-
PDF
- Split View
-
Views
-
Cite
Cite
Antonie Willner, Christian Teske, Thilo Hackert, Thilo Welsch, Effects of early postoperative mobilization following gastrointestinal surgery: systematic review and meta-analysis, BJS Open, Volume 7, Issue 5, October 2023, zrad102, https://doi.org/10.1093/bjsopen/zrad102
Close - Share Icon Share
Abstract
Early postoperative mobilization is considered a key element of enhanced recovery after surgery protocols. The aim of this study was to summarize the effect of early postoperative mobilization following gastrointestinal operations on patient recovery, mobility, the morbidity rate and duration of hospital stay.
A systematic literature search was conducted in December, 2022, using PubMed, Web of Science and the Cochrane Central Register of Controlled Trials. Controlled trials reporting the effects of early postoperative mobilization after gastrointestinal surgery were included. The risk of bias was assessed using a modified Downs and Black tool and the Cochrane Collaboration tool for randomized trials. The outcomes of interest were gastrointestinal recovery (defined passage of first flatus or bowel movements), mobility (step count on postoperative day 3), the morbidity rate and duration of hospital stay.
After elimination of duplicates, 3678 records were identified, and 71 full-text articles were screened. Finally, 15 studies (eight RCTs) reporting on 3538 patients were included. Most trials evaluated early postoperative mobilization after different gastrointestinal operations, including upper gastrointestinal (n = 8 studies), hepatopancreatobiliary (n = 10 studies) and colorectal resections (n = 10 studies). The investigated early postoperative mobilization protocols, operative techniques (minimally invasive or open) and outcome parameters were heterogeneous between the studies. Early postoperative mobilization seemed to significantly accelerate clinical gastrointestinal recovery (mean difference, hours: −11.53 (−22.08, −0.97), P = 0.03). However, early postoperative mobilization did not significantly improve the morbidity rate (risk ratio: 0.93 (0.70, 1.23), P = 0.59), postoperative mobility of patients (step count mean difference: 1009 (−803, 2821), P = 0.28) or shorten the duration of hospital stay (mean difference, days: −0.25 (−0.99,0.43), P = 0.47) in randomized trials.
There is a large heterogeneity among the study cohorts, operations and interventions. The available evidence currently does not support specific early postoperative mobilization protocols as an isolated element to further reduce the morbidity rate and duration of hospital stay. Further well-designed trials are required to identify effective early postoperative mobilization protocols.
Introduction
Enhanced recovery after surgery (ERAS) protocols have been implemented in many centres for gastrointestinal operations, for example for colorectal, pancreatic, gastric or liver surgery1–4. Such protocols include pre-, intra- and postoperative elements to minimize surgical trauma and accelerate patient recovery. Early postoperative mobilization (EPM) is one of these postoperative core elements that is considered by all current ERAS guidelines and is thought to alleviate intestinal paresis, pulmonary complications or pain. In line with this concept, the grade of recommendation for EPM after gastrointestinal surgery is strong but only based on either low or moderate evidence levels1–4. Although there is a consensus on the harmful effects of prolonged bed rest, the optimal protocol for EPM and its effects on mental or physical recovery and morbidity rate are still unclear. However, despite the lack of evidence, enhanced postoperative mobilization is further encouraged by modern concepts: numerous trials are aimed to enhance postoperative patient mobilization by using activity trackers or equivalent wearables/digital systems5–7. Current recommendations on EPM after gastrointestinal surgery basically refer to a systematic review of trials involving early mobilization and published up to 2015, which identified four prospective trials conducted after abdominal and gynaecological operations8. Since then, numerous studies have further focused on EPM effects after gastrointestinal surgery. The objective of this meta-analysis was to provide an updated body of evidence for guidance of effective patient education and early mobilization programmes in gastrointestinal surgery. The central question to be addressed is whether EPM of patients following gastrointestinal operations has advantages in terms of patient recovery, mobility, morbidity rate and length of hospital stay (LOS).
Methods
Eligibility criteria
Studies were included in the review if they fulfilled the following criteria: original full-text article (no congress abstracts) considering adult patients undergoing open or minimally invasive abdominal/gastrointestinal surgery; a specific protocol for early in-hospital mobilization used as an intervention (with out-of-bed activities starting at least within the first two postoperative days); patients who followed a specific EPM protocol were compared with a control group receiving either no structured mobilization protocol (that is mobilization at will) or patients with objectively documented different or delayed mobilization achievements; reported outcome measures of interest as defined and publication in English.
Studies were excluded if they involved mainly (>50 per cent) patients undergoing gynaecological, urological, thoracic, cardiac or orthopaedic procedures and data for gastrointestinal subgroups were not separately reported, the EPM protocol was not reported, and the EPM protocol was not studied in isolation (for example mobilization protocol within a multimodal enhanced recovery programme versus traditional care). Studies in which the control group mobilization protocol stipulated bed rest during the first postoperative days9 were also excluded because this practice is regarded obsolete for the majority of standard gastrointestinal operations.
Outcome measures of interest for the present review included the postoperative morbidity rate, pulmonary function, clinical gastrointestinal function (first flatus or bowel movements), performance-based functional tests or other surrogate markers for recovery (pain intensity or sleep quality). Data on the adherence to the planned exercise protocol and quantitative measures of the amount of physical activity (for example step count) were also extracted if available.
Information sources, search strategy and collection process
This systematic review was performed in accordance with the PRISMA guidelines10. The following electronic databases were searched: PubMed, Web of Science and Cochrane Central Register of Controlled Trials (via Ovid). The last online search was conducted on 20 December, 2022.
The search strategy was built along the targeted cohort, intervention and outcome measures, and the term was adapted according to the respective database terminology (Table S1, Supplementary material). The following search query was used for PubMed:
abdominal OR colon OR rectal OR colorectal OR pancreatic OR liver OR gastrointestinal OR gastric; surgery OR resection OR operation; #1 AND #2; early OR enhanced OR immediate* OR accelerated; postoperative; mobilization OR mobilisation OR ambulat* OR exercise OR walking; #4 AND #5 AND #6; complications OR morbidity OR ‘pulmonary function’ OR pain OR recovery OR ‘hospital stay’ OR ‘bowel movements’; #3 AND #7 AND #8.
