-
PDF
- Split View
-
Views
-
Cite
Cite
Alessandro Parente, Sivesh K Kamarajah, Joseph P Thompson, Charlotte Crook, Sebastian Aspinall, Ross Melvin, Michael J Stechman, Helen Perry, Sabapathy P Balasubramanian, Arslan Pannu, Fausto F Palazzo, Klaas Van Den Heede, Fiona Eatock, Hannah Anderson, Helen Doran, Kelvin Wang, Johnathan Hubbard, Abdulaziz Aldrees, Susannah L Shore, Clare Fung, Alison Waghorn, John Ayuk, Davinia Bennett, Robert P Sutcliffe, on behalf of the UK Phaeo Study Group, Risk factors for postoperative complications after adrenalectomy for phaeochromocytoma: multicentre cohort study, BJS Open, Volume 7, Issue 5, October 2023, zrad090, https://doi.org/10.1093/bjsopen/zrad090
Close - Share Icon Share
Abstract
To determine the incidence and risk factors for postoperative complications and prolonged hospital stay after adrenalectomy for phaeochromocytoma.
Demographics, perioperative outcomes and complications were evaluated for consecutive patients who underwent adrenalectomy for phaeochromocytoma from 2012 to 2020 in nine high-volume UK centres. Odds ratios were calculated using multivariable models. The primary outcome was postoperative complications according to the Clavien–Dindo classification and secondary outcome was duration of hospital stay.
Data were available for 406 patients (female n = 221, 54.4 per cent). Two patients (0.5 per cent) had perioperative death, whilst 148 complications were recorded in 109 (26.8 per cent) patients. On adjusted analysis, the age-adjusted Charlson Co-morbidity Index ≥3 (OR 8.09, 95 per cent c.i. 2.31 to 29.63, P = 0.001), laparoscopic converted to open (OR 10.34, 95 per cent c.i. 3.24 to 36.23, P <0.001), and open surgery (OR 11.69, 95 per cent c.i. 4.52 to 32.55, P <0.001) were independently associated with postoperative complications. Overall, 97 of 430 (22.5 per cent) had a duration of stay ≥5 days and this was associated with an age-adjusted Charlson Co-morbidity Index ≥3 (OR 4.31, 95 per cent c.i. 1.08 to 18.26, P = 0.042), tumour size (OR 1.15, 95 per cent c.i. 1.05 to 1.28, P = 0.006), laparoscopic converted to open (OR 32.11, 95 per cent c.i. 9.2 to 137.77, P <0.001), and open surgery (OR 28.01, 95 per cent c.i. 10.52 to 83.97, P <0.001).
Adrenalectomy for phaeochromocytoma is associated with a very low mortality rate, whilst postoperative complications are common. Several risk factors, including co-morbidities and operative approach, are independently associated with postoperative complications and/or prolonged hospitalization, and should be considered when counselling patients.
Introduction
Phaeochromocytoma is a rare neuroendocrine tumour arising from adrenomedullary chromaffin cells that frequently produces catecholamines, namely epinephrine, norepinephrine, and dopamine1. Surgical treatment is the ‘standard’ therapy for phaeochromocytoma and recommended as being the only curative option2. Robust data on postoperative complications are lacking. To date, several studies have shown that postoperative complications after adrenalectomy develop in a range of 4.0 to 11.5 per cent of patients3–7. However, these series only reported data for Clavien–Dindo classification8 (CDC) grade II or higher and were not focused solely on phaeochromocytoma. A few other studies reported a complication rate of 16.0 to 29.8 per cent9–11 after surgery for phaeochromocytoma, which appears to be higher than any other indication for adrenalectomy. However, these studies did not focus on prolonged length of hospital stay (LOS), which is an important outcome in the era of limited resources. Therefore, the primary aim of this study was to quantify the cumulative burden of postoperative complications after surgery for phaeochromocytoma using both the CDC8 and the comprehensive complications index (CCI)12 in a large multicentre UK data set. The secondary aims were to identify risk factors for postoperative complications and for prolonged duration of hospital stay.
Methods
Data collection and study population
Data for all consecutive adult patients undergoing surgery for phaeochromocytoma between 1 January 2012 and 31 December 2020 were retrospectively reviewed from nine tertiary UK referral institutions. Only surgeons working in high-volume UK centres were invited to contribute data for this study. The database consisted of data from 10 consultant surgeons working in nine UK centres, with a median annual adrenalectomy volume of 24.5 (interquartile range (i.q.r.) 14–33.5) cases per surgeon. This data was obtained from British Association of Endocrine and Thyroid Surgeons (BAETS) audit data from 2019 (https://www.baets.org.uk/audit/). Preoperative diagnosis of phaeochromocytoma was confirmed or suspected based on cross-sectional imaging (CT or magnetic resonance imaging) combined with elevated plasma/urine catecholamine levels according to individual centre protocols. Information on patient sex, age, and body mass index (BMI) was collected. Preoperative co-morbidities were scored according to the age-adjusted Charlson Co-morbidity Index13. In addition, tumour size, location, and underlying genetic conditions were collated. All cases were reviewed and the most recent preoperative urine and/or plasma levels of catecholamine or catecholamine metabolites, including total urinary metanephrines (metanephrines and normetanephrines), urinary catecholamines (epinephrine, norepinephrine, and dopamine), and plasma metanephrine and normetanephrine. The fourth quartile was calculated for each metabolite and the preoperative catecholamine level was treated as a categorical variable (above or below the fourth quartile) in order to maximize the number of patients in the analysis, thus resulting in upper or lower quartiles. The measure of the preoperative catecholamine used in the analysis was a combined measure. The value for each patient was based on only one of the catecholamine measures. The specific measures were ranked based on the frequency of data collected, with the most commonly measured variable given preference. The order of preference was as follows (with cut-off values for upper quartile provided): plasma normetanephrine (n = 234) >7578 pmol/l; plasma metanephrine (n = 196) >1496 pmol/l; urinary normetanephrine (n = 185) >22 μmol/24 h; urinary metadrenaline (n = 181) >1960 nmol/24 h; urinary noradrenaline (n = 112) >2957 nmol/24 h; urinary adrenaline (n = 108) >275 nmol/24 h.
Intra- and postoperative data regarding haemodynamic variables were obtained by review of anaesthetic charts and electronic patient records. Intraoperative hypertension was defined as systolic blood pressure >200 mmHg and/or need for vasodilator therapy. Intraoperative/postoperative hypotension was defined as systolic blood pressure <90 mmHg and/or need for vasopressor therapy. The volume of intravenous fluid administered during and after surgery was also recorded for each patient. Patients with incomplete perioperative data were excluded. Patients were not required to give informed consent to the study because the analysis used anonymous clinical data collected retrospectively. This study was registered with the Clinical Audit departments of all participating centres, and due to its retrospective design, ethical approval was waived.
Perioperative management and surgical technique
Based on centre preference, patients were started on alpha-adrenergic blockade either as outpatients (5 of 9 centres) or inpatients (4 of 9 centres). Additional use of beta-adrenergic blockade was selective by local protocols. Patients were routinely admitted the evening before surgery for intravenous fluid therapy in four centres. Adrenalectomy was routinely performed either with an open approach (OA) or laparoscopic approach (LA). LA was undertaken using either transperitoneal or retroperitoneal techniques according to surgeon preference. Intraoperative and postoperative haemodynamic instability were managed by vasoactive drugs and/or intravenous fluids led by anaesthetists experienced in the perioperative management of phaeochromocytoma. Perioperative complications and postoperative outcomes were evaluated. Complications were considered as ‘any deviation from the normal postoperative course’, according to the CDC8 between the date of surgery and discharge. Each complication was assigned a CDC grade I to V, based on the degree of invasiveness of the required treatment, with grade V representing death. The CCI12 was used to score (0–100) the overall burden of complications between surgery and discharge. LOS was calculated from the day of surgery until the day of discharge. Of note, prolonged vasopressor support, defined as postoperative hypotension (PH) requiring vasopressor support for more than 24 h, was considered as a grade IVa complication based on a previous study14.
Study outcomes
The primary outcome was development of ≥CDC grade I postoperative complications, which gives a CCI score ≥8.7. Secondary outcomes included risk factors for developing any type of postoperative complication and prolonged LOS. The latter was defined as prolonged if the stay was longer than the 75th percentile of the overall cohort based on a previous study3.
