-
PDF
- Split View
-
Views
-
Cite
Cite
Arnaud Pasquer, Quentin Cordier, Jean-Christophe Lifante, Gilles Poncet, Stéphanie Polazzi, Antoine Duclos, on behalf of the TopSurgeons Study Group, Influence of a surgeon’s exposure to operating room turnover delays on patient outcomes, BJS Open, Volume 8, Issue 5, October 2024, zrae117, https://doi.org/10.1093/bjsopen/zrae117
Close - Share Icon Share
Abstract
A surgeon’s daily performance may be affected by operating room organizational factors, potentially impacting patient outcomes. The aim of this study was to investigate the link between a surgeon’s exposure to delays in starting scheduled operations and patient outcomes.
A prospective observational study was conducted from 1 November 2020 to 31 December 2021, across 14 surgical departments in four university hospitals, covering various surgical disciplines. All elective surgeries by 45 attending surgeons were analysed, assessing delays in starting operations and inter-procedural wait times exceeding 1 or 2 h. The primary outcome was major adverse events within 30 days post-surgery. Mixed-effect logistic regression accounted for operation clustering within surgeons, estimating adjusted relative risks and outcome rate differences using marginal standardization.
Among 8844 elective operations, 4.0% started more than 1 h late, associated with an increased rate of adverse events (21.6% versus 14.4%, P = 0.039). Waiting time surpassing 1 h between procedures occurred in 71.4% of operations and was also associated with a higher frequency of adverse events (13.9% versus 5.3%, P < 0.001). After adjustment, delayed operations were associated with an elevated risk of major adverse events (adjusted relative risk 1.37 (95% c.i. 1.06 to 1.85)). The standardized rate of major adverse events was 12.1%, compared with 8.9% (absolute difference of 3.3% (95% c.i. 0.6% to 5.6%)), when a surgeon experienced a delay in operating room scheduling or waiting time between two procedures exceeding 1 h, as opposed to not experiencing such delays.
A surgeon’s exposure to delay before starting elective procedures was associated with an increased occurrence of major adverse events. Optimizing operating room turnover to prevent delayed operations and waiting time is critical for patient safety.
Introduction
The success of surgical procedures is contingent upon the high-risk environment of the operating room where these procedures take place1,2. The organization of the operating room potentially impacts a surgeon’s performance, but there is a lack of prospective research to determine whether the daily flow of operations significantly influences patient outcomes3. Patient turnover in the operating room is crucial in a surgeon’s routine. It mirrors the alignment of the operating flow with scheduled cases and the time between the previous patient’s closure and the next patient’s incision. Efficient turnover requires completing tasks without delay, such as transitioning the previous patient to the post-anaesthesia care unit, preparing the operating room for the next patient, and ensuring timely entrance for the next patient. This is more controllable for elective surgeries than emergency procedures and can be enhanced by reducing bio-cleaning duration between two procedures, anticipating the next patient’s call and transport4.
A fast day in the operating room, with reduced turnover time, may boost provider satisfaction and minimize intraoperative risk5. In contrast, impatient surgeons facing inefficient turnover and extended waiting times may experience irritation and stress, affecting their performance and potentially resulting in unintended harm to the next patient. The hypothesis was that smooth execution of elective surgeries as per schedule is important for safe surgical practice. In this multicentre study, the aim was to investigate the relationship between a surgeon’s exposure to excessive delays in starting scheduled operations and patient outcomes.
Methods
Study population and exposure
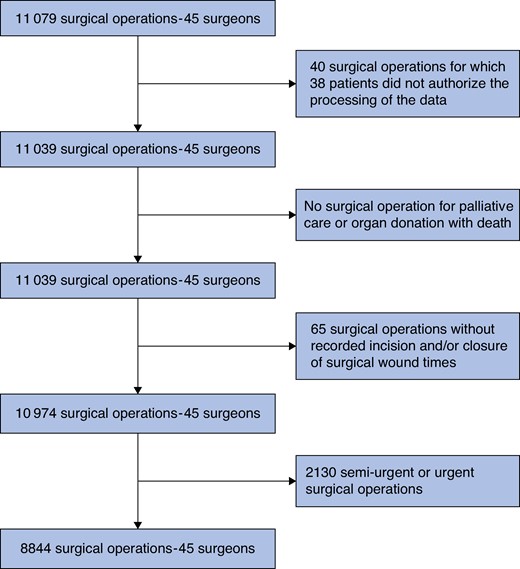
A prospective observational study was conducted across 14 surgical departments in four university hospitals situated in the Metropolis of Lyon in France, specializing in digestive, orthopaedic, gynaecological, urological, cardiac, thoracic, and endocrine surgery. A cohort of 45 attending surgeons, each performing a minimum of 50 procedures per year, was formed. All elective surgical procedures they performed between 1 November 2020 and 31 December 2021 were considered in the final analysis. Operations of patients younger than 18 years old or who had refused to share their personal data were excluded. Urgent and semi-urgent operations, as well as operations for palliative care or organ donation, and with missing operative timestamping, were not analysed.
The association between a surgeon’s exposure to a delay in the operating room turnover and patient outcomes was studied. This delay was categorized as either exceeding 1 h or 2 h and assessed through two complementary metrics. First, a surgeon’s exposure to the delay of a scheduled operation was measured by comparing the planned and actual start time of the operation. The planned start time could have been updated until the previous patient departed from the operating room. Second, a surgeon’s exposure to waiting time between two operations was determined by measuring the duration between the incision for the current operation and the closure time of the previous operation. Waiting time was not considered for the first surgical procedure of the day.
According to European General Data Protection Regulation No. 2016/679, this observational study was based on anonymous data and approved by the French Committee of Expertise in Research, Studies, and Evaluations in the Health Domain (TPS_970330 CEREES), the French National Data Protection Authority (DR-2020-055 CNIL), and the European Research Council Executive Agency (801660 ERCEA). Additionally, this study was deemed exempt from formal oversight by the Mass General Brigham Institutional Review Board (Protocol 2023P002266). Patients were informed that their health data might be reused for research purposes and that they had the opportunity to opt out. Each surgeon in the cohort provided informed consent to participate in the research and for the use of their data.
Data sources and outcomes
For each operation, data were prospectively collected from a homogeneous information system across the Lyon university hospitals, encompassing details regarding the operation, patient, surgeon, and operating room involved. Specifically, data were gathered on the patients’ socio-demographic characteristics (age, sex, social coverage, and median income), care provided during hospital stays, including details about the type of surgery performed (organ, surgical approach, and complexity), and the primary diagnosis linked to the operative indication.
