-
PDF
- Split View
-
Views
-
Cite
Cite
Jiale Gao, Xiaodong Gu, Minghui Pang, Hong Zhang, Yugui Lian, Lei Zhou, Bo Feng, Guiying Wang, Zhicao Zhang, He Huang, Gang Xiao, Fanghai Han, Xinxiang Li, Xiaojun Zhou, Quan Wang, Qian Liu, Haijun Deng, Zhenjun Wang, Wu Song, Zhengqiang Wei, Yong Li, Yong Dai, Moubin Lin, Jianyong Zheng, Bo Tang, Xianli He, Hui Wang, Fanlong Liu, Yongxiang Li, Dongbing Zhou, Wei Zhang, Kefeng Ding, Weidong Tong, Guodong He, Changqing Jing, Bin Wu, Tao Wu, Ming Dong, Zhifei Li, Zhanlong Shen, Hongbo Wei, Lian Bai, Zhiqian Hu, Shiliang Tu, Jian Qiu, Xuejun Sun, Ang Li, Jing Zhuang, Su Yan, Hendrik Bonjer, Jurriaan Tuynman, Hongwei Yao, Zhongtao Zhang, the RHC-SNAPSHOT investigators , Risk factors for anastomotic leak and postoperative morbidity after right hemicolectomy for colon cancer: results from a prospective, multi-centre, snapshot study in China, British Journal of Surgery, Volume 111, Issue 1, January 2024, znad316, https://doi.org/10.1093/bjs/znad316
Close - Share Icon Share
Abstract
Right hemicolectomy is the standard treatment for right-sided colon cancer. There is variation in the technical aspects of performing right hemicolectomy as well as in short-term outcomes. It is therefore necessary to explore best clinical practice following right hemicolectomy in expert centres.
This snapshot study of right hemicolectomy for colon cancer in China was a prospective, multicentre cohort study in which 52 tertiary hospitals participated. Eligible patients with stage I–III right-sided colon cancer who underwent elective right hemicolectomy were consecutively enrolled in all centres over 10 months. The primary endpoint was the incidence of postoperative 30-day anastomotic leak.
Of the 1854 patients, 89.9 per cent underwent laparoscopic surgery and 52.3 per cent underwent D3 lymph node dissection. The overall 30-day morbidity and mortality were 11.7 and 0.2 per cent, respectively. The 30-day anastomotic leak rate was 1.4 per cent. In multivariate analysis, ASA grade > II (P < 0.001), intraoperative blood loss > 50 ml (P = 0.044) and D3 lymph node dissection (P = 0.008) were identified as independent risk factors for postoperative morbidity. Extracorporeal side-to-side anastomosis (P = 0.031), intraoperative blood loss > 50 ml (P = 0.004) and neoadjuvant chemotherapy (P = 0.004) were identified as independent risk factors for anastomotic leak.
In high-volume expert centres in China, laparoscopic resection with D3 lymph node dissection was performed in most patients with right-sided colon cancer, and overall postoperative morbidity and mortality was low. Further studies are needed to explore the optimal technique for right hemicolectomy in order to improve outcomes further.
Introduction
Colorectal cancer is the third commonest malignancy in China1, with right hemicolectomy (RHC) being the standard treatment for right-sided colon cancer. The surgical steps for right hemicolectomy are highly diverse and constantly evolving, with new concepts such as complete mesocolic excision (CME)2–6 and intracorporeal ileocolic anastomosis (IIA)7–9. High-quality studies are needed to further validate these techniques and understand their impact on short-term outcomes.
Anastomotic leak is a major complication after RHC and a risk factor for mortality and postoperative recurrence10–14. Analysis of predictors for anastomotic leak showed an obvious correlation with patient condition (malnutrition), surgical experience and surgical technique (hand-sewn anastomosis, open approach)10,11. However, previous studies have considered multiple factors, and few studies have examined the effect of the technical aspects of surgery, particularly the latest techniques (for example, CME, intracorporeal anastomosis), on complications and anastomotic leak rates.
By prospectively collecting data from high-volume expert centres in China, and in particular documenting the surgical approach, this study describes the implementation of technological advances in RHC and their impact on short-term outcomes (morbidity, anastomotic leak) and provides the direction and foundation for further high-quality studies. This study also serves as the first step of the COLOR IV trial (NCT05493033), an international multicentre study comparing intracorporeal and extracorporeal anastomosis after laparoscopic RHC.
