-
PDF
- Split View
-
Views
-
Cite
Cite
Tiago Ribeiro, Jesse Zuckerman, Shiva Jayaraman, Alice C Wei, Alyson L Mahar, Guillaume Martel, Natalie Coburn, Julie Hallet, Association between surgeon volume and the use of laparoscopic liver resection: retrospective cohort study, BJS Open, Volume 8, Issue 4, August 2024, zrae085, https://doi.org/10.1093/bjsopen/zrae085
Close - Share Icon Share
Introduction
The 2015 Morioka international expert consensus conference established laparoscopic liver resection (LLR) as the standard of care for minor liver resections and as having a growing role for major resections1. Despite these recommendations, and although the literature has addressed the concerns regarding oncological outcomes, learning curves, and high costs, the utilization of LLR remains variable2–11. LLR has not followed the trends of increased use that have been seen for common abdominal surgeries, such as appendectomies and cholecystectomies6,12.
A better understanding of the influence of some surgical practice factors, such as surgeon expertise, on the use of LLR may improve its application. It has been suggested that laparoscopic surgery experience can influence comfort levels and patient outcomes13. Whether overall liver surgery experience influences the utilization of LLR is not known.
The aim of this study was to evaluate the association between surgeon liver resection volume and the use of LLR, with a view to better understand how surgeon experience may be associated with uptake and inform actionable targets to support LLR programmes.
Methods
Design and study population
A retrospective cohort study was performed using administrative data sets at the Institute for Clinical Evaluative Sciences (ICES) in Ontario, Canada. Individuals greater than or equal to 18 years old with hepatobiliary or entero-colorectal malignancy in Ontario between 1 January 2007 and 31 March 2019 were identified. Patients were included if they had undergone an elective hepatectomy up to 180 days before diagnosis; only the first resection per patient was included and emergency surgeries were excluded. This study was approved by the Sunnybrook Health Sciences Centre Research Ethics Board and authorized under section 45 of Ontario’s Personal Health Information Act.
Exposure
For each patient, the surgeon’s annual liver resection volume was captured as the mean annual number of liver resections performed by that surgeon in the 2 years before the date of index surgery. This definition accounts for possible year-to-year variation and reflects the actual surgeon experience leading up to the index surgery14,15.
Outcomes
The primary outcome was the use of LLR, defined using billing codes. If a laparoscopy code was not billed, the resection was presumed to have been open.
Statistical analysis
For clinical interpretability and in alignment with prior surgery volume literature, the exposure was dichotomized guided by data distribution and the association with outcomes16,17. Standardized differences were used to compare the distribution of covariates between groups treated by high- and low-volume surgeons, with a value greater than 10% considered significant18, and are presented alongside P values from chi-squared or Wilcoxon’s rank sum tests, where appropriate. The association between surgeon volume and the use of LLR was adjusted for potential confounders in a multivariable modified Poisson model. The following covariates were chosen a priori based on the literature and clinical reasoning: patient age, sex, and socio-economic status, year of surgery, surgeon’s years in practice, and extent of hepatectomy. A sensitivity analysis handling the annual mean surgeon volume as a continuous variable was performed. Using this sensitivity analysis, the predicted probability of LLR and its 95% confidence interval was plotted against surgeon’s annual liver resections.
Further information regarding data sources (Table S1), cohort selection (Tables S2, S3), covariates, and statistical analysis are available in the Supplementary Methods. Statistical tests were two-sided and P < 0.050 was considered statistically significant. Analyses were conducted using SAS Enterprise Guide 7.1 (SAS Institute, Cary, NC, USA).
Results
Patient and surgeon characteristics
Of 5133 patients, 908 patients (17.7%) underwent LLR. Baseline cohort characteristics for all patients stratified according to whether patients were cared for by a low- or high-volume surgeon are presented in Table 1. There was a non-linear relationship between surgeon liver resection volume and the use of LLR (Fig. S1). Informed by this curve, the 60th percentile was chosen as the cut-off to define low-volume (less than 30 annual resections) and high-volume (greater than or equal to 30 annual resections) surgeons.
