-
PDF
- Split View
-
Views
-
Cite
Cite
Johannes Byrling, Sam Ghazi, Bodil Andersson, Tumour origin, diagnostic accuracy and histopathological evaluation in patients with periampullary cancer: nationwide cohort study, BJS Open, Volume 7, Issue 5, October 2023, zrad104, https://doi.org/10.1093/bjsopen/zrad104
Close - Share Icon Share
Abstract
The prevalence of different periampullary cancers (pancreatic ductal adenocarcinoma, distal cholangiocarcinoma, ampullary cancer and duodenal cancer) is heterogeneous in the literature. During the 2010s, a standardized histopathological protocol for pancreatoduodenectomy specimens based on axial slicing was adopted in Sweden. The present study sought to provide information about periampullary cancers with regard to tumour types in curative and noncurative settings, preoperative diagnostic accuracy and the impact of a standardized evaluation of the surgical specimen on diagnosis, R status and lymph node assessment.
Data from patients diagnosed with periampullary cancer from 2010 to 2019 were retrieved from the Swedish National Registry for Pancreatic and Periampullary Cancer.
Among non-curative patients, 3704 (83.6 per cent) were diagnosed with pancreatic ductal adenocarcinoma. Among patients treated with pancreatoduodenectomy, diagnosis was pancreatic ductal adenocarcinoma in 1380 (50.0 per cent), distal cholangiocarcinoma in 284 (10.3 per cent), ampullary cancer in 376 (13.6 per cent), duodenal cancer in 160 (5.8 per cent) and other diagnoses in 560 (20.3 per cent) patients. The preoperative diagnosis corresponded to the postoperative in 1177 (67.5 per cent) patients for pancreatic ductal adenocarcinoma, 162 (37.4 per cent) patients for distal cholangiocarcinoma, 220 (61.3 per cent) patients for ampullary cancer and 120 (53.6 per cent) patients for duodenal cancer. A higher rate of pancreatic ductal adenocarcinoma was seen in surgical specimens who underwent standardized evaluation, from 56.8 per cent to 64.3 per cent (P = 0.003). After standardization, higher rates of R1 resection (31.7 per cent versus 44.6 per cent, P < 0.001) and N1 stage (62.1 per cent versus 77.0 per cent, P < 0.001) were found.
The proportion of pancreatic ductal adenocarcinoma was higher in patients in a non-curative setting compared with patients who underwent surgery. The rate of misdiagnosis for periampullary cancers was confirmed to be high. Thus, it should be taken into account when preoperative oncological treatment is considered. Standardized evaluation of the surgical specimen has increased pancreatic ductal adenocarcinoma, R1 and N1 rates.
Introduction
Tumours originating anatomically in the periampullary region include pancreatic ductal adenocarcinoma (PDAC), distal cholangiocarcinoma (dCCA), ampullary cancer (AC) and duodenal cancer (DC)1. Pancreatoduodenectomy is always the procedure of choice to achieve oncological radicality, while pre- and postoperative treatment varies greatly according to the type of tumour2–4. To differentiate tumour types is not only difficult preoperatively5,6, but the evaluation of the surgical specimens also has its pitfalls. Indeed, in the current literature there is considerable heterogeneity in tumour type, R1 resection rate and positive lymph node rate described after pancreatoduodenectomy, presumably due to differences in the histopathological assessment of the specimen7. During the 2010s, a standardized protocol for histopathological examination was adopted in Sweden8. This protocol is based on the axial slicing technique described by Verbeke et al.9, which has previously been shown to increase the rate of non-pancreatic periampullary cancers and R1 resections compared with historical controls9,10. Pancreatic surgery was gradually centralized in Sweden during the 2010s11 and in 2016, the decision was made to centralize all pancreatic surgery to six university hospitals/regions.
The aim of the present study was two-fold: to evaluate the tumour type rate and diagnostic accuracy in patients with periampullary carcinomas both in curative and non-curative settings using the Swedish National Registry for Pancreatic and Periampullary Cancer and to assess the impact of a standardized evaluation on final pathology.
Methods
Patients and data collection
This population-based observational study was carried out according to the STROBE guidelines for cohort studies12. The data used in the present study were retrieved from the Swedish National Registry for Pancreatic and Periampullary Cancer. The registry was established in 2009 and prospectively collects data from patients with pancreatic and periampullary malignancies and from patients who undergo pancreatic surgery, regardless of diagnosis. Data regarding the diagnostic process, perioperative data, histopathology, oncological treatment and follow-up are recorded. The registry has shown a high data quality upon validation and coverage >90 per cent compared with the Swedish Cancer Registry since 2014. The registry structure and recorded variables has been previously described11. All consecutive patients diagnosed with a periampullary cancer or who underwent pancreatoduodenectomy due to suspicion of periampullary cancer between 2010 and 2019 were eligible for inclusion.
Definitions
The six regions where pancreatic surgery is performed in Sweden are Stockholm/Gotland (Region 1), Northern (Region 2), Uppsala/Orebro (Region 3), South-eastern (Region 4), Southern (Region 5) and Western (Region 6).
Periampullary cancers were included; for PDAC this implies only pancreatic tumours originating in caput pancreatis (C25.0), and for dCCA, AC and DC all registered cases.
Treatment intention was recorded as curative intent or non-curative intent based on the decision of the multidisciplinary team (MDT) conference and/or responsible physician. Staging was performed in accordance with the American Joint Committee on Cancer (AJCC) cancer staging manual 7th edition13. For comparison purposes, any patients with regional lymph node metastases present were considered N1, and the N2 category that was included for staging duodenal cancer in the AJCC 7th edition was not used for analysis. R1 resection was defined as microscopic cancer <1 mm from the resection margin, except for the anterior surface where growth on the surface (0 mm margin) was considered R1.
Since 2017, the registry required histopathological evaluation to be performed in accordance with the standardized protocol for registration. Prior to 2017, a separate variable coding whether registration was performed in accordance with standardization was recorded. For comparison, patients operated on since 2017 and forward as well as patients operated on between 2010 and 2016 and recorded as standardized were considered in the standardized group. During the study interval, neoadjuvant chemotherapy was not routinely used in Sweden in upfront resectable PDAC patients outside the clinical trial NorPACT-114. Preoperative chemotherapy was considered in borderline resectable patients and selected locally advanced patients according to the national comprehensive cancer network definition15,16.
Statistical analysis
Statistical analysis was performed using Stata MP statistical package version 14.2 for Mac OS X (Stata Corporation LP, College Station, TX, USA). Data are presented as medians with interquartile ranges (i.q.r.) for continuous variables and frequencies with proportions for categorical variables. Contingency tables were used for categorical variables with the associated x2 test. The Kruskal–Wallis H test was used to compare medians. All significance tests were two-tailed, and a P value of 0.05 was considered significant.