Methods
Study design
This study was a multicentre, prospective, observational study to explore the clinical practice and short-term outcomes of right hemicolectomy in high-volume expert centres in China, as well as to establish the risk factors for postoperative morbidity and anastomotic leak. The study was registered at ClinicalTrials.gov (NCT04628182) and was conducted in accordance with the published research protocol (Appendix 1). A total of 71 tertiary hospitals in China were invited, and 52 centres that perform more than 120 colon cancer operations per year were enrolled in this study (Fig. S1). All participating centres were required to enrol consecutive eligible cases for 10 months and complete the 30-day follow-up.
Patients
Patients with stage I–III right-sided colon cancer (caecum, ascending colon, and proximal two-thirds of the transverse colon) who underwent elective RHC were enrolled in this study. Patients with inflammatory bowel disease (Crohn’s disease or ulcerative colitis) requiring emergency surgery or a more extensive procedure (subtotal colectomy, pan proctocolectomy, or planned synchronous abdominal organ resections) were excluded.
Outcomes
The primary outcome was 30-day postoperative anastomotic leak rate. Anastomotic leak was defined as a defect of the intestinal wall at the anastomotic site leading to communication between intra- and extraluminal compartments15,16. The diagnosis of anastomotic leak was initially based on clinical signs (fever, abnormal drain output, leukocytosis, etc.) and confirmed radiologically, endoscopically, or intraoperatively. Patients with suspected anastomotic leak were required to have an abdominal CT scan. The severity of anastomotic leak was classified according to the Clavien–Dindo classification17 and the International Study Group of Rectal Cancer grading (ISREC) standards18.
The secondary outcomes were 30-day postoperative morbidity, mortality, reoperation rate, readmission rate, postoperative bowel function recovery, and length of hospital stay. The severity of complications was assessed according to the Clavien–Dindo classification17.
Data collection
All data were collected in an online database (https://color4.dpclouds.com:8098/login). To ensure data completion, approximately 90 per cent of the data fields were mandatory.
The data were recorded as follows: demographic, preoperative, surgical, pathological, and postoperative information. The detailed surgical data were provided by the surgeons. The superior mesenteric artery (SMA) was classified into four patterns. Pattern I was the independent origin of the ileocolic artery (ICA), right colic artery (RCA), and middle colic artery (MCA). Pattern IIa was a common trunk between the RCA and MCA, pattern IIb was a common trunk between the RCA and ICA, and pattern IIc was a common trunk for the ICA, RCA, and MCA. Pattern III was an absence of RCA. Pattern IV was the presence of accessory arteries19. The classification of Henle’s trunk was recorded according to the Miyazawa criteria (type 0, I, II, III) determined according to the number of colic veins that ran into the main trunk20. The extent of the resection was determined as limited (proximal to hepatic flexure), complete (hepatic flexure to the middle), and extended (middle to distal transverse colon).
Statistical analysis
Categorical variables are presented as numbers and percentages. Continuous variables are presented as the median and 25th–75th percentiles. Variables selected for risk factor analysis were identified by the Least Absolute Shrinkage and Selection Operator (LASSO) technique21. By choosing the optimal penalty parameter (λ) correlated with the minimum 10-fold cross-validation, factors associated with the outcomes of interest were further analysed using multivariate logistic regression. The factors selected by LASSO underwent univariate analysis, and factors with P < 0.1 were subsequently subjected to multivariate analysis. P < 0.05 was considered statistically significant. R software (version 4.2.1) was used to perform the LASSO and logistic regression.
Results
Demographic information
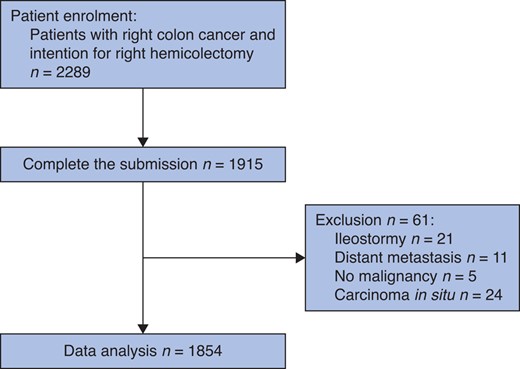
A total of 2289 patients from 52 tertiary hospitals were enrolled from September 2021 to June 2022, of which 1915 (83.7 per cent) completed 30-day follow-up and submitted all data. After excluding 37 patients for metastasis, no malignancy, carcinoma in situ, and formation of ileostomy, 1854 patients were available for analysis (Fig. 1, Fig. S1). The median (i.q.r. age of patients was 64 (55–72) years, and median (i.q.r.) BMI was 23.2 (21.1–25.4) kg/m2. Detailed patient and tumour characteristics are reported in Table 1.
