introgression among Vriesea spp. in the same region, Zanella et al. (2016) found relatively few hybrids between species, with pre-zygotic barriers such as flowering time divergence probably playing a major role.
Such resistance to interspecific gene flow has also been shown in herbaceous species of Costus L. from Costa Rican rainforests (Costus pulverulentus C.Presl and C. scaber Ruiz & Pav., Costaceae) using morphological data, ecological experiments and microsatellites (Kay, 2006; Surget-Groba & Kay, 2013). Both studies found a similarly small degree of introgression to that found in Vriesea, again driven by pollinator-mediated pre-zygotic isolating barriers, highly divergent morphology and selection against hybrids. This low gene flow was evident despite these species being recently divergent, sympatric and producing fertile offspring in greenhouse experiments. This indicates an important role for ecological divergence and reinforcement (i.e. natural selection, which promotes reproductive isolation between species through selection against hybrids; Hopkins, 2013) in these Costus spp.
Mountains
Neotropical mountains are home to some of the most species-rich environments (Rahbek et al., 2019). In particular, the extremely high plant diversity of the Andean páramo was principally accumulated relatively recently through rapid evolutionary radiations (e.g. Lupinus L. (Fabaceae) (Hughes & Eastwood, 2006), Espeletia Bonpl. (Asteraceae) (Madriñán et al., 2013; Cortés et al., 2018) and Campanulaceae (Lagomarsino et al., 2016). The recency of the species radiations reflects the young geological age of the high-elevation páramos, since these environments were not available for colonization before the Pliocene (Hoorn et al., 2010). Their high species diversity may be the result of repeated cycles of allopatry driven by Pleistocene climate changes, periodically allowing páramo species to migrate downslope during cold glacial periods, with upslope movement driving isolation during warmer interglacials. This model of ‘flickering connectivity’ (Flantua et al., 2019) envisages extensive opportunity for secondary contact and interspecific gene flow, and this is now supported by numerous empirical examples (Nevado et al., 2018; Pouchon et al., 2021). This, coupled with the diversity of habitats resulting from the steep ecological clines along mountain slopes, renders hybridization a relatively common occurrence in the Andes.
Hybridization following secondary contact is probably the major mechanism of gene flow in species-rich Andean clades. As such, sensu the ‘combinatorial speciation’ model (Marques et al., 2019), it is likely that genetic variants which have accumulated between diverging lineages are passed around through hybridization (as has been shown for haplotype blocks that undergo minimal recombination; Todesco et al., 2020). Assuming a few of these variants are favoured by selection, this can promote adaptation to novel niches (Meier et al., 2019), of which there are many in the Andes due to large elevational gradients over small spatial scales. This allows hybrid individuals with novel trait combinations to escape competition, adapt and speciate, in a similar fashion to the ‘competitive release’ model explaining island radiations (Wilson, 1961; Yoder et al., 2010).
Asteraceae are one major group in which hybridization is likely to have mediated diversification in the Andean páramos. In this family, Espeletiinae (a subtribe of eight genera with c. 140 species endemic to the Andes) are the prime example of rapid diversification. Species of Espeletiinae are known to have diversified rapidly, within the last c. 2.3 Myr, and show a wide range of growth forms, from herbaceous rosettes to trees (Monasterio & Sarmiento, 1991). Pouchon et al. (2018) used plastid, mitochondrial and nuclear data recovered via genome skimming to show that most diversification in Espeletiinae occurred through climate-driven morphological adaptation, allopatry and ecological speciation (Pouchon et al., 2021) and that hybridization was common between sympatric species. Moreover, they suggested that the broad range of morphological variation in the group and the occurrence of species with admixed phenotypes was probably influenced by the sharing of traits through hybridization that may have been catalysed by repeated periods of habitat connectivity. However, the authors highlight that the impact of hybridization and hybrid speciation on the diversification of Espeletiinae remains to be explored in more detail.
A non-specialized pollination system is common in Asteraceae and likely to be prone to interspecific gene flow. This may explain why many young, species-rich genera of Asteraceae that form a significant part of the páramo flora show evidence of deep phylogenetic reticulation through patterns of phylogenetic incongruence, probably driven by the ‘flickering connectivity’ model. Such incongruence is particularly evident among the c. 111 species of Diplostephium Kunth found in the Andes, as shown using genome skimming and RADseq data (Vargas, Ortiz & Simpson, 2017). Moreover, recent homoploid hybridization was revealed using AFLPs in Andean Senecio L. (Asteraceae), coupled with many species with intermediate growth forms that may have hybrid ancestry (Dušková et al., 2017), suggesting a role for hybridization in phenotypic change during periods of habitat (and genetic) connectivity. However, again, a useful direction for future work would be to examine explicitly the impacts of hybridization, adaptive introgression and hybrid speciation in catalysing species-rich Andean plant radiations, as direct evidence of this is lacking.