This information was supplemented with data collected by clinical research assistants from the patients’ electronic health records to acquire details such as the scheduling of the operation (elective, semi-urgent, or urgent), occurrences of intraoperative and postoperative adverse events, and the preoperative co-morbidities of the patient (critical condition, current pregnancy, obesity (body mass index (BMI) greater than or equal to 30 kg/m²), malnourishment, tobacco/alcohol or other drug addictions, open wound, surgical-site infection, sepsis, endocarditis, cancer, neoadjuvant treatment, immune deficiency, coagulopathy, anticoagulant or antiplatelet treatment, blood transfusion, coma, limb paralysis, other neurological disorder, confusion, dementia, depression, cardiovascular disease, neurovascular disease, peripheral arterial disease, cardiac arrhythmia, chronic heart failure, hypertension, diabetes, dyslipidaemia, pulmonary artery systolic pressure (greater than 60 mmHg), chronic renal failure, acute renal failure, chronic respiratory failure, chronic obstructive pulmonary disease, liver disease, rheumatic pathology, and hypoparathyroidism). Data were also extracted from the anaesthesia records to identify the type of anaesthesia administered and the patients’ American Society of Anesthesiologists (ASA) physical status classification.
Additionally, for each operation, data were used from the operating room management software and the surgical procedure report to determine daily patient turnover. This included the timings of the patient’s entry into the operating room, the incision and closure of the wound, and the primary operating surgeon involved. Analysing the last planned and actual operation timings enabled any scheduling delays and prolonged preoperative waiting times to be identified. Lastly, human resources data were gathered to determine the age and professional status of the operating surgeon. The duration of a typical day in the operating room was 10 h. Operating times were scheduled through dedicated operating room management software, which automatically input the mean operating time for each individual surgeon according to one procedure. The mean was automatically updated after each similar procedure performed by the same surgeon.
The primary outcome was a composite measure encompassing major adverse events during surgery or within the 30-day post-surgery interval. These events included intraoperative or postoperative mortality, extended stay after surgery in the intensive care unit (ICU) for at least two nights or in the intermediate care unit (IMCU) for at least five nights due to organ failure, unplanned reoperation for complications arising from the initial surgery, and severe intraoperative or postoperative complications categorized as detailed in Appendix S1 across various domains: general, infectious, haemorrhagic, parietal, cardiopulmonary, neurological, abdominopelvic, orthopaedic, cervical, and functional. The composite was inspired from the Clavien–Dindo classification, which considers surgical major adverse events in an objective and reproducible manner6. For secondary outcomes, each event was considered separately.
Statistical analysis
Patient characteristics are presented as mean(s.d.) for continuous variables and as n (%) for qualitative variables. Surgical outcomes were compared across each exposure metric using the Rao–Scott chi-squared test, which considered the clustering of operations within surgeons. Mixed logistic regression models with a maximum likelihood estimation based on the Laplace approximation were then employed to assess the association between each exposure metric and surgical outcomes. The operating surgeon’s identity was included in the models as a random effect, while controlling for the surgeon’s age (older than 45 years versus 45 years or younger), the surgeon’s professional status (faculty position as associate/full-time professor versus non-professor), and the patient’s preoperative risk score (log-transformed) as fixed effects.
Preoperative risk scores were created using outcome prediction models developed from an independent data set of patients operated on by the same cohort of surgeons between 1 January 2022 and 31 October 2022 (see Appendix S1 for details). To construct these specific models for each surgical specialty and outcome, stepwise logistic regressions were employed and considered: patient age, sex, socio-economic status, ASA grade, and co-morbidities; surgical specialty, indication, and approach; procedure complexity and scheduling; and the type of anaesthesia.
As ORs tend to provide a biased interpretation of risks for frequent outcome rates (greater than or equal to 10%), the adjusted relative risks (aRRs) of major adverse events associated with the delay in starting a scheduled operation and the waiting time between operations were estimated. aRRs were derived from marginal probabilities of events for exposed (pT = 1) and non-exposed (pT = 0) subjects, and calculated as relative risk = pT = 1/pT = 07. Corresponding 95% confidence intervals were estimated using non-parametric bootstraps based on 1000 replications, picking as endpoints the 2.5th and the 97.5th percentiles. Using the same marginal standardization method, standardized outcome rates and their absolute changes were determined for each exposure group to mitigate case-mix differences among these groups7,8. All reported P values are two-sided and P < 0.050 was considered statistically significant. Data manipulation and analyses were performed using SAS software (SAS Institute Inc., Cary, NC, USA; version 9.4).
Results
The study population included 8844 operations (Fig. 1), involving orthopaedic (27.1%), gynaecological (22.3%), digestive (20.9%), urological (9.2%), endocrine (8.5%), cardiac (7.8%), and thoracic (4.3%) procedures (Table 1). Operations were primarily performed under general anaesthesia (77.3%) and with an open approach (60.0%). Patients had a mean of 2.3 co-morbidities and 22.7% had an ASA grade of III or higher. Compared with the scheduled time, unexpected delays in patient turnover exceeded 1 h for 352 operations (4.0%) and 2 h for 154 (1.8%) operations. Waiting times between surgical cases exceeding 1 and 2 h affected 2955 operations (71.4%) and 654 operations (15.8%) respectively. Major adverse events occurred in 1319 operations (14.9%), categorized as follows: 1228 intraoperative or postoperative complications (13.9%), 392 unplanned reoperations (4.4%), 188 extended ICU/IMCU stays (2.1%), and 49 patient deaths (0.6%).
| Characteristic . | Value . |
|---|---|
| Male | 3731 (42.2) |
| Age (years), mean(s.d.) | 56.5(16.9) |
| Surgical specialty | |
| Cardiac | 691 (7.8) |
| Endocrine | 751 (8.5) |
| Digestive | 1846 (20.9) |
| Gynaecological | 1971 (22.3) |
| Orthopaedic | 2396 (27.1) |
| Thoracic | 377 (4.3) |
| Urological | 812 (9.2) |
| Principal anaesthesia technique (missing n = 6) | |
| General | 6827 (77.2) |
| Regional | 1824 (20.6) |
| Local | 187 (2.1) |
| Initial surgical approach (missing n = 49) | |
| Open | 5280 (60.0) |