| Median age (years) | 64 (55–72) |
| Median BMI (kg/m2) | 23.2 (21.1–25.4) |
| Sex ratio (M:F) | 978:876 |
| ECOG score | |
| 0–1 | 1696 (91.5) |
| 2–4 | 158 (8.5) |
| ASA fitness grade | |
| I–II | 1637 (88.3) |
| III–IV | 217 (11.7) |
| NRS2002 score | |
| <3 | 626 (33.8) |
| ≥3 | 555 (29.9) |
| NA | 673 (36.3) |
| History of abdominal surgery | 458 (24.7) |
| Chronic obstructive pulmonary disease | 55 (3.0) |
| Heart disease | |
| Coronary artery disease | 110 (5.9) |
| Heart arrhythmias | 21 (1.1) |
| Heart failure | 23 (1.2) |
| Heart valve disease | 5 (0.3) |
| Anaemia | |
| Mild | 402 (21.7) |
| Moderate | 616 (33.2) |
| Severe | 260 (14.0) |
| Anaemia treatment | |
| Iron infusion | 106 (5.7) |
| Blood transfusion | 159 (8.6) |
| Albumin (g/l) | |
| >35 | 1383 (74.6) |
| 30–35 | 369 (19.9) |
| 25–30 | 90 (4.9) |
| <25 | 12 (0.6) |
| Diabetes mellitus | 261 (14.1) |
| Diet controlled | 19 (7.3) |
| Tablet controlled | 194 (74.3) |
| Insulin controlled | 48 (18.4) |
| Preoperative intestinal obstruction | 356 (19.2) |
| Preoperative haematochezia | 729 (39.3) |
| Elevated creatinine (>133 µmol/l) | 30 (1.6) |
| Tobacco use | 229 (12.4) |
| Statin use | 82 (4.4) |
| Anticoagulant use | 98 (5.3) |
| Elevated CEA (>5 ng/ml) | 644 (34.7) |
| Neoadjuvant chemotherapy | 70 (3.8) |
| Pathological T category | |
| 1 | 93 (5.0) |
| 2 | 187 (10.0) |
| 3 | 948 (51.1) |
| 4a | 585 (31.6) |
| Not recorded | 41 (2.2) |
| Pathological N category | |
| 0 | 977 (52.7) |
| 1 | 630 (34.0) |
| 2 | 192 (10.4) |
| Not recorded | 55 (3.0) |
| Median age (years) | 64 (55–72) |
| Median BMI (kg/m2) | 23.2 (21.1–25.4) |
| Sex ratio (M:F) | 978:876 |
| ECOG score | |
| 0–1 | 1696 (91.5) |
| 2–4 | 158 (8.5) |
| ASA fitness grade | |
| I–II | 1637 (88.3) |
| III–IV | 217 (11.7) |
| NRS2002 score | |
| <3 | 626 (33.8) |
| ≥3 | 555 (29.9) |
| NA | 673 (36.3) |
| History of abdominal surgery | 458 (24.7) |
| Chronic obstructive pulmonary disease | 55 (3.0) |
| Heart disease | |
| Coronary artery disease | 110 (5.9) |
| Heart arrhythmias | 21 (1.1) |
| Heart failure | 23 (1.2) |
| Heart valve disease | 5 (0.3) |
| Anaemia | |
| Mild | 402 (21.7) |
| Moderate | 616 (33.2) |
| Severe | 260 (14.0) |
| Anaemia treatment | |
| Iron infusion | 106 (5.7) |
| Blood transfusion | 159 (8.6) |
| Albumin (g/l) | |
| >35 | 1383 (74.6) |
| 30–35 | 369 (19.9) |
| 25–30 | 90 (4.9) |
| <25 | 12 (0.6) |
| Diabetes mellitus | 261 (14.1) |
| Diet controlled | 19 (7.3) |
| Tablet controlled | 194 (74.3) |
| Insulin controlled | 48 (18.4) |
| Preoperative intestinal obstruction | 356 (19.2) |
| Preoperative haematochezia | 729 (39.3) |
| Elevated creatinine (>133 µmol/l) | 30 (1.6) |
| Tobacco use | 229 (12.4) |
| Statin use | 82 (4.4) |
| Anticoagulant use | 98 (5.3) |
| Elevated CEA (>5 ng/ml) | 644 (34.7) |
| Neoadjuvant chemotherapy | 70 (3.8) |
| Pathological T category | |
| 1 | 93 (5.0) |
| 2 | 187 (10.0) |
| 3 | 948 (51.1) |
| 4a | 585 (31.6) |
| Not recorded | 41 (2.2) |
| Pathological N category | |
| 0 | 977 (52.7) |
| 1 | 630 (34.0) |
| 2 | 192 (10.4) |
| Not recorded | 55 (3.0) |
Values are n (%) unless otherwise indicated. Anaemia was defined according to WHO criteria. TNM stage adopted the AJCC 8th edition.