Rosaceae are another important family of the Andean páramo, and in a similar fashion to genera of Asteraceae mentioned previously, their evolution may have been impacted by oscillations of habitat connectivity. One group which shows this is the tree genus Polylepis Ruiz & Pav., diversification history of which involved multiple reticulation events, as uncovered using AFLP and morphological data by Schmidt-Lebuhn, Kessler & Kumar (2006). Similarly, in Lachemilla (Focke) Rydb. (Morales-Briones, Liston & Tank, 2018), a phenotypically diverse genus of c. 60 species common in the páramo, hybridization is evident both deep in the phylogenetic tree and at the tips. In particular, Morales-Briones, Liston & Tank (2018) showed, using hundreds of genomic loci, that most phylogenetic incongruence and much of the morphological variation in Lachemilla was caused by hybridization at both shallow and deep temporal scales, with one clade being entirely of ancient hybrid origin. Again, this suggests repeated events of connectivity, divergence and gene flow over geological time, both recently and during earlier interglacial periods.
Evidence of range flux and hybridization is also found in other Andean plant families, although probably with subtler evolutionary impacts. For example, in the Andean radiation of Lupinus (Fabaceae), Nevado et al. (2018) used RADseq data and transcriptomic data from 6013 orthologous loci to provide indications of gene flow both deep in the phylogenetic tree and more recently, similar to the situation observed in Lachemilla. This gene flow was again suggested to be prompted by changes in habitat connectivity among Andean páramos, catalysed by climatic flux. However, in the Lupinus spp. studied, selection against hybrid offspring resulted in heterogeneous rates of introgression across the genome of the studied species. What is more, earlier work with less extensive genomic datasets (e.g. microsatellites and AFLPs) in herbaceous (e.g. Phaedranassa Herb.; Amaryllidaceae) and arborescent (Vasconcellea A.St.-Hil.; Caricaceae) lineages showed that although hybridization does occur, it is relatively restricted to narrow hybrid zones (Van Droogenbroeck et al., 2006; Oleas, Meerow & Francisco-Ortega, 2013).
Outside the páramos, in the southern Andes, García et al. (2017) used a large dataset of nuclear and organellar loci to uncover evidence of hybridization early in the diversification of Hippeastreae, a tribe of Amaryllidaceae comprising 180 species in 13 genera. However, they found ILS and a lack of reticulation events in some subtribes, again suggesting that hybridization may have not been as important for the diversification of this group when compared to those found closer to the Equator.
Inselbergs
Another major portion of Neotropical plant diversity is found on the inselbergs of eastern South America. Inselbergs are large rock outcrops, and many are home to a unique biota due to their inaccessibility and resulting isolation from surrounding environments (Porembski, 2007). Some of the plant groups that have been studied on inselbergs are endemic epiphytes, lithophytes and rupicolous herbs, such as bromeliads and orchids. In these groups, the outcomes of hybridization contrast starkly with those found in the high Andean flora, probably shaped by different responses to abiotic extremes between woody taxa and epiphytes/lithophytes between contrasting environments (Zotz & Hietz, 2001). However, the hybridization outcomes in many inselberg taxa were consistent with other members of the same family from different environments (e.g. Bromeliaceae), again highlighting the phylogenetic conservativeness of hybridization and its outcomes (Whitney et al., 2010).
The major outcome of hybridization in these inselbergs appears to be the maintenance of species boundaries despite frequent hybridization between sympatric epiphytes, lithophytes and rupicolous species. For example, in the species-rich genus Pitcairnia L’Hér. (Bromeliaceae; c. 400 species), barriers between species pairs are maintained through pre-zygotic isolation (e.g. through divergent phenology and floral morphology), despite much morphological evidence of hybridization (e.g. Wendt et al., 2001). In other species pairs in Pitcairnia, isolation is further imposed by selection against hybrid offspring, albeit with hybridization playing a moderate role in maintaining genetic diversity among isolated inselbergs (Mota et al., 2019). Moreover, Dobzhansky–Muller effects appear to contribute to isolation due to asymmetric introgression of some loci (Palma-Silva et al., 2011), with microsatellite data indicating a role for genetic incompatibilities in shaping species boundaries. In addition, in Pitcairnia spp. found in these heterogeneous, harsh inselberg landscapes, shifts from outcrossing to selfing through changes in herkogamy are well known. These shifts in mating system are probably an evolutionary response to reduce costly heterospecific pollination and to assure reproductive success on inselbergs (Palma-Silva et al., 2015).
A similar pattern of divergent selection and the maintenance of species boundaries was also recovered using microsatellites in Epidendrum spp. from the mountains of south-eastern Brazil. Pinheiro et al. (2015, 2016) uncovered a high degree of hybridization between two Epidendrum spp. despite their different ploidies. However, this hybridization is unlikely to persist because of cytonuclear incompatibilities and the formation of unbalanced gametes that prevent introgression and gene flow, resulting in low fertility of hybrid offspring and selection against hybrids, in stark contrast to Epidendrum spp. from other biomes (e.g. Vega et al., 2013; Marques et al., 2014).