No further limits or filters were used regarding time frame, article type or language.
The retrieved items from all sources were imported into reference management software (Zotero, version 6.0.19) to eliminate duplicates. This process was supervised manually. All abstracts were screened independently by two investigators for eligibility criteria. Full-text articles were obtained only from studies that met the inclusion criteria or if inclusion could not be decided upon, based on the abstract data. The remaining full-text articles were then reviewed again in detail by the two investigators according to the inclusion and exclusion criteria. Discordant opinions were resolved by consensus discussion. The relevant data were extracted in an electronic data collection form covering the outcome measures of interest, the study design, number of patients enrolled (intervention and control group), age, gender, diagnosis and field of surgery, type of operation, type of operative technique (minimally invasive or open), description of the mobilization protocol, guidance of the exercise training (patient-based or assisted by professionals), patient compliance to completion of the protocol, physical activity (step counts) and information about devices used to support the mobilization protocol. The effect measures mean/standard deviation, median/interquartile range, mean difference and risk ratio with 95 per cent confidence interval respectively, were considered to compare quantitative variables. If relevant data for quantitative analysis were collected but not reported in the full-text article, the authors were asked to provide the missing data from the original data sets7.
Risk-of-bias assessment
The risk of bias of all included studies was assessed using a modified Downs and Black tool8,11. This tool is based on a checklist to evaluate the methodological quality of both randomized and non-randomized studies. As described in the literature, a modification of the original tool was applied regarding Item 27. Instead of rating according to an available range of study powers (scores 0–5), we rated whether the study did or did not perform a power calculation (scores 0 or 1 respectively). As a result, the maximum score of the tool was 28 instead of 32. In addition, the risk of bias of the included randomized controlled studies was assessed using the Cochrane Collaboration tool12. The assessment was performed independently by two authors (T.W., A.W.), and discrepancies were resolved in a consensus meeting.
Data synthesis
The inclusion criteria allowed the inclusion of retrospective trials if a control group was defined. This was considered important, so that the information of these trials was not lost in the review. First, all included studies were summarized to provide an overview of the study designs, cohorts, operations, mobilization protocols and outcome measures. Subgroups of studies were created according to the following specific endpoints reported: pulmonary function, morbidity rate, LOS, pain, exercise intervention (patient-based or assisted digitally or by professional staff), type of operation, technique (minimally invasive or open) and physical activity (step count, walking tests). Each of these effect sizes is addressed in a narrative review based on the available studies. Because of the heterogeneity of the operations and primary endpoints, a quantitative meta-analysis was intended for the subgroup of studies including colorectal operations and for RCTs reporting the most frequently reported clinically relevant outcomes: LOS, morbidity rate and time to gastrointestinal recovery. Studies that did not report these outcomes as primary or secondary endpoints were not considered for the quantitative meta-analysis. If a mean value was not provided for the effect size of interest, the mean and standard deviation were approximated from the median and interquartile range (i.q.r./1.35) according to the handbook recommendations of the Cochrane Collaboration13.
The R software package (version 3.1.3) was used for statistical calculations and graphs. For comparison of step counts, the Welch two-sample t test was applied. The forest plot and meta-analysis were performed using Review Manager (RevMan, Version 5.4.1, The Cochrane Collaboration, 2020). Because of the heterogeneity of the mobilization protocols and intervention effects across studies, a random-effects model was used for meta-analysis. To investigate considerable heterogeneity, standard strategies according to the Cochrane Handbook for Systematic Reviews of Interventions (version 6.3, 2022) were followed13.
Results
Study selection and characteristics
The primary database search yielded 3678 abstracts after duplicate records were eliminated (Fig. 1). Based on the PubMed search, the number of search results doubled since the last systematic review, which included studies until January 2015: 992 abstracts (1946–2014) and 926 abstracts (2015–2023) (Fig. 2). After screening the abstracts, 71 full-text articles were reviewed for final inclusion, but several studies were further excluded for distinct reasons (Fig. 1). Sixteen articles (15 studies7,14–27) matched the eligibility criteria after careful review, including eight RCTs (Table 1). Two articles reported different outcomes of the identical RCT19 and were not listed separately19,28. The included trials were published between 2013 and 2022, originated in 13 nations and reported on 3538 patients. Most frequently, the studies investigated EPM after different gastrointestinal operations, including upper gastrointestinal, hepatopancreatobiliary and colorectal resections (n = 7). The largest subgroup of studies focusing on a homogeneous surgical cohort reported on colorectal surgery (n = 4). The analysed mobilization interventions and outcomes were also heterogeneous (Table 1).
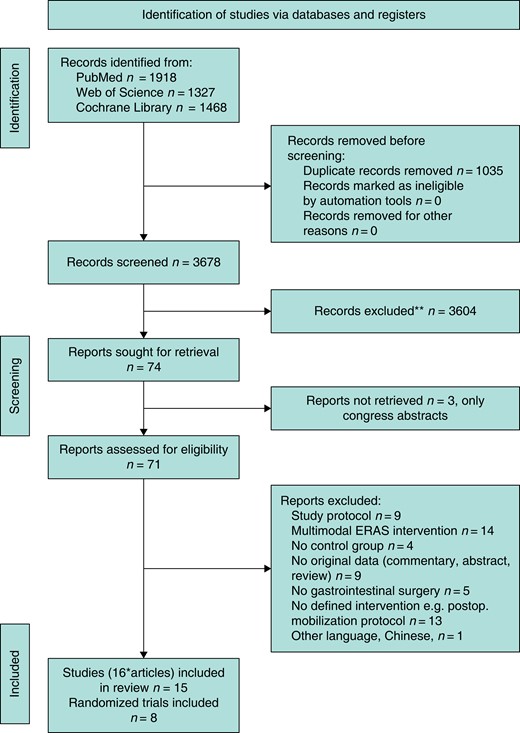
Flow chart for the systematic literature search algorithm (PRISMA 2020 template)
*Two articles reported different outcomes of the identical RCT and were not listed separately. ERAS, enhanced recovery after surgery.
