-
PDF
- Split View
-
Views
-
Cite
Cite
Kiran K Rajan, Katherine Fairhurst, Beth Birkbeck, Shonnelly Novintan, Rebecca Wilson, Jelena Savović, Chris Holcombe, Shelley Potter, Overall survival after mastectomy versus breast-conserving surgery with adjuvant radiotherapy for early-stage breast cancer: meta-analysis, BJS Open, Volume 8, Issue 3, June 2024, zrae040, https://doi.org/10.1093/bjsopen/zrae040
Close - Share Icon Share
Abstract
Breast-conserving surgery with adjuvant radiotherapy and mastectomy are currently offered as equivalent surgical options for early-stage breast cancer based on RCTs from the 1970s and 1980s. However, the treatment of breast cancer has evolved and recent observational studies suggest a survival advantage for breast-conserving surgery with adjuvant radiotherapy. A systematic review and meta-analysis was undertaken to summarize the contemporary evidence regarding survival after breast-conserving surgery with adjuvant radiotherapy versus mastectomy for women with early-stage breast cancer.
A systematic search of MEDLINE, the Cochrane Central Register of Controlled Trials (CENTRAL), and Embase that identified studies published between 1 January 2000 and 18 December 2023 comparing overall survival after breast-conserving surgery with adjuvant radiotherapy versus mastectomy for patients with unilateral stage 1–3 breast cancer was undertaken. The main exclusion criteria were studies evaluating neoadjuvant chemotherapy, rare breast cancer subtypes, and specific breast cancer populations. The ROBINS-I tool was used to assess risk of bias, with the overall certainty of evidence assessed using the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) tool. Studies without critical risk of bias were included in a quantitative meta-analysis.
From 11 750 abstracts, 108 eligible articles were identified, with one article including two studies; 29 studies were excluded from the meta-analysis due to an overall critical risk of bias, 42 studies were excluded due to overlapping study populations, and three studies were excluded due to reporting incompatible results. A total of 35 observational studies reported survival outcomes for 909 077 patients (362 390 patients undergoing mastectomy and 546 687 patients undergoing breast-conserving surgery with adjuvant radiotherapy). The pooled HR was 0.72 (95% c.i. 0.68 to 0.75, P < 0.001), demonstrating improved overall survival for patients undergoing breast-conserving surgery with adjuvant radiotherapy. The overall certainty of the evidence was very low.
This meta-analysis provides evidence suggesting a survival advantage for women undergoing breast-conserving surgery with adjuvant radiotherapy for early-stage breast cancer compared with mastectomy. Although these results should be interpreted with caution, they should be shared with patients to support informed surgical decision-making.
Introduction
Breast cancer is the most common cancer worldwide, with over 2.2 million cases diagnosed in 20201. Treatment for early-stage breast cancer is multimodal and may include chemotherapy, endocrine therapy, and radiotherapy, but almost all women will undergo surgery as part of their treatment.
Currently, mastectomy and breast-conserving surgery with adjuvant radiotherapy (BCS + RT) are offered as comparable surgical options based on the results of two landmark RCTs that showed equivalent long-term survival outcomes for the procedures2,3. Since then, treatment for breast cancer has evolved, with improvements in systemic anticancer therapy and locoregional treatments4, and a recently published large-scale observational study suggests that BCS + RT may provide a survival benefit compared with mastectomy5. If BCS + RT offers women superior oncological outcomes compared with mastectomy, this information should be shared with patients as a key part of the decision-making process and may impact women’s treatment decisions.
The aim of this review was to systematically identify, appraise, and summarize the current literature on survival outcomes for patients undergoing mastectomy and BCS + RT to provide the most up-to-date evidence to support informed decision-making in the treatment of early-stage breast cancer.
Methods
Eligibility
All RCTs and observational studies published between 1 January 2000 and 18 December 2023 that compared overall survival for women undergoing BCS + RT versus mastectomy (with or without radiotherapy) for primary unilateral unifocal stage 1–3 breast cancer and having surgery as their first treatment were eligible for inclusion in the systematic review. Scoping work suggested that many observational studies included patients who did not receive adjuvant radiotherapy as part of the BCS + RT group; only studies for which greater than 95% of the BCS + RT cohort received adjuvant radiotherapy were therefore considered eligible for inclusion in the review. Studies published before 2000 were excluded as they were considered unlikely to reflect current practice. Letters, conference abstracts, reviews, and grey literature were excluded due to difficulty assessing incomplete information. Studies reporting outcomes for patients receiving neoadjuvant therapy were excluded, together with studies specifically reporting outcomes for specified breast cancer groups (for example pregnancy-related breast cancer, occult primary breast cancer, bilateral breast cancer, and prespecified rare subtypes of breast cancer). See Table 1. The titles and abstracts were screened independently by two reviewers (K.K.R., B.B. or S.N.) and the full texts were obtained if there was uncertainty. Disagreement was resolved by discussion with senior members of the team (K.F. and S.P.).