Ethical approval
The study was approved by the Regional Human Ethics Committee in Lund Sweden (Dnr 2015/392 and 2021/00622).
Results
Tumour type in the curative and non-curative setting
During the study interval, a total of 9143 patients with a diagnosis of periampullary tumour were recorded in the registry. At the time of diagnosis, 4668 (51.1 per cent) were eligible for treatment with curative intent, while 4428 (48.4 per cent) were not. Forty-seven patients (0.5 per cent) did not have treatment intention recorded. A flow chart of the patient pathway is presented in Fig. 1. For patients with non-curative intent, 3704 (83.6 per cent) were diagnosed with PDAC, 192 (4.3 per cent) with dCCA, 226 (5.1 per cent) with AC and 306 (6.9 per cent) with DC. Baseline characteristics of patients with non-curative intent at diagnosis are presented in Table 1. Treatment intentions were discussed at an MDT conference for 3401 (76.8 per cent) patients. In approximately half of them (N = 2238; 50.7 per cent), the diagnosis was based on radiological imaging, while in 2177 (49.3 per cent) a cytological/histological confirmation was available.
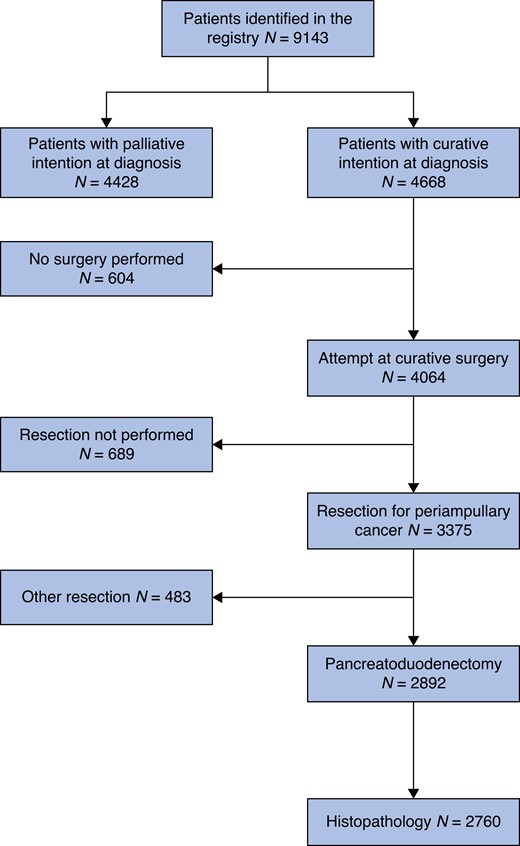
Flow chart describing the event pathway of patients from the Swedish National Registry for Pancreatic and Periampullary Cancer Missing values. Treatment intention 47, Histopathology 132.
Baseline characteristics of patients with periampullary cancer and non-curative treatment intention at diagnosis
| . | Total cohort N = 4428 . | PDAC N = 3704 (83.6) . | dCCA N = 192 (4.3) . | AC N = 226 (5.1) . | DC N = 306 (6.9) . |
|---|---|---|---|---|---|
| Variable | |||||
| Age (years) | 74 (67–81) | 73 (66–80) | 78 (70–83) | 81 (73–85) | 75 (68–83) |
| Female sex | 2230 (50.4) | 1903 (51.4) | 84 (43.8) | 109 (48.2) | 134 (43.8) |
| MDT conference | 3401 (76.8) | 2905 (78.4) | 143 (74.5) | 143 (63.3) | 210 (68.9) |
| Basis for diagnosis | |||||
| Imaging | 2238 (50.7) | 2042 (55.3) | 68 (35.4) | 61 (27.1) | 67 (22.0) |
| Cytology | 668 (15.1) | 575 (15.6) | 55 (28.7) | 20 (8.9) | 18 (5.9) |
| Histopathology | 1509 (34.2) | 1077 (29.2) | 69 (35.9) | 144 (64.0) | 219 (72.0) |
| . | Total cohort N = 4428 . | PDAC N = 3704 (83.6) . | dCCA N = 192 (4.3) . | AC N = 226 (5.1) . | DC N = 306 (6.9) . |
|---|---|---|---|---|---|
| Variable | |||||
| Age (years) | 74 (67–81) | 73 (66–80) | 78 (70–83) | 81 (73–85) | 75 (68–83) |
| Female sex | 2230 (50.4) | 1903 (51.4) | 84 (43.8) | 109 (48.2) | 134 (43.8) |
| MDT conference | 3401 (76.8) | 2905 (78.4) | 143 (74.5) | 143 (63.3) | 210 (68.9) |
| Basis for diagnosis | |||||
| Imaging | 2238 (50.7) | 2042 (55.3) | 68 (35.4) | 61 (27.1) | 67 (22.0) |
| Cytology | 668 (15.1) | 575 (15.6) | 55 (28.7) | 20 (8.9) | 18 (5.9) |
| Histopathology | 1509 (34.2) | 1077 (29.2) | 69 (35.9) | 144 (64.0) | 219 (72.0) |
Data are presented as absolute number (percentage) for categorical variables and median (interquartile range) for continuous variables. Missing: MDT conference 1, basis for diagnosis 13. AC, ampullary cancer; dCCA, distal cholangiocarcinoma; DC, duodenal cancer; MDT, multidisciplinary team; PDAC, pancreatic ductal adenocarcinoma.
Baseline characteristics of patients with periampullary cancer and non-curative treatment intention at diagnosis
| . | Total cohort N = 4428 . | PDAC N = 3704 (83.6) . | dCCA N = 192 (4.3) . | AC N = 226 (5.1) . | DC N = 306 (6.9) . |
|---|---|---|---|---|---|
| Variable | |||||
| Age (years) | 74 (67–81) | 73 (66–80) | 78 (70–83) | 81 (73–85) | 75 (68–83) |
| Female sex | 2230 (50.4) | 1903 (51.4) | 84 (43.8) | 109 (48.2) | 134 (43.8) |
| MDT conference | 3401 (76.8) | 2905 (78.4) | 143 (74.5) | 143 (63.3) | 210 (68.9) |
| Basis for diagnosis | |||||
| Imaging | 2238 (50.7) | 2042 (55.3) | 68 (35.4) | 61 (27.1) | 67 (22.0) |
| Cytology | 668 (15.1) | 575 (15.6) | 55 (28.7) | 20 (8.9) | 18 (5.9) |
| Histopathology | 1509 (34.2) | 1077 (29.2) | 69 (35.9) | 144 (64.0) | 219 (72.0) |
| . | Total cohort N = 4428 . | PDAC N = 3704 (83.6) . | dCCA N = 192 (4.3) . | AC N = 226 (5.1) . | DC N = 306 (6.9) . |
|---|---|---|---|---|---|
| Variable | |||||
| Age (years) | 74 (67–81) | 73 (66–80) | 78 (70–83) | 81 (73–85) | 75 (68–83) |
| Female sex | 2230 (50.4) | 1903 (51.4) | 84 (43.8) | 109 (48.2) | 134 (43.8) |
| MDT conference | 3401 (76.8) | 2905 (78.4) | 143 (74.5) | 143 (63.3) | 210 (68.9) |
| Basis for diagnosis | |||||
| Imaging | 2238 (50.7) | 2042 (55.3) | 68 (35.4) | 61 (27.1) | 67 (22.0) |
| Cytology | 668 (15.1) | 575 (15.6) | 55 (28.7) | 20 (8.9) | 18 (5.9) |
| Histopathology | 1509 (34.2) | 1077 (29.2) | 69 (35.9) | 144 (64.0) | 219 (72.0) |
Data are presented as absolute number (percentage) for categorical variables and median (interquartile range) for continuous variables. Missing: MDT conference 1, basis for diagnosis 13. AC, ampullary cancer; dCCA, distal cholangiocarcinoma; DC, duodenal cancer; MDT, multidisciplinary team; PDAC, pancreatic ductal adenocarcinoma.