| Robotic | 489 (5.6) |
| Videoscopic | 2111 (24.0) |
| Endoscopic | 915 (10.4) |
| ASA grade | |
| I | 2488 (28.1) |
| II | 4349 (49.2) |
| III | 1949 (22.0) |
| IV | 56 (0.6) |
| V | 2 (0.0) |
| Median income in the city of residence in €*(x1000) (missing n = 24), mean(s.d.) | 23.1(3.6) |
| Precarity | 706 (8.0) |
| Inpatient surgery (missing n = 1) | 6499 (73.5) |
| Co-morbidities†, mean(s.d.) | 2.3(2.0) |
| HFRS frailty score of the patient during index stay, mean(s.d.) | 0.6(1.7) |
| Age of the attending surgeon at the time of the operation >45 years | 3797 (42.9) |
| Faculty position of the attending surgeon | 5326 (60.2) |
| Delayed operation‡ >1 h (missing n = 86) | 352 (4.0) |
| Delayed operation‡ >2 h (missing n = 86) | 154 (1.8) |
| Waiting time§ >1 h (not applicable n = 4705) | 2955 (71.4) |
| Waiting time§ >2 h (not applicable n = 4705) | 654 (15.8) |
| At least one among delayed operation‡ >1 h and waiting time§ >1 h (not applicable n = 4554) | 3129 (72.9) |
| At least one among delayed operation‡ >2 h and waiting time§ >2 h (not applicable n = 4626) | 751 (17.8) |
| Characteristic . | Value . |
|---|---|
| Male | 3731 (42.2) |
| Age (years), mean(s.d.) | 56.5(16.9) |
| Surgical specialty | |
| Cardiac | 691 (7.8) |
| Endocrine | 751 (8.5) |
| Digestive | 1846 (20.9) |
| Gynaecological | 1971 (22.3) |
| Orthopaedic | 2396 (27.1) |
| Thoracic | 377 (4.3) |
| Urological | 812 (9.2) |
| Principal anaesthesia technique (missing n = 6) | |
| General | 6827 (77.2) |
| Regional | 1824 (20.6) |
| Local | 187 (2.1) |
| Initial surgical approach (missing n = 49) | |
| Open | 5280 (60.0) |
| Robotic | 489 (5.6) |
| Videoscopic | 2111 (24.0) |
| Endoscopic | 915 (10.4) |
| ASA grade | |
| I | 2488 (28.1) |
| II | 4349 (49.2) |
| III | 1949 (22.0) |
| IV | 56 (0.6) |
| V | 2 (0.0) |
| Median income in the city of residence in €*(x1000) (missing n = 24), mean(s.d.) | 23.1(3.6) |
| Precarity | 706 (8.0) |
| Inpatient surgery (missing n = 1) | 6499 (73.5) |
| Co-morbidities†, mean(s.d.) | 2.3(2.0) |
| HFRS frailty score of the patient during index stay, mean(s.d.) | 0.6(1.7) |
| Age of the attending surgeon at the time of the operation >45 years | 3797 (42.9) |
| Faculty position of the attending surgeon | 5326 (60.2) |
| Delayed operation‡ >1 h (missing n = 86) | 352 (4.0) |
| Delayed operation‡ >2 h (missing n = 86) | 154 (1.8) |
| Waiting time§ >1 h (not applicable n = 4705) | 2955 (71.4) |
| Waiting time§ >2 h (not applicable n = 4705) | 654 (15.8) |
| At least one among delayed operation‡ >1 h and waiting time§ >1 h (not applicable n = 4554) | 3129 (72.9) |
| At least one among delayed operation‡ >2 h and waiting time§ >2 h (not applicable n = 4626) | 751 (17.8) |
Values are n (%) unless otherwise indicated. Percentages might not add up to 100 because of rounding. *€1.00 equivalent to £0.87 (US$1.07) as of November 2023. †To take into account the preoperative risk of each operation, a risk score was constructed based on all the variables presented in this table, as well as the following co-morbidities: current pregnancy, obesity (BMI greater than or equal to 30 kg/m²), malnourishment, tobacco addiction, alcohol addiction, other addiction, open wound, surgical-site infection, sepsis, endocarditis, cancer, neoadjuvant treatment, immune deficiency, coagulopathy, anticoagulant treatment, antiaggregation treatment, blood transfusion, coma, limb paralysis, other neurological disorder, confusion, dementia, depression, cardiovascular disease, neurovascular disease, peripheral arterial disease, cardiac arrhythmia, chronic heart failure, hypertension, diabetes, dyslipidaemia, pulmonary artery systolic pressure (greater than 60 mmHg), chronic renal failure, acute renal failure, chronic respiratory failure, chronic obstructive pulmonary disease, liver disease, rheumatic pathology, and hypoparathyroidism. ‡Delay of a scheduled operation was measured by comparing the actual start time of the current operation (marked by the patient’s entry into the operating room) with the planned start time for that operation. §Waiting time between operations was determined by measuring the duration between the incision for the current operation and the closure time of the previous operation. Waiting time was not calculated for the first surgical procedure of the day. ASA, American Society of Anesthesiologists; HFRS, Hospital Frailty Risk Score.
| Characteristic . | Value . |
|---|---|
| Male | 3731 (42.2) |
| Age (years), mean(s.d.) | 56.5(16.9) |
| Surgical specialty | |
| Cardiac | 691 (7.8) |
| Endocrine | 751 (8.5) |
| Digestive | 1846 (20.9) |
| Gynaecological | 1971 (22.3) |
| Orthopaedic | 2396 (27.1) |
| Thoracic | 377 (4.3) |
| Urological | 812 (9.2) |
| Principal anaesthesia technique (missing n = 6) | |
| General | 6827 (77.2) |
| Regional | 1824 (20.6) |
| Local | 187 (2.1) |
| Initial surgical approach (missing n = 49) | |
| Open | 5280 (60.0) |
| Robotic | 489 (5.6) |
| Videoscopic | 2111 (24.0) |
| Endoscopic | 915 (10.4) |
| ASA grade | |
| I | 2488 (28.1) |
| II | 4349 (49.2) |
| III | 1949 (22.0) |
| IV | 56 (0.6) |
| V | 2 (0.0) |
| Median income in the city of residence in €*(x1000) (missing n = 24), mean(s.d.) | 23.1(3.6) |
| Precarity | 706 (8.0) |
| Inpatient surgery (missing n = 1) | 6499 (73.5) |
| Co-morbidities†, mean(s.d.) | 2.3(2.0) |
| HFRS frailty score of the patient during index stay, mean(s.d.) | 0.6(1.7) |
| Age of the attending surgeon at the time of the operation >45 years | 3797 (42.9) |
| Faculty position of the attending surgeon | 5326 (60.2) |
| Delayed operation‡ >1 h (missing n = 86) | 352 (4.0) |
| Delayed operation‡ >2 h (missing n = 86) | 154 (1.8) |
| Waiting time§ >1 h (not applicable n = 4705) | 2955 (71.4) |
| Waiting time§ >2 h (not applicable n = 4705) | 654 (15.8) |
| At least one among delayed operation‡ >1 h and waiting time§ >1 h (not applicable n = 4554) | 3129 (72.9) |
| At least one among delayed operation‡ >2 h and waiting time§ >2 h (not applicable n = 4626) | 751 (17.8) |
| Characteristic . | Value . |
|---|---|
| Male | 3731 (42.2) |
| Age (years), mean(s.d.) | 56.5(16.9) |
| Surgical specialty | |
| Cardiac | 691 (7.8) |
| Endocrine | 751 (8.5) |
| Digestive | 1846 (20.9) |
| Gynaecological | 1971 (22.3) |
| Orthopaedic | 2396 (27.1) |
| Thoracic | 377 (4.3) |
| Urological | 812 (9.2) |
| Principal anaesthesia technique (missing n = 6) | |
| General | 6827 (77.2) |
| Regional | 1824 (20.6) |
| Local | 187 (2.1) |
| Initial surgical approach (missing n = 49) | |
| Open | 5280 (60.0) |
| Robotic | 489 (5.6) |
| Videoscopic | 2111 (24.0) |
| Endoscopic | 915 (10.4) |
| ASA grade | |
| I | 2488 (28.1) |
| II | 4349 (49.2) |
| III | 1949 (22.0) |
| IV | 56 (0.6) |
| V | 2 (0.0) |
| Median income in the city of residence in €*(x1000) (missing n = 24), mean(s.d.) | 23.1(3.6) |
| Precarity | 706 (8.0) |
| Inpatient surgery (missing n = 1) | 6499 (73.5) |
| Co-morbidities†, mean(s.d.) | 2.3(2.0) |
| HFRS frailty score of the patient during index stay, mean(s.d.) | 0.6(1.7) |
| Age of the attending surgeon at the time of the operation >45 years | 3797 (42.9) |