| Letters, correspondence, comments, conference reports, abstracts, and grey literature |
| Study not primarily comparing survival outcomes for women undergoing breast-conserving surgery with adjuvant radiotherapy versus mastectomy for early-stage (1–3) breast cancer with curative intent |
| Study published before 1 January 2000 |
| All study participants treated or diagnosed before 1 January 1990 |
| No oncological outcomes reported in the published article |
| Study not in humans |
| Study not published in English |
| Study sample size <10 |
| Studies solely reporting outcomes for specific patient groups |
| Patients receiving neoadjuvant therapy |
| Patients with occult breast cancers |
| Patients with bilateral, multifocal, or multicentric breast cancer |
| Patients with recurrent breast cancer |
| Patients with inflammatory breast cancer |
| Patients with pregnancy-related breast cancer |
| Patients with pre-specified rare subtypes of breast cancers |
| Patients with hereditary breast cancer |
| Patients with breast sarcomas |
| Patients undergoing breast-conserving surgery without adjuvant radiotherapy (studies excluded if ≤95% of the breast-conserving surgery with adjuvant radiotherapy cohort received adjuvant radiotherapy) |
| Patients not receiving standard of care (for example radical mastectomy) |
| Letters, correspondence, comments, conference reports, abstracts, and grey literature |
| Study not primarily comparing survival outcomes for women undergoing breast-conserving surgery with adjuvant radiotherapy versus mastectomy for early-stage (1–3) breast cancer with curative intent |
| Study published before 1 January 2000 |
| All study participants treated or diagnosed before 1 January 1990 |
| No oncological outcomes reported in the published article |
| Study not in humans |
| Study not published in English |
| Study sample size <10 |
| Studies solely reporting outcomes for specific patient groups |
| Patients receiving neoadjuvant therapy |
| Patients with occult breast cancers |
| Patients with bilateral, multifocal, or multicentric breast cancer |
| Patients with recurrent breast cancer |
| Patients with inflammatory breast cancer |
| Patients with pregnancy-related breast cancer |
| Patients with pre-specified rare subtypes of breast cancers |
| Patients with hereditary breast cancer |
| Patients with breast sarcomas |
| Patients undergoing breast-conserving surgery without adjuvant radiotherapy (studies excluded if ≤95% of the breast-conserving surgery with adjuvant radiotherapy cohort received adjuvant radiotherapy) |
| Patients not receiving standard of care (for example radical mastectomy) |
| Letters, correspondence, comments, conference reports, abstracts, and grey literature |
| Study not primarily comparing survival outcomes for women undergoing breast-conserving surgery with adjuvant radiotherapy versus mastectomy for early-stage (1–3) breast cancer with curative intent |
| Study published before 1 January 2000 |
| All study participants treated or diagnosed before 1 January 1990 |
| No oncological outcomes reported in the published article |
| Study not in humans |
| Study not published in English |
| Study sample size <10 |
| Studies solely reporting outcomes for specific patient groups |
| Patients receiving neoadjuvant therapy |
| Patients with occult breast cancers |
| Patients with bilateral, multifocal, or multicentric breast cancer |
| Patients with recurrent breast cancer |
| Patients with inflammatory breast cancer |
| Patients with pregnancy-related breast cancer |
| Patients with pre-specified rare subtypes of breast cancers |
| Patients with hereditary breast cancer |
| Patients with breast sarcomas |
| Patients undergoing breast-conserving surgery without adjuvant radiotherapy (studies excluded if ≤95% of the breast-conserving surgery with adjuvant radiotherapy cohort received adjuvant radiotherapy) |
| Patients not receiving standard of care (for example radical mastectomy) |
| Letters, correspondence, comments, conference reports, abstracts, and grey literature |
| Study not primarily comparing survival outcomes for women undergoing breast-conserving surgery with adjuvant radiotherapy versus mastectomy for early-stage (1–3) breast cancer with curative intent |
| Study published before 1 January 2000 |
| All study participants treated or diagnosed before 1 January 1990 |
| No oncological outcomes reported in the published article |
| Study not in humans |
| Study not published in English |
| Study sample size <10 |
| Studies solely reporting outcomes for specific patient groups |
| Patients receiving neoadjuvant therapy |
| Patients with occult breast cancers |
| Patients with bilateral, multifocal, or multicentric breast cancer |
| Patients with recurrent breast cancer |
| Patients with inflammatory breast cancer |
| Patients with pregnancy-related breast cancer |
| Patients with pre-specified rare subtypes of breast cancers |
| Patients with hereditary breast cancer |
| Patients with breast sarcomas |
| Patients undergoing breast-conserving surgery without adjuvant radiotherapy (studies excluded if ≤95% of the breast-conserving surgery with adjuvant radiotherapy cohort received adjuvant radiotherapy) |
| Patients not receiving standard of care (for example radical mastectomy) |
Search strategy
This systematic review was prospectively registered in PROSPERO, the international prospective register of systematic reviews (CRD42021248849)6, and is reported according to PRISMA guidelines7. The PRISMA checklist is available in the Supplementary material. A search of MEDLINE, the Cochrane Central Register of Controlled Trials (CENTRAL), and Embase was conducted on 18 December 2023. The search strategy was developed based on the eligibility criteria in collaboration with a specialist librarian. The search included terms for ‘breast cancer’ in combination with terms for surgical procedures of interest and ‘overall survival’ as the outcome of interest. The full search strategy is detailed in the Supplementary Methods.
Data extraction
A data extraction form was iteratively developed in Microsoft Excel by a clinician and a methodologist with expertise in evidence synthesis (R.W. and J.S.). Data collected included study characteristics, type of study, type of data collection, aim of study, study interval, population and setting studied, inclusion and exclusion criteria, sample size, treatment details, demographic data, statistical analysis performed, and reported overall survival. The completed data extraction forms were double-checked by the second reviewer (S.N.).
Bias assessment
The Risk of Bias 2 (RoB 2)8 and ROBINS-I9 tools were used to assess the risk of bias in RCTs and observational studies respectively. The bias was evaluated by two reviewers (K.K.R. and S.N.), with any disagreements resolved by discussion with senior members of the team (K.F. and S.P.). The Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) tool10 was used to assess the overall certainty of the evidence.
Outcomes
The primary outcome was overall survival for patients undergoing mastectomy and BCS + RT for early-stage breast cancer.
Statistical analysis
All studies, excluding those considered to be at critical risk of bias, were included in a quantitative meta-analysis. When studies reported on the same or overlapping population, such as registry-based studies, the study with the most recent and largest population was selected for inclusion. The primary meta-analysis included all eligible studies and used random effects with a common-effect inverse-variance model to produce a combined HR. A fixed-effect model was also conducted as a sensitivity analysis. A total of four subgroup analyses were conducted to explore survival outcomes for specific patient populations of interest: studies including patients with triple-negative breast cancer only; studies reporting outcomes for BCS + RT and mastectomy without radiotherapy; studies reporting outcomes for BCS + RT and mastectomy with radiotherapy; and studies of young patients (defined as those less than 50 years old at diagnosis). If a study reported outcomes for these defined groups separately the results were also included in the subgroup analyses. If studies reported outcomes other than HRs, and no data transformation was possible, they were excluded. Studies with unclear or inconsistent numbers of enrolled participants were also excluded from the meta-analysis. When results were presented separately in a study, they were combined using appropriate statistical methods, so that they could be included in the meta-analysis. Details of this process can be found in the Supplementary Methods. All analyses were conducted in STATA1711.
Results
Study selection
After de-duplication, 11 750 abstracts were identified; 189 underwent full-text review and, of these, 108 papers met the inclusion criteria. A paper by Kim et al.12 included separate analyses on two distinct databases and is therefore counted as two studies; these are referred to as Kim et al.12, 2021 (Korean Breast Cancer Registry) and Kim et al.12, 2021 (Asan Medical Center database) (see Table 2). No RCTs were identified. A total of 29 studies45–73 were excluded from the meta-analysis due to an overall critical risk of bias; 42 studies74–115 were excluded due to overlapping study populations and three studies116–118 were excluded due to reporting results incompatible with inclusion in the meta-analysis. Details regarding study selection are provided in Fig. 1.