| Median age (years) | 64 (55–72) |
| Median BMI (kg/m2) | 23.2 (21.1–25.4) |
| Sex ratio (M:F) | 978:876 |
| ECOG score | |
| 0–1 | 1696 (91.5) |
| 2–4 | 158 (8.5) |
| ASA fitness grade | |
| I–II | 1637 (88.3) |
| III–IV | 217 (11.7) |
| NRS2002 score | |
| <3 | 626 (33.8) |
| ≥3 | 555 (29.9) |
| NA | 673 (36.3) |
| History of abdominal surgery | 458 (24.7) |
| Chronic obstructive pulmonary disease | 55 (3.0) |
| Heart disease | |
| Coronary artery disease | 110 (5.9) |
| Heart arrhythmias | 21 (1.1) |
| Heart failure | 23 (1.2) |
| Heart valve disease | 5 (0.3) |
| Anaemia | |
| Mild | 402 (21.7) |
| Moderate | 616 (33.2) |
| Severe | 260 (14.0) |
| Anaemia treatment | |
| Iron infusion | 106 (5.7) |
| Blood transfusion | 159 (8.6) |
| Albumin (g/l) | |
| >35 | 1383 (74.6) |
| 30–35 | 369 (19.9) |
| 25–30 | 90 (4.9) |
| <25 | 12 (0.6) |
| Diabetes mellitus | 261 (14.1) |
| Diet controlled | 19 (7.3) |
| Tablet controlled | 194 (74.3) |
| Insulin controlled | 48 (18.4) |
| Preoperative intestinal obstruction | 356 (19.2) |
| Preoperative haematochezia | 729 (39.3) |
| Elevated creatinine (>133 µmol/l) | 30 (1.6) |
| Tobacco use | 229 (12.4) |
| Statin use | 82 (4.4) |
| Anticoagulant use | 98 (5.3) |
| Elevated CEA (>5 ng/ml) | 644 (34.7) |
| Neoadjuvant chemotherapy | 70 (3.8) |
| Pathological T category | |
| 1 | 93 (5.0) |
| 2 | 187 (10.0) |
| 3 | 948 (51.1) |
| 4a | 585 (31.6) |
| Not recorded | 41 (2.2) |
| Pathological N category | |
| 0 | 977 (52.7) |
| 1 | 630 (34.0) |
| 2 | 192 (10.4) |
| Not recorded | 55 (3.0) |
| Median age (years) | 64 (55–72) |
| Median BMI (kg/m2) | 23.2 (21.1–25.4) |
| Sex ratio (M:F) | 978:876 |
| ECOG score | |
| 0–1 | 1696 (91.5) |
| 2–4 | 158 (8.5) |
| ASA fitness grade | |
| I–II | 1637 (88.3) |
| III–IV | 217 (11.7) |
| NRS2002 score | |
| <3 | 626 (33.8) |
| ≥3 | 555 (29.9) |
| NA | 673 (36.3) |
| History of abdominal surgery | 458 (24.7) |
| Chronic obstructive pulmonary disease | 55 (3.0) |
| Heart disease | |
| Coronary artery disease | 110 (5.9) |
| Heart arrhythmias | 21 (1.1) |
| Heart failure | 23 (1.2) |
| Heart valve disease | 5 (0.3) |
| Anaemia | |
| Mild | 402 (21.7) |
| Moderate | 616 (33.2) |
| Severe | 260 (14.0) |
| Anaemia treatment | |
| Iron infusion | 106 (5.7) |
| Blood transfusion | 159 (8.6) |
| Albumin (g/l) | |
| >35 | 1383 (74.6) |
| 30–35 | 369 (19.9) |
| 25–30 | 90 (4.9) |
| <25 | 12 (0.6) |
| Diabetes mellitus | 261 (14.1) |
| Diet controlled | 19 (7.3) |
| Tablet controlled | 194 (74.3) |
| Insulin controlled | 48 (18.4) |
| Preoperative intestinal obstruction | 356 (19.2) |
| Preoperative haematochezia | 729 (39.3) |
| Elevated creatinine (>133 µmol/l) | 30 (1.6) |
| Tobacco use | 229 (12.4) |
| Statin use | 82 (4.4) |
| Anticoagulant use | 98 (5.3) |
| Elevated CEA (>5 ng/ml) | 644 (34.7) |
| Neoadjuvant chemotherapy | 70 (3.8) |
| Pathological T category | |
| 1 | 93 (5.0) |
| 2 | 187 (10.0) |
| 3 | 948 (51.1) |
| 4a | 585 (31.6) |
| Not recorded | 41 (2.2) |
| Pathological N category | |
| 0 | 977 (52.7) |
| 1 | 630 (34.0) |
| 2 | 192 (10.4) |
| Not recorded | 55 (3.0) |
Values are n (%) unless otherwise indicated. Anaemia was defined according to WHO criteria. TNM stage adopted the AJCC 8th edition.