In patients eligible for surgery, the preoperative diagnosis was PDAC in 3057 (65.5 per cent), dCCA in 624 (13.4 per cent), AC in 480 (10.3 per cent) and DC in 507 (10.9 per cent). Treatment was discussed at an MDT conference for 4392 (94.1 per cent) patients. Some 4064 (87.1 per cent) patients underwent surgical exploration, 3375 (83.0 per cent) of which underwent resection. Some 3836 (94.1 per cent) patients had a histological diagnosis (either based on biopsy or resected specimen) recorded. Data on histological diagnosis stratified on whether resection was performed or not is presented in Table S1. Notably, 435 (73.7 per cent) patients who were unresectable at surgical exploration were diagnosed with PDAC compared with 1597 (49.2 per cent) of resected patients.
Tumour type in patients who underwent pancreatoduodenectomy
Pancreatoduodenectomy for presumed periampullary cancers was performed in 2892 patients, 2760 (95.4 per cent) of which had available histopathological data. The final histopathological diagnosis was PDAC in 1380 (50.0 per cent), dCCA in 284 (10.3 per cent), AC in 376 (13.6 per cent) and DC in 160 (5.8 per cent) patients. Endocrine cancer and metastases from other cancers were found in 44 (1.6 per cent) and 24 (0.9 per cent) patients respectively. Benign or premalignant conditions were found in 435 (15.7 per cent) patients. Other diagnoses accounted for 57 (2.1 per cent) patients. Baseline characteristics of the patients with resected periampullary cancers are presented in Table 2. Preoperative chemotherapy was administered in 69 (5.0 per cent) PDAC, 7 (2.5 per cent) dCCA, 6 (1.6 per cent) AC and 14 (8.8 per cent) DC patients. In patients who received preoperative treatment, the final histopathological diagnosis was concordant with preoperative diagnosis in 64 of 70 (91.4 per cent) PDAC patients, 3 of 7 (42.9 per cent) dCCA patients, 2 of 3 (66.7 per cent) AC patients and 13 of 16 (81.3 per cent) DC patients.
Clinicopathological characteristics of patients who underwent pancreatoduodenectomy for periampullary cancer confirmed at final pathology
| . | PDAC N = 1380 . | dCCA N = 284 . | AC N = 376 . | DC N = 160 . |
|---|---|---|---|---|
| Clinical data | ||||
| Age (years) | 69 (63–74) | 69 (63–74) | 69 (62–74) | 68 (64–72) |
| Female sex | 662 (48.0) | 108 (38.0) | 161 (42.8) | 67 (41.9) |
| BMI (kg/m2) | 25 (22–27) | 25 (23–28) | 24 (22–28) | 25 (23–28) |
| Weight loss | 821 (60.4) | 149 (52.8) | 202 (55.0) | 75 (48.4) |
| Smoking history | 215 (16.0) | 43 (15.5) | 47 (13.1) | 20 (13.5) |
| Diabetes mellitus | 320 (23.3) | 53 (18.8) | 72 (19.5) | 17 (10.8) |
| PBD | 1026 (74.7) | 245 (86.6) | 273 (74.0) | 36 (22.9) |
| ASA score > II | 358 (26.0) | 73 (26.0) | 99 (26.9) | 44 (27.7) |
| Preoperative therapy | 69 (5.0) | 7 (2.5) | 6 (1.6) | 14 (8.8) |
| Cystic tumour | 79 (6.0) | 3 (1.2) | 7 (2.1) | 4 (2.7) |
| Preoperative lab | ||||
| Haemoglobin (g/l) | 128 (118–136) | 130 (120–141) | 128 (118–137) | 120 (104–134) |
| Bilirubin (µmol/l) | 20 (10–45) | 16 (10–38) | 15 (8–36) | 7 (5–14) |
| CRP (mg/l) | 6 (3–14) | 7 (2–15) | 5 (2–13) | 4 (2–12) |
| CA 19-9 (kU/l) | 139 (36–503) | 69 (24–177) | 38 (14–131) | 16 (8–71) |
| Histopathology | ||||
| T stage | ||||
| I | 66 (4.8) | 24 (8.5) | 53 (14.2) | 11 (7.6) |
| II | 259 (18.9) | 52 (18.4) | 95 (25.5) | 21 (14.5) |
| III | 1014 (73.8) | 202 (71.4) | 148 (39.7) | 43 (29.7) |
| IV | 35 (2.6) | 5 (1.8) | 77 (20.6) | 70 (48.3) |
| N stage | ||||
| I | 1089 (79.3) | 181 (64.2) | 245 (65.2) | 101 (64.7) |
| No. of lymph node metastases | 4 (2–7) | 3 (2–5) | 3 (2–6) | 4 (2–7) |
| No. of resected nodes | 22 (16–30) | 21 (16–29) | 21 (15–28) | 20 (15–29) |
| R1 resection | 716 (52.4) | 104 (37.0) | 57 (15.4) | 34 (21.7) |
| . | PDAC N = 1380 . | dCCA N = 284 . | AC N = 376 . | DC N = 160 . |
|---|---|---|---|---|
| Clinical data | ||||
| Age (years) | 69 (63–74) | 69 (63–74) | 69 (62–74) | 68 (64–72) |
| Female sex | 662 (48.0) | 108 (38.0) | 161 (42.8) | 67 (41.9) |
| BMI (kg/m2) | 25 (22–27) | 25 (23–28) | 24 (22–28) | 25 (23–28) |
| Weight loss | 821 (60.4) | 149 (52.8) | 202 (55.0) | 75 (48.4) |
| Smoking history | 215 (16.0) | 43 (15.5) | 47 (13.1) | 20 (13.5) |
| Diabetes mellitus | 320 (23.3) | 53 (18.8) | 72 (19.5) | 17 (10.8) |