Statistical analysis
Normally distributed data were presented as mean(s.d.) and non-normally distributed data were presented as median and i.q.r. Categorical variables were compared using the chi-squared test. Continuous variables were analysed using an independent Student’s t test or a Mann–Whitney U test, based on their distribution. Multivariable analyses used binary logistic regression to develop adjusted odds ratio and 95 per cent confidence intervals for primary and secondary outcomes as described above. The authors hypothesized that postoperative hypotension requiring vasopressor >24 h would be a common complication after adrenalectomy for phaeochromocytoma, therefore sensitivity analysis was performed excluding this specific complication in order to better understand the overall burden of postoperative surgical complications and risk factors after adrenalectomy for phaeochromocytoma. A two-sided P value of <0.05 was considered statistically significant. Data analysis was performed using R Foundation Statistical software (R 3.2.2) with TableOne and finalfit packages (R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient demographics
Four hundred and thirty patients underwent surgery for phaeochromocytoma among nine UK centres during the study interval. The median annual volume of adrenalectomy for phaeochromocytoma for participating centres was 7.3 (range 3.1–12.3). The mean(s.d.) age at surgery was 54.1(16.2) years and 54.4 per cent (n = 234) of patients were female. Metastatic disease was found in five (1.2 per cent) patients before surgery, whereas four patients (0.9 per cent) had bilateral disease. The median tumour size was 4.0 cm (i.q.r. 2.9–6) and the diagnosis of phaeochromocytoma was confirmed at histology in the entire cohort. Genetic conditions were present in 68 patients (15.8 per cent), including Multiple Endocrine Neoplasia (n = 21), neurofibromatosis (n = 18), succinate dehydrogenase (SDH) mutations (n = 13) and von Hippel Lindau (n = 11).
Peri- and postoperative outcomes
Data regarding operative approach and postoperative outcomes was available for 406 patients. The majority of patients (321 of 406, 79.1 per cent) underwent LA and 58 patients (14.2 per cent) underwent upfront OA. Of the 321 laparoscopic procedures, 23 (7.1 per cent) were converted to open surgery due to technical difficulty (n = 13), intraoperative bleeding (n = 8), or adhesions (n = 2). Retroperitoneal approach was performed in 41 patients (12 per cent) of whom only one was converted to open. None of the patients underwent robotic procedures in our cohort. The main reasons for upfront OA were tumour size (median 6 cm (range 3–10) compared with median 4 cm (range 2.8–5.5) in the LA group (P = 0.0002)), and known extra adrenal metastasis (present in five (7.5 per cent) patients). Of 395 patients, 385 (97.5 per cent) received preoperative alpha-adrenergic blockade, predominantly using either phenoxybenzamine (n = 296) or doxazosin (n = 89). Of 392 patients, 148 (37.8 per cent) received beta-adrenergic blockade, either b1-selective (n = 61) or non-selective (n = 87). Baseline characteristics between LA and OA in the entire cohort are detailed in Table 1.
Baseline characteristics of patients undergoing adrenalectomy for phaeochromocytoma based on surgical approach (laparoscopic versus open)
| Variable . | N . | Total . | Lap = 340 . | Open = 66 . | P . |
|---|---|---|---|---|---|
| Age (years), mean(s.d.) | 430 | 54.1(16.2) | 54.7(16.2) | 50.4(16.2) | P = 0.059 |
| Women, n (%) | 430 | 234 (54.4%) | 190 (55.8%) | 44 (66.6%) | P = 0.064 |
| BMI (kg/m2), mean(s.d.) | 363 | 27.9(6.2) | 27.8(6.2) | 28.4(6.5) | P = 0.548 |
| Charlson score§ | |||||
| 0–2, n (%) | 406 | 273 (67.2%) | 232 (68.2%) | 41 (62.1%) | P = 0.366 |
| ≥3, n (%) | 133 (32.7%) | 108 (31.7%) | 25 (37.8%) | ||
| Tumour size (cm), median (i.q.r.) | 404 | 4 (2.9–6) | 4 (2.8–5.5) | 6 (3–10) | P = 0.002 |
| Extra-adrenal metastases, n (%) | 408 | 5 (1.2%) | 0 (0%) | 5 (7.5%) | P <0.001 |
| Bilateral disease, n (%) | 430 | 4 (0.9%) | 4 (1.1%) | 0 (0%) | P <0.001 |
| Genetic mutation, n (%)* | 430 | 68 (15.8%) | 52 (15.2%) | 16 (24.2%) | P = 0.062 |
| Mean arterial pressure (mmHg), mean(s.d.)† | 348 | 96.3(15.3) | 96(15.5) | 98.4(14.2) | P = 0.307 |
| Alpha-blocker used, n (%) | 395 | 385 (97.5%) | 331 (97.3%) | 54 (94.7%) | P = 0.164 |
| Doxazosin, n (%) | 89 (22.5%) | 78 (22.9%) | 11 (19.3%) | P = 0.097 | |
| Phenoxybenzamine, n (%) | 296 (74.9%) | 256 (75.3%) | 40 (70.2%) | ||
| Beta-blocker user, n (%) | 392 | 148 (37.8%) | 127 (37.3%) | 21 (37.5%) | P = 0.987 |
| Propranolol, n (%) | 87 (58.7%) | 71 (56%) | 16 (76.2%) | P = 0.301 | |
| β1-selective, n (%)‡ | 61 (41.2%) | 52 (41%) | 9 (42.8%) |
| Variable . | N . | Total . | Lap = 340 . | Open = 66 . | P . |
|---|---|---|---|---|---|
| Age (years), mean(s.d.) | 430 | 54.1(16.2) | 54.7(16.2) | 50.4(16.2) | P = 0.059 |
| Women, n (%) | 430 | 234 (54.4%) | 190 (55.8%) | 44 (66.6%) | P = 0.064 |
| BMI (kg/m2), mean(s.d.) | 363 | 27.9(6.2) | 27.8(6.2) | 28.4(6.5) | P = 0.548 |
| Charlson score§ | |||||
| 0–2, n (%) | 406 | 273 (67.2%) | 232 (68.2%) | 41 (62.1%) | P = 0.366 |
| ≥3, n (%) | 133 (32.7%) | 108 (31.7%) | 25 (37.8%) | ||
| Tumour size (cm), median (i.q.r.) | 404 | 4 (2.9–6) | 4 (2.8–5.5) | 6 (3–10) | P = 0.002 |
| Extra-adrenal metastases, n (%) | 408 | 5 (1.2%) | 0 (0%) | 5 (7.5%) | P <0.001 |
| Bilateral disease, n (%) | 430 | 4 (0.9%) | 4 (1.1%) | 0 (0%) | P <0.001 |
| Genetic mutation, n (%)* | 430 | 68 (15.8%) | 52 (15.2%) | 16 (24.2%) | P = 0.062 |
| Mean arterial pressure (mmHg), mean(s.d.)† | 348 | 96.3(15.3) | 96(15.5) | 98.4(14.2) | P = 0.307 |
| Alpha-blocker used, n (%) | 395 | 385 (97.5%) | 331 (97.3%) | 54 (94.7%) | P = 0.164 |
| Doxazosin, n (%) | 89 (22.5%) | 78 (22.9%) | 11 (19.3%) | P = 0.097 | |
| Phenoxybenzamine, n (%) | 296 (74.9%) | 256 (75.3%) | 40 (70.2%) | ||
| Beta-blocker user, n (%) | 392 | 148 (37.8%) | 127 (37.3%) | 21 (37.5%) | P = 0.987 |
| Propranolol, n (%) | 87 (58.7%) | 71 (56%) | 16 (76.2%) | P = 0.301 | |
| β1-selective, n (%)‡ | 61 (41.2%) | 52 (41%) | 9 (42.8%) |
*These included: multiple endocrine neoplasia, neurofibromatosis, SDH mutations, and von Hippel Lindau. †Preoperative mean arterial pressure. ‡β1-selective include atenolol, bisoprolol, metoprolol. §Age-adjusted Charlson Co-morbidity Index. Bold values represent statistical significance.