Baseline cohort characteristics for all patients and stratified according to whether patients were cared for by a low- or high-volume surgeon
| . | All patients (n = 5133) . | Patients cared for by a low-volume surgeon (<30 liver resections per year) (n = 3218) . | Patients cared for by a high-volume surgeon (≥30 liver resections per year) (n = 1915) . | Standardized difference*, % . | P . |
|---|---|---|---|---|---|
| Age (years), median (i.q.r.) | 64 (56–72) | 64 (56–72) | 64 (55–71) | 3 | 0.295 |
| Sex | 0.730 | ||||
| Male | 3149 (61.3) | 1980 (61.5) | 1169 (61.0) | 1 | |
| Female | 1984 (38.7) | 1238 (38.5) | 746 (39.0) | 1 | |
| Socio-economic status† | <0.001 | ||||
| 1st (highest) | 1033 (20.2) | 694 (21.6) | 339 (17.7) | 10 | |
| 2nd | 1044 (20.3) | 629 (19.4) | 415 (21.7) | 5 | |
| 3rd | 1044 (20.3) | 684 (21.3) | 360 (18.8) | 6 | |
| 4th | 998 (19.4) | 592 (18.4) | 406 (21.2) | 7 | |
| 5th (lowest) | 969 (18.9) | 591 (18.4) | 378 (19.7) | 3 | |
| Missing | 45 (0.9) | 28 (0.9) | 17 (0.9) | 0 | |
| Co-morbidity burden | 0.021 | ||||
| High | 401 (7.8) | 230 (7.1) | 171 (8.9) | 7 | |
| Low | 4732 (92.2) | 2988 (92.9) | 1744 (91.1) | 7 | |
| Diagnosis | 0.521 | ||||
| Entero-colorectal cancer | 3314 (64.6) | 2067 (64.2) | 1247 (65.1) | 2 | |
| Hepatobiliary cancer | 1819 (35.4) | 1151 (35.8) | 668 (34.9) | 2 | |
| Extent of hepatectomy | 0.004 | ||||
| Minor (<3 segments) | 1985 (38.7) | 1196 (37.2) | 789 (41.2) | 8 | |
| Major (≥3 segments) | 3148 (61.3) | 2022 (62.8) | 1126 (58.8) | 8 | |
| Surgeon’s time in practice (years), median (i.q.r.) | 9.2 (5.3–15.0) | 10.4 (4.7–18.7) | 8.2 (5.8–11.5) | 25 | <0.001 |
| Year of surgery | <0.001 | ||||
| 2007 | 108 | 72 (2.2) | 36 (1.9) | 3 | |
| 2008 | 249 | 191 (5.9) | 58 (3.0) | 14 | |
| 2009 | 335 | 254 (7.9) | 81 (4.2) | 15 | |
| 2010 | 328 | 257 (8.0) | 71 (3.7) | 18 | |
| 2011 | 410 | 339 (10.5) | 71 (3.7) | 27 | |
| 2012 | 475 | 357 (11.1) | 118 (6.2) | 18 | |
| 2013 | 504 | 311 (9.7) | 193 (10.1) | 1 | |
| 2014 | 487 | 248 (7.7) | 239 (12.5) | 16 | |
| 2015 | 508 | 270 (8.4) | 238 (12.4) | 13 | |
| 2016 | 517 | 287 (8.9) | 230 (12.0) | 1 | |
| 2017 | 519 | 284 (8.8) | 235 (12.3) | 11 | |
| 2018 | 548 | 270 (8.4) | 278 (14.5) | 19 | |
| 2019 | 145 | 78 (2.4) | 67 (3.5) | 6 |