| Faculty position of the attending surgeon | 5326 (60.2) |
| Delayed operation‡ >1 h (missing n = 86) | 352 (4.0) |
| Delayed operation‡ >2 h (missing n = 86) | 154 (1.8) |
| Waiting time§ >1 h (not applicable n = 4705) | 2955 (71.4) |
| Waiting time§ >2 h (not applicable n = 4705) | 654 (15.8) |
| At least one among delayed operation‡ >1 h and waiting time§ >1 h (not applicable n = 4554) | 3129 (72.9) |
| At least one among delayed operation‡ >2 h and waiting time§ >2 h (not applicable n = 4626) | 751 (17.8) |
Values are n (%) unless otherwise indicated. Percentages might not add up to 100 because of rounding. *€1.00 equivalent to £0.87 (US$1.07) as of November 2023. †To take into account the preoperative risk of each operation, a risk score was constructed based on all the variables presented in this table, as well as the following co-morbidities: current pregnancy, obesity (BMI greater than or equal to 30 kg/m²), malnourishment, tobacco addiction, alcohol addiction, other addiction, open wound, surgical-site infection, sepsis, endocarditis, cancer, neoadjuvant treatment, immune deficiency, coagulopathy, anticoagulant treatment, antiaggregation treatment, blood transfusion, coma, limb paralysis, other neurological disorder, confusion, dementia, depression, cardiovascular disease, neurovascular disease, peripheral arterial disease, cardiac arrhythmia, chronic heart failure, hypertension, diabetes, dyslipidaemia, pulmonary artery systolic pressure (greater than 60 mmHg), chronic renal failure, acute renal failure, chronic respiratory failure, chronic obstructive pulmonary disease, liver disease, rheumatic pathology, and hypoparathyroidism. ‡Delay of a scheduled operation was measured by comparing the actual start time of the current operation (marked by the patient’s entry into the operating room) with the planned start time for that operation. §Waiting time between operations was determined by measuring the duration between the incision for the current operation and the closure time of the previous operation. Waiting time was not calculated for the first surgical procedure of the day. ASA, American Society of Anesthesiologists; HFRS, Hospital Frailty Risk Score.
Table 2 shows a higher frequency of major adverse events when a surgeon experienced exposure to operating room delays versus their absence. A delay in starting a scheduled operation of more than 1 h was linked to an increased rate of the primary composite outcome (21.6% versus 14.4%, P = 0.039), as was a delay of more than 2 h (26.0% versus 14.4%, P = 0.003). Waiting times surpassing 1 h were associated with a higher rate of the composite outcome (13.9% versus 5.3%, P < 0.0001), as were delays exceeding 2 h (20.2% versus 9.8%, P = 0.001). These findings remained consistent when analysing each type of exposure and adverse event separately.
Frequency of major adverse events depending on a surgeon’s exposure to delayed operation and waiting time
| . | . | Composite . | Complications . | Unplanned reoperation . | Extended ICU/IMCU stay . | Death . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Frequency . | P . | Frequency . | P . | Frequency . | P . | Frequency . | P . | Frequency . | P . | ||
| Delayed operation* >1 h n = 8758 | Yes n = 352 | 76 (21.6) | 0.039 | 67 (19.0) | 0.0862 | 27 (7.7) | 0.091 | 22 (6.3) | 0.0269 | 8 (2.3) | 0.053 |
| No n= 8406 | 1207 (14.4) | 1132 (13.5) | 348 (4.1) | 155 (1.8) | 37 (0.4) | ||||||
| Delayed operation* >2 h n = 8758 | Yes n = 154 | 40 (26.0) | 0.003 | 32 (20.8) | 0.0158 | 18 (11.7) | 0.039 | 13 (8.4) | 0.0164 | 5 (3.2) | 0.068 |
| No n= 8604 | 1243 (14.4) | 1167 (13.6) | 357 (4.1) | 164 (1.9) | 40 (0.5) | ||||||
| Waiting time† >1 h n = 4139 | Yes n= 2955 | 412 (13.9) | <0.001 | 387 (13.1) | <0.0001 | 110 (3.7) | 0.001 | 41 (1.4) | 0.0110 | 11 (0.4) | NA |
| No n = 1184 | 63 (5.3) | 56 (4.7) | 19 (1.6) | 3 (0.3) | 0 (0.0) | ||||||
| Waiting time† >2 h n = 4139 | Yes n = 654 | 132 (20.2) | 0.001 | 123 (18.8) | 0.0012 | 38 (5.8) | 0.018 | 27 (4.1) | 0.0197 | 9 (1.4) | 0.024 |
| No n = 3485 | 343 (9.8) | 320 (9.2) | 91 (2.6) | 17 (0.5) | 2 (0.1) | ||||||
| At least one among delayed operation* >1 h and waiting time† >1 h n = 4290 | Yes n = 3129 | 446 (14.3) | <0.001 | 416 (13.3) | <0.0001 | 123 (3.9) | 0.001 | 52 (1.7) | 0.0076 | 16 (0.5) | NA |
| No n = 1161 | 63 (5.4%) | 56 (4.8) | 19 (1.6) | 3 (0.3) | 0 (0.0) | ||||||
| At least one among delayed operation* >2 h and waiting time† >2 h n = 4218 | Yes n = 751 | 158 (21.0) | 0.001 | 145 (19.3) | 0.0005 | 49 (6.5) | 0.012 | 35 (4.7) | 0.0076 | 13 (1.7) | 0.013 |
| No n = 3467 | 339 (9.8) | 316 (9.1) | 91 (2.6) | 17 (0.5) | 2 (0.1) | ||||||
| . | . | Composite . | Complications . | Unplanned reoperation . | Extended ICU/IMCU stay . | Death . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Frequency . | P . | Frequency . | P . | Frequency . | P . | Frequency . | P . | Frequency . | P . | ||
| Delayed operation* >1 h n = 8758 | Yes n = 352 | 76 (21.6) | 0.039 | 67 (19.0) | 0.0862 | 27 (7.7) | 0.091 | 22 (6.3) | 0.0269 | 8 (2.3) | 0.053 |
| No n= 8406 | 1207 (14.4) | 1132 (13.5) | 348 (4.1) | 155 (1.8) | 37 (0.4) | ||||||
| Delayed operation* >2 h n = 8758 | Yes n = 154 | 40 (26.0) | 0.003 | 32 (20.8) | 0.0158 | 18 (11.7) | 0.039 | 13 (8.4) | 0.0164 | 5 (3.2) | 0.068 |
| No n= 8604 | 1243 (14.4) | 1167 (13.6) | 357 (4.1) | 164 (1.9) | 40 (0.5) | ||||||
| Waiting time† >1 h n = 4139 | Yes n= 2955 | 412 (13.9) | <0.001 | 387 (13.1) | <0.0001 | 110 (3.7) | 0.001 | 41 (1.4) | 0.0110 | 11 (0.4) | NA |
| No n = 1184 | 63 (5.3) | 56 (4.7) | 19 (1.6) | 3 (0.3) | 0 (0.0) | ||||||
| Waiting time† >2 h n = 4139 | Yes n = 654 | 132 (20.2) | 0.001 | 123 (18.8) | 0.0012 | 38 (5.8) | 0.018 | 27 (4.1) | 0.0197 | 9 (1.4) | 0.024 |
| No n = 3485 | 343 (9.8) | 320 (9.2) | 91 (2.6) | 17 (0.5) | 2 (0.1) | ||||||
| At least one among delayed operation* >1 h and waiting time† >1 h n = 4290 | Yes n = 3129 | 446 (14.3) | <0.001 | 416 (13.3) | <0.0001 | 123 (3.9) | 0.001 | 52 (1.7) | 0.0076 | 16 (0.5) | NA |
| No n = 1161 | 63 (5.4%) | 56 (4.8) | 19 (1.6) | 3 (0.3) | 0 (0.0) | ||||||
| At least one among delayed operation* >2 h and waiting time† >2 h n = 4218 | Yes n = 751 | 158 (21.0) | 0.001 | 145 (19.3) | 0.0005 | 49 (6.5) | 0.012 | 35 (4.7) | 0.0076 | 13 (1.7) | 0.013 |
| No n = 3467 | 339 (9.8) | 316 (9.1) | 91 (2.6) | 17 (0.5) | 2 (0.1) | ||||||
Outcome proportions in the presence/absence of each predictor were compared through a Rao–Scott chi-squared test to account for clustering within surgeons. *Delay of a scheduled operation was measured by comparing the actual start time of the current operation (marked by the patient’s entry into the operating room) with the planned start time for that operation. †Waiting time between operations was determined by measuring the duration between the incision for the current operation and the closure time of the previous operation. Waiting time was not calculated for the first surgical procedure of the day. ICU, intensive care unit; IMCU, intermediate care unit; NA, non applicable.