**No automation tools were used.
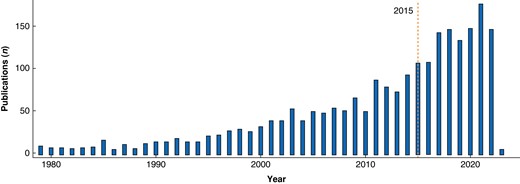
Number of publications per year captured by the MEDLINE search term (PubMed) on early postoperative mobilization after gastrointestinal surgery (total number of retrieved records: 1918)
The dashed vertical orange line indicates the year 2015.
| Study . | Country . | Design . | Included patients (n) . | Field of surgery . | Technique* . | Intervention† (duration/assistance/type) . | Primary outcome (sec. outcomes) . |
|---|---|---|---|---|---|---|---|
| Ahn 2013 | Korea | RCT | 41 | Colon | o, l (77), r (6) | POD 1–dis/P/stretching and ambulation twice daily | LOS (time to flatus, morbidity rate, walking distance) |
| Fiore 2017, Balvardi 2021§ | Canada | RCT | 100 | Colorectal | o, l (81) | POD 0–dis/P/ambulation 3 times daily | 6MWT‡ (FVC, FEV1, PPC, step count, LOS, GI recovery, morbidity rate) |
| Bhatt 2017 | Ireland | Prosp. observ. | 60 | UGI, hepatobiliary, colorectal | o, l | POD 2–dis/P/pedal exerciser twice daily | PPC (dyspnoea score, LOS) |
| Boerrigter 2022 | Netherlands | Retro. observ. | 373 | UGI, HPB, colorectal | n.a. | POD 1–3/P/increased ambulation targets (max. 1125 m daily) | Ceiling effect of target goals (LOS) |
| de Almeida 2017 | Brazil | RCT | 108 | UGI, HPB, colorectal, urological (3–6%) | o, l (22) | POD 1–dis/P/physical exercises including walking/strengthening exercise/cycle ergometry twice daily | Inability to ambulate at POD 5 (6MWT, fatigue, QoL, morbidity rate, LOS) |
| Fagevik Olsén 2021 | Sweden | RCT | 83 | Pancreatic | o | POD 0–dis/P/ambulation and cycle ergometry (2–3 times daily) | Oxygenation (pulmonary function, pneumonia rate, LOS) |
| Grass 2018 | Switzerland | Retro. | 1170 | Colorectal | o, l (58) | POD 1–3/E/ambulation out of bed ≥6 h on POD 1 | Compliance to mobilization (morbidity rate, LOS) |
| Koyuncu 2021 | Turkey | Prosp. | 42 | UGI, pancreatic, colorectal | n.a. | POD 0/E/ambulation 2 h out of bed on POD 0 | Mobilization time (sleep quality, bowel function, LOS) |
| Ni 2018 | China | RCT | 120 | Hepatic | n.a. | POD 1–5/P/ambulation beginning POD 2, 2–5 times daily | Step count (pain, sleeping state, bowel function, morbidity rate, LOS) |
| Ni 2022 | China | RCT | 120 | Hepatic | n.a. | POD 1–5/P/ambulation beginning POD 2, 2–5 times daily | Sleep quality (pain, bowel function, step count, LOS) |
| Nishijima 2020 | Japan | Retro. | 718 | UGI (excl. oesophagus), HPB, colon | o, l (50) | POD 1–dis/P/ambulation >20 m (during POD 1–2 versus later) | Morbidity rate (LOS) |
| Rosowicz 2022 | USA | Retro. | 291 | Colorectal | o, l (76) | POD 0–dis/E/ambulation (within 24 h versus later begin) | Morbidity rate (LOS) |
| Van der Leeden 2016 | Netherlands | Retro. | 116 | UGI, hepatic, colorectal, other | o, l (24) | POD 0–5/P/ambulation target goals (scheduled distance) | PPC (LOS, 6MWT) |
| Wiklund 2015 | Sweden | RCT | 66 | Gastric bypass (bariatric) | l | POD 1–7/D, E/ambulation (target step count) | Step count (bowel function, LOS) |
| Wolk 2019 | Germany | RCT | 132 | UGI, HPB, colorectal | o, l (51) | POD 1–5/D, P/ambulation (target step count) | Step count (morbidity rate, LOS) |
| Study . | Country . | Design . | Included patients (n) . | Field of surgery . | Technique* . | Intervention† (duration/assistance/type) . | Primary outcome (sec. outcomes) . |
|---|---|---|---|---|---|---|---|
| Ahn 2013 | Korea | RCT | 41 | Colon | o, l (77), r (6) | POD 1–dis/P/stretching and ambulation twice daily | LOS (time to flatus, morbidity rate, walking distance) |
| Fiore 2017, Balvardi 2021§ | Canada | RCT | 100 | Colorectal | o, l (81) | POD 0–dis/P/ambulation 3 times daily | 6MWT‡ (FVC, FEV1, PPC, step count, LOS, GI recovery, morbidity rate) |
| Bhatt 2017 | Ireland | Prosp. observ. | 60 | UGI, hepatobiliary, colorectal | o, l | POD 2–dis/P/pedal exerciser twice daily | PPC (dyspnoea score, LOS) |
| Boerrigter 2022 | Netherlands | Retro. observ. | 373 | UGI, HPB, colorectal | n.a. | POD 1–3/P/increased ambulation targets (max. 1125 m daily) | Ceiling effect of target goals (LOS) |
| de Almeida 2017 | Brazil | RCT | 108 | UGI, HPB, colorectal, urological (3–6%) | o, l (22) | POD 1–dis/P/physical exercises including walking/strengthening exercise/cycle ergometry twice daily | Inability to ambulate at POD 5 (6MWT, fatigue, QoL, morbidity rate, LOS) |
| Fagevik Olsén 2021 | Sweden | RCT | 83 | Pancreatic | o | POD 0–dis/P/ambulation and cycle ergometry (2–3 times daily) | Oxygenation (pulmonary function, pneumonia rate, LOS) |
| Grass 2018 | Switzerland | Retro. | 1170 | Colorectal | o, l (58) | POD 1–3/E/ambulation out of bed ≥6 h on POD 1 | Compliance to mobilization (morbidity rate, LOS) |
| Koyuncu 2021 | Turkey | Prosp. | 42 | UGI, pancreatic, colorectal | n.a. | POD 0/E/ambulation 2 h out of bed on POD 0 | Mobilization time (sleep quality, bowel function, LOS) |
| Ni 2018 | China | RCT | 120 | Hepatic | n.a. | POD 1–5/P/ambulation beginning POD 2, 2–5 times daily | Step count (pain, sleeping state, bowel function, morbidity rate, LOS) |