| PBD | 1026 (74.7) | 245 (86.6) | 273 (74.0) | 36 (22.9) |
| ASA score > II | 358 (26.0) | 73 (26.0) | 99 (26.9) | 44 (27.7) |
| Preoperative therapy | 69 (5.0) | 7 (2.5) | 6 (1.6) | 14 (8.8) |
| Cystic tumour | 79 (6.0) | 3 (1.2) | 7 (2.1) | 4 (2.7) |
| Preoperative lab | ||||
| Haemoglobin (g/l) | 128 (118–136) | 130 (120–141) | 128 (118–137) | 120 (104–134) |
| Bilirubin (µmol/l) | 20 (10–45) | 16 (10–38) | 15 (8–36) | 7 (5–14) |
| CRP (mg/l) | 6 (3–14) | 7 (2–15) | 5 (2–13) | 4 (2–12) |
| CA 19-9 (kU/l) | 139 (36–503) | 69 (24–177) | 38 (14–131) | 16 (8–71) |
| Histopathology | ||||
| T stage | ||||
| I | 66 (4.8) | 24 (8.5) | 53 (14.2) | 11 (7.6) |
| II | 259 (18.9) | 52 (18.4) | 95 (25.5) | 21 (14.5) |
| III | 1014 (73.8) | 202 (71.4) | 148 (39.7) | 43 (29.7) |
| IV | 35 (2.6) | 5 (1.8) | 77 (20.6) | 70 (48.3) |
| N stage | ||||
| I | 1089 (79.3) | 181 (64.2) | 245 (65.2) | 101 (64.7) |
| No. of lymph node metastases | 4 (2–7) | 3 (2–5) | 3 (2–6) | 4 (2–7) |
| No. of resected nodes | 22 (16–30) | 21 (16–29) | 21 (15–28) | 20 (15–29) |
| R1 resection | 716 (52.4) | 104 (37.0) | 57 (15.4) | 34 (21.7) |
Data are presented as absolute number (percentage) for categorical variables and median (interquartile range) for continuous variables. Missing values: BMI 83, weight loss 36, smoking history 67, diabetes mellitus 16, PBD 17, ASA score 17, preoperative therapy 5, cystic lesion 148, haemoglobin 76, bilirubin 90, CRP 194, CA 19-9 658, T stage 14, N stage 67, number of lymph node metastases 24, number of resected nodes 13, R1 resection 25. AC, ampullary cancer; ASA, American Society of Anesthesiologists; BMI, body mass index; CA 19-9, carbohydrate antigen; CRP, C-reactive protein; dCCA, distal cholangiocarcinoma; DC, duodenal cancer; PDAC, pancreatic ductal adenocarcinoma; PBD, preoperative biliary drainage.
Clinicopathological characteristics of patients who underwent pancreatoduodenectomy for periampullary cancer confirmed at final pathology
| . | PDAC N = 1380 . | dCCA N = 284 . | AC N = 376 . | DC N = 160 . |
|---|---|---|---|---|
| Clinical data | ||||
| Age (years) | 69 (63–74) | 69 (63–74) | 69 (62–74) | 68 (64–72) |
| Female sex | 662 (48.0) | 108 (38.0) | 161 (42.8) | 67 (41.9) |
| BMI (kg/m2) | 25 (22–27) | 25 (23–28) | 24 (22–28) | 25 (23–28) |
| Weight loss | 821 (60.4) | 149 (52.8) | 202 (55.0) | 75 (48.4) |
| Smoking history | 215 (16.0) | 43 (15.5) | 47 (13.1) | 20 (13.5) |
| Diabetes mellitus | 320 (23.3) | 53 (18.8) | 72 (19.5) | 17 (10.8) |
| PBD | 1026 (74.7) | 245 (86.6) | 273 (74.0) | 36 (22.9) |
| ASA score > II | 358 (26.0) | 73 (26.0) | 99 (26.9) | 44 (27.7) |
| Preoperative therapy | 69 (5.0) | 7 (2.5) | 6 (1.6) | 14 (8.8) |
| Cystic tumour | 79 (6.0) | 3 (1.2) | 7 (2.1) | 4 (2.7) |
| Preoperative lab | ||||
| Haemoglobin (g/l) | 128 (118–136) | 130 (120–141) | 128 (118–137) | 120 (104–134) |
| Bilirubin (µmol/l) | 20 (10–45) | 16 (10–38) | 15 (8–36) | 7 (5–14) |
| CRP (mg/l) | 6 (3–14) | 7 (2–15) | 5 (2–13) | 4 (2–12) |
| CA 19-9 (kU/l) | 139 (36–503) | 69 (24–177) | 38 (14–131) | 16 (8–71) |
| Histopathology | ||||
| T stage | ||||
| I | 66 (4.8) | 24 (8.5) | 53 (14.2) | 11 (7.6) |
| II | 259 (18.9) | 52 (18.4) | 95 (25.5) | 21 (14.5) |
| III | 1014 (73.8) | 202 (71.4) | 148 (39.7) | 43 (29.7) |
| IV | 35 (2.6) | 5 (1.8) | 77 (20.6) | 70 (48.3) |
| N stage | ||||
| I | 1089 (79.3) | 181 (64.2) | 245 (65.2) | 101 (64.7) |
| No. of lymph node metastases | 4 (2–7) | 3 (2–5) | 3 (2–6) | 4 (2–7) |
| No. of resected nodes | 22 (16–30) | 21 (16–29) | 21 (15–28) | 20 (15–29) |
| R1 resection | 716 (52.4) | 104 (37.0) | 57 (15.4) | 34 (21.7) |
| . | PDAC N = 1380 . | dCCA N = 284 . | AC N = 376 . | DC N = 160 . |
|---|---|---|---|---|
| Clinical data | ||||
| Age (years) | 69 (63–74) | 69 (63–74) | 69 (62–74) | 68 (64–72) |
| Female sex | 662 (48.0) | 108 (38.0) | 161 (42.8) | 67 (41.9) |
| BMI (kg/m2) | 25 (22–27) | 25 (23–28) | 24 (22–28) | 25 (23–28) |
| Weight loss | 821 (60.4) | 149 (52.8) | 202 (55.0) | 75 (48.4) |
| Smoking history | 215 (16.0) | 43 (15.5) | 47 (13.1) | 20 (13.5) |
| Diabetes mellitus | 320 (23.3) | 53 (18.8) | 72 (19.5) | 17 (10.8) |
| PBD | 1026 (74.7) | 245 (86.6) | 273 (74.0) | 36 (22.9) |