Frequency of major adverse events depending on a surgeon’s exposure to delayed operation and waiting time
| . | . | Composite . | Complications . | Unplanned reoperation . | Extended ICU/IMCU stay . | Death . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Frequency . | P . | Frequency . | P . | Frequency . | P . | Frequency . | P . | Frequency . | P . | ||
| Delayed operation* >1 h n = 8758 | Yes n = 352 | 76 (21.6) | 0.039 | 67 (19.0) | 0.0862 | 27 (7.7) | 0.091 | 22 (6.3) | 0.0269 | 8 (2.3) | 0.053 |
| No n= 8406 | 1207 (14.4) | 1132 (13.5) | 348 (4.1) | 155 (1.8) | 37 (0.4) | ||||||
| Delayed operation* >2 h n = 8758 | Yes n = 154 | 40 (26.0) | 0.003 | 32 (20.8) | 0.0158 | 18 (11.7) | 0.039 | 13 (8.4) | 0.0164 | 5 (3.2) | 0.068 |
| No n= 8604 | 1243 (14.4) | 1167 (13.6) | 357 (4.1) | 164 (1.9) | 40 (0.5) | ||||||
| Waiting time† >1 h n = 4139 | Yes n= 2955 | 412 (13.9) | <0.001 | 387 (13.1) | <0.0001 | 110 (3.7) | 0.001 | 41 (1.4) | 0.0110 | 11 (0.4) | NA |
| No n = 1184 | 63 (5.3) | 56 (4.7) | 19 (1.6) | 3 (0.3) | 0 (0.0) | ||||||
| Waiting time† >2 h n = 4139 | Yes n = 654 | 132 (20.2) | 0.001 | 123 (18.8) | 0.0012 | 38 (5.8) | 0.018 | 27 (4.1) | 0.0197 | 9 (1.4) | 0.024 |
| No n = 3485 | 343 (9.8) | 320 (9.2) | 91 (2.6) | 17 (0.5) | 2 (0.1) | ||||||
| At least one among delayed operation* >1 h and waiting time† >1 h n = 4290 | Yes n = 3129 | 446 (14.3) | <0.001 | 416 (13.3) | <0.0001 | 123 (3.9) | 0.001 | 52 (1.7) | 0.0076 | 16 (0.5) | NA |
| No n = 1161 | 63 (5.4%) | 56 (4.8) | 19 (1.6) | 3 (0.3) | 0 (0.0) | ||||||
| At least one among delayed operation* >2 h and waiting time† >2 h n = 4218 | Yes n = 751 | 158 (21.0) | 0.001 | 145 (19.3) | 0.0005 | 49 (6.5) | 0.012 | 35 (4.7) | 0.0076 | 13 (1.7) | 0.013 |
| No n = 3467 | 339 (9.8) | 316 (9.1) | 91 (2.6) | 17 (0.5) | 2 (0.1) | ||||||
| . | . | Composite . | Complications . | Unplanned reoperation . | Extended ICU/IMCU stay . | Death . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Frequency . | P . | Frequency . | P . | Frequency . | P . | Frequency . | P . | Frequency . | P . | ||
| Delayed operation* >1 h n = 8758 | Yes n = 352 | 76 (21.6) | 0.039 | 67 (19.0) | 0.0862 | 27 (7.7) | 0.091 | 22 (6.3) | 0.0269 | 8 (2.3) | 0.053 |
| No n= 8406 | 1207 (14.4) | 1132 (13.5) | 348 (4.1) | 155 (1.8) | 37 (0.4) | ||||||
| Delayed operation* >2 h n = 8758 | Yes n = 154 | 40 (26.0) | 0.003 | 32 (20.8) | 0.0158 | 18 (11.7) | 0.039 | 13 (8.4) | 0.0164 | 5 (3.2) | 0.068 |
| No n= 8604 | 1243 (14.4) | 1167 (13.6) | 357 (4.1) | 164 (1.9) | 40 (0.5) | ||||||
| Waiting time† >1 h n = 4139 | Yes n= 2955 | 412 (13.9) | <0.001 | 387 (13.1) | <0.0001 | 110 (3.7) | 0.001 | 41 (1.4) | 0.0110 | 11 (0.4) | NA |
| No n = 1184 | 63 (5.3) | 56 (4.7) | 19 (1.6) | 3 (0.3) | 0 (0.0) | ||||||
| Waiting time† >2 h n = 4139 | Yes n = 654 | 132 (20.2) | 0.001 | 123 (18.8) | 0.0012 | 38 (5.8) | 0.018 | 27 (4.1) | 0.0197 | 9 (1.4) | 0.024 |
| No n = 3485 | 343 (9.8) | 320 (9.2) | 91 (2.6) | 17 (0.5) | 2 (0.1) | ||||||
| At least one among delayed operation* >1 h and waiting time† >1 h n = 4290 | Yes n = 3129 | 446 (14.3) | <0.001 | 416 (13.3) | <0.0001 | 123 (3.9) | 0.001 | 52 (1.7) | 0.0076 | 16 (0.5) | NA |
| No n = 1161 | 63 (5.4%) | 56 (4.8) | 19 (1.6) | 3 (0.3) | 0 (0.0) | ||||||
| At least one among delayed operation* >2 h and waiting time† >2 h n = 4218 | Yes n = 751 | 158 (21.0) | 0.001 | 145 (19.3) | 0.0005 | 49 (6.5) | 0.012 | 35 (4.7) | 0.0076 | 13 (1.7) | 0.013 |
| No n = 3467 | 339 (9.8) | 316 (9.1) | 91 (2.6) | 17 (0.5) | 2 (0.1) | ||||||
Outcome proportions in the presence/absence of each predictor were compared through a Rao–Scott chi-squared test to account for clustering within surgeons. *Delay of a scheduled operation was measured by comparing the actual start time of the current operation (marked by the patient’s entry into the operating room) with the planned start time for that operation. †Waiting time between operations was determined by measuring the duration between the incision for the current operation and the closure time of the previous operation. Waiting time was not calculated for the first surgical procedure of the day. ICU, intensive care unit; IMCU, intermediate care unit; NA, non applicable.