Baseline characteristics of patients undergoing adrenalectomy for phaeochromocytoma based on surgical approach (laparoscopic versus open)
| Variable . | N . | Total . | Lap = 340 . | Open = 66 . | P . |
|---|---|---|---|---|---|
| Age (years), mean(s.d.) | 430 | 54.1(16.2) | 54.7(16.2) | 50.4(16.2) | P = 0.059 |
| Women, n (%) | 430 | 234 (54.4%) | 190 (55.8%) | 44 (66.6%) | P = 0.064 |
| BMI (kg/m2), mean(s.d.) | 363 | 27.9(6.2) | 27.8(6.2) | 28.4(6.5) | P = 0.548 |
| Charlson score§ | |||||
| 0–2, n (%) | 406 | 273 (67.2%) | 232 (68.2%) | 41 (62.1%) | P = 0.366 |
| ≥3, n (%) | 133 (32.7%) | 108 (31.7%) | 25 (37.8%) | ||
| Tumour size (cm), median (i.q.r.) | 404 | 4 (2.9–6) | 4 (2.8–5.5) | 6 (3–10) | P = 0.002 |
| Extra-adrenal metastases, n (%) | 408 | 5 (1.2%) | 0 (0%) | 5 (7.5%) | P <0.001 |
| Bilateral disease, n (%) | 430 | 4 (0.9%) | 4 (1.1%) | 0 (0%) | P <0.001 |
| Genetic mutation, n (%)* | 430 | 68 (15.8%) | 52 (15.2%) | 16 (24.2%) | P = 0.062 |
| Mean arterial pressure (mmHg), mean(s.d.)† | 348 | 96.3(15.3) | 96(15.5) | 98.4(14.2) | P = 0.307 |
| Alpha-blocker used, n (%) | 395 | 385 (97.5%) | 331 (97.3%) | 54 (94.7%) | P = 0.164 |
| Doxazosin, n (%) | 89 (22.5%) | 78 (22.9%) | 11 (19.3%) | P = 0.097 | |
| Phenoxybenzamine, n (%) | 296 (74.9%) | 256 (75.3%) | 40 (70.2%) | ||
| Beta-blocker user, n (%) | 392 | 148 (37.8%) | 127 (37.3%) | 21 (37.5%) | P = 0.987 |
| Propranolol, n (%) | 87 (58.7%) | 71 (56%) | 16 (76.2%) | P = 0.301 | |
| β1-selective, n (%)‡ | 61 (41.2%) | 52 (41%) | 9 (42.8%) |
| Variable . | N . | Total . | Lap = 340 . | Open = 66 . | P . |
|---|---|---|---|---|---|
| Age (years), mean(s.d.) | 430 | 54.1(16.2) | 54.7(16.2) | 50.4(16.2) | P = 0.059 |
| Women, n (%) | 430 | 234 (54.4%) | 190 (55.8%) | 44 (66.6%) | P = 0.064 |
| BMI (kg/m2), mean(s.d.) | 363 | 27.9(6.2) | 27.8(6.2) | 28.4(6.5) | P = 0.548 |
| Charlson score§ | |||||
| 0–2, n (%) | 406 | 273 (67.2%) | 232 (68.2%) | 41 (62.1%) | P = 0.366 |
| ≥3, n (%) | 133 (32.7%) | 108 (31.7%) | 25 (37.8%) | ||
| Tumour size (cm), median (i.q.r.) | 404 | 4 (2.9–6) | 4 (2.8–5.5) | 6 (3–10) | P = 0.002 |
| Extra-adrenal metastases, n (%) | 408 | 5 (1.2%) | 0 (0%) | 5 (7.5%) | P <0.001 |
| Bilateral disease, n (%) | 430 | 4 (0.9%) | 4 (1.1%) | 0 (0%) | P <0.001 |
| Genetic mutation, n (%)* | 430 | 68 (15.8%) | 52 (15.2%) | 16 (24.2%) | P = 0.062 |
| Mean arterial pressure (mmHg), mean(s.d.)† | 348 | 96.3(15.3) | 96(15.5) | 98.4(14.2) | P = 0.307 |
| Alpha-blocker used, n (%) | 395 | 385 (97.5%) | 331 (97.3%) | 54 (94.7%) | P = 0.164 |
| Doxazosin, n (%) | 89 (22.5%) | 78 (22.9%) | 11 (19.3%) | P = 0.097 | |
| Phenoxybenzamine, n (%) | 296 (74.9%) | 256 (75.3%) | 40 (70.2%) | ||
| Beta-blocker user, n (%) | 392 | 148 (37.8%) | 127 (37.3%) | 21 (37.5%) | P = 0.987 |
| Propranolol, n (%) | 87 (58.7%) | 71 (56%) | 16 (76.2%) | P = 0.301 | |
| β1-selective, n (%)‡ | 61 (41.2%) | 52 (41%) | 9 (42.8%) |
*These included: multiple endocrine neoplasia, neurofibromatosis, SDH mutations, and von Hippel Lindau. †Preoperative mean arterial pressure. ‡β1-selective include atenolol, bisoprolol, metoprolol. §Age-adjusted Charlson Co-morbidity Index. Bold values represent statistical significance.
Data regarding intraoperative haemodynamic instability was available for 274 patients (63.7 per cent). A total of 149 patients (54.4 per cent) experienced intraoperative hypertension (systolic blood pressure >200 mmHg), of whom 135 patients received vasodilator therapy. The most common vasodilator agents used were glycerol trinitrate (n = 52) and sodium nitroprusside (n = 46). On univariable analysis, female sex (61.0 per cent versus 45.9 per cent; P = 0.014), preoperative plasma normetanephrine levels (4655 versus 2164 pmol/l; P <0.001), and preoperative beta-adrenergic blockade (51.4 per cent versus 28.9 per cent; P <0.001) were significantly associated with intraoperative hypertension. There were no significant associations between tumour size (4.5 versus 4.0 cm; P = 0.079), epidural analgesia (13.0 per cent versus 13.1 per cent; P = 0.980), open surgery (16.4 per cent versus 15.6 per cent; P = 0.848) and intraoperative hypertension. Among the 274 patients, 254 (92.7 per cent) experienced intraoperative hypotension (systolic blood pressure <90 mmHg), of whom 231 patients received vasopressor therapy. Noradrenaline (n = 114) and metaraminol (n = 102) were the most frequently used vasopressors. The median volume of intravenous fluids administered intraoperatively was 2.5 litres (range 0.5–10).
Of 406 patients, 109 patients (26.8 per cent) developed one or more complications (grade I = 10, grade II = 80, grade IIIa = 1, grade IIIb = 3, grade IVa = 52, grade IVb = 0, grade V = 2), and the median CCI was 0 (i.q.r. 0–20.9). The most frequent complications were prolonged hypotension (n = 48; defined as vasopressor requirements for >24 h), haemorrhage requiring perioperative transfusion (n = 20), pneumonia (n = 19), wound infection (n = 12), postoperative ileus (n = 8), and urinary tract infection (n = 7). Of note, prolonged hypotension was the only complication in 31 patients. There were two perioperative deaths (0.5 per cent), due to myocardial infarct and respiratory failure respectively. A detailed description of the complications is summarized in Tables S1 and S2 and visualized in Fig. 1.