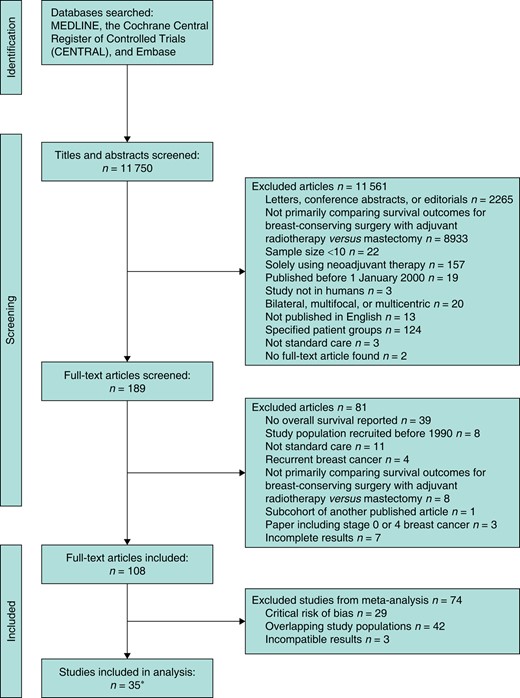
PRISMA flow diagram of the systematic review
*Paper by Kim et al.12 included separate analyses on two distinct databases and is therefore counted as two studies.
| Study . | Population setting . | No. of patients in total . | No. of patients undergoing breast-conserving surgery with adjuvant radiotherapy . | No. of patients undergoing mastectomy . | Breast cancer stage(s) . | Comparison intervention . | Overall bias . | HR (95% c.i.) . |
|---|---|---|---|---|---|---|---|---|
| Abdulkarim et al.13, 2011 | Alberta, Canada | 768 | 319 | 449 | 1, 2, and 3 | Mastectomy with or without radiotherapy | Serious | 0.92 (0.66, 1.26) |
| Adkins et al.14, 2011 | USA | 1325 | 651 | 674 | 1, 2, and 3 | Mastectomy with or without radiotherapy | Serious | 0.82 (0.68, 0.98) |
| Almahariq et al.15, 2020 | USA | 231 642 | 144 263 | 87 379 | 1 and 2 | Mastectomy alone | Serious | 0.71 (0.69, 0.73) |
| Bhoo-Pathy et al.16, 2015 | Asia | 1138 | 366 | 772 | 1, 2, and 3 | Mastectomy with radiotherapy | Serious | 0.60 (0.38, 2.45) |
| Braunstein et al.17, 2015 | USA | 98 | 46 | 52 | 1, 2, and 3 | Mastectomy with radiotherapy | Serious | 0.94 (0.36, 2.45) |
| Cao et al.18, 2014 | Canada | 965 | 616 | 349 | 1 and 2 | Mastectomy with or without radiotherapy | Serious | 0.88 (0.66, 1.18) |
| Chu et al.19, 2022 | USA | 214 128 | 125 025 | 89 103 | 1 and 2 | Mastectomy with or without radiotherapy | Serious | 0.69 (0.66, 0.72) |
| Corradini et al.20, 2019 | Munich, Germany | 7565 | 6412 | 1153 | 1 and 2 | Mastectomy alone | Serious | 0.79 (0.66, 0.95) |
| de Boniface et al.21, 2018 | Sweden | 2767 | 2338 | 429 | 1 and 3 | Mastectomy alone | Serious | 0.59 (0.48, 0.73) |
| de Boniface et al.22, 2021 | Sweden | 48 986 | 29 367 | 19 619 | 1, 2, and 3 | Mastectomy with or without radiotherapy | Serious | 0.64 (0.60, 0.68) |
| De Lorenzi et al.23, 2016 | Italy | 579 | 193 | 386 | 2 and 3 | Mastectomy with or without radiotherapy | Serious | 0.91 (0.52, 1.60) |
| De-la-Cruz-Ku et al.24, 2020 | Peru | 263 | 101 | 162 | 1 and 2 | Not stated | Serious | 0.79 (0.37, 1.67) |
| Grover et al.25, 2017 | USA | 150 171 | 98 952 | 51 219 | 1 and 2 | Mastectomy alone | Serious | 0.73 (0.70, 0.75) |
| Hartmann-Johnsen et al.26, 2015 | Norway | 13 015 | 8065 | 4950 | 1 and 2 | Mastectomy with or without radiotherapy | Serious | 0.61 (0.55, 0.67) |
| Horiguchi et al.27, 2002 | Japan | 228 | 119 | 109 | 1 and 2 | Mastectomy alone | Serious | 0.89 (0.36, 2.17) |
| Kim et al.12, 2021 (Korean Breast Cancer Registry)* | South Korea | 45 770 | 28 623 | 17 147 | 1 and 2 | Mastectomy alone | Serious | 0.56 (0.51, 0.60) |
| Kim et al.12, 2021 (Asan Medical Center database)* | South Korea | 10 016 | 6383 | 3633 | 1 and 2 | Mastectomy alone | Serious | 0.61 (0.51, 0.73) |
| Kim et al.28, 2011 | South Korea | 490 | 125 | 365 | 2 | Mastectomy alone | Serious | 0.54 (0.28, 1.07) |
| Lagendijk et al.5, 2018 | The Netherlands | 129 692 | 72 993 | 56 699 | 1, 2, and 3 | Mastectomy with or without radiotherapy | Serious | 0.72 (0.70, 0.74) |
| Laurberg et al.29, 2016 | Denmark | 813 | 298 | 515 | 1 and 2 | Mastectomy alone | Serious | 1.13 (0.86, 1.47) |
| Magnoni et al.30, 2023 | Italy | 3940 | 1970 | 1970 | 1, 2, and 3 | Mastectomy alone | Serious | 1.10 (0.90, 1.33) |
| Maishman et al.31, 2017 | UK | 2429 | 1240 | 1189 | 1, 2, and 3 | Mastectomy with or without radiotherapy | Serious | 0.79 (0.61, 1.03) |
| Parker et al.32, 2010 | USA | 202 | 61 | 141 | 1, 2, and 3 | Mastectomy with or without radiotherapy | Serious | 0.54 (0.22, 1.34) |
| Rapiti et al.33, 2003 | Switzerland | 989 | 780 | 209 | 1 | Not stated | Serious | 0.69 (0.46, 1.03) |
| Ratosa et al.34, 2021 | Slovenia | 1360 | 1021 | 339 | 1 and 2 | Mastectomy alone | Serious | 0.94 (0.50, 1.77) |
| Sayed et al.35, 2020 | Egypt | 434 | 254 | 180 | 1 and 2 | Mastectomy alone | Serious | 0.35 (0.15, 0.84) |
| Sinnadurai et al.36, 2019 | Malaysia, Singapore, and Hong Kong | 3536 | 1291 | 2245 | 1 and 2 | Mastectomy with or without radiotherapy | Serious | 0.81 (0.64, 1.03) |
| Sun et al.37, 2020 | China | 4262 | 404 | 3858 | 2 | Mastectomy with or without radiotherapy | Serious | 1.19 (0.72, 1.99) |
| Vasilyeva et al.38, 2023 | Canada | 13 914 | 8228 | 5686 | 1, 2, and 3 | Mastectomy with or without radiotherapy | Serious | 0.72 (0.65, 0.80) |
| Wan et al.39, 2021 | China | 7905 | 2956 | 4949 | 1, 2, and 3 | Mastectomy with or without radiotherapy | Serious | 0.58 (0.50, 0.68) |
| Wang et al.40, 2018 | China | 6137 | 1296 | 4841 | 1 and 2 | Mastectomy alone | Serious | 0.62 (0.40, 0.97) |
| Wen et al.41, 2020 | Australia | 214 | 168 | 46 | 1, 2, and 3 | Mastectomy with radiotherapy | Serious | 0.48 (0.18, 1.28) |
| Yoo et al.42, 2019 | South Korea | 1074 | 676 | 398 | 2 and 3 | Mastectomy alone | Serious | 0.60 (0.30, 1.20) |
| Yood et al.43, 2008 | USA | 1616 | 639 | 977 | 1 and 2 | Not stated | Serious | 1.00 (0.82, 1.21) |
| Zumsteg et al.44, 2013 | New York, USA | 646 | 448 | 198 | 1 and 2 | Mastectomy alone | Serious | 0.92 (0.53, 1.59) |