Surgical details
More than 95 per cent of RHCs were completed by the chief or associate chief surgeon. In addition, 89.9 per cent of patients underwent laparoscopic surgery with a 4 per cent conversion rate. D3 lymph node dissection was performed for 52.3 per cent patients, and D2 lymph node dissection for 47.0 per cent. The median (i.q.r.) duration of surgery was 180 (140–210) minutes. Intraoperative complications occurred in 4.9 per cent of patients, the most common of which were bleeding (3.1 per cent) and vascular injury (1.1 per cent) (Table S1). Technical details of the surgery performed are reported in Table 2. The superior mesenteric artery was identified in 90 per cent of patients, with type 1 being the most common (41.3 per cent). Similarly, Henle’s trunk was identified in 86.8 per cent, with almost half (48.7 per cent) being type I (Table S2).
| Performing surgeon | |
| Chief surgeon | 1209 (65.2) |
| Associate chief surgeon | 594 (32.0) |
| Attending surgeon | 51 (2.8) |
| Type of right hemicolectomy | |
| Limited (C1–3) | 76 (4.1) |
| Complete (C4–5) | 1482 (79.9) |
| Extended (C6–7) | 296 (16.0) |
| Surgical approach | |
| Open | 188 (10.1) |
| Laparoscopic | 1666 (89.9) |
| Convert to hand-assisted laparoscopic surgery* | 11 (0.7) |
| Convert to open surgery* | 55 (3.3) |
| Resection approach | |
| Medial | 809 (43.3) |
| Lateral | 336 (18.2) |
| Mixed | 709 (38.4) |
| Lymph-node dissection | |
| D1 | 14 (0.8) |
| D2 | 871 (47.0) |
| D3 | 969 (52.3) |
| Middle colic artery ligation | |
| Right branch | 1156 (62.4) |
| Root | 512 (27.6) |
| Not recorded | 186 (10.0) |
| Right gastro-epiploic vein ligation | 522 (28.2) |
| Energy device used | |
| Ultrasonic dissector | 1361 (73.4) |
| Monopolar/bipolar diathermy | 76 (4.1) |
| Both | 417 (22.5) |
| Abdominal incision | |
| Upper median | 1140 (61.5) |
| Umbilical | 219 (11.8) |
| Lower median | 159 (8.6) |
| Transverse | 96 (5.2) |
| Other | 240 (12.9) |
| Mesenteric defect closure | 783 (42.2) |
| Intraoperative blood loss (ml) | |
| 0–50 | 1238 (66.8) |
| 50–200 | 567 (30.6) |
| >200 | 49 (2.6) |
| Surgical duration† (min) | 180 (140–210) |
| Performing surgeon | |
| Chief surgeon | 1209 (65.2) |
| Associate chief surgeon | 594 (32.0) |
| Attending surgeon | 51 (2.8) |
| Type of right hemicolectomy | |
| Limited (C1–3) | 76 (4.1) |
| Complete (C4–5) | 1482 (79.9) |
| Extended (C6–7) | 296 (16.0) |
| Surgical approach | |
| Open | 188 (10.1) |
| Laparoscopic | 1666 (89.9) |
| Convert to hand-assisted laparoscopic surgery* | 11 (0.7) |
| Convert to open surgery* | 55 (3.3) |
| Resection approach | |
| Medial | 809 (43.3) |
| Lateral | 336 (18.2) |
| Mixed | 709 (38.4) |
| Lymph-node dissection | |
| D1 | 14 (0.8) |
| D2 | 871 (47.0) |
| D3 | 969 (52.3) |
| Middle colic artery ligation | |
| Right branch | 1156 (62.4) |
| Root | 512 (27.6) |
| Not recorded | 186 (10.0) |
| Right gastro-epiploic vein ligation | 522 (28.2) |
| Energy device used | |
| Ultrasonic dissector | 1361 (73.4) |
| Monopolar/bipolar diathermy | 76 (4.1) |
| Both | 417 (22.5) |
| Abdominal incision | |
| Upper median | 1140 (61.5) |
| Umbilical | 219 (11.8) |
| Lower median | 159 (8.6) |