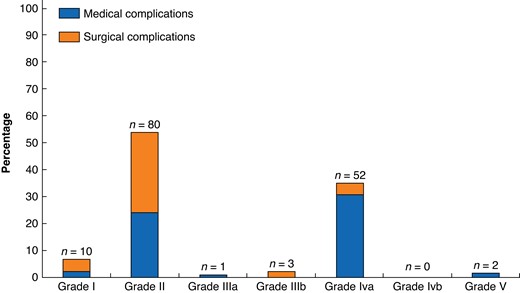
Percentages and numbers of postoperative complications after adrenalectomy for phaeochromocytoma according to Clavien–Dindo classification. Number of 148 complications in 109 patients according to Clavien–Dindo classification. Data available for 406 patients
Risk factors for postoperative complications
Age-adjusted Charlson Co-morbidity Index (P = 0.025), tumour size (P = 0.006), and conversion to open surgery (P <0.001) were significantly higher in patients who experienced postoperative complications (Table 2). There were no statistically significant differences in preoperative catecholamines or usage of alpha/beta blockade among the groups of patients who developed complications versus the ones who did not (Table 2). The age-adjusted Charlson Co-morbidity Index ≥ 3 (OR 8.09, 95 per cent c.i. 2.31 to 29.63, P = 0.001), conversion to open surgery (OR 10.34, 95 per cent c.i. 3.24 to 36.23, P <0.001), and open surgery (OR 11.69, 95 per cent c.i. 4.52 to 32.55, P <0.001) were all found to be independently associated with postoperative complications on multivariable analysis (Table 3). Of these, tumour size was found to correlate on univariate analysis (OR 1.11, 95 per cent c.i. 1.03 to 1.20, P = 0.004), but this was not confirmed by multivariable analysis (OR 1.03, 95 per cent c.i. 0.93 to 1.14, P = 0.53) (Table 4). At sensitivity analysis, which excluded prolonged hypotension requiring vasopressor support as a complication, the age-adjusted Charlson Co-morbidity Index ≥ 3 (OR 5.28 95 per cent c.i. 1.35 to 21.98, P = 0.019), conversion to open surgery (OR 6.59 95 per cent c.i. 2.06 to 21.10, P = 0.001), and open surgery (OR 8.59 95 per cent c.i. 3.30 to 23.22, P < 0.001) remained significantly associated with an increased risk of postoperative complications in the multivariable model (Table S3).
| . | Postoperative complication . | . | |
|---|---|---|---|
| . | No n = 297 . | Yes n = 109 . | P . |
| Age (years), mean(s.d.) | 55.2(7.2) | 56.7(6.1) | 0.38 |
| BMI (kg/m2), median (i.q.r.) | 27 (24–30) | 26 (24–30) | 0.975 |
| Sex | |||
| Women, n (%) | 160 (53.9) | 61 (56) | 0.793 |
| Men, n (%) | 137 (46.1) | 48 (44) | |
| Charlson index, median (i.q.r.)† | 1 (0–3) | 2 (1–3) | 0.025 |
| Alpha-blockers | |||
| Yes, n (%) | 274 (92.3) | 108 (99.1) | 0.36 |
| No, n (%) | 9 (3.0) | 0 (0.0) | |
| Beta-blockers | |||
| Yes, n (%) | 99 (33.0) | 47 (43.1) | 0.137 |
| No, n (%) | 183 (61.6) | 60 (55.0) | |
| Catecholamines | |||
| Lower quartile, n (%) | 217 (73.0) | 71 (65.1) | 0.055 |
| Upper quartile, n (%) | 62 (20.8) | 34 (31.1) | |
| Tumour size (cm), median (i.q.r.) | 4 (2.8–5.5) | 4.8 (3.2–7) | 0.006 |
| Tumour location | |||
| Right, n (%) | 147 (49.5) | 45 (41.3) | 0.251 |
| Left, n (%) | 137 (46.1) | 55 (50.5) | |
| Unknown, n (%) | 13 (4.3) | 9 (8.3) | |
| Approach‡ | |||
| Laparoscopic, n (%) | 264/321 (82.2) | 57/321 (17.8) | <0.001 |
| Converted to open, n (%) | 10/23 (43.4) | 13/23 (56.6) | |
| Open, n (%) | 21/58 (36.2) | 37/58 (63.8) | |
| . | Postoperative complication . | . | |
|---|---|---|---|
| . | No n = 297 . | Yes n = 109 . | P . |
| Age (years), mean(s.d.) | 55.2(7.2) | 56.7(6.1) | 0.38 |
| BMI (kg/m2), median (i.q.r.) | 27 (24–30) | 26 (24–30) | 0.975 |
| Sex | |||
| Women, n (%) | 160 (53.9) | 61 (56) | 0.793 |
| Men, n (%) | 137 (46.1) | 48 (44) | |
| Charlson index, median (i.q.r.)† | 1 (0–3) | 2 (1–3) | 0.025 |
| Alpha-blockers | |||
| Yes, n (%) | 274 (92.3) | 108 (99.1) | 0.36 |
| No, n (%) | 9 (3.0) | 0 (0.0) | |
| Beta-blockers | |||
| Yes, n (%) | 99 (33.0) | 47 (43.1) | 0.137 |
| No, n (%) | 183 (61.6) | 60 (55.0) | |
| Catecholamines | |||
| Lower quartile, n (%) | 217 (73.0) | 71 (65.1) | 0.055 |
| Upper quartile, n (%) | 62 (20.8) | 34 (31.1) | |
| Tumour size (cm), median (i.q.r.) | 4 (2.8–5.5) | 4.8 (3.2–7) | 0.006 |
| Tumour location | |||
| Right, n (%) | 147 (49.5) | 45 (41.3) | 0.251 |
| Left, n (%) | 137 (46.1) | 55 (50.5) | |
| Unknown, n (%) | 13 (4.3) | 9 (8.3) | |
| Approach‡ | |||
| Laparoscopic, n (%) | 264/321 (82.2) | 57/321 (17.8) | <0.001 |
| Converted to open, n (%) | 10/23 (43.4) | 13/23 (56.6) | |
| Open, n (%) | 21/58 (36.2) | 37/58 (63.8) | |
*Data available for 406 patients. †Age-adjusted Charlson Co-morbidity Index. ‡Data for surgical approach are presented horizontally, calculating the percentages on the total number of procedures available. Bold values represent statistical significance.
| . | Postoperative complication . | . | |
|---|---|---|---|
| . | No n = 297 . | Yes n = 109 . | P . |
| Age (years), mean(s.d.) | 55.2(7.2) | 56.7(6.1) | 0.38 |
| BMI (kg/m2), median (i.q.r.) | 27 (24–30) | 26 (24–30) | 0.975 |
| Sex | |||
| Women, n (%) | 160 (53.9) | 61 (56) | 0.793 |
| Men, n (%) | 137 (46.1) | 48 (44) | |
| Charlson index, median (i.q.r.)† | 1 (0–3) | 2 (1–3) | 0.025 |
| Alpha-blockers | |||
| Yes, n (%) | 274 (92.3) | 108 (99.1) | 0.36 |
| No, n (%) | 9 (3.0) | 0 (0.0) | |
| Beta-blockers | |||
| Yes, n (%) | 99 (33.0) | 47 (43.1) | 0.137 |
| No, n (%) | 183 (61.6) | 60 (55.0) | |
| Catecholamines | |||
| Lower quartile, n (%) | 217 (73.0) | 71 (65.1) | 0.055 |
| Upper quartile, n (%) | 62 (20.8) | 34 (31.1) | |
| Tumour size (cm), median (i.q.r.) | 4 (2.8–5.5) | 4.8 (3.2–7) | 0.006 |
| Tumour location | |||
| Right, n (%) | 147 (49.5) | 45 (41.3) | 0.251 |
| Left, n (%) | 137 (46.1) | 55 (50.5) | |
| Unknown, n (%) | 13 (4.3) | 9 (8.3) | |
| Approach‡ | |||
| Laparoscopic, n (%) | 264/321 (82.2) | 57/321 (17.8) | <0.001 |
| Converted to open, n (%) | 10/23 (43.4) | 13/23 (56.6) | |
| Open, n (%) | 21/58 (36.2) | 37/58 (63.8) | |
| . | Postoperative complication . | . | |
|---|---|---|---|
| . | No n = 297 . | Yes n = 109 . | P . |
| Age (years), mean(s.d.) | 55.2(7.2) | 56.7(6.1) | 0.38 |
| BMI (kg/m2), median (i.q.r.) | 27 (24–30) | 26 (24–30) | 0.975 |
| Sex | |||
| Women, n (%) | 160 (53.9) | 61 (56) | 0.793 |
| Men, n (%) | 137 (46.1) | 48 (44) | |
| Charlson index, median (i.q.r.)† | 1 (0–3) | 2 (1–3) | 0.025 |
| Alpha-blockers | |||
| Yes, n (%) | 274 (92.3) | 108 (99.1) | 0.36 |
| No, n (%) | 9 (3.0) | 0 (0.0) | |
| Beta-blockers | |||
| Yes, n (%) | 99 (33.0) | 47 (43.1) | 0.137 |
| No, n (%) | 183 (61.6) | 60 (55.0) | |
| Catecholamines | |||
| Lower quartile, n (%) | 217 (73.0) | 71 (65.1) | 0.055 |
| Upper quartile, n (%) | 62 (20.8) | 34 (31.1) | |
| Tumour size (cm), median (i.q.r.) | 4 (2.8–5.5) | 4.8 (3.2–7) | 0.006 |
| Tumour location | |||
| Right, n (%) | 147 (49.5) | 45 (41.3) | 0.251 |
| Left, n (%) | 137 (46.1) | 55 (50.5) | |
| Unknown, n (%) | 13 (4.3) | 9 (8.3) | |
| Approach‡ | |||
| Laparoscopic, n (%) | 264/321 (82.2) | 57/321 (17.8) | <0.001 |
| Converted to open, n (%) | 10/23 (43.4) | 13/23 (56.6) | |
| Open, n (%) | 21/58 (36.2) | 37/58 (63.8) | |
*Data available for 406 patients. †Age-adjusted Charlson Co-morbidity Index. ‡Data for surgical approach are presented horizontally, calculating the percentages on the total number of procedures available. Bold values represent statistical significance.