| Ni 2022 | China | RCT | 120 | Hepatic | n.a. | POD 1–5/P/ambulation beginning POD 2, 2–5 times daily | Sleep quality (pain, bowel function, step count, LOS) |
| Nishijima 2020 | Japan | Retro. | 718 | UGI (excl. oesophagus), HPB, colon | o, l (50) | POD 1–dis/P/ambulation >20 m (during POD 1–2 versus later) | Morbidity rate (LOS) |
| Rosowicz 2022 | USA | Retro. | 291 | Colorectal | o, l (76) | POD 0–dis/E/ambulation (within 24 h versus later begin) | Morbidity rate (LOS) |
| Van der Leeden 2016 | Netherlands | Retro. | 116 | UGI, hepatic, colorectal, other | o, l (24) | POD 0–5/P/ambulation target goals (scheduled distance) | PPC (LOS, 6MWT) |
| Wiklund 2015 | Sweden | RCT | 66 | Gastric bypass (bariatric) | l | POD 1–7/D, E/ambulation (target step count) | Step count (bowel function, LOS) |
| Wolk 2019 | Germany | RCT | 132 | UGI, HPB, colorectal | o, l (51) | POD 1–5/D, P/ambulation (target step count) | Step count (morbidity rate, LOS) |
6MWT, 6-minute walk test; dis, discharge from hospital; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; GI, gastrointestinal; HPB, hepatopancreatobiliary; LOS, duration of hospital stay; n.a., not assessed; oberv., observational; POD, postoperative day; PPC, postoperative pulmonary complications; prosp., prospective; QoL, quality of life; RCT, randomized clinical trial; retro., retrospective; UGI, upper gastrointestinal. *o, open; l, laparoscopic; r, robot-assisted (percentage of the respective minimally invasive technique referred to the total cohort if the data were available). †Mobilization protocol was encouraged during the indicated postoperative days by education only (E), digital advice (D) or healthcare professionals (P). Details of the mobilization protocols in RCTs are presented in Table S3, Supplementary material. ‡Assessed 4 weeks after surgery. §These articles both report the results of the same RCT and were combined in this table.
| Study . | Country . | Design . | Included patients (n) . | Field of surgery . | Technique* . | Intervention† (duration/assistance/type) . | Primary outcome (sec. outcomes) . |
|---|---|---|---|---|---|---|---|
| Ahn 2013 | Korea | RCT | 41 | Colon | o, l (77), r (6) | POD 1–dis/P/stretching and ambulation twice daily | LOS (time to flatus, morbidity rate, walking distance) |
| Fiore 2017, Balvardi 2021§ | Canada | RCT | 100 | Colorectal | o, l (81) | POD 0–dis/P/ambulation 3 times daily | 6MWT‡ (FVC, FEV1, PPC, step count, LOS, GI recovery, morbidity rate) |
| Bhatt 2017 | Ireland | Prosp. observ. | 60 | UGI, hepatobiliary, colorectal | o, l | POD 2–dis/P/pedal exerciser twice daily | PPC (dyspnoea score, LOS) |
| Boerrigter 2022 | Netherlands | Retro. observ. | 373 | UGI, HPB, colorectal | n.a. | POD 1–3/P/increased ambulation targets (max. 1125 m daily) | Ceiling effect of target goals (LOS) |
| de Almeida 2017 | Brazil | RCT | 108 | UGI, HPB, colorectal, urological (3–6%) | o, l (22) | POD 1–dis/P/physical exercises including walking/strengthening exercise/cycle ergometry twice daily | Inability to ambulate at POD 5 (6MWT, fatigue, QoL, morbidity rate, LOS) |
| Fagevik Olsén 2021 | Sweden | RCT | 83 | Pancreatic | o | POD 0–dis/P/ambulation and cycle ergometry (2–3 times daily) | Oxygenation (pulmonary function, pneumonia rate, LOS) |
| Grass 2018 | Switzerland | Retro. | 1170 | Colorectal | o, l (58) | POD 1–3/E/ambulation out of bed ≥6 h on POD 1 | Compliance to mobilization (morbidity rate, LOS) |
| Koyuncu 2021 | Turkey | Prosp. | 42 | UGI, pancreatic, colorectal | n.a. | POD 0/E/ambulation 2 h out of bed on POD 0 | Mobilization time (sleep quality, bowel function, LOS) |
| Ni 2018 | China | RCT | 120 | Hepatic | n.a. | POD 1–5/P/ambulation beginning POD 2, 2–5 times daily | Step count (pain, sleeping state, bowel function, morbidity rate, LOS) |
| Ni 2022 | China | RCT | 120 | Hepatic | n.a. | POD 1–5/P/ambulation beginning POD 2, 2–5 times daily | Sleep quality (pain, bowel function, step count, LOS) |
| Nishijima 2020 | Japan | Retro. | 718 | UGI (excl. oesophagus), HPB, colon | o, l (50) | POD 1–dis/P/ambulation >20 m (during POD 1–2 versus later) | Morbidity rate (LOS) |
| Rosowicz 2022 | USA | Retro. | 291 | Colorectal | o, l (76) | POD 0–dis/E/ambulation (within 24 h versus later begin) | Morbidity rate (LOS) |
| Van der Leeden 2016 | Netherlands | Retro. | 116 | UGI, hepatic, colorectal, other | o, l (24) | POD 0–5/P/ambulation target goals (scheduled distance) | PPC (LOS, 6MWT) |
| Wiklund 2015 | Sweden | RCT | 66 | Gastric bypass (bariatric) | l | POD 1–7/D, E/ambulation (target step count) | Step count (bowel function, LOS) |
| Wolk 2019 | Germany | RCT | 132 | UGI, HPB, colorectal | o, l (51) | POD 1–5/D, P/ambulation (target step count) | Step count (morbidity rate, LOS) |
| Study . | Country . | Design . | Included patients (n) . | Field of surgery . | Technique* . | Intervention† (duration/assistance/type) . | Primary outcome (sec. outcomes) . |
|---|---|---|---|---|---|---|---|