| Transverse | 96 (5.2) |
| Other | 240 (12.9) |
| Mesenteric defect closure | 783 (42.2) |
| Intraoperative blood loss (ml) | |
| 0–50 | 1238 (66.8) |
| 50–200 | 567 (30.6) |
| >200 | 49 (2.6) |
| Surgical duration† (min) | 180 (140–210) |
Values are n (%) unless otherwise indicated. *Conversion rate was calculated by dividing the number of converted surgeries by the number of laparoscopic surgeries. †Data presented as median (range).
| Performing surgeon | |
| Chief surgeon | 1209 (65.2) |
| Associate chief surgeon | 594 (32.0) |
| Attending surgeon | 51 (2.8) |
| Type of right hemicolectomy | |
| Limited (C1–3) | 76 (4.1) |
| Complete (C4–5) | 1482 (79.9) |
| Extended (C6–7) | 296 (16.0) |
| Surgical approach | |
| Open | 188 (10.1) |
| Laparoscopic | 1666 (89.9) |
| Convert to hand-assisted laparoscopic surgery* | 11 (0.7) |
| Convert to open surgery* | 55 (3.3) |
| Resection approach | |
| Medial | 809 (43.3) |
| Lateral | 336 (18.2) |
| Mixed | 709 (38.4) |
| Lymph-node dissection | |
| D1 | 14 (0.8) |
| D2 | 871 (47.0) |
| D3 | 969 (52.3) |
| Middle colic artery ligation | |
| Right branch | 1156 (62.4) |
| Root | 512 (27.6) |
| Not recorded | 186 (10.0) |
| Right gastro-epiploic vein ligation | 522 (28.2) |
| Energy device used | |
| Ultrasonic dissector | 1361 (73.4) |
| Monopolar/bipolar diathermy | 76 (4.1) |
| Both | 417 (22.5) |
| Abdominal incision | |
| Upper median | 1140 (61.5) |
| Umbilical | 219 (11.8) |
| Lower median | 159 (8.6) |
| Transverse | 96 (5.2) |
| Other | 240 (12.9) |
| Mesenteric defect closure | 783 (42.2) |
| Intraoperative blood loss (ml) | |
| 0–50 | 1238 (66.8) |
| 50–200 | 567 (30.6) |
| >200 | 49 (2.6) |
| Surgical duration† (min) | 180 (140–210) |
| Performing surgeon | |
| Chief surgeon | 1209 (65.2) |
| Associate chief surgeon | 594 (32.0) |
| Attending surgeon | 51 (2.8) |
| Type of right hemicolectomy | |
| Limited (C1–3) | 76 (4.1) |
| Complete (C4–5) | 1482 (79.9) |
| Extended (C6–7) | 296 (16.0) |
| Surgical approach | |
| Open | 188 (10.1) |
| Laparoscopic | 1666 (89.9) |
| Convert to hand-assisted laparoscopic surgery* | 11 (0.7) |
| Convert to open surgery* | 55 (3.3) |
| Resection approach | |
| Medial | 809 (43.3) |
| Lateral | 336 (18.2) |
| Mixed | 709 (38.4) |
| Lymph-node dissection | |
| D1 | 14 (0.8) |
| D2 | 871 (47.0) |
| D3 | 969 (52.3) |
| Middle colic artery ligation | |
| Right branch | 1156 (62.4) |
| Root | 512 (27.6) |
| Not recorded | 186 (10.0) |
| Right gastro-epiploic vein ligation | 522 (28.2) |
| Energy device used | |
| Ultrasonic dissector | 1361 (73.4) |
| Monopolar/bipolar diathermy | 76 (4.1) |
| Both | 417 (22.5) |
| Abdominal incision | |
| Upper median | 1140 (61.5) |
| Umbilical | 219 (11.8) |
| Lower median | 159 (8.6) |
| Transverse | 96 (5.2) |
| Other | 240 (12.9) |
| Mesenteric defect closure | 783 (42.2) |
| Intraoperative blood loss (ml) | |
| 0–50 | 1238 (66.8) |
| 50–200 | 567 (30.6) |
| >200 | 49 (2.6) |
| Surgical duration† (min) | 180 (140–210) |
Values are n (%) unless otherwise indicated. *Conversion rate was calculated by dividing the number of converted surgeries by the number of laparoscopic surgeries. †Data presented as median (range).