| ASA score > II | 358 (26.0) | 73 (26.0) | 99 (26.9) | 44 (27.7) |
| Preoperative therapy | 69 (5.0) | 7 (2.5) | 6 (1.6) | 14 (8.8) |
| Cystic tumour | 79 (6.0) | 3 (1.2) | 7 (2.1) | 4 (2.7) |
| Preoperative lab | ||||
| Haemoglobin (g/l) | 128 (118–136) | 130 (120–141) | 128 (118–137) | 120 (104–134) |
| Bilirubin (µmol/l) | 20 (10–45) | 16 (10–38) | 15 (8–36) | 7 (5–14) |
| CRP (mg/l) | 6 (3–14) | 7 (2–15) | 5 (2–13) | 4 (2–12) |
| CA 19-9 (kU/l) | 139 (36–503) | 69 (24–177) | 38 (14–131) | 16 (8–71) |
| Histopathology | ||||
| T stage | ||||
| I | 66 (4.8) | 24 (8.5) | 53 (14.2) | 11 (7.6) |
| II | 259 (18.9) | 52 (18.4) | 95 (25.5) | 21 (14.5) |
| III | 1014 (73.8) | 202 (71.4) | 148 (39.7) | 43 (29.7) |
| IV | 35 (2.6) | 5 (1.8) | 77 (20.6) | 70 (48.3) |
| N stage | ||||
| I | 1089 (79.3) | 181 (64.2) | 245 (65.2) | 101 (64.7) |
| No. of lymph node metastases | 4 (2–7) | 3 (2–5) | 3 (2–6) | 4 (2–7) |
| No. of resected nodes | 22 (16–30) | 21 (16–29) | 21 (15–28) | 20 (15–29) |
| R1 resection | 716 (52.4) | 104 (37.0) | 57 (15.4) | 34 (21.7) |
Data are presented as absolute number (percentage) for categorical variables and median (interquartile range) for continuous variables. Missing values: BMI 83, weight loss 36, smoking history 67, diabetes mellitus 16, PBD 17, ASA score 17, preoperative therapy 5, cystic lesion 148, haemoglobin 76, bilirubin 90, CRP 194, CA 19-9 658, T stage 14, N stage 67, number of lymph node metastases 24, number of resected nodes 13, R1 resection 25. AC, ampullary cancer; ASA, American Society of Anesthesiologists; BMI, body mass index; CA 19-9, carbohydrate antigen; CRP, C-reactive protein; dCCA, distal cholangiocarcinoma; DC, duodenal cancer; PDAC, pancreatic ductal adenocarcinoma; PBD, preoperative biliary drainage.
Diagnostic accuracy
The preoperative diagnosis corresponded to the histopathological diagnosis in 1177 of 1744 (67.5 per cent) patients for PDAC, 162 of 433 (37.4 per cent) patients for dCCA, 220 of 359 (61.3 per cent) patients for AC and 120 of 224 (53.6 per cent) patients for DC (Table 3). The diagnostic accuracy increased slightly when restricting the analysis to tumours classified as solid lesions preoperatively (Table 4). Among patients with PDAC on final histology 203 of 1380 (14.7 per cent) had a preoperative diagnosis of non-pancreatic periampullary cancer. Among patients with non-pancreatic periampullary cancer 186 of 820 (22.7 per cent) had PDAC as a preoperative diagnosis.
Cross tabulation of pre- and postoperative diagnosis for patients treated with pancreatoduodenectomy
| Postoperative diagnosis . | PDAC N = 1380 . | dCCA N = 284 . | AC N = 376 . | DC N = 160 . | Endocrine cancer/metastases from other cancers N = 68 . | Premalignant/benign N = 435 . | Other N = 57 . |
|---|---|---|---|---|---|---|---|
| Preoperative diagnosis | |||||||
| PDAC N = 1744 | 1177 (67.5) | 106 (6.1) | 66 (3.8) | 14 (0.8) | 55 (3.2) | 300 (17.2) | 26 (1.5) |
| dCCA N = 433 | 128 (29.6) | 162 (37.4) | 58 (13.4) | 4 (0.9) | 6 (1.4) | 60 (13.9) | 15 (3.5) |
| AC N = 359 | 51 (14.2) | 14 (3.9) | 220 (61.3) | 22 (6.1) | 1 (0.3) | 45 (12.5) | 6 (1.7) |
| DC N = 224 | 24 (10.7) | 2 (0.9) | 32 (14.3) | 120 (53.6) | 6 (2.7) | 30 (13.4) | 10 (4.5) |
| Postoperative diagnosis . | PDAC N = 1380 . | dCCA N = 284 . | AC N = 376 . | DC N = 160 . | Endocrine cancer/metastases from other cancers N = 68 . | Premalignant/benign N = 435 . | Other N = 57 . |
|---|---|---|---|---|---|---|---|
| Preoperative diagnosis | |||||||
| PDAC N = 1744 | 1177 (67.5) | 106 (6.1) | 66 (3.8) | 14 (0.8) | 55 (3.2) | 300 (17.2) | 26 (1.5) |
| dCCA N = 433 | 128 (29.6) | 162 (37.4) | 58 (13.4) | 4 (0.9) | 6 (1.4) | 60 (13.9) | 15 (3.5) |
| AC N = 359 | 51 (14.2) | 14 (3.9) | 220 (61.3) | 22 (6.1) | 1 (0.3) | 45 (12.5) | 6 (1.7) |
| DC N = 224 | 24 (10.7) | 2 (0.9) | 32 (14.3) | 120 (53.6) | 6 (2.7) | 30 (13.4) | 10 (4.5) |
Data are presented as absolute number (percentage). The percentage in the cells is the preoperative diagnosis concordance with postoperative histopathological diagnosis. The concordance ratio for each cancer type is highlighted in bold. AC, ampullary cancer; dCCA, distal cholangiocarcinoma; DC, duodenal cancer; PDAC, pancreatic ductal adenocarcinoma.