| Ahn 2013 | Korea | RCT | 41 | Colon | o, l (77), r (6) | POD 1–dis/P/stretching and ambulation twice daily | LOS (time to flatus, morbidity rate, walking distance) |
| Fiore 2017, Balvardi 2021§ | Canada | RCT | 100 | Colorectal | o, l (81) | POD 0–dis/P/ambulation 3 times daily | 6MWT‡ (FVC, FEV1, PPC, step count, LOS, GI recovery, morbidity rate) |
| Bhatt 2017 | Ireland | Prosp. observ. | 60 | UGI, hepatobiliary, colorectal | o, l | POD 2–dis/P/pedal exerciser twice daily | PPC (dyspnoea score, LOS) |
| Boerrigter 2022 | Netherlands | Retro. observ. | 373 | UGI, HPB, colorectal | n.a. | POD 1–3/P/increased ambulation targets (max. 1125 m daily) | Ceiling effect of target goals (LOS) |
| de Almeida 2017 | Brazil | RCT | 108 | UGI, HPB, colorectal, urological (3–6%) | o, l (22) | POD 1–dis/P/physical exercises including walking/strengthening exercise/cycle ergometry twice daily | Inability to ambulate at POD 5 (6MWT, fatigue, QoL, morbidity rate, LOS) |
| Fagevik Olsén 2021 | Sweden | RCT | 83 | Pancreatic | o | POD 0–dis/P/ambulation and cycle ergometry (2–3 times daily) | Oxygenation (pulmonary function, pneumonia rate, LOS) |
| Grass 2018 | Switzerland | Retro. | 1170 | Colorectal | o, l (58) | POD 1–3/E/ambulation out of bed ≥6 h on POD 1 | Compliance to mobilization (morbidity rate, LOS) |
| Koyuncu 2021 | Turkey | Prosp. | 42 | UGI, pancreatic, colorectal | n.a. | POD 0/E/ambulation 2 h out of bed on POD 0 | Mobilization time (sleep quality, bowel function, LOS) |
| Ni 2018 | China | RCT | 120 | Hepatic | n.a. | POD 1–5/P/ambulation beginning POD 2, 2–5 times daily | Step count (pain, sleeping state, bowel function, morbidity rate, LOS) |
| Ni 2022 | China | RCT | 120 | Hepatic | n.a. | POD 1–5/P/ambulation beginning POD 2, 2–5 times daily | Sleep quality (pain, bowel function, step count, LOS) |
| Nishijima 2020 | Japan | Retro. | 718 | UGI (excl. oesophagus), HPB, colon | o, l (50) | POD 1–dis/P/ambulation >20 m (during POD 1–2 versus later) | Morbidity rate (LOS) |
| Rosowicz 2022 | USA | Retro. | 291 | Colorectal | o, l (76) | POD 0–dis/E/ambulation (within 24 h versus later begin) | Morbidity rate (LOS) |
| Van der Leeden 2016 | Netherlands | Retro. | 116 | UGI, hepatic, colorectal, other | o, l (24) | POD 0–5/P/ambulation target goals (scheduled distance) | PPC (LOS, 6MWT) |
| Wiklund 2015 | Sweden | RCT | 66 | Gastric bypass (bariatric) | l | POD 1–7/D, E/ambulation (target step count) | Step count (bowel function, LOS) |
| Wolk 2019 | Germany | RCT | 132 | UGI, HPB, colorectal | o, l (51) | POD 1–5/D, P/ambulation (target step count) | Step count (morbidity rate, LOS) |
6MWT, 6-minute walk test; dis, discharge from hospital; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; GI, gastrointestinal; HPB, hepatopancreatobiliary; LOS, duration of hospital stay; n.a., not assessed; oberv., observational; POD, postoperative day; PPC, postoperative pulmonary complications; prosp., prospective; QoL, quality of life; RCT, randomized clinical trial; retro., retrospective; UGI, upper gastrointestinal. *o, open; l, laparoscopic; r, robot-assisted (percentage of the respective minimally invasive technique referred to the total cohort if the data were available). †Mobilization protocol was encouraged during the indicated postoperative days by education only (E), digital advice (D) or healthcare professionals (P). Details of the mobilization protocols in RCTs are presented in Table S3, Supplementary material. ‡Assessed 4 weeks after surgery. §These articles both report the results of the same RCT and were combined in this table.
Risk of bias in studies
The risk-of-bias assessment of all included trials is shown in Table S2 (Supplementary material). The RCT by de Almeida et al.17 had the highest methodological quality, achieving a score of 24/28 on the modified Downs and Black tool. The study by Nishijima et al.24 was identified as the study with the lowest quality (score 14/28). Common methodologic issues observed in the included studies were strong reporting data on adverse events due to intervention15,18,26, ensuring external validity7,22,23,26,27, blinding study subjects to the intervention as well as blinding those measuring the outcome parameters17, ensuring concealment of allocation7,17–19,22,23,27,28 and providing information on statistical power14,17,19–22,25,27,28. Relevant characteristics and variables of the intervention and control subgroups in retrospective studies were further extracted for comparison (Table S3, Supplementary material).
A further bias assessment of the included RCTs was performed according to the Cochrane Collaboration tool (Fig. 3). Selection bias (random sequence generation and allocation concealment), attrition bias and reporting bias were evaluated as low risk in all included RCTs7,14,17–19,22,23,27,28. Performance bias was determined to be high risk in all studies except for the study by de Almeida et al.7,14,17,18,19,22,23,27. Detection bias was present in four further trials 7,22,23,27.