Montane forests and woodlands
The montane forests of the tropical Americas are typified by consistent rainfall and their high elevation, often > 1800 m. Most studies examining hybridization in montane forests have focused along the volcanic ranges of Central America. Many of these studies examine hybridization dynamics in well-documented hybrid complexes found in Quercus L. (Fagaceae) woodlands, largely exploring how hybridization occurs along elevational and environmental clines using microsatellites, RAPD markers and morphological data (González-Rodríguez et al., 2004; Albarrán-Lara et al., 2010; Ramos-Ortiz et al., 2016). Quercus is also a well-studied genus in temperate woodlands due to its tendency to hybridize (Petit et al., 2004; Hipp et al., 2020).
In many Central American Quercus hybrid zones, there is broad morphological intergradation between hybridizing species pairs adapted to different environments along elevational gradients (González-Rodríguez et al., 2004). Hybrid individuals appear to persist mainly in intermediate habitats when compared to parental species, with selection favouring parental genotypes at either end of the hybrid cline (Albarrán-Lara et al., 2010). This is likely to be modulated by differential introgression across the genome resulting from selection against hybrids in different environments (Ramos-Ortiz et al., 2016; Pérez-Pedraza et al., 2021). In other Central American Quercus spp., evidence of older reticulation at deep phylogenetic scale has also been shown with RADseq data, albeit on a localized basis (Eaton et al., 2015), and that hybridization can alter expression of secondary metabolites and generate variation in these traits (Castillo-Mendoza et al., 2019).
Brahea Mart. (Arecaceae) from montane Mexican forests exhibit similar patterns of geographically structured admixture to other lineages such as Quercus (Ramírez-Rodríguez et al., 2011). Between two Brahea spp., Ramírez-Rodríguez et al. used nuclear loci, RAPD data and morphology to recover a mosaic of parental, transgressive and intermediate phenotypes and some asymmetric introgression between rarer and more common species. Comparably, in species of Cedrela P.Browne (Meliaceae) from the yungas forests of Bolivia and Argentina, AFLP data recovered a wide range of hybrid ancestries, suggesting persistent hybridization over generations in multiple hybrid zones (Zelener et al., 2016). Moreover, species of Pinus L. found in similar montane woodland communities show comparable levels of introgression when examined with microsatellite data (Delgado et al., 2007). However, in these Pinus spp. there is also evidence of novel hybrid lineages, again suggesting a role for hybridization as an evolutionary stimulus in arborescent taxa.
Among herbaceous plants found in montane tropical forests, there are even more significant evolutionary outcomes from hybridization. Recent transcriptomic work by Roberts & Roalson (2018) uncovered hybridization between ten of 24 species of Achimenes Pers. (Gesneriaceae), coupled with introgression among species pairs that share pollinators and produce viable hybrid offspring, suggesting that novel hybrid lineages may persist in these environments. This persistence of hybrid lineages was reflected in work on Mexican mistletoes (Psittacanthus Mart., Loranthaceae) using microsatellites. This work also suggested that the diversity of environments found in Central American mountain ranges may have facilitated colonization of adjacent habitats and even speciation via new hybrid lineages (Baena-Díaz, Ramírez-Barahona & Ornelas, 2018). Introgression between Psittacanthus lineages in pine forest and those in cloud forest precipitated the evolution of a third, hybrid lineage adapted to lowland, SDTFs. What is more, this novel hybrid lineage also showed a different host plant preference when compared to the parental species. This hybridization was again probably facilitated by Pleistocene climatic shifts, similar to the fluxes of habitat connectivity driving speciation in Andean taxa, which the authors found also influenced the population genetic and demographic history of the mistletoe species involved.
Similarly, in Solanum L. (Solanaceae) species from both subtropical forests of the yungas and the southern Andes, hybridization between parental species adapted to climatically contrasting environments (i.e. wet vs. dry) facilitated the formation of a novel lineage. AFLP data suggested that hybridization caused genome rearrangements and high levels of polymorphism in parental and hybrid species, allowing establishment of novel epigenetic patterns and facilitated adaptation to a novel environment in the descendent lineage (Cara, Marfil & Masuelli, 2013). Although the formation of novel hybrid lineages may also be evident in other herbaceous species found in montane Neotropical forests (e.g. in Guatemalan Cypripedium L.; Orchidaceae; Szlachetko et al., 2017), more ecological and genomic work must be undertaken to understand its evolutionary significance.
Savannas and grasslands
A large area of eastern South America is occupied by savanna vegetation, the most notable extensions of which are the cerrado of Brazil and the llanos of Venezuela, Colombia and Guyana (Pennington & Ratter, 2006). Savanna biomes are often maintained by fire, resulting in a landscape dominated by C4 grasses (Edwards et al., 2010; Lehmann et al., 2011) and relatively few trees. Savannas are relatively young biomes in the Neotropics, only forming in the last 10 Myr or so, and as a result many endemic species are the result of recent radiations (Pennington & Lavin, 2016). As such, because hybridization has been implicated in other recent plant radiations and due to the likelihood of minimal divergence among species in these savanna radiations, we suspect that hybridization may be relatively widespread in this biome, as suggested by the taxonomic complexity, morphological intermediacy and genetic evidence in groups such as Comanthera L.B.Sm. (Eriocaulaceae) (Ribeiro et al., 2018). However, to date, relatively few studies have examined the impacts of hybridization in this biome.