After adjusting for patient preoperative risk and surgeon attributes (see Fig. 2 and Appendix S2a), an increased risk of unplanned reoperation was observed when a surgeon faced a delayed operation exceeding 2 h (aRR 1.83 (95% c.i. 1.00 to 2.99)), as well as for the primary composite outcome (aRR 1.39 (95% c.i. 1.07 to 1.85)) and severe surgical complications (aRR 1.41 (95% c.i. 1.07 to 1.93)) with waiting times exceeding 1 h. These risks persisted when exposed to a disturbance in patient turnover exceeding 1 h (aRR 1.37 (95% c.i. 1.06 to 1.85) and 1.38 (95% c.i. 1.04 to 1.90) respectively) and 2 h (aRR 1.29 (95% c.i. 1.04 to 1.62) and 1.28 (95% c.i. 1.02 to 1.61) respectively). Though not always reaching statistical significance, this pattern remained consistent across various exposures and secondary outcomes.
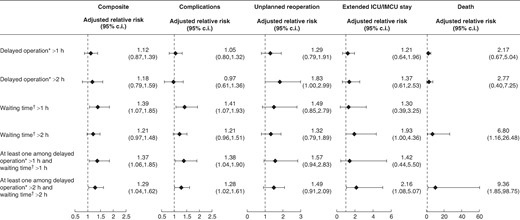
Adjusted risk of major adverse events associated with a surgeon’s exposure to delayed operation and waiting time
Adjusted relative risks were derived from mixed logistic regression models to capture surgeon variations and obtained from marginal probabilities of events for exposed (pT = 1) and non-exposed (pT = 0) subjects. They were calculated as RR = pT = 1/pT = 0. Confidence intervals were estimated using non-parametric bootstraps based on 1000 replications, picking as endpoints the 2.5th and the 97.5th percentiles. Models were adjusted with surgeon’s age (older than 45 years versus 45 years or younger), surgeon’s professional status (associate/full-time professor versus non-professor), and preoperative risk scores calculated for each outcome and derived from risk models developed on data from these same surgeons, but during another phase of the project. These risk scores took into account the surgical specialty, the surgical procedure complexity, the ICD-10 chapter of the surgical indication, the scheduling (urgent, semi-urgent, or elective), the type of anaesthesia, the surgical approach, and patient factors (age, sex, socio-economic status (median income of the municipality of residence in quartiles and precarious situation), American Society of Anesthesiologists (ASA) grade, and the following co-morbidities: current pregnancy, obesity (body mass index (BMI) greater than or equal to 30 kg/m²), malnourishment, tobacco addiction, alcohol addiction, other addiction, open wound, surgical-site infection, sepsis, endocarditis, cancer, neoadjuvant treatment, immune deficiency, coagulopathy, anticoagulant treatment, antiaggregation treatment, blood transfusion, coma, limb paralysis, other neurological disorder, confusion, dementia, depression, cardiovascular disease, neurovascular disease, peripheral arterial disease, cardiac arrhythmia, chronic heart failure, hypertension, diabetes, dyslipidaemia, pulmonary artery systolic pressure (greater than 60 mmHg), chronic renal failure, acute renal failure, chronic respiratory failure, chronic obstructive pulmonary disease, liver disease, rheumatic pathology, and hypoparathyroidism). A detailed explanation of the construction of the risk scores is provided in Appendix S1. *Delay of a scheduled operation was measured by comparing the actual start time of the current operation (marked by the patient’s entry into the operating room) with the planned start time for that operation. †Waiting time between operations was determined by measuring the duration between the incision for the current operation and the closure time of the previous operation. Waiting time was not calculated for the first surgical procedure of the day. ICU, intensive care unit; IMCU, intermediate care unit.
Figure 3 and Appendix S2b show the corresponding standardized outcome rates. The standardized rate of major adverse events was 12.1%, compared with 8.9% (absolute difference of 3.3% (95% c.i. 0.6% to 5.6%)), when a surgeon experienced a delay in operating room scheduling or waiting time between two procedures exceeding 1 h, as opposed to not experiencing such delays.
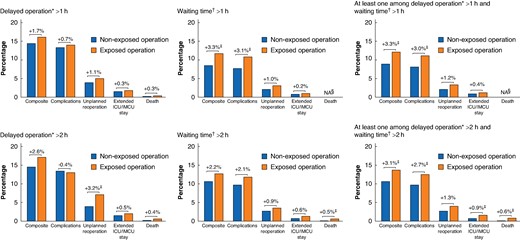
Difference in standardized outcome rates between exposed and non-exposed operations to delays
The bar charts represent the standardized outcome rates between non-exposed and exposed operations. These standardized rates were calculated using estimated coefficients obtained from the mixed logistic regression models and a marginal standardization method to control case-mix differences between non-exposed and exposed operations. Differences above brackets indicate absolute differences in standardized rates between the groups. The population of waiting time (n = 4139) was very different from that of delayed operation (n = 8758), notably with the exclusion of highly complex operations that lasted the entire day. Therefore, event rates associated with or without long waiting times cannot be compared with those for delayed operations. *Delay of a scheduled operation was measured by comparing the actual start time of the current operation (marked by the patient’s entry into the operating room) with the planned start time for that operation. †Waiting time between operations was determined by measuring the duration between the incision for the current operation and the closure time of the previous operation. Waiting time was not calculated for the first surgical procedure of the day. ‡P < 0.050. §Since mixed logistic regression models did not converge, it was not possible to calculate standardized outcome rates and standardized differences. NA, non applicable; ICU, intensive care unit; IMCU, intermediate care unit.
Discussion
This multispecialty and multicentre prospective study underscores the safety risks in the operating room associated with a surgeon’s exposure to delays in patient turnover before starting an operation. Delays exceeding 1 or 2 h were significantly linked to a higher risk of major adverse events, including severe complications, unplanned reoperation, extended ICU/IMCU stay due to organ failure, and patient mortality. A disparity was found between the rate of delayed operation and waiting time metrics. This difference was explained because of the rarity of delayed operation for the first patient of the day, but also because, even when waiting time is important (1 or 2 h), as the time between closure of the first patient to the incision of the second one was measured, when the previous procedure was shorter than scheduled, there was no delay longer than 1 h after 1 h of waiting time. Considering the high frequency of excessive delays in the operating room, addressing this organizational issue could substantially improve patient outcomes.