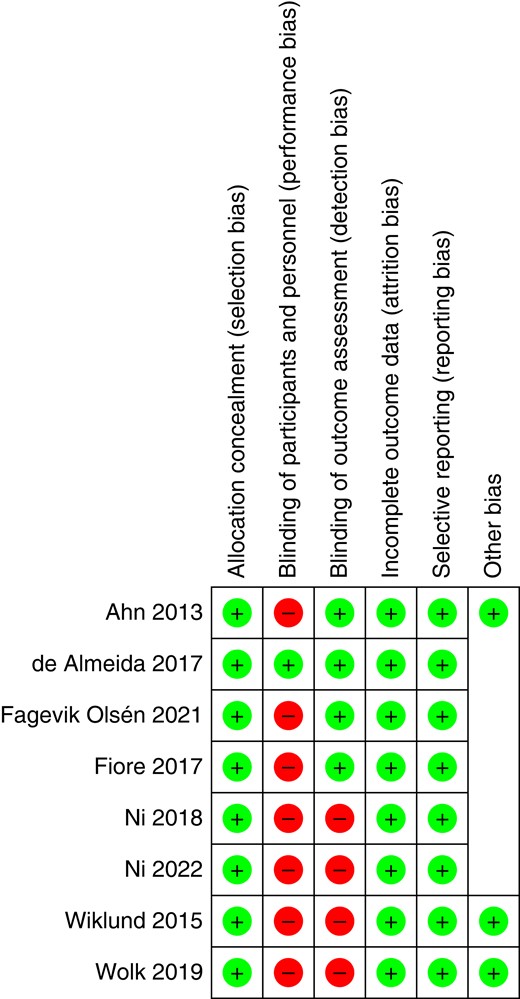
Risk-of-bias assessment of the included RCTs according to the Cochrane Collaboration tool
Types and characteristics of early mobilization protocols
The specific mobilization protocols of each RCT are described in detail in Table S4 (Supplementary material). All except one trial15 started patient mobilization before the second POD. Two studies started with a structured EPM programme as soon as the day of surgery (POD 0); study participants were visited to reinforce mobilization goals19 or were mobilized in or out of bed18,19 if haemodynamically stable. All trials provided different structured EPM protocols, including physical exercises/stretching, ambulation/walking or cycle ergometry/pedal exerciser (n = 3), which were supervised and facilitated either by trained health professionals (for example physiotherapists/graduate trainees), surgical fellows or study nurses. Activity tracking devices were used to quantify postoperative mobilization in several trials7,22,23,27.
Impact of early mobilization on postoperative physical activity and step count
In theory, EPM protocols should enhance patient mobilization and subsequently shorten the recovery process and lower complications. On the other hand, many patients receiving gastrointestinal operations are of advanced age, have substantial co-morbidities and sustained major surgical trauma, all of which can mitigate enforced mobilization. Several studies therefore intended to measure the extent of postoperative physical activity after early enhanced mobilization compared with standard protocols. Physical activity as a surrogate parameter for recovery can be objectively recorded and studied using activity tracking devices or by standardized exercise tests.
Eight of the included studies (seven RCTs) objectively assessed postoperative activity, either by recording step counts7,19,22,23,27, by conducting exercise tests (for example 6-minute walking test (6MWT)17,19,26) or by measuring walking distance14.
Studies with prospectively documented step counts were further investigated. Patients who underwent EPM protocols had a mean step count of approximately 700, 2500 and 3600 during postoperative days 1, 3 and 5 respectively (Fig. 4). Not all studies reported the respective step counts on each postoperative day, which limited the analysis.
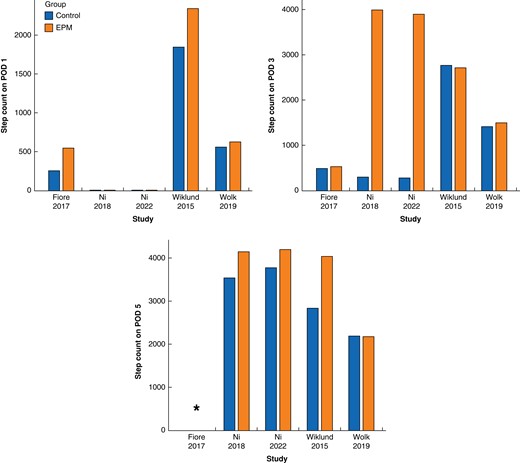
Mean step count on postoperative days (POD) 1 (top left), 3 (top right) and 5 (bottom) in the early postoperative mobilization (EPM, intervention) and the control group respectively, reported by RCTs
*Step counts on POD 5 were not assessed in this study. EPM, early postoperative mobilization.
Interestingly, five of the eight studies provided evidence that EPM significantly enhanced the step count or 6MWT performance during the first postoperative days (de Almeida 2017: 6MWT on POD 5; Fiore 2017: POD 1 and 2; Ni 2018: POD 2–5; Ni 2022: POD 2–5; Wiklund 2015: POD 1–7). No improved 6MWT on the day of discharge was noted by van der Leeden26 and no significantly increased step count after EPM was observed during POD 1–5 in two RCTs7,14. However, the latter trial did show a significant increase after enforced mobilization only in the laparoscopic group7. A meta-analysis was attempted based on five RCTs reporting step counts on postoperative day 3 (mean difference: 1009 (−803, 2821) steps, I2 = 99 per cent, P = 0.28), but the results were limited by a large heterogeneity (Fig. S1, Supplementary material).
The percentage of a minimally invasive approach ranged from 22 to 83 per cent in studies that included both techniques (Table 1). But only a few studies investigated the impact of the minimally invasive approach on enhanced mobilization. According to one study7, early mobilization of patients led to a higher mean step count on POD 5 after minimally invasive (laparoscopic) operations compared with open surgery: 2725 versus 1572 (P = 0.0747). Likewise, the cumulative steps during POD 1–5 were significantly increased after minimally invasive surgery: 4635 versus 9867 (P = 0.003)7. Other randomized studies did not discriminate between the minimally invasive and open approaches, but retrospective data including 718 patients after gastrointestinal and hepatobiliary operations also supported the conclusion that laparoscopic surgery may significantly facilitate early mobilization24. In contrast, the percentage of the minimally invasive approach (58 per cent) in the subgroups of patients who achieved early mobilization goals on postoperative day 1 after colorectal surgery was not significantly different20.