| . | All patients (n = 5133) . | Patients cared for by a low-volume surgeon (<30 liver resections per year) (n = 3218) . | Patients cared for by a high-volume surgeon (≥30 liver resections per year) (n = 1915) . | Standardized difference*, % . | P . |
|---|---|---|---|---|---|
| Age (years), median (i.q.r.) | 64 (56–72) | 64 (56–72) | 64 (55–71) | 3 | 0.295 |
| Sex | 0.730 | ||||
| Male | 3149 (61.3) | 1980 (61.5) | 1169 (61.0) | 1 | |
| Female | 1984 (38.7) | 1238 (38.5) | 746 (39.0) | 1 | |
| Socio-economic status† | <0.001 | ||||
| 1st (highest) | 1033 (20.2) | 694 (21.6) | 339 (17.7) | 10 | |
| 2nd | 1044 (20.3) | 629 (19.4) | 415 (21.7) | 5 | |
| 3rd | 1044 (20.3) | 684 (21.3) | 360 (18.8) | 6 | |
| 4th | 998 (19.4) | 592 (18.4) | 406 (21.2) | 7 | |
| 5th (lowest) | 969 (18.9) | 591 (18.4) | 378 (19.7) | 3 | |
| Missing | 45 (0.9) | 28 (0.9) | 17 (0.9) | 0 | |
| Co-morbidity burden | 0.021 | ||||
| High | 401 (7.8) | 230 (7.1) | 171 (8.9) | 7 | |
| Low | 4732 (92.2) | 2988 (92.9) | 1744 (91.1) | 7 | |
| Diagnosis | 0.521 | ||||
| Entero-colorectal cancer | 3314 (64.6) | 2067 (64.2) | 1247 (65.1) | 2 | |
| Hepatobiliary cancer | 1819 (35.4) | 1151 (35.8) | 668 (34.9) | 2 | |
| Extent of hepatectomy | 0.004 | ||||
| Minor (<3 segments) | 1985 (38.7) | 1196 (37.2) | 789 (41.2) | 8 | |
| Major (≥3 segments) | 3148 (61.3) | 2022 (62.8) | 1126 (58.8) | 8 | |
| Surgeon’s time in practice (years), median (i.q.r.) | 9.2 (5.3–15.0) | 10.4 (4.7–18.7) | 8.2 (5.8–11.5) | 25 | <0.001 |
| Year of surgery | <0.001 | ||||
| 2007 | 108 | 72 (2.2) | 36 (1.9) | 3 | |
| 2008 | 249 | 191 (5.9) | 58 (3.0) | 14 | |
| 2009 | 335 | 254 (7.9) | 81 (4.2) | 15 | |
| 2010 | 328 | 257 (8.0) | 71 (3.7) | 18 | |
| 2011 | 410 | 339 (10.5) | 71 (3.7) | 27 | |
| 2012 | 475 | 357 (11.1) | 118 (6.2) | 18 | |
| 2013 | 504 | 311 (9.7) | 193 (10.1) | 1 | |
| 2014 | 487 | 248 (7.7) | 239 (12.5) | 16 | |
| 2015 | 508 | 270 (8.4) | 238 (12.4) | 13 | |
| 2016 | 517 | 287 (8.9) | 230 (12.0) | 1 | |
| 2017 | 519 | 284 (8.8) | 235 (12.3) | 11 | |
| 2018 | 548 | 270 (8.4) | 278 (14.5) | 19 | |
| 2019 | 145 | 78 (2.4) | 67 (3.5) | 6 |
Values are n (%) unless otherwise indicated. *A standardized mean difference greater than 10% was considered statistically significant. †Assessed via a multidimensional ecological measure incorporating socio-economic factors, such as education and income19. i.q.r., interquartile range.