Multivariable logistic models to identify risk factors for postoperative complications and prolonged hospital stay ≥5 days
| Postoperative complications . | . | . | . | . |
|---|---|---|---|---|
| Variable . | OR univariate (95% c.i.) . | P . | OR multivariate (95% c.i.) . | P . |
| Age, years | 1.01 (1.00–1.02) | 0.426 | 0.97 (0.94–1.00) | 0.052 |
| Sex, female | 1.09 (0.70–1.70) | 0.708 | 1.26 (0.71–2.25) | 0.428 |
| BMI, kg/m2 | 1.00 (0.96–1.03) | 0.801 | 0.98 (0.93–1.03) | 0.427 |
| Charlson Index ≥3 | 2.05 (1.14–3.74) | 0.017 | 8.09 (2.31–29.63) | 0.001 |
| Alpha blockade | 3.55 (0.66–6.81, P = 0.232) | 0.232 | 6.30 (0.95–12.69, P = 0.109) | 0.109 |
| Beta blockade | 1.45 (0.92–2.28, P = 0.110) | 0.11 | 1.68 (0.95–2.96, P = 0.073) | 0.73 |
| Tumour size (cm) | 1.11 (1.03–1.20) | 0.004 | 1.03 (0.93–1.14) | 0.53 |
| Approach | ||||
| Laparoscopic | REF | <0.001 | REF | <0.001 |
| Converted to open | 6.37 (2.47–17.14) | <0.001 | 10.34 (3.24–36.23) | <0.001 |
| Open | 7.60 (4.31–13.64) | 11.69 (4.52–32.55) | ||
| Postoperative duration of hospital stay ≥ 5 days . | . | . | . | . |
| Variable . | OR univariate (95% c.i.) . | P . | OR multivariate (95% c.i.) . | P . |
| Age, years | 1.00 (0.99–1.02) | 0.841 | 0.99 (0.96–1.02) | 0.531 |
| Sex, female | 0.79 (0.50–1.25) | 0.31 | 0.85 (0.45–1.61) | 0.612 |
| BMI, kg/m2 | 1.00 (0.96–1.04) | 0.99 | 0.98 (0.93–1.03) | 0.434 |
| Charlson Index ≥ 3 | 1.37 (0.76–2.51) | 0.295 | 4.31 (1.08–18.26) | 0.042 |
| Alpha blockade | 1.28 (0.31–8.59) | 0.756 | 3.35 (0.53–31.99) | 0.238 |
| Beta blockade | 1.06 (0.65–1.71) | 0.819 | 1.24 (0.63–2.44) | 0.527 |
| Tumour size (cm) | 1.26 (1.16–1.37) | <0.001 | 1.15 (1.05–1.28) | 0.006 |
| Approach | ||||
| Laparoscopic | REF | <0.001 | REF | <0.001 |
| Converted to open | 15.51 (5.77–46.44) | <0.001 | 32.11 (9.2–133.77) | <0.001 |
| Open | 18.29 (9.8–35.54) | 28.01 (10.52–83.97) |
| Postoperative complications . | . | . | . | . |
|---|---|---|---|---|
| Variable . | OR univariate (95% c.i.) . | P . | OR multivariate (95% c.i.) . | P . |
| Age, years | 1.01 (1.00–1.02) | 0.426 | 0.97 (0.94–1.00) | 0.052 |
| Sex, female | 1.09 (0.70–1.70) | 0.708 | 1.26 (0.71–2.25) | 0.428 |
| BMI, kg/m2 | 1.00 (0.96–1.03) | 0.801 | 0.98 (0.93–1.03) | 0.427 |
| Charlson Index ≥3 | 2.05 (1.14–3.74) | 0.017 | 8.09 (2.31–29.63) | 0.001 |
| Alpha blockade | 3.55 (0.66–6.81, P = 0.232) | 0.232 | 6.30 (0.95–12.69, P = 0.109) | 0.109 |
| Beta blockade | 1.45 (0.92–2.28, P = 0.110) | 0.11 | 1.68 (0.95–2.96, P = 0.073) | 0.73 |
| Tumour size (cm) | 1.11 (1.03–1.20) | 0.004 | 1.03 (0.93–1.14) | 0.53 |
| Approach | ||||
| Laparoscopic | REF | <0.001 | REF | <0.001 |
| Converted to open | 6.37 (2.47–17.14) | <0.001 | 10.34 (3.24–36.23) | <0.001 |
| Open | 7.60 (4.31–13.64) | 11.69 (4.52–32.55) | ||
| Postoperative duration of hospital stay ≥ 5 days . | . | . | . | . |
| Variable . | OR univariate (95% c.i.) . | P . | OR multivariate (95% c.i.) . | P . |
| Age, years | 1.00 (0.99–1.02) | 0.841 | 0.99 (0.96–1.02) | 0.531 |
| Sex, female | 0.79 (0.50–1.25) | 0.31 | 0.85 (0.45–1.61) | 0.612 |
| BMI, kg/m2 | 1.00 (0.96–1.04) | 0.99 | 0.98 (0.93–1.03) | 0.434 |
| Charlson Index ≥ 3 | 1.37 (0.76–2.51) | 0.295 | 4.31 (1.08–18.26) | 0.042 |
| Alpha blockade | 1.28 (0.31–8.59) | 0.756 | 3.35 (0.53–31.99) | 0.238 |
| Beta blockade | 1.06 (0.65–1.71) | 0.819 | 1.24 (0.63–2.44) | 0.527 |
| Tumour size (cm) | 1.26 (1.16–1.37) | <0.001 | 1.15 (1.05–1.28) | 0.006 |
| Approach | ||||
| Laparoscopic | REF | <0.001 | REF | <0.001 |
| Converted to open | 15.51 (5.77–46.44) | <0.001 | 32.11 (9.2–133.77) | <0.001 |
| Open | 18.29 (9.8–35.54) | 28.01 (10.52–83.97) |
Bold values represent statistical significance. REF, reference category.