One of the dominant tree genera of the Brazilian cerrado is Kielmeyera Mart. (Calophyllaceae; c. 50 species,). Caddah et al. (2013) used microsatellites to examine hybridization between two co-occurring species with a complex taxonomic history probably resulting from hybridization. In these two species, they uncovered evidence of hybridization between species with low levels of divergence. Moreover, they showed that there was some degree of asymmetric introgression between species, probably mediated through selection favouring unidirectional backcrossing through epistatic interactions. Although allozymes and morphological work, e.g. on Chamaecrista Moench (Fabaceae), have provided some evidence of early-generation hybrids in other woody taxa (Conceiçao, De Queiroz & Borba, 2008), there is evidently still much work required to understand gene flow among woody taxa in the cerrado, given the paucity of studies with such a focus.
One of the few other studies of plant hybridization in the drylands of Brazil focuses on the well-studied orchid genus Epidendrum. Much like the patterns of hybridization uncovered in Epidendrum spp. from Brazilian inselbergs, Arida et al. (2021) used pollination experiments, fruit set and seed viability assays and cytogenetic methods to show that reproductive barriers between species were maintained by strong pre-zygotic barriers and hybrid sterility. Arida et al., examined a hybrid zone between two terrestrial Epidendrum spp., one of which occupies cerrado and coastal vegetation and the other is adapted to the much drier caatinga, and showed that isolation by environment (Wang & Bradburd, 2014) was an important pre-zygotic barrier. From this, they concluded that hybrid sterility probably prevented species collapse and was caused by differences in chromosome number, along with differential tolerances to flooding and shading.
In other lineages from the arid portions of Central and South America it appears that hybridization does not lead to negative evolutionary outcomes. For example, recent work on a species pair in Petunia Juss. (Solanaceae) from the grasslands of the pampas found moderate bidirectional gene exchange between ecologically divergent species using microsatellite loci, leading to introgression in sympatry and frequent backcrossing (Turchetto, Schnitzler & Freitas, 2019). This study builds on the previous finding of a novel hybrid lineage, coupled with an increase in floral polymorphism, resulting from hybridization among two other Petunia spp. growing on Brazilian rock outcrops (Turchetto et al., 2019). This suggests that in terrestrial species hybrid offspring are more likely to persist for multiple generations and undergo backcrossing, eventually making detectable contributions to the genetic composition of parental species, as also shown in terrestrial Epidendrum spp. (Sujii et al., 2019; Leal et al., 2020) and Pitcairnia spp. (Wendt et al., 2001) on inselbergs. Persistence of hybrid genotypes is also known in Central American Tithonia Desf. ex Juss. (Asteraceae), which undergoes bidirectional gene flow between co-occurring species, detected using RAPD markers. Moreover, in Tithonia, hybrid offspring have much higher genetic diversity than parental species which may allow them to colonize and dominate new environments, including those subject to anthropogenic disturbance (Tovar-Sánchez et al., 2012).
Deserts and tropical dry forests
Deserts, in contrast with savannas, cover a smaller area of the Neotropics and are not fire-prone. In the Neotropics, the most floristically significant desert regions include the Sonoran Desert of Mexico and the Atacama Desert of the Pacific coast of Peru. These coastal deserts include the lomas vegetation, a fog-watered, seasonal formation with many ephemeral endemic species (Rundel et al., 1991). Most studies of reticulate evolution in this biome have focused on Cactaceae, which are known to hybridize extensively (Machado, 2008).
Deserts are among the most challenging environments on Earth, and specialized adaptations are required to survive in such habitats, e.g. ephemerality (Mulroy & Rundel, 1977) and succulence (Griffiths & Males, 2017). Multiple studies exist that show hybridization occurs between desert plant species. In the restricted and ephemeral desert lomas vegetation of Peru, which are watered by seasonal fog from the Pacific Ocean, continued hybridization was shown in Palaua Cav. (Malvaceae), a genus of 15 species that are among the dominant shrubs in the lomas. Schneider et al. (2011) used two nuclear markers to show that there were multiple interspecific hybrids between subclades in Palaua along a narrow hybrid zone, which were persistent enough to result in phylogenetic incongruence. They inferred that this pattern was probably shaped by interannual fluctuation of humidity and fog in the lomas, resulting in contracted expansion of this vegetation type.
Similarly, in the large genus Opuntia Mill. (Cactaceae; c. 130 species), Granados Aguilar et al. (2020) found significant levels of hybridization between nine co-occurring lineages in the Tehuacán-Cuicatlán Valley of southern Mexico using two nuclear markers. This study also showed that multiple lineages in this region were probably the result of hybrid speciation, and that the high degree of morphological variability and phylogenetic incongruence in this group were probably caused by extensive hybridization.