Anastomotic technique
Overall, 97.8 per cent of patients underwent stapled anastomosis, and 84.3 per cent underwent extracorporeal anastomosis. Side-to-side and end-to-side anastomosis accounted for 57.2 and 41.2 per cent, respectively. All intracorporeal anastomoses were side-to-side, with 87.3 per cent being isoperistaltic compared to 54.8 per cent of those performed extracorporeally. Further details of anastomotic technique are reported in Table 3.
| Anastomotic approach | |
| Extracorporeal | 1562 (84.3) |
| Intracorporeal | 292 (15.7) |
| Anastomotic technique | |
| Hand sewn | 41 (2.2) |
| Stapled | 1813 (97.8) |
| Anastomotic type | |
| Side-to-side (intracorporeal) | 292 (15.7) |
| Side-to-side (extracorporeal) | 769 (41.5) |
| End-to-side | 764 (41.2) |
| End-to-end | 29 (1.6) |
| Anastomotic type in side-to-side anastomosis* | |
| Isoperistaltic | 677 (63.8) |
| Antiperistaltic | 384 (36.2) |
| Reinforcement | |
| Seromuscular layer suture | 801 (43.2) |
| Full-thickness suture | 710 (38.3) |
| No reinforcement | 168 (9.1) |
| Not recorded | 175 (9.4) |
| Fluoresence (indocyanine green) use | 49 (2.6) |
| Anastomotic approach | |
| Extracorporeal | 1562 (84.3) |
| Intracorporeal | 292 (15.7) |
| Anastomotic technique | |
| Hand sewn | 41 (2.2) |
| Stapled | 1813 (97.8) |
| Anastomotic type | |
| Side-to-side (intracorporeal) | 292 (15.7) |
| Side-to-side (extracorporeal) | 769 (41.5) |
| End-to-side | 764 (41.2) |
| End-to-end | 29 (1.6) |
| Anastomotic type in side-to-side anastomosis* | |
| Isoperistaltic | 677 (63.8) |
| Antiperistaltic | 384 (36.2) |
| Reinforcement | |
| Seromuscular layer suture | 801 (43.2) |
| Full-thickness suture | 710 (38.3) |
| No reinforcement | 168 (9.1) |
| Not recorded | 175 (9.4) |
| Fluoresence (indocyanine green) use | 49 (2.6) |
Values are n (%) unless otherwise indicated. *Anastomotic type in side-to-side anastomosis was calculated by dividing the number of anastomosis in category of interest by the total number of side-to-side anastomoses.