Multivariable logistic models to identify risk factors for postoperative complications and prolonged hospital stay ≥5 days
| Postoperative complications . | . | . | . | . |
|---|---|---|---|---|
| Variable . | OR univariate (95% c.i.) . | P . | OR multivariate (95% c.i.) . | P . |
| Age, years | 1.01 (1.00–1.02) | 0.426 | 0.97 (0.94–1.00) | 0.052 |
| Sex, female | 1.09 (0.70–1.70) | 0.708 | 1.26 (0.71–2.25) | 0.428 |
| BMI, kg/m2 | 1.00 (0.96–1.03) | 0.801 | 0.98 (0.93–1.03) | 0.427 |
| Charlson Index ≥3 | 2.05 (1.14–3.74) | 0.017 | 8.09 (2.31–29.63) | 0.001 |
| Alpha blockade | 3.55 (0.66–6.81, P = 0.232) | 0.232 | 6.30 (0.95–12.69, P = 0.109) | 0.109 |
| Beta blockade | 1.45 (0.92–2.28, P = 0.110) | 0.11 | 1.68 (0.95–2.96, P = 0.073) | 0.73 |
| Tumour size (cm) | 1.11 (1.03–1.20) | 0.004 | 1.03 (0.93–1.14) | 0.53 |
| Approach | ||||
| Laparoscopic | REF | <0.001 | REF | <0.001 |
| Converted to open | 6.37 (2.47–17.14) | <0.001 | 10.34 (3.24–36.23) | <0.001 |
| Open | 7.60 (4.31–13.64) | 11.69 (4.52–32.55) | ||
| Postoperative duration of hospital stay ≥ 5 days . | . | . | . | . |
| Variable . | OR univariate (95% c.i.) . | P . | OR multivariate (95% c.i.) . | P . |
| Age, years | 1.00 (0.99–1.02) | 0.841 | 0.99 (0.96–1.02) | 0.531 |
| Sex, female | 0.79 (0.50–1.25) | 0.31 | 0.85 (0.45–1.61) | 0.612 |
| BMI, kg/m2 | 1.00 (0.96–1.04) | 0.99 | 0.98 (0.93–1.03) | 0.434 |
| Charlson Index ≥ 3 | 1.37 (0.76–2.51) | 0.295 | 4.31 (1.08–18.26) | 0.042 |
| Alpha blockade | 1.28 (0.31–8.59) | 0.756 | 3.35 (0.53–31.99) | 0.238 |
| Beta blockade | 1.06 (0.65–1.71) | 0.819 | 1.24 (0.63–2.44) | 0.527 |
| Tumour size (cm) | 1.26 (1.16–1.37) | <0.001 | 1.15 (1.05–1.28) | 0.006 |
| Approach | ||||
| Laparoscopic | REF | <0.001 | REF | <0.001 |
| Converted to open | 15.51 (5.77–46.44) | <0.001 | 32.11 (9.2–133.77) | <0.001 |
| Open | 18.29 (9.8–35.54) | 28.01 (10.52–83.97) |
| Postoperative complications . | . | . | . | . |
|---|---|---|---|---|
| Variable . | OR univariate (95% c.i.) . | P . | OR multivariate (95% c.i.) . | P . |
| Age, years | 1.01 (1.00–1.02) | 0.426 | 0.97 (0.94–1.00) | 0.052 |
| Sex, female | 1.09 (0.70–1.70) | 0.708 | 1.26 (0.71–2.25) | 0.428 |
| BMI, kg/m2 | 1.00 (0.96–1.03) | 0.801 | 0.98 (0.93–1.03) | 0.427 |
| Charlson Index ≥3 | 2.05 (1.14–3.74) | 0.017 | 8.09 (2.31–29.63) | 0.001 |
| Alpha blockade | 3.55 (0.66–6.81, P = 0.232) | 0.232 | 6.30 (0.95–12.69, P = 0.109) | 0.109 |
| Beta blockade | 1.45 (0.92–2.28, P = 0.110) | 0.11 | 1.68 (0.95–2.96, P = 0.073) | 0.73 |
| Tumour size (cm) | 1.11 (1.03–1.20) | 0.004 | 1.03 (0.93–1.14) | 0.53 |
| Approach | ||||
| Laparoscopic | REF | <0.001 | REF | <0.001 |
| Converted to open | 6.37 (2.47–17.14) | <0.001 | 10.34 (3.24–36.23) | <0.001 |
| Open | 7.60 (4.31–13.64) | 11.69 (4.52–32.55) | ||
| Postoperative duration of hospital stay ≥ 5 days . | . | . | . | . |
| Variable . | OR univariate (95% c.i.) . | P . | OR multivariate (95% c.i.) . | P . |
| Age, years | 1.00 (0.99–1.02) | 0.841 | 0.99 (0.96–1.02) | 0.531 |
| Sex, female | 0.79 (0.50–1.25) | 0.31 | 0.85 (0.45–1.61) | 0.612 |
| BMI, kg/m2 | 1.00 (0.96–1.04) | 0.99 | 0.98 (0.93–1.03) | 0.434 |
| Charlson Index ≥ 3 | 1.37 (0.76–2.51) | 0.295 | 4.31 (1.08–18.26) | 0.042 |
| Alpha blockade | 1.28 (0.31–8.59) | 0.756 | 3.35 (0.53–31.99) | 0.238 |
| Beta blockade | 1.06 (0.65–1.71) | 0.819 | 1.24 (0.63–2.44) | 0.527 |
| Tumour size (cm) | 1.26 (1.16–1.37) | <0.001 | 1.15 (1.05–1.28) | 0.006 |
| Approach | ||||
| Laparoscopic | REF | <0.001 | REF | <0.001 |
| Converted to open | 15.51 (5.77–46.44) | <0.001 | 32.11 (9.2–133.77) | <0.001 |
| Open | 18.29 (9.8–35.54) | 28.01 (10.52–83.97) |
Bold values represent statistical significance. REF, reference category.
| . | Postoperative duration of hospital stay ≥ 5 days . | . | |
|---|---|---|---|
| . | No N = 297 . | Yes N = 97 . | P . |
| Age, years (mean, s.d.) | 55.2(7.2) | 55.5(6.3) | 0.83 |
| BMI, kg/m2 (median, i.q.r.) | 27 (24–30) | 27 (25–30) | 0.663 |
| Sex | |||
| Women, n (%) | 165 (55.6) | 48 (49.5) | 0.369 |
| Men, n (%) | 132 (44.4) | 49 (50.5) | |
| Charlson Index (median, i.q.r.)* | 2 (0–3) | 2 (1–3) | 0.395 |
| Alpha-blockers | |||
| Yes, n (%) | 284 (95.6) | 91 (93.8) | 0.658 |
| No, n (%) | 13 (4.4) | 6 (6.2) | |
| Beta-blockers | |||
| Yes, n (%) | 110 (37.0) | 36 (37.1) | 0.215 |
| No, n (%) | 187 (63.0) | 61 (62.9) | |
| Catecholamines† | |||
| Lower quartile, n (%) | 221 (77) | 67 (69.1) | 0.15 |
| Upper quartile, n (%) | 66 (20) | 30 (30.9) | |
| Tumour size (cm) (median, i.q.r.) | 3.8 (2.7–5.2) | 5.2 (3.2–8.5) | <0.001 |
| Tumour location | |||
| Right, n (%) | 139 (47.1) | 48 (49.5) | 0.001 |
| Left, n (%) | 148 (49.8) | 37 (38.1) | |
| Unknown, n (%) | 9 (3.0) | 12 (12.4) | |
| Approach | |||
| Laparoscopic, n (%) | 272 (87.9) | 38 (39.2) | <0.001 |
| Converted to open, n (%) | 6 (2.0) | 13 (13.4) | |
| Open, n (%) | 18 (6.1) | 46 (47.4) | |
| . | Postoperative duration of hospital stay ≥ 5 days . | . | |
|---|---|---|---|
| . | No N = 297 . | Yes N = 97 . | P . |
| Age, years (mean, s.d.) | 55.2(7.2) | 55.5(6.3) | 0.83 |
| BMI, kg/m2 (median, i.q.r.) | 27 (24–30) | 27 (25–30) | 0.663 |
| Sex | |||
| Women, n (%) | 165 (55.6) | 48 (49.5) | 0.369 |
| Men, n (%) | 132 (44.4) | 49 (50.5) | |
| Charlson Index (median, i.q.r.)* | 2 (0–3) | 2 (1–3) | 0.395 |
| Alpha-blockers | |||
| Yes, n (%) | 284 (95.6) | 91 (93.8) | 0.658 |
| No, n (%) | 13 (4.4) | 6 (6.2) | |
| Beta-blockers | |||
| Yes, n (%) | 110 (37.0) | 36 (37.1) | 0.215 |
| No, n (%) | 187 (63.0) | 61 (62.9) | |
| Catecholamines† | |||
| Lower quartile, n (%) | 221 (77) | 67 (69.1) | 0.15 |
| Upper quartile, n (%) | 66 (20) | 30 (30.9) | |
| Tumour size (cm) (median, i.q.r.) | 3.8 (2.7–5.2) | 5.2 (3.2–8.5) | <0.001 |
| Tumour location | |||
| Right, n (%) | 139 (47.1) | 48 (49.5) | 0.001 |
| Left, n (%) | 148 (49.8) | 37 (38.1) | |
| Unknown, n (%) | 9 (3.0) | 12 (12.4) | |
| Approach | |||
| Laparoscopic, n (%) | 272 (87.9) | 38 (39.2) | <0.001 |
| Converted to open, n (%) | 6 (2.0) | 13 (13.4) | |
| Open, n (%) | 18 (6.1) | 46 (47.4) | |
*Age-adjusted Charlson Co-morbidity Index. †The fourth quartile was calculated for each metabolite and the preoperative catecholamine level was treated as a categorical variable (above or below the fourth quartile) in order to maximize the number of patients in the analysis. The measure of the preoperative catecholamine used in the analysis was a combined measure. The value for each patient was based on only one of the catecholamine measures. The specific measures were ranked based on the frequency of data collected, with the most commonly measured variable given preference. The order of preference was as follows (with cut-off values for upper quartile provided): plasma normetanephrine (n = 234) > 7578 pmol/l; plasma metanephrine (n = 196) > 1496 pmol/l; urinary normetanephrine (n = 185) > 22 μmol/24 h; urinary metadrenaline (n = 181) > 1960 nmol/24 h; urinary noradrenaline (n = 112) > 2957 nmol/24 h; urinary adrenaline (n = 108) > 275 nmol/24. ‡Prolonged postoperative length of hospital stay was defined as ≥5 days (75th centile). Data available for 394 patients. Bold values represent statistical significance.