However, a directly contrasting outcome of hybridization is evident in another cactus genus found in the rocky outcrops of the Morro do Chapéu in the Brazilian caatinga. Khan et al. (2020) used RADseq data to show that, despite extensive hybridization between five species of Melocactus Link & Otto in a hybrid zone, there were extremely low levels of introgression coupled with strong selection against hybrids, building on previous morphological and allozyme evidence of hybridization in other Melocactus spp. (Lambert et al., 2006). Indeed, the authors indicated that selection was favouring pure parental genotypes along genetic clines, suggesting that the region of Brazil in which these species come into contact represents a tension hybrid zone, where dispersal and recurrent hybridization into the zone is constrained by selection against hybrids.
The SDTF of the tropical Americas are typified by seasonal rainfall, an absence of fire and many narrowly endemic woody taxa, which are often deciduous (Pennington & Ratter, 2006). Plant species from SDTF are characterized by their evolutionary uniqueness, their small population sizes and their strong reproductive isolation from other species (Pennington & Lavin, 2016), but despite these reproductive barriers, hybridization in certain genera is evident. One genus in which hybridization is fairly well known in SDTF species is Leucaena Benth. (Fabaceae). Leucaena also includes multiple allopolyploid species resulting from hybridization events, as evidenced by cytogenetic work and the taxonomic complexity of the genus (Cardoso, Schifino-Wittmann & Bodanese-Zanettini, 2000). Building on this work, Govindarajulu et al. (2011) used two nuclear and four plastid Sanger-sequenced loci to infer gene trees for Leucaena spp., and by comparing topologies between nuclear and plastid loci they were able to infer potential parental species for the four allopolyploid species in their study. Their results indicated that these novel tetraploid lineages were the result of hybridization through artificial sympatry, incurred by the ancient cultivation of Leucaena by humans.
Mangroves and wetlands
Mangrove ecosystems are found on nearly every tropical coastline and are one of the most ecologically important environments for marine biodiversity, as well as protecting coastlines from erosion in the face of sea level rise (Tomlinson, 2016). In the Americas, mangroves are found on the Pacific coast of South and Central America, extending from Mexico to Peru and on its Atlantic coast from Brazil to Venezuela. In addition, mangrove habitats fringe the coasts of the Caribbean Sea and its many islands and parts of the southern USA (Giri et al., 2011).
Mangrove tree species belong to multiple families that convergently adapted to coastal environments (Tomlinson, 2016). It has been well documented that mangroves disperse over extremely long distances since their viviparous propagules are carried by ocean currents and have the ability to initiate growth as soon as they make landfall (Rabinowitz, 1978). Accordingly, there is ample gene flow between mangroves, with this gene flow being largely structured by ocean currents (Triest, 2008). This dispersal may also bring related species into contact, providing a chance for hybridization to occur.
In Neotropical mangrove species there is evidence of persistent and evolutionarily significant hybridization. In Avicennia L. (Acanthaceae), Mori, Zucchi & Sousa (2015) indicated that there was sustained hybridization between multiple species, their microsatellite data revealing the existence of fertile hybrids between three co-occurring Avicennia spp. with overlapping phenology. Given the ancient introgression in the ITS locus also observed in Central American Avicennia (Nettel et al., 2008), the authors suggested that interactions between hybridization and extrinsic factors (such as salinity and flooding tolerance) probably modulate diversification through passage of variants conferring adaptation to such extreme environments, as suggested in other species (e.g. Epidendrum: Pinheiro et al., 2010; Sujii et al., 2019; Leal et al., 2020).
In another microsatellite study of a distantly related group of mangroves (Rhizophora Pers., Rhizophoraceae), Cerón-Souza et al. (2010) recovered a similar reticulate evolutionary pattern resulting from hybridization. In three Neotropical Rhizophora spp., the authors found evidence of persistent introgressive hybridization deep in the phylogenetic tree, coupled with geographical clustering shaped by ocean currents. In addition, similar to multiple other woody and herbaceous lineages discussed before, Cerón-Souza et al. (2010) showed that species relationships clustered by geography rather than morphology, as would be expected if genetic variants were being passed between sympatric, outcrossing species (as in Schley et al., 2020). This was corroborated by work on Rhizophora from the Brazilian coast, in which microsatellite data revealed that backcrossing was common, probably through the formation of fertile hybrids, and that asymmetric introgression was common (Francisco et al., 2018).
Finally, in a fashion similar to mangroves, Neotropical wetlands facilitate long-distance dispersal of seeds and propagules, e.g. through hydrochory (Fernández, Schneider & Zilli, 2019) or ichthyochory (Correa et al., 2016). Some of the most notable wetlands of the Neotropics include the Pantanal of Brazil and the coastal marshes of Central America. Studies of hybridization in these environments are, however, scarce, and focus on Central American marshes. One study that examined genetic and morphometric patterns of reticulation in species of Eleocharis R.Br. (Cyperaceae) showed that three Eleocharis spp. were undergoing persistent hybridization in Belizean marshes (Košnar et al., 2010). Despite their possible affinities to mangrove environments, however, this study did not find evidence of introgression hybridization or of any major evolutionary outcome from this reticulation. This highlights the need for studies of hybridization in Neotropical wetlands such as the Pantanal, which is among the largest and most species-rich wetlands on Earth (Junk et al., 2006).