Reliable scheduling of operations and a seamless patient turnover are keystones to operating room organization. Nonetheless, a notable knowledge gap exists regarding the objective influence of surgical delays on patient safety. The present study aims to fill this gap by augmenting existing evidence, emphasizing the clinical benefits of achieving reliable operation scheduling and optimal patient turnover. This extends beyond the traditional goals of enhancing operating room operational and financial gains. Previous literature, to the authors’ understanding, has predominantly focused on the relationship between patient turnover and operating room efficiency.
Effective monitoring of the operative schedule depends on the number of procedures performed per day9. Notable variations in start times for second cases highlight the complexity of operation scheduling beyond the initial case, emphasizing the need for enhanced planning to optimize timeliness and resource utilization in the operating room10. Interventional studies previously aimed to enhance first case on-time starts and reduce patient flow variability, recognizing their potential influence on operating room scheduling and economic performance11,12. Turnover time optimization between surgical procedures is critical for hospital systems facing cost implications of delays. Viewing turnover time as a series of essential tasks, rather than a singular entity, enables focused assessment and improvement. Turnover time is influenced by factors like the time of day, staffing availability, and scheduling practices, with consecutive specialty cases and surgeon involvement identified as reducing turnover time13–15. Strategic planning, such as the presence of surgeon-preferred staff and scheduling consecutive cases with a consistent team, may also improve operating room efficiency16,17.
This multicentre study has both strengths and weaknesses. Accurate data were prospectively collected across various specialties by clinical research assistants using a rigorous collection process. These data were then cross-checked with information extracted from the hospital database to ensure their validity. The analysis yielded robust results across various exposures and outcome metrics. Rate ratios were calculated from model estimates to prevent overstatement of risks based solely on ORs and absolute differences in outcome rates are presented using the same approach for insightful clinical interpretations. In this analysis, the clustering of patients operated on by different surgeons was considered and various attributes related to the attending surgeon were accounted for. A comprehensive score was also devised using an independent data set to assess preoperative patient risk.
Despite considering various patient-, operation-, and surgeon-specific variables in the adjustment scheme, not all potential confounders can be controlled for, threatening comparability between exposure groups. Causal mechanisms explaining the relationship between a surgeon’s exposure to operating room delays and poor patient outcomes remain unclear. Furthermore, the generalizability of these findings is limited by the French context within a specific geographical area. While the COVID-19 pandemic may have influenced care during the study interval, it primarily affected hospitals in the first part of 2020, with a noticeable learning effect observed by the year’s end, returning to normal in 202118.
The present study establishes a direct link between excessive delays before initiating scheduled operations and patient outcomes. This underscores the importance of testing potentially life-saving organizational interventions to enhance patient flow in the operating room. Redesigning effective perioperative systems and teamwork is key to address time discrepancies between planned and executed cases19,20. It can be catalysed with verbal goal management to promote adherence to the operational timelines specified by the initial scheduling21 or through a nurse relay approach, coordinating activities outside the operating room to prepare the next patient22. The strategic allocation of dedicated operating rooms and stable surgical teams, tailored to specific specialties or procedures, is also identified as a promising approach to minimize delayed operations or waiting times3. Enhancing timeliness at each operational step and patient turnover, this approach has the potential to save resources and prevent patient harm23,24. Anticipating a surgeon’s workload and complex cases throughout the day25, as well as considering trainee involvement during operations26 and managing turnover within the nursing team27, are also recognized as key factors in improving operating room efficiency and safety.
Concurrent with organizational interventions, ensuring reliable case scheduling by accurately predicting surgical times and patient turnover in the operating room is crucial. This demands a comprehensive assessment of factors, such as patient, procedure, surgeon, team, operating room, and perioperative care organization factors. The current accessibility of large databases and powerful artificial intelligence modelling approaches now makes this goal achievable28.
The exposure of surgeons to delays and waiting times in the operating room scheduling before starting operations is associated with an increased risk of adverse events for patients. Efforts directed towards implementing efficient interventions to enhance patient flow and ensure reliable operation turnover are critical for safe surgical practices and better patient outcomes.
Collaborators
TopSurgeons Study Group
Jake Awtry (Center for Surgery and Public Health, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts, USA), Lionel Badet (Department of Urological Surgery, Edouard Herriot Hospital, Hospices Civils de Lyon, Lyon, France), Lucie Bonin-Crepet (Department of Gynaecologic Surgery, Lyon Sud Hospital, Hospices Civils de Lyon, Lyon, France), Olivier Cannarella (COLLIGARE, Sceaux, France), Damien Carnicelli (Department of Urological Surgery, Lyon Sud Hospital, Hospices Civils de Lyon, Lyon, France), Martin Carrerre (Mon Coach Lyonnais, Caluire, France), Keyne Charlot (Research on Healthcare Performance RESHAPE, Inserm U1290, Université Claude Bernard Lyon 1, Lyon, France), Philippe Chaudier (Department of Orthopaedic Surgery, Lyon Sud Hospital, Hospices Civils de Lyon, Lyon, France), Gautier Chene (Department of Gynaecologic Surgery, Femme Mère Enfant Hospital, Hospices Civils de Lyon, Lyon, France), François Chollet (Research on Healthcare Performance RESHAPE, Inserm U1290, Université Claude Bernard Lyon 1, Lyon, France), Virginie Cloud (Department of Gynaecologic Surgery, Lyon Sud Hospital, Hospices Civils de Lyon, Lyon, France), Quentin Cordier (Research on Healthcare Performance RESHAPE, Inserm U1290, Université Claude Bernard Lyon 1, Lyon, France), Ethan Cormont (professional athlete, Paris, France), Marion Cortet (Department of Gynaecologic Surgery, Croix Rousse Hospital, Hospices Civils de Lyon, Lyon, France), Eddy Cotte (Department of Digestive Surgery, Lyon Sud Hospital, Hospices Civils de Lyon, Lyon, France), Sébastien Crouzet (Department of Urological Surgery, Edouard Herriot