Impact of early mobilization on postoperative morbidity rate
All except 416,21,22,27 of the 15 trials reported results on the postoperative morbidity rate. Whereas most studies listed standard surgical and non-surgical complications (within 30 days) graded in accordance with the Clavien–Dindo classification (CDC), three studies concentrated on defined postoperative pulmonary complications (PPC) only15,18,26. The three studies with the largest number of included patients (n = 291–1170) were retrospective and described a significantly lower complication rate in patients with EPM, mainly after colorectal surgery20,24,25. Consequently, a subgroup meta-analysis of morbidity rate after EPM in colorectal patients (n = 4 studies)14,19,20,25 resulted in a significant relative risk (RR) reduction in postoperative complications (RR 0.60 (0.47, 0.77), P < 0.01, Fig. S2, Supplementary material). However, the retrospective design cannot exclude that the occurrence of complications negatively impacts patient mobility and co-morbidities were not balanced in all retrospective subgroups (Table S3, Supplementary material). Therefore, the meta-analysis was focused on morbidity rate of RCTs only.
To this end, morbidity rate results could be extracted from six RCTs (including colorectal, upper gastrointestinal and hepatopancreatic surgery) for meta-analysis, which showed no significant effect of EPM protocols on the postoperative morbidity rate (RR 0.93 (0.70, 1.23), P = 0.59), Fig. 57,14,17–19,23. Most of the analysed trials included all relevant complications graded according to the CDC. However, one trial18 only reported the rate of postoperative pneumonia. Exclusion of this RCT from the meta-analysis did not significantly alter the result (RR 0.94 (0.71, 1.25), P = 0.94). Further subgroup meta-analyses focusing on morbidity rate after specific operations other than colorectal (for example pancreatic (one study included pancreatic resections only) or upper gastrointestinal surgery (one study included bariatric resections only)) are currently not conclusive because of the paucity of available data.
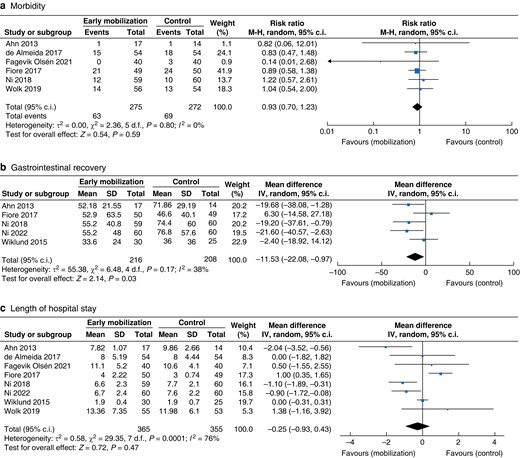
Meta-analysis results (forest plots) of different comparisons in RCTs evaluating early postoperative versus standard mobilization after gastrointestinal surgery
a Postoperative 30-day morbidity rate (CDC I–V7,14,19,23, CDC III–V17, pneumonia18). b Gastrointestinal recovery (time to first flatus or stool in hours). c Length of hospital stay (days). CDC: Clavien-Dindo classification of complications; M-H: Mantel-Haenszel.
Only three studies considered adverse events related to mobilization or the study intervention (for example collapse, falling, accidental drain removal), but no such adverse events were observed 15,18,26.
Impact of early mobilization on pulmonary and gastrointestinal function
Three prospective studies (one case control, two RCTs) assessed short-term pulmonary function after EPM by measuring oxygenation (for example oxygen saturation (SaO2)), spirometry data (for example forced vital capacity (FVC) and forced expiratory volume in one second (FEV1)) or the Borg breathlessness scale 15,18,28. In addition, postoperative pulmonary complications were recorded. The two RCTs did not discover significantly different postoperative spirometry results after pancreatic or colorectal surgery with EPM 18,28. However, in one trial, the SaO2/FiO2 ratio was significantly higher at 18.00 on the day of surgery in a same-day mobilization group 18.
Recovery of gastrointestinal function (first time to flatus or defaecation) was investigated in six studies as secondary outcome parameters14,19,21–23,27. A significantly shorter recovery of gastrointestinal function was demonstrated in four studies14,21–23. Across these studies, the mean time to gastrointestinal recovery was 53.5 h (approximately 2 days) after early mobilization, and 68.3 h (approximately 2.8 days) in the control groups. A meta-analysis of the RCTs revealed a significantly earlier gastrointestinal recovery (mean difference: −11.53 (−22.08, −0.97) hours, P = 0.03) (Fig. 5b).
Impact of early mobilization on postoperative pain and sleep quality
Only two of the included studies reported postoperative pain intensity22,23. From days 4 or 5 following liver resection, the pain scores in the early mobilization groups were significantly lower than those in the control groups (mean difference on day 5: −0.54 (−1.44, −0.04), P = 0.03; Fig. S3, Supplementary material). Additionally, sleeping times were longer on POD 322 and POD 522,23 than in the standard mobilization groups. As an additional parameter to evaluate recovery after major abdominal surgery, one study recorded the incidence and intensity of fatigue measured by the revised Piper fatigue scale (PFS-R). The results showed that patients undergoing the EPM programme had a lower incidence of fatigue at POD 5 than did the control group17.
Impact of early mobilization on duration of hospital stay
The duration of postoperative hospital stay is one of the most relevant clinical and economic outcome parameters for the evaluation of ERAS and mobilization protocols. Accordingly, the LOS was reported in all included studies, although LOS was assessed as the primary endpoint in only one trial14. Nine studies described a positive effect of EPM protocols, resulting in a significantly shorter LOS7,14,15,20–25. However, a meta-analysis of RCTs demonstrated no significant difference in LOS after EPM (mean difference: −0.25 (−0.99, 0.43), P = 0.47) (Fig. 5). Of note, there was considerable heterogeneity among the LOS results (see also the funnel plot, Fig. S4, Supplementary material). Reasons for the heterogeneity are most likely the various included operations (from laparoscopic bariatric bypass to open pancreatic resection), patient characteristics and the different intervention protocols.