HYBRIDIZATION: CREATOR OR DESTROYER OF NEOTROPICAL PLANT DIVERSITY?
Almost the entire spectrum of evolutionary impacts deriving from hybridization are evident in the Neotropical flora, as evidenced by DNA sequence data in the recent empirical studies that we have reviewed. Finding the effects of hybridization in so many studies is perhaps surprising since in taxonomic monographs and floras for plant groups, at least those found in lowland biomes (e.g. rainforest, savanna and dry forest), few researchers discuss hybrids or invoke hybridization. This lack of emphasis on tropical hybridization contrasts with temperate floras where hybrids are frequently noted and discussed, with even a ‘Hybrid Flora’ published for the British Isles (Stace, Preston & Pearman, 2015). It might reflect that tropical taxonomists have been influenced by the paradigm that hybridization is rare in the Tropics (Ashton, 1969; Ehrendorfer, 1970; Gentry, 1982: see discussion before); one does not find what one is not looking for. Alternatively, it could be a reflection of the fact that in tropical plants, species boundaries are often not well-defined, or that there is lower recorder effort in the Tropics where specialist, detailed, local knowledge of floras is often lacking. Conceivably, lower detection of hybridization in the Tropics based on morphological patterns might reflect mechanisms now better understood through genetic data, which invoke interspecific gene flow but that do not result in phenotypically ‘intermediate’ individuals easily identifiable as hybrids, e.g. the ‘islands of divergence’ model (Turner et al., 2005).
More empirical studies using genetic data such as those that we have reviewed here will be needed to understand if hybridization is less frequent in tropical floras and reasons for this. However, our review finds effects of hybridization in the Neotropical flora ranging from generation of phenotypic variation and broadened ecological amplitude, which may influence the evolution of novel lineages (Marques et al., 2014) through to the reinforcement of species boundaries (Kay, 2006; Mota et al., 2019) and erosion of divergence between species (Vega et al., 2013). Across this range of outcomes, certain patterns emerge in groups of taxa in certain environments, and these are discussed next.
Hybridization in species-rich montane radiations
One emergent theme among studies of Neotropical hybridization is the impact of deep, phylogenetic reticulation and more recent hybridization on species-rich Andean plant radiations. Such radiations are typified by their relatively low degree of genetic divergence, different niche preferences and propensity for hybridization in secondary contact (Ackermann, Achatz & Weigend, 2008; Nevado et al., 2018; Morales-Briones et al., 2018). This secondary contact is likely to occur in hybrid zones located along elevational gradients among starkly different plant communities which are structured by differences in temperature, rainfall and geology, as found on mountains elsewhere (Abbott & Brennan, 2014). This meeting of different environments along mountainsides may allow ‘bridges’ of gene flow between species within hybrid zones along adjacent species ranges (e.g. in Senecio on Mt. Etna; Filatov, Osborne & Papadopulos, 2016). In the tropical Andes the shifting of these bridges and the connection of high-elevation species isolated on adjacent mountains is probably mediated by the ‘flickering connectivity’ of montane habitats driven by climatic flux (Flantua et al., 2019).
Moreover, due to recombination and transgressive effects, hybrid zones often contain individuals with a broader range of phenotypic variation than that found in the parental species (Rieseberg & Willis, 2007). This may then allow colonization and diversification into novel niches along elevation gradients, probably contributing to rapid diversification, as found in multiple Andean groups, e.g. Espeletiinae (Pouchon et al., 2018), Diplostephium (Vargas et al., 2017), Polylepis (Schmidt-Lebuhn et al., 2006) and Lachemilla (Morales-Briones et al., 2018). In many ways, these types of rapid radiation are analogous to those found on oceanic islands, e.g. in Hawaiian silverswords (Witter & Carr, 1988; Baldwin & Sanderson, 1998) and Dubautia Gaudich. (Caraway, Carr & Morden, 2001; Remington & Robichaux, 2007) given the range of environments present and the potential to escape competition and evolve towards new fitness peaks. Dispersal and adaptation to novel environments in the absence of parent species may also render homoploid hybrid speciation more likely, without the necessity for instantaneous reproductive isolation as in polyploid hybrid speciation (Coyne & Orr, 2004; Gross & Rieseberg, 2005), and this may be a fruitful direction for future research.
Hybridization in the neotropics vs. other tropical regions
Hybridization appears to also be relatively understudied in other tropical regions, with this paucity of work being highlighted by Parnell et al. (2013) for the flora of Thailand. Parnell et al. mentioned that it is more likely that the lack of hybrid taxa evident in tropical floras is due to the fact they are understudied and to the difficulty of identifying hybrids using morphology alone. Similarly, in their review of studies focusing on plant hybrid zones, Abbott (2017) highlighted the relative lack of hybrid zone studies from Africa and South America, and implicated differences in scientific resources, rather than a lower frequency of hybrid zones, in causing this difference.