| Study . | Population setting . | No. of patients in total . | No. of patients undergoing breast-conserving surgery with adjuvant radiotherapy . | No. of patients undergoing mastectomy . | Breast cancer stage(s) . | Comparison intervention . | Overall bias . | HR (95% c.i.) . |
|---|---|---|---|---|---|---|---|---|
| Abdulkarim et al.13, 2011 | Alberta, Canada | 768 | 319 | 449 | 1, 2, and 3 | Mastectomy with or without radiotherapy | Serious | 0.92 (0.66, 1.26) |
| Adkins et al.14, 2011 | USA | 1325 | 651 | 674 | 1, 2, and 3 | Mastectomy with or without radiotherapy | Serious | 0.82 (0.68, 0.98) |
| Almahariq et al.15, 2020 | USA | 231 642 | 144 263 | 87 379 | 1 and 2 | Mastectomy alone | Serious | 0.71 (0.69, 0.73) |
| Bhoo-Pathy et al.16, 2015 | Asia | 1138 | 366 | 772 | 1, 2, and 3 | Mastectomy with radiotherapy | Serious | 0.60 (0.38, 2.45) |
| Braunstein et al.17, 2015 | USA | 98 | 46 | 52 | 1, 2, and 3 | Mastectomy with radiotherapy | Serious | 0.94 (0.36, 2.45) |
| Cao et al.18, 2014 | Canada | 965 | 616 | 349 | 1 and 2 | Mastectomy with or without radiotherapy | Serious | 0.88 (0.66, 1.18) |
| Chu et al.19, 2022 | USA | 214 128 | 125 025 | 89 103 | 1 and 2 | Mastectomy with or without radiotherapy | Serious | 0.69 (0.66, 0.72) |
| Corradini et al.20, 2019 | Munich, Germany | 7565 | 6412 | 1153 | 1 and 2 | Mastectomy alone | Serious | 0.79 (0.66, 0.95) |
| de Boniface et al.21, 2018 | Sweden | 2767 | 2338 | 429 | 1 and 3 | Mastectomy alone | Serious | 0.59 (0.48, 0.73) |
| de Boniface et al.22, 2021 | Sweden | 48 986 | 29 367 | 19 619 | 1, 2, and 3 | Mastectomy with or without radiotherapy | Serious | 0.64 (0.60, 0.68) |
| De Lorenzi et al.23, 2016 | Italy | 579 | 193 | 386 | 2 and 3 | Mastectomy with or without radiotherapy | Serious | 0.91 (0.52, 1.60) |
| De-la-Cruz-Ku et al.24, 2020 | Peru | 263 | 101 | 162 | 1 and 2 | Not stated | Serious | 0.79 (0.37, 1.67) |
| Grover et al.25, 2017 | USA | 150 171 | 98 952 | 51 219 | 1 and 2 | Mastectomy alone | Serious | 0.73 (0.70, 0.75) |
| Hartmann-Johnsen et al.26, 2015 | Norway | 13 015 | 8065 | 4950 | 1 and 2 | Mastectomy with or without radiotherapy | Serious | 0.61 (0.55, 0.67) |
| Horiguchi et al.27, 2002 | Japan | 228 | 119 | 109 | 1 and 2 | Mastectomy alone | Serious | 0.89 (0.36, 2.17) |
| Kim et al.12, 2021 (Korean Breast Cancer Registry)* | South Korea | 45 770 | 28 623 | 17 147 | 1 and 2 | Mastectomy alone | Serious | 0.56 (0.51, 0.60) |
| Kim et al.12, 2021 (Asan Medical Center database)* | South Korea | 10 016 | 6383 | 3633 | 1 and 2 | Mastectomy alone | Serious | 0.61 (0.51, 0.73) |
| Kim et al.28, 2011 | South Korea | 490 | 125 | 365 | 2 | Mastectomy alone | Serious | 0.54 (0.28, 1.07) |
| Lagendijk et al.5, 2018 | The Netherlands | 129 692 | 72 993 | 56 699 | 1, 2, and 3 | Mastectomy with or without radiotherapy | Serious | 0.72 (0.70, 0.74) |
| Laurberg et al.29, 2016 | Denmark | 813 | 298 | 515 | 1 and 2 | Mastectomy alone | Serious | 1.13 (0.86, 1.47) |
| Magnoni et al.30, 2023 | Italy | 3940 | 1970 | 1970 | 1, 2, and 3 | Mastectomy alone | Serious | 1.10 (0.90, 1.33) |
| Maishman et al.31, 2017 | UK | 2429 | 1240 | 1189 | 1, 2, and 3 | Mastectomy with or without radiotherapy | Serious | 0.79 (0.61, 1.03) |
| Parker et al.32, 2010 | USA | 202 | 61 | 141 | 1, 2, and 3 | Mastectomy with or without radiotherapy | Serious | 0.54 (0.22, 1.34) |
| Rapiti et al.33, 2003 | Switzerland | 989 | 780 | 209 | 1 | Not stated | Serious | 0.69 (0.46, 1.03) |
| Ratosa et al.34, 2021 | Slovenia | 1360 | 1021 | 339 | 1 and 2 | Mastectomy alone | Serious | 0.94 (0.50, 1.77) |
| Sayed et al.35, 2020 | Egypt | 434 | 254 | 180 | 1 and 2 | Mastectomy alone | Serious | 0.35 (0.15, 0.84) |
| Sinnadurai et al.36, 2019 | Malaysia, Singapore, and Hong Kong | 3536 | 1291 | 2245 | 1 and 2 | Mastectomy with or without radiotherapy | Serious | 0.81 (0.64, 1.03) |
| Sun et al.37, 2020 | China | 4262 | 404 | 3858 | 2 | Mastectomy with or without radiotherapy | Serious | 1.19 (0.72, 1.99) |
| Vasilyeva et al.38, 2023 | Canada | 13 914 | 8228 | 5686 | 1, 2, and 3 | Mastectomy with or without radiotherapy | Serious | 0.72 (0.65, 0.80) |
| Wan et al.39, 2021 | China | 7905 | 2956 | 4949 | 1, 2, and 3 | Mastectomy with or without radiotherapy | Serious | 0.58 (0.50, 0.68) |
| Wang et al.40, 2018 | China | 6137 | 1296 | 4841 | 1 and 2 | Mastectomy alone | Serious | 0.62 (0.40, 0.97) |
| Wen et al.41, 2020 | Australia | 214 | 168 | 46 | 1, 2, and 3 | Mastectomy with radiotherapy | Serious | 0.48 (0.18, 1.28) |
| Yoo et al.42, 2019 | South Korea | 1074 | 676 | 398 | 2 and 3 | Mastectomy alone | Serious | 0.60 (0.30, 1.20) |
| Yood et al.43, 2008 | USA | 1616 | 639 | 977 | 1 and 2 | Not stated | Serious | 1.00 (0.82, 1.21) |
| Zumsteg et al.44, 2013 | New York, USA | 646 | 448 | 198 | 1 and 2 | Mastectomy alone | Serious | 0.92 (0.53, 1.59) |
*Paper by Kim et al.12 included separate analyses on two distinct databases and is therefore counted as two studies.