| . | Postoperative duration of hospital stay ≥ 5 days . | . | |
|---|---|---|---|
| . | No N = 297 . | Yes N = 97 . | P . |
| Age, years (mean, s.d.) | 55.2(7.2) | 55.5(6.3) | 0.83 |
| BMI, kg/m2 (median, i.q.r.) | 27 (24–30) | 27 (25–30) | 0.663 |
| Sex | |||
| Women, n (%) | 165 (55.6) | 48 (49.5) | 0.369 |
| Men, n (%) | 132 (44.4) | 49 (50.5) | |
| Charlson Index (median, i.q.r.)* | 2 (0–3) | 2 (1–3) | 0.395 |
| Alpha-blockers | |||
| Yes, n (%) | 284 (95.6) | 91 (93.8) | 0.658 |
| No, n (%) | 13 (4.4) | 6 (6.2) | |
| Beta-blockers | |||
| Yes, n (%) | 110 (37.0) | 36 (37.1) | 0.215 |
| No, n (%) | 187 (63.0) | 61 (62.9) | |
| Catecholamines† | |||
| Lower quartile, n (%) | 221 (77) | 67 (69.1) | 0.15 |
| Upper quartile, n (%) | 66 (20) | 30 (30.9) | |
| Tumour size (cm) (median, i.q.r.) | 3.8 (2.7–5.2) | 5.2 (3.2–8.5) | <0.001 |
| Tumour location | |||
| Right, n (%) | 139 (47.1) | 48 (49.5) | 0.001 |
| Left, n (%) | 148 (49.8) | 37 (38.1) | |
| Unknown, n (%) | 9 (3.0) | 12 (12.4) | |
| Approach | |||
| Laparoscopic, n (%) | 272 (87.9) | 38 (39.2) | <0.001 |
| Converted to open, n (%) | 6 (2.0) | 13 (13.4) | |
| Open, n (%) | 18 (6.1) | 46 (47.4) | |
| . | Postoperative duration of hospital stay ≥ 5 days . | . | |
|---|---|---|---|
| . | No N = 297 . | Yes N = 97 . | P . |
| Age, years (mean, s.d.) | 55.2(7.2) | 55.5(6.3) | 0.83 |
| BMI, kg/m2 (median, i.q.r.) | 27 (24–30) | 27 (25–30) | 0.663 |
| Sex | |||
| Women, n (%) | 165 (55.6) | 48 (49.5) | 0.369 |
| Men, n (%) | 132 (44.4) | 49 (50.5) | |
| Charlson Index (median, i.q.r.)* | 2 (0–3) | 2 (1–3) | 0.395 |
| Alpha-blockers | |||
| Yes, n (%) | 284 (95.6) | 91 (93.8) | 0.658 |
| No, n (%) | 13 (4.4) | 6 (6.2) | |
| Beta-blockers | |||
| Yes, n (%) | 110 (37.0) | 36 (37.1) | 0.215 |
| No, n (%) | 187 (63.0) | 61 (62.9) | |
| Catecholamines† | |||
| Lower quartile, n (%) | 221 (77) | 67 (69.1) | 0.15 |
| Upper quartile, n (%) | 66 (20) | 30 (30.9) | |
| Tumour size (cm) (median, i.q.r.) | 3.8 (2.7–5.2) | 5.2 (3.2–8.5) | <0.001 |
| Tumour location | |||
| Right, n (%) | 139 (47.1) | 48 (49.5) | 0.001 |
| Left, n (%) | 148 (49.8) | 37 (38.1) | |
| Unknown, n (%) | 9 (3.0) | 12 (12.4) | |
| Approach | |||
| Laparoscopic, n (%) | 272 (87.9) | 38 (39.2) | <0.001 |
| Converted to open, n (%) | 6 (2.0) | 13 (13.4) | |
| Open, n (%) | 18 (6.1) | 46 (47.4) | |
*Age-adjusted Charlson Co-morbidity Index. †The fourth quartile was calculated for each metabolite and the preoperative catecholamine level was treated as a categorical variable (above or below the fourth quartile) in order to maximize the number of patients in the analysis. The measure of the preoperative catecholamine used in the analysis was a combined measure. The value for each patient was based on only one of the catecholamine measures. The specific measures were ranked based on the frequency of data collected, with the most commonly measured variable given preference. The order of preference was as follows (with cut-off values for upper quartile provided): plasma normetanephrine (n = 234) > 7578 pmol/l; plasma metanephrine (n = 196) > 1496 pmol/l; urinary normetanephrine (n = 185) > 22 μmol/24 h; urinary metadrenaline (n = 181) > 1960 nmol/24 h; urinary noradrenaline (n = 112) > 2957 nmol/24 h; urinary adrenaline (n = 108) > 275 nmol/24. ‡Prolonged postoperative length of hospital stay was defined as ≥5 days (75th centile). Data available for 394 patients. Bold values represent statistical significance.
Risk factors for prolonged duration of hospital stay
Data for postoperative LOS were available in 394 patients. Overall, median postoperative LOS was 4 days (i.q.r. 2–5). Prolonged LOS was defined as ≥5th centile (5 days). Tumour size (P <0.001) and open surgery (P <0.001) were associated with a prolonged LOS on univariable analysis (Table 4). There were no statistically significant differences in preoperative catecholamines or usage of alpha/beta blockade among the two groups (Table 4). Ninety-seven of 394 patients (24.6 per cent) had prolonged LOS and this was significantly associated with CCI ≥3 (OR 4.31, 95 per cent c.i. 1.08 to 18.26, P = 0.042), tumour size (OR 1.15, 95 per cent c.i. 1.05 to 1.28, P = 0.006), laparoscopic converted to open (OR 32.11, 95 per cent c.i. 9.2 to 137.77, P < 0.001), and open surgery (OR 28.01, 95 per cent c.i. 10.52 to 83.97, P < 0.001) in the adjusted multivariable model (Table 3).
Discussion
This multicentre study represents a large cohort reporting the overall burden of postoperative complications for patients undergoing adrenalectomy for phaeochromocytoma. The authors have demonstrated that surgery appears to be safe in these patients with a very low mortality rate. Postoperative complications are relatively common, occurring in over one-quarter of patients, and lead to prolonged hospital stay. Patient co-morbidity, tumour size, laparoscopic converted to open, and open surgery were all found to be significant risk factors associated with adverse short-term outcomes. These findings warrant a thorough patient assessment when considering adrenalectomy for phaeochromocytoma, and the study identified several risk factors useful for clinicians when counselling patients.