Studies of hybridization in other tropical regions draw striking parallels with work on Neotropical species, first and foremost in the patterns of incongruence between gene trees in several genera from tropical Asia. The patterns of incongruence evident in these taxa reflect the hybridization at deep phylogenetic levels uncovered in Neotropical plants, e.g. Lachemilla (Morales-Briones et al., 2018). A notable example of this is in Annonaceae in West Malesia, in which Guo, Thomas & Saunders (2018) uncovered intergeneric hybridization between Dasymaschalon (Hook.f. & Thomson) Dalla Torre & Harms and Friesodielsia Steenis at deep phylogenetic timescales. Similarly, in the pitcher plant genus Nepenthes L. genome skimming data revealed extensive plastid-nuclear incongruence (Nauheimer et al., 2019), an unsurprising result since Nepenthes is known to hybridize extensively in cultivation. Moreover, in a similar fashion to patterns observed in Brownea (Schley et al., 2020), Boom et al (2020) showed that plastome lineages of Brachystegia Benth. (Fabaceae), a key component of Miombo woodland in southern Africa, clustered by geography rather than taxonomy, for which they implicated hybridization between closely related species as the distribution of Miombo woodlands shifted over the course of the Neogene.
More recent hybridization among taxa from Miombo woodlands has also been shown using population genomic methods. For example, in C4 and non-C4 forms of the grass Alloteropsis semialata (R.Br.) Hitchc., hybridization has been shown to occur, but hybrids are usually selected against (as in Melocactus; Khan et al., 2020). It is likely that this barrier, coupled with different ploidies among forms, has allowed co-existence and maintained reproductive boundaries after the forms came into secondary contact following re-expansion of Miombo woodlands (Olofsson et al., 2021).
More generally, the flux of biomes in tropical Africa has probably also resulted in dispersal and invasion of novel regions in rainforest taxa, usually resulting in interspecific gene flow and asymmetric introgression by the invader, e.g. in Haumania J.Léonard (Marantaceae) (Ley & Hardy, 2017) and Milicia Sim (Moracaeae) (Daïnou et al., 2017). Moreover, Duminil et al. (2012) used microsatellites to reveal frequent hybridization explaining the morphological variability in species complexes of African Carapa, resulting in similar taxonomic confusion as that shown in Brazilian Kielmeyera. Much like in studies of Neotropical Carapa (Scotti-Saintagne et al., 2013b), the dipterocarp genus Shorea Roxb. ex C.F.Gaertn. in Southeast Asia also undergoes frequent hybridization between four species, facilitated by overlapping flowering periods and persistence of hybrid seedlings (Kamiya et al., 2011; Kenzo et al., 2019). Indeed, this persistence of hybrid genotypes in Asian dipterocarps has led to the discussion of ‘genomic mutualists’ existing in ‘syngameons’ of interfertile species. This theory posits that hybridization between closely related tropical tree species may furnish parental populations with novel genetic variation at minimal cost to fitness. This improved genetic diversity can help alleviate Allee effects and inbreeding depression in tropical tree species, which tend to exist at low population densities (Cannon & Lerdau, 2015). Such an effect has also been observed in temperate tree species (Cronk & Suarez-Gonzalez, 2018).
The similarities in hybridization outcomes across tropical regions are particularly evident in convergently adapted lineages. Just as in the Neotropical species of the mangrove genera Avicennia and Rhizophora, population-level studies of Rhizophora mangroves in Asia and the Pacific (Ng & Szmidt, 2015) uncovered persistent hybrids in mangrove populations. Similarly, Lo (2010) showed that hybridization was common among Rhizophora spp., with the potential for nearly all species to act as maternal parents. However, the work of Lo (2010) also suggested that later-generation hybrids were absent from all sites, and that little dispersal of hybrid individuals occurred beyond hybrid zones, contrasting with dispersal patterns in Neotropical mangroves (Cerón-Souza et al., 2010; Mori et al., 2015). Given these similarities, in addition to their caveats, further exploration of hybridization in tropical floras may allow general themes and processes underlying speciation to be uncovered in diverse tropical systems with the advent of new data and analytical approaches.
FUTURE DIRECTIONS
Without a doubt, the study of hybridization in many Neotropical taxa has been made more accessible with the advent of next-generation sequencing technologies (Twyford & Ennos, 2012). In addition to the ever-decreasing cost of these technologies, the wealth of new resources that have become available for studying species-rich Neotropical plant groups are further facilitating hybridization research. For example, the availability of taxon-specific sets of nuclear loci for targeted enrichment and sequencing for specific plant groups, e.g. Costus (Vargas et al., 2020) and Inga (Nicholls et al., 2015), and those designed for use across large plant clades, e.g. for all angiosperms (Johnson et al., 2019), can yield hundreds of informative loci at relatively low cost. Moreover, this targeted enrichment method allows the use of even historical plant material from herbaria and other sources.