| Study . | Population setting . | No. of patients in total . | No. of patients undergoing breast-conserving surgery with adjuvant radiotherapy . | No. of patients undergoing mastectomy . | Breast cancer stage(s) . | Comparison intervention . | Overall bias . | HR (95% c.i.) . |
|---|---|---|---|---|---|---|---|---|
| Abdulkarim et al.13, 2011 | Alberta, Canada | 768 | 319 | 449 | 1, 2, and 3 | Mastectomy with or without radiotherapy | Serious | 0.92 (0.66, 1.26) |
| Adkins et al.14, 2011 | USA | 1325 | 651 | 674 | 1, 2, and 3 | Mastectomy with or without radiotherapy | Serious | 0.82 (0.68, 0.98) |
| Almahariq et al.15, 2020 | USA | 231 642 | 144 263 | 87 379 | 1 and 2 | Mastectomy alone | Serious | 0.71 (0.69, 0.73) |
| Bhoo-Pathy et al.16, 2015 | Asia | 1138 | 366 | 772 | 1, 2, and 3 | Mastectomy with radiotherapy | Serious | 0.60 (0.38, 2.45) |
| Braunstein et al.17, 2015 | USA | 98 | 46 | 52 | 1, 2, and 3 | Mastectomy with radiotherapy | Serious | 0.94 (0.36, 2.45) |
| Cao et al.18, 2014 | Canada | 965 | 616 | 349 | 1 and 2 | Mastectomy with or without radiotherapy | Serious | 0.88 (0.66, 1.18) |
| Chu et al.19, 2022 | USA | 214 128 | 125 025 | 89 103 | 1 and 2 | Mastectomy with or without radiotherapy | Serious | 0.69 (0.66, 0.72) |
| Corradini et al.20, 2019 | Munich, Germany | 7565 | 6412 | 1153 | 1 and 2 | Mastectomy alone | Serious | 0.79 (0.66, 0.95) |
| de Boniface et al.21, 2018 | Sweden | 2767 | 2338 | 429 | 1 and 3 | Mastectomy alone | Serious | 0.59 (0.48, 0.73) |
| de Boniface et al.22, 2021 | Sweden | 48 986 | 29 367 | 19 619 | 1, 2, and 3 | Mastectomy with or without radiotherapy | Serious | 0.64 (0.60, 0.68) |
| De Lorenzi et al.23, 2016 | Italy | 579 | 193 | 386 | 2 and 3 | Mastectomy with or without radiotherapy | Serious | 0.91 (0.52, 1.60) |
| De-la-Cruz-Ku et al.24, 2020 | Peru | 263 | 101 | 162 | 1 and 2 | Not stated | Serious | 0.79 (0.37, 1.67) |
| Grover et al.25, 2017 | USA | 150 171 | 98 952 | 51 219 | 1 and 2 | Mastectomy alone | Serious | 0.73 (0.70, 0.75) |
| Hartmann-Johnsen et al.26, 2015 | Norway | 13 015 | 8065 | 4950 | 1 and 2 | Mastectomy with or without radiotherapy | Serious | 0.61 (0.55, 0.67) |
| Horiguchi et al.27, 2002 | Japan | 228 | 119 | 109 | 1 and 2 | Mastectomy alone | Serious | 0.89 (0.36, 2.17) |
| Kim et al.12, 2021 (Korean Breast Cancer Registry)* | South Korea | 45 770 | 28 623 | 17 147 | 1 and 2 | Mastectomy alone | Serious | 0.56 (0.51, 0.60) |
| Kim et al.12, 2021 (Asan Medical Center database)* | South Korea | 10 016 | 6383 | 3633 | 1 and 2 | Mastectomy alone | Serious | 0.61 (0.51, 0.73) |
| Kim et al.28, 2011 | South Korea | 490 | 125 | 365 | 2 | Mastectomy alone | Serious | 0.54 (0.28, 1.07) |
| Lagendijk et al.5, 2018 | The Netherlands | 129 692 | 72 993 | 56 699 | 1, 2, and 3 | Mastectomy with or without radiotherapy | Serious | 0.72 (0.70, 0.74) |
| Laurberg et al.29, 2016 | Denmark | 813 | 298 | 515 | 1 and 2 | Mastectomy alone | Serious | 1.13 (0.86, 1.47) |
| Magnoni et al.30, 2023 | Italy | 3940 | 1970 | 1970 | 1, 2, and 3 | Mastectomy alone | Serious | 1.10 (0.90, 1.33) |
| Maishman et al.31, 2017 | UK | 2429 | 1240 | 1189 | 1, 2, and 3 | Mastectomy with or without radiotherapy | Serious | 0.79 (0.61, 1.03) |
| Parker et al.32, 2010 | USA | 202 | 61 | 141 | 1, 2, and 3 | Mastectomy with or without radiotherapy | Serious | 0.54 (0.22, 1.34) |
| Rapiti et al.33, 2003 | Switzerland | 989 | 780 | 209 | 1 | Not stated | Serious | 0.69 (0.46, 1.03) |
| Ratosa et al.34, 2021 | Slovenia | 1360 | 1021 | 339 | 1 and 2 | Mastectomy alone | Serious | 0.94 (0.50, 1.77) |
| Sayed et al.35, 2020 | Egypt | 434 | 254 | 180 | 1 and 2 | Mastectomy alone | Serious | 0.35 (0.15, 0.84) |
| Sinnadurai et al.36, 2019 | Malaysia, Singapore, and Hong Kong | 3536 | 1291 | 2245 | 1 and 2 | Mastectomy with or without radiotherapy | Serious | 0.81 (0.64, 1.03) |
| Sun et al.37, 2020 | China | 4262 | 404 | 3858 | 2 | Mastectomy with or without radiotherapy | Serious | 1.19 (0.72, 1.99) |
| Vasilyeva et al.38, 2023 | Canada | 13 914 | 8228 | 5686 | 1, 2, and 3 | Mastectomy with or without radiotherapy | Serious | 0.72 (0.65, 0.80) |
| Wan et al.39, 2021 | China | 7905 | 2956 | 4949 | 1, 2, and 3 | Mastectomy with or without radiotherapy | Serious | 0.58 (0.50, 0.68) |
| Wang et al.40, 2018 | China | 6137 | 1296 | 4841 | 1 and 2 | Mastectomy alone | Serious | 0.62 (0.40, 0.97) |
| Wen et al.41, 2020 | Australia | 214 | 168 | 46 | 1, 2, and 3 | Mastectomy with radiotherapy | Serious | 0.48 (0.18, 1.28) |
| Yoo et al.42, 2019 | South Korea | 1074 | 676 | 398 | 2 and 3 | Mastectomy alone | Serious | 0.60 (0.30, 1.20) |
| Yood et al.43, 2008 | USA | 1616 | 639 | 977 | 1 and 2 | Not stated | Serious | 1.00 (0.82, 1.21) |
| Zumsteg et al.44, 2013 | New York, USA | 646 | 448 | 198 | 1 and 2 | Mastectomy alone | Serious | 0.92 (0.53, 1.59) |