Hospital, Hospices Civils de Lyon, Lyon, France), Filippo Dagnino (Center for Surgery and Public Health, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts, USA), Hassan Demian (Department of Digestive Surgery, Croix Rousse Hospital, Hospices Civils de Lyon, Lyon, France), Tanujit Dey (Center for Surgery and Public Health, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts, USA), Antoine Duclos (Center for Surgery and Public Health, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts, USA), Xavier Dutheil (VERBATIM CONSEIL, LEVALLOIS PERRET, France), Fadi Farhat (Department of Cardiac Surgery, Louis Pradel Hospital, Hospices Civils de Lyon, Lyon, France), Jean-Baptiste Fassier (Department of Occupational Medicine, Hospices Civils de Lyon, France), Yves François (Department of Digestive Surgery, Lyon Sud Hospital, Hospices Civils de Lyon, Lyon, France), Witold Gertych (Department of Gynaecologic Surgery, Lyon Sud Hospital, Hospices Civils de Lyon, Lyon, France), François Golfier (Department of Gynaecologic Surgery, Lyon Sud Hospital, Hospices Civils de Lyon, Lyon, France), Romain Gorioux (Department of Orthopaedic Surgery, Edouard Herriot Hospital, Hospices Civils de Lyon, Lyon, France), Claire-Angéline Goutard (Department of Digestive Surgery, Lyon Sud Hospital, Hospices Civils de Lyon, Lyon, France), Stanislas Gunst (Department of Orthopaedic Surgery, Edouard Herriot Hospital, Hospices Civils de Lyon, Lyon, France), Muriel Hermine (coaching, Sarguemines, France), Nathalie Hoen (Department of Gynaecologic Surgery, Lyon Sud Hospital, Hospices Civils de Lyon, Lyon, France), Vahan Képénékian (Department of Digestive Surgery, Lyon Sud Hospital, Hospices Civils de Lyon, Lyon, France), Gery Lamblin (Department of Gynaecologic Surgery, Femme Mère Enfant Hospital, Hospices Civils de Lyon, Lyon, France), Mickaël Lesurtel (Department of Digestive Surgery, Croix Rousse Hospital, Hospices Civils de Lyon, Lyon, France), Jean-Christophe Lifante (Department of Digestive Surgery, Lyon Sud Hospital, Hospices Civils de Lyon, Lyon, France), Lucie Louboutin (Department of Orthopaedic Surgery, Lyon Sud Hospital, Hospices Civils de Lyon, Lyon, France), Sébastien Lustig (Department of Orthopaedic Surgery, Croix Rousse Hospital, Hospices Civils de Lyon, Lyon, France), Jean-Yves Mabrut (Department of Digestive Surgery, Croix Rousse Hospital, Hospices Civils de Lyon, Lyon, France), Laure Maillard (Department of Digestive Surgery, Lyon Sud Hospital, Hospices Civils de Lyon, Lyon, France), Jean-Michel Maury (Department of Thoracic Surgery, Louis Pradel Hospital, Hospices Civils de Lyon, Lyon, France), Stéphanie Mazza (Research on Healthcare Performance RESHAPE, Inserm U1290, Université Claude Bernard Lyon 1, Lyon, France), Kayvan Mohkam (Department of Digestive Surgery, Croix Rousse Hospital, Hospices Civils de Lyon, Lyon, France), Nicolas Morel-Journel (Department of Urological Surgery, Lyon Sud Hospital, Hospices Civils de Lyon, Lyon, France), Erdogan Nohuz (Department of Gynaecologic Surgery, Femme Mère Enfant Hospital, Hospices Civils de Lyon, Lyon, France), Andréa Nunes (Lyon Sud Hospital, Hospices Civils de Lyon, Lyon, France), Jean-François Obadia (Department of Cardiac Surgery, Louis Pradel Hospital, Hospices Civils de Lyon, Lyon, France), Léa Pascal (Research on Healthcare Performance RESHAPE, Inserm U1290, Université Claude Bernard Lyon 1, Lyon, France), Arnaud Pasquer (Department of Digestive Surgery, Edouard Herriot Hospital, Hospices Civils de Lyon, Lyon, France), Guillaume Passot (Department of Digestive Surgery, Lyon Sud Hospital, Hospices Civils de Lyon, Lyon, France), Elise Pelascini (Department of Digestive Surgery, Edouard Herriot Hospital, Hospices Civils de Lyon, Lyon, France), Charles-André Philip (Department of Gynaecologic Surgery, Femme Mère Enfant Hospital, Hospices Civils de Lyon, Lyon, France), Vincent Pibarot (Department of Orthopaedic Surgery, Edouard Herriot Hospital, Hospices Civils de Lyon, Lyon, France), Stéphanie Polazzi (Research on Healthcare Performance RESHAPE, Inserm U1290, Université Claude Bernard Lyon 1, Lyon, France), Gilles Poncet (Department of Digestive Surgery, Edouard Herriot Hospital, Hospices Civils de Lyon, Lyon, France), Matteo Pozzi (Department of Cardiac Surgery, Louis Pradel Hospital, Hospices Civils de Lyon, Lyon, France), Hugo Prieur (data management, Hospices Civils de Lyon, Lyon, France), Maud Robert (Department of Digestive Surgery, Edouard Herriot Hospital, Hospices Civils de Lyon, Lyon, France), Frédéric Rongieras (Department of Orthopaedic Surgery, Edouard Herriot Hospital, Hospices Civils de Lyon, Lyon, France), Alain Ruffion (Department of Urological Surgery, Lyon Sud Hospital, Hospices Civils de Lyon, Lyon, France), Sophie Schlatter (Research on Healthcare Performance RESHAPE, Inserm U1290, Université Claude Bernard Lyon 1, Lyon, France), Sofia Sebaoui (Clinical Research Department, Hospices Civils de LYon, Lyon, France), Elvire Servien (Department of Orthopaedic Surgery, Croix Rousse Hospital, Hospices Civils de Lyon, Lyon, France), Sarah Skinner (Research on Healthcare Performance RESHAPE, Inserm U1290, Université Claude Bernard Lyon 1, Lyon, France), Stefanie Soelling (Center for Surgery and Public Health, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts, USA), Daniel Stelzl (Center for Surgery and Public Health, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts, USA), François Tronc (Department of Thoracic Surgery, Louis Pradel Hospital, Hospices Civils de Lyon, Lyon, France), Delphine Vaudoyer (Department of Digestive Surgery, Lyon Sud Hospital, Hospices Civils de Lyon, Lyon, France), Laurent Villeneuve (Lyon Sud Hospital, Hospices Civils de Lyon, Lyon, France), Anthony Viste (Department of Orthopaedic Surgery, Lyon Sud Hospital, Hospices Civils de Lyon, Lyon, France), Marco Vola (Department of Cardiac Surgery, Louis Pradel Hospital, Hospices Civils de Lyon, Lyon, France), Sophie Warembourg (Department of Gynaecologic Surgery, Croix Rousse Hospital, Hospices Civils de Lyon, Lyon, France).
Funding
This project received a European Research Council (ERC) Starting Grant under the European Union’s Horizon 2020 research and innovation programme (Grant Agreement No. 801660—TopSurgeons—ERC-2018-STG).
Author contributions
Arnaud Pasquer (Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing—original draft), Quentin Cordier (Conceptualization, Data curation, Formal analysis, Methodology, Validation, Writing—review & editing), Jean-Christophe Lifante (Data curation, Formal analysis, Investigation, Project administration, Writing—review & editing), Gilles Poncet (Data curation, Formal analysis, Supervision, Writing—review & editing), Stéphanie Polazzi (Data curation, Formal analysis, Methodology, Writing—original draft, Writing—review & editing), and Antoine Duclos (Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing—original draft, Writing—review & editing)
Disclosure
The authors declare no conflict of interest.
Supplementary material
Supplementary material is available at BJS Open online.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
Author notes
Members of the TopSurgeons Study Group are co-authors of this study and are listed under the heading Collaborators.