Discussion
It is believed that EPM after gastrointestinal surgery is an important element of the ERAS concept, although detailed descriptions of exercise protocols are scarce. Surgeons often recommend early ambulation to overcome postoperative gastroparesis and drive propulsive gut motility. However, experimental data revealed that early ambulation did not significantly affect gastrointestinal myoelectric activity29. The physiologic link between postoperative patient mobility and gastrointestinal recovery is of peculiar interest in gastrointestinal surgery because of the frequently altered gastrointestinal anatomy and anastomotic healing. Interestingly, the present meta-analysis now indicates that EPM positively correlates with a faster clinical gastrointestinal recovery.
In addition, patients scheduled for gastrointestinal operations are often advanced in age and have co-morbidities, which may limit physical fitness and motivation6. On the other hand, patients accept the need for early ambulation and experience early mobilization as an important part of care with an impact on recovery and well-being6,30. New tools such as digital apps or wearables are now supporting patient motivation, education and training, and help to objectively assess postoperative mobility5,7,31,32. The increasing use of minimally invasive (laparoscopic and robotic) approaches to gastrointestinal surgery reduces surgical trauma and will further facilitate enforced ambulation7.
The present review and meta-analysis demonstrated that current trials frequently include heterogeneous patient cohorts, operations, mobilization protocols and outcome measures, which contribute to the fact that we cannot clearly describe the effects of EPM protocols. The start of EPM programmes should begin on the day of surgery or the first postoperative day, which was respected by almost all included trials. There are discrepant observations between retrospective studies and prospective RCTs regarding the effect of EPM on morbidity rate and LOS. Larger retrospective studies have described a reduction in postoperative morbidity rate and LOS for patients with enhanced mobilization, but it remains unclear whether the control group patients did not ambulate because of emerging complications20,24,25. Thus, an RCT in a homogeneous patient cohort is a more appropriate design to minimize selection bias.
Based on the present review, the largest homogeneous subgroup of studies consists of patients evaluated after colorectal surgery. However, only a few trials analysed the intervention ‘patient mobilization’ in isolation and could be included in this review. Overall, there seems to be a significant link between EPM and reduced morbidity rate after colorectal surgery, but this has not been confirmed in RCTs so far. One could argue that the intervention ‘mobilization’ alone has significant effects only in conjunction with additional ERAS elements. However, further RCTs tested a combined intervention comprising mobilization and diet after colorectal operations but also failed to show a significant reduction in morbidity rate and LOS33,34.
In sum, the present study tested the effect of EPM after gastrointestinal surgery. This systematic review underscores that EPM is a rapidly growing and important research field in gastrointestinal surgery that is generating randomized data. Based on this finding, the study indicates that EPM may improve patient oxygenation during the first postoperative hours, which is additionally confirmed by RCTs including other than gastrointestinal operations35. Moreover, EPM significantly accelerates clinical gastrointestinal recovery (for example first time to flatus). Single-centre reports may also support reduced pain intensity and improved sleep quality22,23. However, EPM in isolation was not found to improve pulmonary function, or reduce morbidity rate or LOS in this meta-analysis. The review also summarized the mean realistic amount of ambulation after gastrointestinal surgery, which was up to 4000 steps on POD 5. This mobilization target may be even higher in cohorts of fitter patients and patients evaluated after minimally invasive surgery.
Several limitations of the present study must be taken into account, which are mainly founded on the designs and heterogeneity (different operations, open and minimally invasive techniques, different mobilization protocols) of the included trials. First, patients with both oncological and bariatric surgery were analysed. Second, the study cohort sizes of the RCTs were maximally 50–60 patients in the intervention subgroups and not always calculated for the clinically relevant endpoints of morbidity rate or LOS. Third, many trials did not report the compliance of the patients for the specific mobilization protocols, so it is unknown how many participants in the intervention groups completed the respective protocols. Finally, poor evidence is provided for the effectiveness of the mobilization protocols because the protocols did not lead to increased activity (for example step count) in all studies. A further limitation is the review process itself, which systematically excluded all trials that investigated EPM within a multimodal ERAS setting. According to the literature research term, studies reporting an EPM starting after POD 1 were included in the analysis, although mobilization after POD 1 is ‘standard’ rather than ‘early’. The authors decided to include these studies as well to provide an overview of what is currently considered EPM in the scientific community.
The number of retrospective studies and RCTs investigating EPM after gastrointestinal surgery has relevantly increased during the last 8 years. However, the presently available evidence, including randomized data, does not currently support specific EPM protocols with additionally allocated resources or training elements as an isolated element to further reduce the morbidity rate and LOS. However, an effect in combination with other ERAS elements cannot be excluded. In order to generate a solid ground of evidence, further well-designed RCTs are needed to identify feasible, effective and procedure- or patient-specific EPM programmes. These trials should focus on more homogeneous cohorts (for example minimally invasive colon surgery) in an established ERAS setting, a consistent study cohort size calculation for clinically relevant endpoints and a clearly defined mobilization protocol beginning on the day of surgery.
Funding
The authors have no funding to declare.
Acknowledgements
This systematic review was not preregistered in an institutional registry. A separate review protocol was not prepared.
Disclosure
The authors declare no conflict of interest.
Supplementary material
Supplementary material is available at BJS Open online.
Data availability
The raw data of the presented analysis are available upon request from the corresponding author.
Author contributions
Antonie Willner (Conceptualization, Data curation, Formal analysis, Investigation, Software, Visualization, Writing—original draft, Writing—review & editing), Christian Teske (Data curation, Formal analysis, Software, Writing—review & editing), Thilo Hackert (Supervision, Writing—review & editing) and Thilo Welsch (Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing—original draft, Writing—review & editing).