Cross tabulation of pre- and postoperative diagnosis for patients treated with pancreatoduodenectomy
| Postoperative diagnosis . | PDAC N = 1380 . | dCCA N = 284 . | AC N = 376 . | DC N = 160 . | Endocrine cancer/metastases from other cancers N = 68 . | Premalignant/benign N = 435 . | Other N = 57 . |
|---|---|---|---|---|---|---|---|
| Preoperative diagnosis | |||||||
| PDAC N = 1744 | 1177 (67.5) | 106 (6.1) | 66 (3.8) | 14 (0.8) | 55 (3.2) | 300 (17.2) | 26 (1.5) |
| dCCA N = 433 | 128 (29.6) | 162 (37.4) | 58 (13.4) | 4 (0.9) | 6 (1.4) | 60 (13.9) | 15 (3.5) |
| AC N = 359 | 51 (14.2) | 14 (3.9) | 220 (61.3) | 22 (6.1) | 1 (0.3) | 45 (12.5) | 6 (1.7) |
| DC N = 224 | 24 (10.7) | 2 (0.9) | 32 (14.3) | 120 (53.6) | 6 (2.7) | 30 (13.4) | 10 (4.5) |
| Postoperative diagnosis . | PDAC N = 1380 . | dCCA N = 284 . | AC N = 376 . | DC N = 160 . | Endocrine cancer/metastases from other cancers N = 68 . | Premalignant/benign N = 435 . | Other N = 57 . |
|---|---|---|---|---|---|---|---|
| Preoperative diagnosis | |||||||
| PDAC N = 1744 | 1177 (67.5) | 106 (6.1) | 66 (3.8) | 14 (0.8) | 55 (3.2) | 300 (17.2) | 26 (1.5) |
| dCCA N = 433 | 128 (29.6) | 162 (37.4) | 58 (13.4) | 4 (0.9) | 6 (1.4) | 60 (13.9) | 15 (3.5) |
| AC N = 359 | 51 (14.2) | 14 (3.9) | 220 (61.3) | 22 (6.1) | 1 (0.3) | 45 (12.5) | 6 (1.7) |
| DC N = 224 | 24 (10.7) | 2 (0.9) | 32 (14.3) | 120 (53.6) | 6 (2.7) | 30 (13.4) | 10 (4.5) |
Data are presented as absolute number (percentage). The percentage in the cells is the preoperative diagnosis concordance with postoperative histopathological diagnosis. The concordance ratio for each cancer type is highlighted in bold. AC, ampullary cancer; dCCA, distal cholangiocarcinoma; DC, duodenal cancer; PDAC, pancreatic ductal adenocarcinoma.
Cross-tabulation of pre- and postoperative diagnosis for patients treated with pancreatoduodenectomy restricted to patients with a solid tumour preoperatively
| Postoperative diagnosis . | PDAC N = 1239 . | dCCA N = 247 . | AC N = 331 . | DC N = 142 . | Endocrine cancer/metastases from other cancers N = 62 . | Premalignant/benign N = 213 . | Other N = 40 . |
|---|---|---|---|---|---|---|---|
| Preoperative diagnosis | |||||||
| PDAC N = 1380 | 1059 (76.7) | 99 (7.2) | 55 (4.0) | 13 (0.9) | 50 (3.6) | 92 (6.7) | 12 (0.9) |
| dCCA N = 370 | 114 (30.8) | 133 (36.0) | 47 (12.7) | 3 (0.8) | 5 (1.4) | 55 (14.9) | 13 (3.5) |
| AC N = 322 | 44 (13.7) | 13 (4.0) | 200 (62.1) | 19 (5.9) | 1 (0.3) | 40 (12.4) | 5 (1.6) |
| DC N = 202 | 22 (10.9) | 2 (1.0) | 29 (14.4) | 107 (53.0) | 6 (3.0) | 26 (12.9) | 10 (5.0) |
| Postoperative diagnosis . | PDAC N = 1239 . | dCCA N = 247 . | AC N = 331 . | DC N = 142 . | Endocrine cancer/metastases from other cancers N = 62 . | Premalignant/benign N = 213 . | Other N = 40 . |
|---|---|---|---|---|---|---|---|
| Preoperative diagnosis | |||||||
| PDAC N = 1380 | 1059 (76.7) | 99 (7.2) | 55 (4.0) | 13 (0.9) | 50 (3.6) | 92 (6.7) | 12 (0.9) |
| dCCA N = 370 | 114 (30.8) | 133 (36.0) | 47 (12.7) | 3 (0.8) | 5 (1.4) | 55 (14.9) | 13 (3.5) |
| AC N = 322 | 44 (13.7) | 13 (4.0) | 200 (62.1) | 19 (5.9) | 1 (0.3) | 40 (12.4) | 5 (1.6) |
| DC N = 202 | 22 (10.9) | 2 (1.0) | 29 (14.4) | 107 (53.0) | 6 (3.0) | 26 (12.9) | 10 (5.0) |
Data are presented as absolute number (percentage). The percentage in the cells is the preoperative diagnosis concordance with postoperative histopathological diagnosis. The concordance ratio for each cancer type is highlighted in bold. AC, ampullary cancer; dCCA, distal cholangiocarcinoma; DC, duodenal cancer; PDAC, pancreatic ductal adenocarcinoma.
Cross-tabulation of pre- and postoperative diagnosis for patients treated with pancreatoduodenectomy restricted to patients with a solid tumour preoperatively
| Postoperative diagnosis . | PDAC N = 1239 . | dCCA N = 247 . | AC N = 331 . | DC N = 142 . | Endocrine cancer/metastases from other cancers N = 62 . | Premalignant/benign N = 213 . | Other N = 40 . |
|---|---|---|---|---|---|---|---|
| Preoperative diagnosis | |||||||
| PDAC N = 1380 | 1059 (76.7) | 99 (7.2) | 55 (4.0) | 13 (0.9) | 50 (3.6) | 92 (6.7) | 12 (0.9) |
| dCCA N = 370 | 114 (30.8) | 133 (36.0) | 47 (12.7) | 3 (0.8) | 5 (1.4) | 55 (14.9) | 13 (3.5) |
| AC N = 322 | 44 (13.7) | 13 (4.0) | 200 (62.1) | 19 (5.9) | 1 (0.3) | 40 (12.4) | 5 (1.6) |
| DC N = 202 | 22 (10.9) | 2 (1.0) | 29 (14.4) | 107 (53.0) | 6 (3.0) | 26 (12.9) | 10 (5.0) |
| Postoperative diagnosis . | PDAC N = 1239 . | dCCA N = 247 . | AC N = 331 . | DC N = 142 . | Endocrine cancer/metastases from other cancers N = 62 . | Premalignant/benign N = 213 . | Other N = 40 . |
|---|---|---|---|---|---|---|---|
| Preoperative diagnosis | |||||||
| PDAC N = 1380 | 1059 (76.7) | 99 (7.2) | 55 (4.0) | 13 (0.9) | 50 (3.6) | 92 (6.7) | 12 (0.9) |
| dCCA N = 370 | 114 (30.8) | 133 (36.0) | 47 (12.7) | 3 (0.8) | 5 (1.4) | 55 (14.9) | 13 (3.5) |
| AC N = 322 | 44 (13.7) | 13 (4.0) | 200 (62.1) | 19 (5.9) | 1 (0.3) | 40 (12.4) | 5 (1.6) |
| DC N = 202 | 22 (10.9) | 2 (1.0) | 29 (14.4) | 107 (53.0) | 6 (3.0) | 26 (12.9) | 10 (5.0) |
Data are presented as absolute number (percentage). The percentage in the cells is the preoperative diagnosis concordance with postoperative histopathological diagnosis. The concordance ratio for each cancer type is highlighted in bold. AC, ampullary cancer; dCCA, distal cholangiocarcinoma; DC, duodenal cancer; PDAC, pancreatic ductal adenocarcinoma.