| Study . | Population setting . | No. of patients in total . | No. of patients undergoing breast-conserving surgery with adjuvant radiotherapy . | No. of patients undergoing mastectomy . | Breast cancer stage(s) . | Comparison intervention . | Overall bias . | HR (95% c.i.) . |
|---|---|---|---|---|---|---|---|---|
| Abdulkarim et al.13, 2011 | Alberta, Canada | 768 | 319 | 449 | 1, 2, and 3 | Mastectomy with or without radiotherapy | Serious | 0.92 (0.66, 1.26) |
| Adkins et al.14, 2011 | USA | 1325 | 651 | 674 | 1, 2, and 3 | Mastectomy with or without radiotherapy | Serious | 0.82 (0.68, 0.98) |
| Almahariq et al.15, 2020 | USA | 231 642 | 144 263 | 87 379 | 1 and 2 | Mastectomy alone | Serious | 0.71 (0.69, 0.73) |
| Bhoo-Pathy et al.16, 2015 | Asia | 1138 | 366 | 772 | 1, 2, and 3 | Mastectomy with radiotherapy | Serious | 0.60 (0.38, 2.45) |
| Braunstein et al.17, 2015 | USA | 98 | 46 | 52 | 1, 2, and 3 | Mastectomy with radiotherapy | Serious | 0.94 (0.36, 2.45) |
| Cao et al.18, 2014 | Canada | 965 | 616 | 349 | 1 and 2 | Mastectomy with or without radiotherapy | Serious | 0.88 (0.66, 1.18) |
| Chu et al.19, 2022 | USA | 214 128 | 125 025 | 89 103 | 1 and 2 | Mastectomy with or without radiotherapy | Serious | 0.69 (0.66, 0.72) |
| Corradini et al.20, 2019 | Munich, Germany | 7565 | 6412 | 1153 | 1 and 2 | Mastectomy alone | Serious | 0.79 (0.66, 0.95) |
| de Boniface et al.21, 2018 | Sweden | 2767 | 2338 | 429 | 1 and 3 | Mastectomy alone | Serious | 0.59 (0.48, 0.73) |
| de Boniface et al.22, 2021 | Sweden | 48 986 | 29 367 | 19 619 | 1, 2, and 3 | Mastectomy with or without radiotherapy | Serious | 0.64 (0.60, 0.68) |
| De Lorenzi et al.23, 2016 | Italy | 579 | 193 | 386 | 2 and 3 | Mastectomy with or without radiotherapy | Serious | 0.91 (0.52, 1.60) |
| De-la-Cruz-Ku et al.24, 2020 | Peru | 263 | 101 | 162 | 1 and 2 | Not stated | Serious | 0.79 (0.37, 1.67) |
| Grover et al.25, 2017 | USA | 150 171 | 98 952 | 51 219 | 1 and 2 | Mastectomy alone | Serious | 0.73 (0.70, 0.75) |
| Hartmann-Johnsen et al.26, 2015 | Norway | 13 015 | 8065 | 4950 | 1 and 2 | Mastectomy with or without radiotherapy | Serious | 0.61 (0.55, 0.67) |
| Horiguchi et al.27, 2002 | Japan | 228 | 119 | 109 | 1 and 2 | Mastectomy alone | Serious | 0.89 (0.36, 2.17) |
| Kim et al.12, 2021 (Korean Breast Cancer Registry)* | South Korea | 45 770 | 28 623 | 17 147 | 1 and 2 | Mastectomy alone | Serious | 0.56 (0.51, 0.60) |
| Kim et al.12, 2021 (Asan Medical Center database)* | South Korea | 10 016 | 6383 | 3633 | 1 and 2 | Mastectomy alone | Serious | 0.61 (0.51, 0.73) |
| Kim et al.28, 2011 | South Korea | 490 | 125 | 365 | 2 | Mastectomy alone | Serious | 0.54 (0.28, 1.07) |
| Lagendijk et al.5, 2018 | The Netherlands | 129 692 | 72 993 | 56 699 | 1, 2, and 3 | Mastectomy with or without radiotherapy | Serious | 0.72 (0.70, 0.74) |
| Laurberg et al.29, 2016 | Denmark | 813 | 298 | 515 | 1 and 2 | Mastectomy alone | Serious | 1.13 (0.86, 1.47) |
| Magnoni et al.30, 2023 | Italy | 3940 | 1970 | 1970 | 1, 2, and 3 | Mastectomy alone | Serious | 1.10 (0.90, 1.33) |
| Maishman et al.31, 2017 | UK | 2429 | 1240 | 1189 | 1, 2, and 3 | Mastectomy with or without radiotherapy | Serious | 0.79 (0.61, 1.03) |
| Parker et al.32, 2010 | USA | 202 | 61 | 141 | 1, 2, and 3 | Mastectomy with or without radiotherapy | Serious | 0.54 (0.22, 1.34) |
| Rapiti et al.33, 2003 | Switzerland | 989 | 780 | 209 | 1 | Not stated | Serious | 0.69 (0.46, 1.03) |
| Ratosa et al.34, 2021 | Slovenia | 1360 | 1021 | 339 | 1 and 2 | Mastectomy alone | Serious | 0.94 (0.50, 1.77) |
| Sayed et al.35, 2020 | Egypt | 434 | 254 | 180 | 1 and 2 | Mastectomy alone | Serious | 0.35 (0.15, 0.84) |
| Sinnadurai et al.36, 2019 | Malaysia, Singapore, and Hong Kong | 3536 | 1291 | 2245 | 1 and 2 | Mastectomy with or without radiotherapy | Serious | 0.81 (0.64, 1.03) |
| Sun et al.37, 2020 | China | 4262 | 404 | 3858 | 2 | Mastectomy with or without radiotherapy | Serious | 1.19 (0.72, 1.99) |
| Vasilyeva et al.38, 2023 | Canada | 13 914 | 8228 | 5686 | 1, 2, and 3 | Mastectomy with or without radiotherapy | Serious | 0.72 (0.65, 0.80) |
| Wan et al.39, 2021 | China | 7905 | 2956 | 4949 | 1, 2, and 3 | Mastectomy with or without radiotherapy | Serious | 0.58 (0.50, 0.68) |
| Wang et al.40, 2018 | China | 6137 | 1296 | 4841 | 1 and 2 | Mastectomy alone | Serious | 0.62 (0.40, 0.97) |
| Wen et al.41, 2020 | Australia | 214 | 168 | 46 | 1, 2, and 3 | Mastectomy with radiotherapy | Serious | 0.48 (0.18, 1.28) |
| Yoo et al.42, 2019 | South Korea | 1074 | 676 | 398 | 2 and 3 | Mastectomy alone | Serious | 0.60 (0.30, 1.20) |
| Yood et al.43, 2008 | USA | 1616 | 639 | 977 | 1 and 2 | Not stated | Serious | 1.00 (0.82, 1.21) |
| Zumsteg et al.44, 2013 | New York, USA | 646 | 448 | 198 | 1 and 2 | Mastectomy alone | Serious | 0.92 (0.53, 1.59) |
*Paper by Kim et al.12 included separate analyses on two distinct databases and is therefore counted as two studies.