Several studies have reported a postoperative complications rate for adrenalectomy in the range of 4–15.6 per cent3–7, with a mortality rate ranging from 0 to 0.8 per cent6,7,15,16. The low mortality rate observed in our cohort seems to be in line with the overall literature reported for all adrenalectomies and supports the finding that adrenalectomy for phaeochromocytoma is a safe procedure. Chen et al.3 demonstrated, in a large retrospective study not solely focused on phaeochromocytoma, that the most common complications after adrenalectomy were bleeding and respiratory tract infection. The authors identified American Society of Anesthesiologists (ASA) class III or IV, conversion to hand-assisted or open surgery, diagnosis of phaeochromocytoma, and a tumour size of 6 cm or greater as factors associated with increased complication rates. We found similar results, with 20 patients of 109 (18.3 per cent) developing postoperative anaemia requiring perioperative transfusion. In this setting, intraoperative bleeding led to conversion to open technique in eight cases, but only one postoperative bleeding case required reoperation on, underlining the fact that bleeding after adrenalectomy is rare and most bleeding cases can be managed without surgical intervention. In line with their results, we have found that patient co-morbidity, tumour size, laparoscopic converted to open, and open surgery were consistently associated with increased complication rates. These remained significant after sensitivity analysis excluding prolonged hypotension. Notably, Chen et al.3 found that prolonged hypotension was present in 14 (2.2 per cent) patients among all adrenalectomies, of whom only 12 were diagnosed with phaeochromocytoma. In contrast to that study, we found that prolonged hypotension occurred in 48 (11.8 per cent) patients, and we deemed this a grade IVa complication when vasopressors were required for >24 h. These conflicting results may be related to the difference in time intervals and the multicentre nature of our study, where patients could have been managed with different policies among the centres. Looking at secondary outcomes, Chen et al.3 found that prolonged LOS was associated with age 65 years or older, an ASA class III–IV, any procedural conversion, and tumour size of 4 cm or larger, consistent with our results. However, age was associated with prolonged LOS in our cohort (OR 0.99, c.i.95 per cent, 0.96–1.02, P = 0.531). In this regard, the age-adjusted Charlson Index incorporates age in its score and an index ≥3 is deemed significantly associated with the development of postoperative complications and prolonged LOS. In doing so, patients’ co-morbidities are incorporated, as there is collinearity between age and morbidity rate which might not be evaluated by the ASA score17. Nevertheless, the authors acknowledge that the reasons behind such differences may well reflect the variability in patient management policies between centres.
Recently, Hallin Thompson et al. reported data from the Eurocrine® database showing that among 551 patients diagnosed with phaeochromocytoma, laparoscopic adrenalectomy was performed in 483 patients (89.1 per cent) and conversion rate to open surgery was 4.6 per cent7. This is a slightly lower rate than in this study, where the authors reported a conversion rate of 7 per cent. Although median tumour size was similar in this study, this discrepancy could be related to centre-specific experience or intraoperative events that were not recorded. Interestingly, they observed 46 complications in 22 patients representing a low rate of 4 per cent. These results are not in line with the ones reported in the current study, where the overall burden of postoperative complications reached 26.8 per cent of the study population. There could be several factors explaining this discrepancy. The authors reported a registry database therefore some detailed data may have been missed. In fact, a comprehensive evaluation of complications and their management was not performed. Conversely, although some of the current data might have been missing due to the retrospective nature of this study, our methodology allowed for granular disease-specific data and the authors believe that this study gives a more accurate estimate of the cumulative burden of postoperative complications after adrenalectomy for phaeochromocytoma. Also, in contrast with Hallin Thompson et al. the authors considered postoperative hypotension requiring vasopressor >24 h as a complication grade IVa, which accounted for 48 cases. When removing this specific complication with the sensitivity analysis, postoperative complications still remained high, with haemorrhage, respiratory infection, and wound complications being the most frequent ones. This sensitivity analysis has highlighted that there are risk factors that need to be considered when counselling the patients.
Interestingly, in this study tumour size was not associated with the development of postoperative complications in multivariable analysis, which is in contrast with data from other studies18,19. Although larger tumour size may be related to higher technical difficulties, the reasons behind our findings could be due to the evolution in the management of phaeochromocytoma excision in recent decades20. In fact, this study interval has a narrow window of 9 years and includes only high-volume centres, with a median annual volume of adrenalectomy of 24.5 (i.q.r. 14–33.5) cases per surgeon and median 7.3 cases/annually of solely phaeochromocytoma. Notably, some studies have linked phaeochromocytoma diagnosis with a higher risk of conversion rates during laparoscopic adrenalectomy, ranging between 4.1 per cent and 6.7 per cent21,22,23. The authors' conversion rate appears slightly higher in the range of 7 per cent, which could be explained by the technical difficulties that are specific to phaeochromocytoma surgery. However, it remains debatable which minimal invasive approach yields better outcomes. This data has demonstrated that the surgical approach was an independent factor for postoperative complications, as one might expect. Indeed, a recent meta-analysis has demonstrated superiority of LA compared with OA for phaeochromocytoma resection24. Importantly, the outcome after laparoscopic converted to open was not inferior to (upfront) open surgery, and therefore, it may be concluded that conversion does not disadvantage patients. Based on this, an initial attempt at laparoscopic resection may be justified even for patients at high risk of conversion, depending on surgeon experience.
Postoperative complications and CCI were found to be associated with poor outcomes, in terms of patient survival and tumour recurrence in several oncological conditions, such as colorectal metastases25, perihilar cholangiocarcinoma26 and retroperitoneal sarcoma27. The prognosis of benign phaeochromocytoma appears to be 90 per cent on a 5-year survival rate28. However, phaeochromocytoma has a malignancy rate of approximately 10 per cent and prognosis is generally poor, with less than 60 per cent 5-year overall survival29. In our study we were not able to draw conclusions regarding the association between complications and long-term outcomes, as follow-up was limited to the time of discharge from hospital stay after surgery. Nevertheless, correlation between surgical outcomes of phaeochromocytoma excision and long-term prognosis would be of great interest for future studies.
The present study has a number of strengths, including the large sample size for a relatively rare disease, and the fact that it was specifically focused on phaeochromocytoma without including other indications for adrenalectomy. Nevertheless, the results must be interpreted within their limitations. First, data on postoperative complications were collected retrospectively, and therefore some data might have been inherently lost. This is likely to have introduced some bias, as there could be other unmeasured complications, which would result in confounding. Second, although being a multicentre study with a large number of patients from nine tertiary referral centres, all were based in the UK, hence the results might not be generalizable to other countries, particularly those where healthcare system and population demographics differ considerably from the UK. Third, different patient management policies among centres could have contributed to biases, especially when evaluating LOS and specific complications, such as hypotension requiring vasopressor support.
Funding
The authors have no funding to declare.
Disclosure
The authors declare no conflict of interest.
Supplementary material
Supplementary material is available at BJS Open online.
Data availability
The data sets generated and/or analysed during the present study are available from the corresponding author upon reasonable request.
Author contributions
Alessandro Parente (Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing—original draft, Writing—review & editing), Sivesh K. Kamarajah (Data curation, Formal analysis, Writing—review & editing), Joseph P. Thompson (Data curation, Writing—review & editing), Charlotte Crook (Data curation, Writing—review & editing), Sebastian Aspinall (Data curation, Writing—review & editing), Ross Melvin (Data curation, Writing—review & editing), Michael J. Stechman (Data curation, Writing—review & editing), Helen Perry (Data curation, Writing—review & editing), Sabapathy P. Balasubramanian (Data curation, Writing—review & editing), Arslan Pannu (Data curation, Writing—review & editing), Fausto F. Palazzo (Data curation, Writing—review & editing), Klaas Van Den Heede (Data curation, Writing—review & editing), Fiona Eatock (Data curation, Writing—review & editing), Hannah Anderson (Data curation, Writing—review & editing), Helen Doran (Data curation, Writing—review & editing), Kelvin Wang (Data curation, Writing—review & editing), Johnathan Hubbard (Data curation, Writing—review & editing), Abdulaziz Aldrees (Data curation, Writing—review & editing), Susannah L. Shore (Data curation, Writing—review & editing), Clare Fung (Data curation, Writing—review & editing), Alison Waghorn (Data curation, Writing—review & editing), John Ayuk (Data curation, Writing—review & editing), Davinia Bennett (Data curation, Writing—review & editing), and Robert P. Sutcliffe (Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing—original draft, Writing—review & editing).