Standardized evaluation of the surgical specimen
In 1739 patients, the standardized histopathological evaluation protocol was used. Cancer type rates were significantly different (P = 0.032) when the histopathological standardization was applied. Some 1118 (64.3 per cent) versus 262 (56.8 per cent) patients were diagnosed with PDAC, 217 (12.5 per cent) versus 67 (14.5 per cent) with dCCA, 284 (16.3 per cent) versus 92 (20.0 per cent) with AC and 120 (6.9 per cent) versus 40 (8.7 per cent) with DC. When stratified by region, the cancer type rates ranged widely (Table 5).
Histopathological diagnosis of patients treated with pancreatoduodenectomy for periampullary cancer stratified by region in Sweden
| . | PDAC . | dCCA . | AC . | DC . |
|---|---|---|---|---|
| Overall N = 2200 | 1380 (62.7) | 284 (12.9) | 376 (17.1) | 160 (7.3) |
| Region 1 N = 541 | 362 (66.9) | 50 (9.2) | 81 (15.0) | 48 (8.9) |
| Region 2 N = 165 | 105 (63.6) | 20 (12.1) | 35 (21.2) | 5 (3.0) |
| Region 3 N = 395 | 250 (63.3) | 49 (12.4) | 61 (15.4) | 35 (8.9) |
| Region 4 N = 362 | 255 (70.4) | 38 (10.5) | 50 (13.8) | 19 (5.3) |
| Region 5 N = 371 | 176 (47.4) | 91 (24.5) | 72 (19.4) | 32 (8.6) |
| Region 6 N = 365 | 231 (63.3) | 36 (9.9) | 77 (21.1) | 21 (5.8) |
| . | PDAC . | dCCA . | AC . | DC . |
|---|---|---|---|---|
| Overall N = 2200 | 1380 (62.7) | 284 (12.9) | 376 (17.1) | 160 (7.3) |
| Region 1 N = 541 | 362 (66.9) | 50 (9.2) | 81 (15.0) | 48 (8.9) |
| Region 2 N = 165 | 105 (63.6) | 20 (12.1) | 35 (21.2) | 5 (3.0) |
| Region 3 N = 395 | 250 (63.3) | 49 (12.4) | 61 (15.4) | 35 (8.9) |
| Region 4 N = 362 | 255 (70.4) | 38 (10.5) | 50 (13.8) | 19 (5.3) |
| Region 5 N = 371 | 176 (47.4) | 91 (24.5) | 72 (19.4) | 32 (8.6) |
| Region 6 N = 365 | 231 (63.3) | 36 (9.9) | 77 (21.1) | 21 (5.8) |
Data are presented as absolute number (percentage). AC, ampullary cancer; dCCA, distal cholangiocarcinoma; DC, duodenal cancer; PDAC, pancreatic ductal adenocarcinoma.
Histopathological diagnosis of patients treated with pancreatoduodenectomy for periampullary cancer stratified by region in Sweden
| . | PDAC . | dCCA . | AC . | DC . |
|---|---|---|---|---|
| Overall N = 2200 | 1380 (62.7) | 284 (12.9) | 376 (17.1) | 160 (7.3) |
| Region 1 N = 541 | 362 (66.9) | 50 (9.2) | 81 (15.0) | 48 (8.9) |
| Region 2 N = 165 | 105 (63.6) | 20 (12.1) | 35 (21.2) | 5 (3.0) |
| Region 3 N = 395 | 250 (63.3) | 49 (12.4) | 61 (15.4) | 35 (8.9) |
| Region 4 N = 362 | 255 (70.4) | 38 (10.5) | 50 (13.8) | 19 (5.3) |
| Region 5 N = 371 | 176 (47.4) | 91 (24.5) | 72 (19.4) | 32 (8.6) |
| Region 6 N = 365 | 231 (63.3) | 36 (9.9) | 77 (21.1) | 21 (5.8) |
| . | PDAC . | dCCA . | AC . | DC . |
|---|---|---|---|---|
| Overall N = 2200 | 1380 (62.7) | 284 (12.9) | 376 (17.1) | 160 (7.3) |
| Region 1 N = 541 | 362 (66.9) | 50 (9.2) | 81 (15.0) | 48 (8.9) |
| Region 2 N = 165 | 105 (63.6) | 20 (12.1) | 35 (21.2) | 5 (3.0) |
| Region 3 N = 395 | 250 (63.3) | 49 (12.4) | 61 (15.4) | 35 (8.9) |
| Region 4 N = 362 | 255 (70.4) | 38 (10.5) | 50 (13.8) | 19 (5.3) |
| Region 5 N = 371 | 176 (47.4) | 91 (24.5) | 72 (19.4) | 32 (8.6) |
| Region 6 N = 365 | 231 (63.3) | 36 (9.9) | 77 (21.1) | 21 (5.8) |
Data are presented as absolute number (percentage). AC, ampullary cancer; dCCA, distal cholangiocarcinoma; DC, duodenal cancer; PDAC, pancreatic ductal adenocarcinoma.
The rate of R1 resection after pancreatoduodenectomy for periampullary cancers was 911 (41.9 per cent), 716 (52.4 per cent) for PDAC, 104 (37.0 per cent) for dCCA, 57 (15.4 per cent) for AC and 34 (22.7 per cent) for DC patients. When stratified by region, the range of R1 resection ranged between 11.7 and 64.9 per cent (Table S2). When histopathological evaluation was performed in accordance with the standardized protocol, the overall R1 rate increased significantly from 144 (31.7 per cent) to 767 (44.6 per cent) (P < 0.001). The R1 rate before and after standardization was 105 (40.5 per cent) versus 611 (55.2 per cent) (P < 0.001) for PDAC, 20 (30.3 per cent) versus 84 (39.1 per cent) (P = 0.197) for dCCA, 11 (12.1 per cent) versus 46 (16.4 per cent) (P = 0.318) for AC and 8 (21.1 per cent) versus 26 (21.9 per cent) (P = 0.917) for DC.
The majority of patients with periampullary cancer had N1 stage disease (N = 1616; 73.9 per cent). When stratified by region, the N1 rate ranged between 52.4 and 81.9 per cent (Table S3). The N1 rate increased after standardization, with 284 (62.1 per cent) versus 1332 (77.0 per cent) (P <0.001): 180 (69.2 per cent) versus 909 (81.6 per cent) (P <0.001) for PDAC, 31 (47.7 per cent) versus 150 (69.1 per cent) (P = 0.002) for dCCA, 53 (57.6 per cent) versus 192 (67.6 per cent) (P = 0.08) for AC and 20 (50.0 per cent) versus 81 (69.8 per cent) for DC (P = 0.024). Additionally, the number of evaluated lymph nodes after pancreatoduodenectomy increased significantly after standardization, with 19 (13–26) versus 22 (17–30) (P <0.001). Also, among node-positive patients, the number of positive lymph nodes increased after standardization, with 3 (2–6) versus 4 (2–7) (P = 0.043).