Study characteristics
The 35 studies (11 multicentre observational studies17,20,21,27,31,32,34,36,37,41,42, 10 single-centre observational studies12,14,23,24,28,30,35,39,40,44, and 14 registry-based observational studies5,12,13,15,16,18,19,22,25,26,29,33,38,43) included in the meta-analysis are summarized in Table 2 and reported overall survival for 909 077 patients (546 687 patients undergoing BCS + RT and 362 390 patients undergoing mastectomy with or without radiotherapy). A single study30 used a propensity score-matched patient cohort and another study23 matched patients by age, year of surgery, number of positive axillary lymph nodes, and tumour subtype.
Risk-of-bias assessment
All 35 included studies had a serious overall risk of bias (Table 2). Most studies (34 of 35 studies) were at high risk of bias due to confounding. Full details of the risk-of-bias assessment are provided in Table S1.
Survival outcomes for all studies comparing breast-conserving surgery with adjuvant radiotherapy versus mastectomy
The main random-effects model of the 35 included studies identified an overall survival advantage for patients undergoing BCS + RT compared with those undergoing mastectomy (pooled HR 0.72, 95% c.i. 0.68 to 0.75, P < 0.001, I2 76.3%) (Fig. 2). Similar results were seen in the fixed-effect model sensitivity analysis (HR 0.70, 95% c.i. 0.69 to 0.71, P < 0.001, I2 76.3%), but the overall evidence was deemed to be of very low certainty due to the serious risk of bias and inconsistency of reported results.
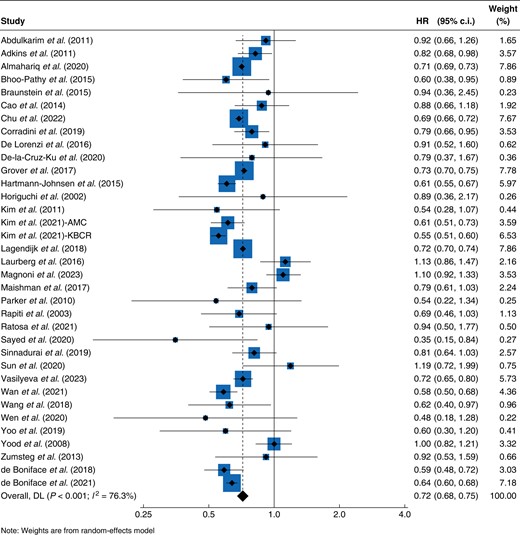
Forest plot of HRs for overall survival for breast-conserving surgery with adjuvant radiotherapy versus mastectomy
AMC, Asan Medical Center database; KBCR, Korean Breast Cancer Registry; DL, DerSimonian and Laird Method.
Survival outcomes for breast-conserving surgery with adjuvant radiotherapy versus mastectomy for triple-negative breast cancer
A total of nine studies13,14,16,19,24,32,36,41,44 with a total 26 530 patients (13 060 patients undergoing mastectomy and 13 470 patients undergoing BCS + RT) reported survival for patients with triple-negative breast cancer only, all of which had a serious overall risk of bias. Similar survival benefits were demonstrated for patients undergoing BCS + RT compared with those undergoing mastectomy in this group (HR 0.73, 95% c.i. 0.68 to 0.79, P < 0.001, I2 0.0%), consistent with the main analysis (Fig. S1).
Survival outcomes for breast-conserving surgery with adjuvant radiotherapy versus mastectomy with and without radiotherapy
A total of 21 studies12,13,15,16,20–22,25,27–30,34,35,39,40,42,44,101,113 including a total of 688 394 patients compared overall survival for 467 283 patients undergoing BCS + RT versus 221 111 patients undergoing mastectomy without radiotherapy. A study101 excluded from the main meta-analysis due to overlapping registry data was included in this subgroup analysis due to the other study38 not explicitly reporting outcomes for mastectomy without radiotherapy. All studies had a serious overall risk of bias. Again, similar survival benefits were seen for patients undergoing BCS + RT compared with patients undergoing mastectomy without radiotherapy (HR 0.70, 95% c.i. 0.65 to 0.75, P < 0.001, I2 84.2%) (Fig. S2).
A total of nine studies13,16,17,22,39,41,82,93,113 including a total of 218 392 patients specifically reported overall survival for women undergoing BCS + RT (194 368 patients) versus mastectomy with radiotherapy (24 024 patients). Three studies82,93,113 that were excluded from the main meta-analysis due to overlapping registry data were included in this subgroup analysis due to the other four studies15,19,25,43 not explicitly reporting outcomes for patients undergoing mastectomy and radiotherapy. All studies had a serious overall risk of bias. A survival benefit was also seen for patients undergoing BCS + RT compared with patients undergoing mastectomy with radiotherapy (HR 0.74, 95% c.i. 0.66 to 0.83, P < 0.001, I2 69.4%) (Fig. S3).
Survival outcomes for breast-conserving surgery with adjuvant radiotherapy versus mastectomy for young patients
A total of ten studies16,18,26,29,31,36,75,93,94,113 including a total of 63 976 patients specifically reported overall survival for younger women (less than 50 years old) undergoing BCS + RT (36 658 patients) versus mastectomy (27 318 patients). Three studies75,93,94 that were excluded from the main meta-analysis due to overlapping registry data were included in this subgroup analysis due to the other studies12,15,19,25 not explicitly reporting outcomes for younger patients. All studies had a serious overall risk of bias. Again, a survival benefit for young women undergoing BCS + RT compared with those undergoing mastectomy (HR 0.82, 95% c.i. 0.73 to 0.93, P = 0.002, I2 68.0%) was seen (Fig. S4).