Baseline cohort characteristics for all patients and stratified according to whether patients were cared for by a low- or high-volume surgeon
| . | All patients (n = 5133) . | Patients cared for by a low-volume surgeon (<30 liver resections per year) (n = 3218) . | Patients cared for by a high-volume surgeon (≥30 liver resections per year) (n = 1915) . | Standardized difference*, % . | P . |
|---|---|---|---|---|---|
| Age (years), median (i.q.r.) | 64 (56–72) | 64 (56–72) | 64 (55–71) | 3 | 0.295 |
| Sex | 0.730 | ||||
| Male | 3149 (61.3) | 1980 (61.5) | 1169 (61.0) | 1 | |
| Female | 1984 (38.7) | 1238 (38.5) | 746 (39.0) | 1 | |
| Socio-economic status† | <0.001 | ||||
| 1st (highest) | 1033 (20.2) | 694 (21.6) | 339 (17.7) | 10 | |
| 2nd | 1044 (20.3) | 629 (19.4) | 415 (21.7) | 5 | |
| 3rd | 1044 (20.3) | 684 (21.3) | 360 (18.8) | 6 | |
| 4th | 998 (19.4) | 592 (18.4) | 406 (21.2) | 7 | |
| 5th (lowest) | 969 (18.9) | 591 (18.4) | 378 (19.7) | 3 | |
| Missing | 45 (0.9) | 28 (0.9) | 17 (0.9) | 0 | |
| Co-morbidity burden | 0.021 | ||||
| High | 401 (7.8) | 230 (7.1) | 171 (8.9) | 7 | |
| Low | 4732 (92.2) | 2988 (92.9) | 1744 (91.1) | 7 | |
| Diagnosis | 0.521 | ||||
| Entero-colorectal cancer | 3314 (64.6) | 2067 (64.2) | 1247 (65.1) | 2 | |
| Hepatobiliary cancer | 1819 (35.4) | 1151 (35.8) | 668 (34.9) | 2 | |
| Extent of hepatectomy | 0.004 | ||||
| Minor (<3 segments) | 1985 (38.7) | 1196 (37.2) | 789 (41.2) | 8 | |
| Major (≥3 segments) | 3148 (61.3) | 2022 (62.8) | 1126 (58.8) | 8 | |
| Surgeon’s time in practice (years), median (i.q.r.) | 9.2 (5.3–15.0) | 10.4 (4.7–18.7) | 8.2 (5.8–11.5) | 25 | <0.001 |
| Year of surgery | <0.001 | ||||
| 2007 | 108 | 72 (2.2) | 36 (1.9) | 3 | |
| 2008 | 249 | 191 (5.9) | 58 (3.0) | 14 | |
| 2009 | 335 | 254 (7.9) | 81 (4.2) | 15 | |
| 2010 | 328 | 257 (8.0) | 71 (3.7) | 18 | |
| 2011 | 410 | 339 (10.5) | 71 (3.7) | 27 | |
| 2012 | 475 | 357 (11.1) | 118 (6.2) | 18 | |
| 2013 | 504 | 311 (9.7) | 193 (10.1) | 1 | |
| 2014 | 487 | 248 (7.7) | 239 (12.5) | 16 | |
| 2015 | 508 | 270 (8.4) | 238 (12.4) | 13 | |
| 2016 | 517 | 287 (8.9) | 230 (12.0) | 1 | |
| 2017 | 519 | 284 (8.8) | 235 (12.3) | 11 | |
| 2018 | 548 | 270 (8.4) | 278 (14.5) | 19 | |
| 2019 | 145 | 78 (2.4) | 67 (3.5) | 6 |
| . | All patients (n = 5133) . | Patients cared for by a low-volume surgeon (<30 liver resections per year) (n = 3218) . | Patients cared for by a high-volume surgeon (≥30 liver resections per year) (n = 1915) . | Standardized difference*, % . | P . |
|---|---|---|---|---|---|
| Age (years), median (i.q.r.) | 64 (56–72) | 64 (56–72) | 64 (55–71) | 3 | 0.295 |
| Sex | 0.730 | ||||
| Male | 3149 (61.3) | 1980 (61.5) | 1169 (61.0) | 1 | |