Discussion
This nationwide study showed that among periampullary cancers PDAC was more frequently diagnosed in the non-curative compared with the curative setting. Clinical misdiagnosis of periampullary tumour origin was frequent, and standardization of histopathological evaluation correlated with increased PDAC, R1 and N1 rates.
Patients in the non-curative setting had a higher rate of PDAC diagnosis compared with those who underwent pancreatoduodenectomy with periampullary cancer on final pathology (83.7 per cent versus 62.6 per cent). Few studies have compared rates of periampullary cancers in curative and non-curative settings. In a national cohort of patients from The Netherlands receiving first-line palliative chemotherapy for periampullary cancers from 2015 to 2019, 85 per cent had PDAC17, which is similar to the present study. The higher rate of PDAC diagnosis in non-curative patients could be attributed to the difficulty of diagnosing non-pancreatic periampullary cancer without histopathological analysis of a resected specimen18. Additionally, a higher likelihood of resection in non-pancreatic cancers due to less aggressive tumour biology could contribute19. In the present study, 50.7 per cent of non-curative patients had imaging as the basis of diagnosis and 49.2 per cent cytology/histopathology. Although cytology/histopathology can provide information on the histopathological subtype (intestinal or pancreatobiliary) this cannot differentiate between pancreatobiliary dCCA and PDAC, or intestinal AC and DC20,21. There is reason to believe that non-pancreatic periampullary cancers are underdiagnosed in unresected patients. Current systemic treatment options are based on tumour origin, however, there is an overlap in regimens used which makes it difficult to estimate the impact of misclassification17. Given the difficulty in correctly estimating tumour origin in non-curative patients, additional efforts to identify biologically relevant and reproducible criteria for systemic treatment selection should be encouraged. Histological differentiation (intestinal versus pancreatobiliary) has been suggested as superior to tumour origin22.
In resected patients with PDAC, 14.7 per cent had a preoperative diagnosis of non-pancreatic periampullary cancer, and 22.7 per cent of non-pancreatic periampullary cancer patients had a preoperative pancreatic cancer diagnosis. These results are similar to a national cohort from The Netherlands with misdiagnosis rates of 13 per cent for PDAC and 21 per cent for nonpancreatic periampullary cancers respectively23. This diagnostic uncertainty should be considered in patients considered for preoperative therapy and in clinical trials. Similarly to the noncurative situation, efforts to identify reproducible, biologically relevant criteria for neoadjuvant treatment selection should be encouraged. The registry data did not allow subgroup analysis on whether preoperative histology was performed, which could have influenced the diagnostic certainty.
In the present study, standardization of the evaluation of the surgical specimen implicated a slightly higher frequency of PDAC and lower frequency of non-pancreatic periampullary cancer compared with non-standardized evaluation, conversely to what has been reported in the current literature7,24,25.The discrepancy could be due to differences in examination protocols. The Swedish protocol8 is based on the axial slicing technique9,10. However, both commonly used techniques (axial slicing and bivalving) have shown higher rates of non-pancreatic origin7,24, with no significant differences in tumour origin frequency when compared25. Other protocol differences such as number of examined tissue blocks and lymph nodes could impact the outcome. In addition to protocol differences, previous studies have presented meticulously revaluated cohorts from tertiary referral centres compared with the present study presenting a real-world national cohort.
Regional differences in the frequency range of the four periampullary cancers were evident, a relatively higher rate of dCCA diagnosis and lower rate of PDAC in Region 5 compared with other regions contributed substantially to the differences identified. Varying adherence and interpretation of the protocol could explain regional differences. Further harmonization is required to increase concordance between centres in Sweden. There is reason to believe that this situation is seen not only in Sweden but throughout the world.
An increased rate of R1 resection after histopathological standardization was found to be 55.2 per cent for PDAC. It is worth noting that several previous studies have presented R1 rates of >70 per cent for PDAC with standardized histopathological evaluation, margin defined as 1 mm and grossing done by axial slicing, suggesting further quality improvement could increase the detection of R1 resection. A considerable variation in the R1 resection rate (12–65 per cent) between centres is worrying and highlights the difficulties and need for additional work within pancreatic cancer networks. The N1 rate likewise increased after standardization. This N1 rate is similar to several previous studies utilizing standardized axial slicing9,25–28, suggesting that adequate nodal staging is performed. However, with regard to nodal staging, unmotivated regional differences were also observed. These results show that a national protocol for histopathological pancreatoduodenectomy specimen examination improves quality, however, active quality improvement efforts are required in order to obtain consistent outcome between centres with regards to tumour origin, R1 resection and lymph node assessment.
The strength of the present study is the large, validated national cohort included during a modern timespan. Study limitations are mostly inherent to registry data, for example the quality of the source data and the amount of missing data. Oncological treatment data had too low a coverage rate to allow analysis. Additionally, there was a lack of specific data, such as details on preoperative diagnostic evaluation and differential diagnostic reasoning.
In conclusion, the proportion of non-pancreatic periampullary cancer is substantially higher in patients with postoperative histopathological diagnosis than in those with clinical diagnosis. The clinical misdiagnosis of periampullary cancer origin occurs in a clinically relevant proportion of patients and needs to be considered in patients with a preoperative oncological treatment plan. Histopathological standardization in Sweden has correlated with an increased rate of PDAC diagnosis, R1 resection and N1 stage, with regional differences evident.
Funding
This research work was supported by the Swedish Cancer Foundation, Maggie Stephens Foundation, Bengt Ihre foundation and Fru Berta Kamprads foundation.
Acknowledgements
We would like to thank all Swedish pancreatic surgeons for providing information and maintaining high coverage of the data in the Swedish National Pancreatic and Periampullary Cancer Registry. The research methodology used was not preregistered.
Disclosure
The authors declare no conflict of interest.
Supplementary material
Supplementary material is available at BJS Open online.
Data availability
Data underlying this study will be shared upon reasonable request to the corresponding author.
References
Author notes
Poster presented to a meeting of the15th International Hepato-Pancreato-Biliary Association (IHPBA) World Congress, New York City, USA, 30 March to 2 April, 2022.
A previous version of this manuscript has been included in the doctoral thesis of J.B.; Lund University, Faculty of Medicine, 2021. 76 s. (Lund University, Faculty of Medicine Doctoral Dissertation Series; 2021:122).