Discussion
Mastectomy and BCS + RT have long been accepted as equivalent oncological options on the basis of landmark RCTs from the 1970s and 1980s2,3. However, the present meta-analysis of 35 contemporary studies published between 1 January 2000 and 18 December 2023 suggests that BCS + RT may confer a survival benefit for patients compared with mastectomy in the context of modern systemic and locoregional management of early-stage breast cancer. These results were consistent in studies of triple-negative breast cancer, in studies of patients less than 50 years old, and irrespective of whether or not patients with mastectomy received radiotherapy. The evidence overall was considered to be of very low certainty due to the high risk of bias in the included studies and high heterogeneity. Therefore, these findings should be interpreted with caution.
Despite this, the present analysis adds to an emerging body of evidence that consistently suggests superior survival outcomes for patients undergoing BCS + RT compared with those for patients undergoing mastectomy119. Another recent meta-analysis120 including all studies published up to October 2021 similarly showed improved survival for BCS + RT, with a relative risk of 0.64 (95% c.i. 0.55 to 0.74). The present study, by contrast, only included studies published between 1 January 2000 and 18 December 2023, with no study cohort solely treated before 1 January 1990, to ensure patients received modern treatments for breast cancer. The findings of the present study are consistent with a recent meta-analysis of 25 population cohort studies published from 2010 and onward, which also showed BCS + RT to be associated with better overall survival (HR 1.34, 95% c.i. 1.20 to 1.51) and breast cancer-specific survival (HR 1.38, 95% c.i. 1.29 to 1.47) compared with mastectomy121.
Similarly consistent results have been reported for specific patient subgroups. For example, a meta-analysis of seven studies122 exploring oncological outcomes for breast-conserving surgery versus mastectomy for patients with triple-negative breast cancer found a significantly lower mortality for breast-conserving surgery, as well as lower rates of locoregional recurrence and distant metastases. However, unlike the present study, only a minority of included studies reported outcomes for patients having adjuvant radiotherapy after mastectomy. While a meta-analysis of five studies exploring survival for patients under 40 years old undergoing BCT + RT versus mastectomy reported no significant survival advantage (HR 0.90, 95% c.i. 0.81 to 1.00, P = 0.150) for the BCS + RT group123, the present study that included patients receiving modern treatments from 10 studies did suggest a survival benefit for this subgroup. Therefore, it is possible that the earlier review123 was underpowered to detect a difference between the groups. The HR and 95% c.i. in the present study (HR 0.82, 95% c.i. 0.73 to 0.93, P = 0.002) remain closer to 1.00, indicating a potentially smaller, but still statistically significant, survival benefit for BCS + RT for young patients.
The mechanism for the survival benefits of BCS + RT compared with mastectomy is unclear. Improvements in systemic anticancer treatment and locoregional therapies in recent years may partially explain the benefits seen, but are unlikely to be the only driver for the survival advantage that is consistently demonstrated for BCS + RT; another potential explanation may be that breast-conserving surgery represents a smaller surgical insult, with fewer complications124 and less immune impairment, which is correlated with decreased proliferation of tumour cells and metastatic seeding125,126. Although the exact mechanism is not completely understood127, the present meta-analysis supports the hypothesis that more radical surgery in the form of total mastectomy is associated with worse overall survival.
Although the present meta-analysis provides further evidence to support survival benefits for BCS + RT compared with mastectomy, the results should be interpreted with caution. In the main analysis, significant heterogeneity between studies was seen. The included studies were selected from a time span of approximately 24 years and include different geographical locations, patient populations, study designs, such as registry-based versus single-institute retrospective observational reviews, and selection criteria for included patients. All of the studies included in the present review are observational, so there are likely to be inherent biases regarding the ways in which patients were selected for BCS + RT versus mastectomy. Patients are not randomized to treatment groups and therefore there may be many potential confounders, not all of which can be adjusted for in multivariable analyses. Important potential confounders, including socio-economic status, educational level, hospital volume, and geographical location, have been identified in a more detailed database128, and one North American study suggested that the cost of surgery was one of the main factors influencing women’s decision-making regarding the treatment of breast cancer129. Most studies did not adjust for all of these potential confounders, often because these data were not collected or available in the data set used. This is reflected in the risk-of-bias assessment, with all but one study having a severe risk of confounding. However, the consistent findings of this and other systematic reviews, in combination with the findings of large-scale studies5,38, suggest that these findings warrant further consideration.
The potential survival benefits highlighted in the present study have important implications for informed consent and surgical decision-making for patients with early-stage breast cancer. If the oncological outcomes after mastectomy are indeed inferior to those after BCS + RT, mastectomy should no longer be offered as an equivalent treatment option for those patients suitable for BCS + RT. However, more high-quality evidence is needed. RCTs comparing surgical techniques in this setting are no longer feasible or ethical, so well-designed prospective observational cohort studies, with careful consideration of potential confounders, will be needed to generate much-needed data. As large numbers of patients will need to be followed up over an extended interval of time, prospective studies involving linkage to routinely collected data sets may be one way in which this could be achieved.
The present review adds to the growing body of evidence suggesting that BCS + RT may be associated with a survival advantage compared with mastectomy for patients with early-stage breast cancer. While these findings must be interpreted with caution due to the high risk of bias associated with observational data, the consistency of findings across multiple studies is increasingly compelling. In addition to mastectomy offering potentially worse long-term survival compared with BCS + RT, mastectomy has a higher rate of complications124 and women undergoing mastectomy report worse quality of life than those undergoing BCS + RT, even if reconstruction is performed130. Therefore, these data should be discussed and shared with all patients who require breast cancer surgery to help them make fully informed decisions about their treatment options.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. This work was supported by the National Institute for Health and Care Research (NIHR) Biomedical Research Centre at University Hospitals Bristol and Weston NHS Foundation Trust and the University of Bristol. S.P. is an NIHR Clinician Scientist (CS-2016-16-019) and K.F. is an NIHR Academic Clinical Lecturer (CL-2020-25-002). J.S. and R.W.’s time was partly supported by the NIHR Applied Research Collaboration West (ARC West) at University Hospitals Bristol and Weston NHS Foundation Trust. The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care.
Author contributions
Kiran K. Rajan (Conceptualization, Investigation, Methodology, Validation, Writing—original draft), Katherine Fairhurst (Supervision, Methodology, Writing—review & editing), Beth Birkbeck (Investigation, Methodology, Writing—review & editing), Shonelly Novintan (Investigation, Methodology, Writing—review & editing), Rebecca Wilson (Conceptualization, Methodology, Writing—review & editing), Jelena Savović (Conceptualization, Methodology, Writing—review & editing), Chris Holcombe (Conceptualization, Writing—review & editing), and Shelley Potter (Conceptualization, Funding acquisition, Supervision, Methodology, Writing—review & editing)
Disclosure
The authors declare no conflict of interest.
Supplementary material
Supplementary material is available at BJS Open online.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its Supplementary material.
References
Author notes
Presented as a Spotlight Poster discussion at the San Antonio Breast Cancer Symposium, December 2022 and as an oral presentation at the Association of Breast Surgery Conference, Belfast, May 2023.