Similarly, the advent and reduced cost of transcriptome sequencing and whole-genome sequencing has allowed the databasing of reference transcriptomes, as well as reference genomes, from many thousands of species, e.g. the ENSEMBL database (https://www.ensembl.org/index.html) and the NCBI Genome database (https://www.ncbi.nlm.nih.gov/genome). Although this can make in-depth population genomic and functional genomic studies possible for species that have reference genomes, for Neotropical plants the majority of reference genomes are of economically important species such as potato (Potato Genome Sequencing Consortium, 2011), papaya (Ming et al., 2008) and cacao (Argout et al., 2011), and do not yet represent the diversity of this region. However, major genome sequencing projects, including the Earth Biogenome Project (Lewin et al., 2018) and the 10 000 Plant Genome Project (Cheng et al., 2018), hope to fill this major gap. The drive towards high-quality reference genomes (Twyford, 2018), initially for all plant families, and eventually for all genera and species, will facilitate evolutionary and ecological genomic studies of diverse hybridizing Neotropical plant taxa.
Given these advances, work on taxa that are endemic to remote tropical regions or which are rare in their native ranges has been made much simpler by the potential to leverage large natural history collections using techniques such as targeted enrichment (Albert et al., 2007; Gnirke et al., 2009). In addition, the expansion by many orders of magnitude in the amount of data that can be generated from a single specimen has provided much finer resolution on hybridization processes across the genome, allowing inference of selection, demographic history and admixture patterns across timescales (e.g. Filatov et al., 2016; Nevado et al., 2018; Nevado et al., 2020). Thus, with such an expansion of methodologies available to infer introgression and reticulation, e.g. PhyloNetworks (Solís-Lemus et al., 2017) Dsuite (Malinsky, Matschiner & Svardal, 2021) and Twisst (Martin & Van Belleghem, 2017), it is useful to consider potential new research directions to be followed in the Neotropics, where the flora is relatively understudied considering its staggering diversity.
To begin with, both existing datasets and those produced by future studies, even by work not focusing on hybridization, should be actively interrogated for any signatures of reticulation using the analytical methods outlined previously. Such an approach has been taken in recent phylogenetic work on the diverse bromeliad genus Vriesea using genome skimming data, revealing hybrid ancestry in c. 11% of species and discordance among nuclear, plastid and mitochondrial genomes (Loiseau et al., 2021). Indeed, more generally, inferring phylogenetic networks rather than phylogenetic trees as standard practice may be a more accurate way of representing the often-reticulate relationships between species in diverse groups of organisms (Solís-Lemus, Yang & Ané, 2016).
Another useful direction to be taken with these novel analytical methods could be to leverage existing sampling of plant diversity to examine hybridization in understudied Neotropical biomes. The varying numbers of studies in different biomes covered in this review highlight that our knowledge of hybridization in Neotropical plants is sparse. Given the high degree of endemism, the expanse and ecological importance of many lesser-known Neotropical biomes, it would be particularly useful to explore hybridization between taxa found in such regions. As an example, of all studies summarized in this review, only one dealt with tree species in the extensive Brazilian cerrado. Moreover, no study examined any species from the Pantanal, one of the most species-rich wetlands. In a similar vein, in this review we focused on seed plant species due to the relative paucity of studies on other groups of plants, including ferns and bryophytes. This further underlines the future use of genomic methods to investigate hybridization in more cryptic or difficult-to-study plant groups with complex, reticulate evolutionary histories, as is typical of ferns (Barrington, Haufler & Werth, 1989) and bryophytes (Natcheva & Cronberg, 2004).
As genomic tools shed light on the evolutionary significance of hybridization across an ever-increasing range of taxa, it will become possible to look for underlying evolutionary patterns of hybridization and to understand its role in generating tropical diversity. The principal mechanisms driving hybridization at the regional level have already begun to be investigated in some regional and insular floras, many of which have detailed (or, in the case of the British flora, near-complete) knowledge of hybrids (Mitchell et al., 2019). Better data on hybridization in the Tropics would allow comparative analyses of hybridization patterns across species-rich tropical floras and less diverse temperate floras, helping to understand the underpinnings of the latitudinal biodiversity gradient. More generally, this would allow the exploration of major questions for the evolution of plant diversity as a whole, such as whether polyploid hybridization and hybrid speciation are uncommon or just an overlooked and important process for the origin of species.
SUPPORTING INFORMATION
Additional Supporting Information may be found in the online version of this article at the publisher's web-site:
Supplementary Information 1. Table of all studies of hybridization in Neotropical plants summarized in this review. Columns of the table represent the biome in which the study was carried out, growth form and taxonomic classification of the study group, the type of data used in the study, the total number of species in the group, the number of species examined in the study, the major evolutionary outcomes of hybridization and the citation of the study. In addition, the number of studies are plotted as bar plots by biome, growth form, plant family and genus.
ACKNOWLEDGEMENTS
Thanks to Meng Lu for her helpful comments on the manuscript and chats about hybridization. We thank four anonymous reviewers for detailed and constructive input that greatly improved this paper, and financial support from NERC grant NE/V012258/1.
REFERENCES