| Female | 1984 (38.7) | 1238 (38.5) | 746 (39.0) | 1 | |
| Socio-economic status† | <0.001 | ||||
| 1st (highest) | 1033 (20.2) | 694 (21.6) | 339 (17.7) | 10 | |
| 2nd | 1044 (20.3) | 629 (19.4) | 415 (21.7) | 5 | |
| 3rd | 1044 (20.3) | 684 (21.3) | 360 (18.8) | 6 | |
| 4th | 998 (19.4) | 592 (18.4) | 406 (21.2) | 7 | |
| 5th (lowest) | 969 (18.9) | 591 (18.4) | 378 (19.7) | 3 | |
| Missing | 45 (0.9) | 28 (0.9) | 17 (0.9) | 0 | |
| Co-morbidity burden | 0.021 | ||||
| High | 401 (7.8) | 230 (7.1) | 171 (8.9) | 7 | |
| Low | 4732 (92.2) | 2988 (92.9) | 1744 (91.1) | 7 | |
| Diagnosis | 0.521 | ||||
| Entero-colorectal cancer | 3314 (64.6) | 2067 (64.2) | 1247 (65.1) | 2 | |
| Hepatobiliary cancer | 1819 (35.4) | 1151 (35.8) | 668 (34.9) | 2 | |
| Extent of hepatectomy | 0.004 | ||||
| Minor (<3 segments) | 1985 (38.7) | 1196 (37.2) | 789 (41.2) | 8 | |
| Major (≥3 segments) | 3148 (61.3) | 2022 (62.8) | 1126 (58.8) | 8 | |
| Surgeon’s time in practice (years), median (i.q.r.) | 9.2 (5.3–15.0) | 10.4 (4.7–18.7) | 8.2 (5.8–11.5) | 25 | <0.001 |
| Year of surgery | <0.001 | ||||
| 2007 | 108 | 72 (2.2) | 36 (1.9) | 3 | |
| 2008 | 249 | 191 (5.9) | 58 (3.0) | 14 | |
| 2009 | 335 | 254 (7.9) | 81 (4.2) | 15 | |
| 2010 | 328 | 257 (8.0) | 71 (3.7) | 18 | |
| 2011 | 410 | 339 (10.5) | 71 (3.7) | 27 | |
| 2012 | 475 | 357 (11.1) | 118 (6.2) | 18 | |
| 2013 | 504 | 311 (9.7) | 193 (10.1) | 1 | |
| 2014 | 487 | 248 (7.7) | 239 (12.5) | 16 | |
| 2015 | 508 | 270 (8.4) | 238 (12.4) | 13 | |
| 2016 | 517 | 287 (8.9) | 230 (12.0) | 1 | |
| 2017 | 519 | 284 (8.8) | 235 (12.3) | 11 | |
| 2018 | 548 | 270 (8.4) | 278 (14.5) | 19 | |
| 2019 | 145 | 78 (2.4) | 67 (3.5) | 6 |
Values are n (%) unless otherwise indicated. *A standardized mean difference greater than 10% was considered statistically significant. †Assessed via a multidimensional ecological measure incorporating socio-economic factors, such as education and income19. i.q.r., interquartile range.
Association between surgeon resection volume and the use of laparoscopic liver resection
The unadjusted association is reported in the Supplementary Results. After adjustment, care by a high-volume surgeon was independently associated with increased use of LLR (relative risk 1.30 (95% c.i. 1.14 to 1.48)) (Table S4). When handled as a continuous variable, the annual surgeon liver resection volume was also independently associated with higher use of LLR (Table S5). Every incremental increase of 10 cases per year was associated with a 6% increase in the likelihood of using LLR (relative risk 1.06 (95% c.i. 1.04 to 1.09)). The adjusted predicted probabilities for LLR showed a positive relationship with surgeon liver resection volume (Fig. 1).
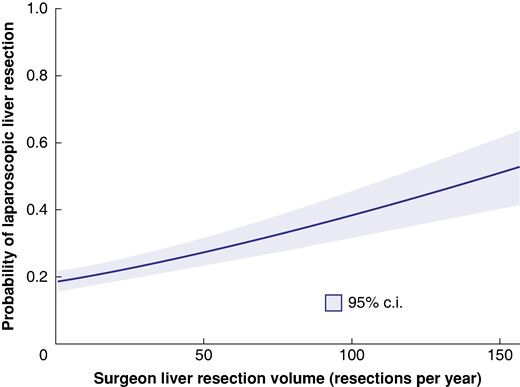
Probability of laparoscopic liver resection by annual surgeon liver resection volume
Adjusted for patient age, sex, socio-economic status, year of surgery, surgeon’s years in practice, and extent of hepatectomy.
Discussion
In this population-based study, surgeon liver resection volume is associated with an increased likelihood of LLR, in patients undergoing hepatectomy for gastrointestinal cancer. Patients cared for by high-volume liver surgeons (greater than or equal to 30 liver resections per year) were 30% more likely to have their hepatectomy performed laparoscopically. This points towards higher liver resection volume, and therefore experience, potentially facilitating the use of LLR in clinical practice.
Although the literature has dispelled many of the initial concerns surrounding compromised surgical and oncological outcomes, the use of LLR remains variable9,20. Prior examinations of LLR uptake have focused on the role of the learning curve and of support by expert opinion leaders1,13,21,22. Although surgeon experience regarding liver resection has been suggested to be important for LLR uptake, it had never been formally examined13. The present findings provide new information and potential actionable targets towards improving the uptake of LLR by leveraging the experience of high-volume surgeons.
In addition to institutional support, efforts to increase LLR could start with directing complex liver surgery to high-volume surgeons. The adoption of LLR by low-volume surgeons may first involve uptake for minor resections until further expertise is attained. There may be concerns that directing complex liver resections to high-volume surgeons could result in care disparities due to differential access to care. However, health systems can be organized such that high-volume surgeons can practice in various settings, from urban to rural institutions, such that all patients can benefit from increased uptake of LLR.
This work supports policy changes directing liver surgery to high-volume surgeons, with the aim of increasing LLR within hospitals and health systems. Future work should consider the relationship between volume and LLR stratified by liver resection complexity to inform more targeted approaches to directing liver surgery.
This study has some limitations. Due to the retrospective design, some factors were not available for analysis and residual confounding was unavoidable. The volume definitions were critical for this analysis and the cut-off identified was dependent on the existing surgeons’ volumes.
Higher surgeon liver resection volume is independently associated with increased use of LLR for gastrointestinal cancer.
Funding
This study received funding from a Sunnybrook Alternative Funding Plan Innovation Grant, a Canadian Institutes of Health Research New Investigator Award, and a Canadian Institutes of Health Research Project Grant (FRN #154131). The funding sources had no role in the design and conduct of the study, the collection, management, analysis, and interpretation of the data, the preparation, review, or approval of the manuscript, and the decision to submit the manuscript for publication.
Author contributions
Tiago Ribeiro (Investigation, Project administration, Visualization, Writing—original draft, Writing—review & editing), Jesse Zuckerman (Conceptualization, Formal analysis, Methodology, Project administration, Visualization, Writing—original draft, Writing—review & editing), Shiva Jayaraman (Supervision, Writing—review & editing), Alice C. Wei (Supervision, Writing—review & editing), Alyson L. Mahar (Methodology, Writing—review & editing), Guillaume Martel (Supervision, Writing—review & editing), Natalie Coburn (Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Supervision, Writing—review & editing), and Julie Hallet (Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing—review & editing)
Disclosure
J.H. has received speaking honoraria from Ipsen and Novartis. The authors declare no other conflict of interest.
Supplementary material
Supplementary material is available at BJS Open online.
Data availability
All relevant results are included in the paper or the Supplementary material. Due to privacy regulations at ICES, raw data will not be made publicly available.
References
Author notes
Part of this work was presented to a meeting of the International Laparoscopic Liver Society held virtually in June 2021.