| Anastomotic approach | |
| Extracorporeal | 1562 (84.3) |
| Intracorporeal | 292 (15.7) |
| Anastomotic technique | |
| Hand sewn | 41 (2.2) |
| Stapled | 1813 (97.8) |
| Anastomotic type | |
| Side-to-side (intracorporeal) | 292 (15.7) |
| Side-to-side (extracorporeal) | 769 (41.5) |
| End-to-side | 764 (41.2) |
| End-to-end | 29 (1.6) |
| Anastomotic type in side-to-side anastomosis* | |
| Isoperistaltic | 677 (63.8) |
| Antiperistaltic | 384 (36.2) |
| Reinforcement | |
| Seromuscular layer suture | 801 (43.2) |
| Full-thickness suture | 710 (38.3) |
| No reinforcement | 168 (9.1) |
| Not recorded | 175 (9.4) |
| Fluoresence (indocyanine green) use | 49 (2.6) |
| Anastomotic approach | |
| Extracorporeal | 1562 (84.3) |
| Intracorporeal | 292 (15.7) |
| Anastomotic technique | |
| Hand sewn | 41 (2.2) |
| Stapled | 1813 (97.8) |
| Anastomotic type | |
| Side-to-side (intracorporeal) | 292 (15.7) |
| Side-to-side (extracorporeal) | 769 (41.5) |
| End-to-side | 764 (41.2) |
| End-to-end | 29 (1.6) |
| Anastomotic type in side-to-side anastomosis* | |
| Isoperistaltic | 677 (63.8) |
| Antiperistaltic | 384 (36.2) |
| Reinforcement | |
| Seromuscular layer suture | 801 (43.2) |
| Full-thickness suture | 710 (38.3) |
| No reinforcement | 168 (9.1) |
| Not recorded | 175 (9.4) |
| Fluoresence (indocyanine green) use | 49 (2.6) |
Values are n (%) unless otherwise indicated. *Anastomotic type in side-to-side anastomosis was calculated by dividing the number of anastomosis in category of interest by the total number of side-to-side anastomoses.
Postoperative outcomes
Overall 30-day morbidity and mortality were 11.7 and 0.2 per cent, respectively. The overall median (i.q.r.) length of hospital stay was 13 (10–16) days, and the postoperative hospital stay was 8 (6–10) days. The median (i.q.r.) first passage of flatus and stool were 2 (2–3) days and 4 (3–5) days, respectively. Detailed postoperative information is reported in Table 4.
| Reoperation | 16 (0.9) |
| Readmission | 23 (1.2) |
| Mortality | 3 (0.2) |
| Morbidity | 216 (11.7) |
| Postoperative complications | |
| Anastomotic leak | 26 (1.4) |
| Abdominal abscess | 10 (0.5) |
| Wound infection | 41 (2.2) |
| Chyle leak | 34 (3.4) |
| Intra-abdominal bleeding | 12 (0.6) |
| Intestinal bleeding | 9 (0.5) |
| Ileus | 47 (2.5) |
| Other | 78 (4.2) |
| Clavien–Dindo classification | |
| I | 89 (4.8) |
| II | 124 (6.7) |
| III | 36 (1.9) |
| IV | 5 (0.3) |
| V | 3 (0.2) |
| Time to first flatus* | 2 (2–3) |
| Time to first stool* | 4 (3–5) |
| Time to first diet* | 3 (2–4) |
| Length of hospital stay* | 13 (10–16) |
| Length of postoperative hospital stay* | 8 (6–10) |
| Reoperation | 16 (0.9) |
| Readmission | 23 (1.2) |
| Mortality | 3 (0.2) |
| Morbidity | 216 (11.7) |
| Postoperative complications | |
| Anastomotic leak | 26 (1.4) |
| Abdominal abscess | 10 (0.5) |
| Wound infection | 41 (2.2) |
| Chyle leak | 34 (3.4) |
| Intra-abdominal bleeding | 12 (0.6) |
| Intestinal bleeding | 9 (0.5) |
| Ileus | 47 (2.5) |
| Other | 78 (4.2) |
| Clavien–Dindo classification | |
| I | 89 (4.8) |
| II | 124 (6.7) |
| III | 36 (1.9) |
| IV | 5 (0.3) |
| V | 3 (0.2) |
| Time to first flatus* | 2 (2–3) |
| Time to first stool* | 4 (3–5) |
| Time to first diet* | 3 (2–4) |
| Length of hospital stay* | 13 (10–16) |
| Length of postoperative hospital stay* | 8 (6–10) |
Values are n (%) unless otherwise indicated. *Data are presented as median (range) number of days.
| Reoperation | 16 (0.9) |
| Readmission | 23 (1.2) |
| Mortality | 3 (0.2) |
| Morbidity | 216 (11.7) |
| Postoperative complications | |
| Anastomotic leak | 26 (1.4) |
| Abdominal abscess | 10 (0.5) |
| Wound infection | 41 (2.2) |
| Chyle leak | 34 (3.4) |
| Intra-abdominal bleeding | 12 (0.6) |
| Intestinal bleeding | 9 (0.5) |
| Ileus | 47 (2.5) |
| Other | 78 (4.2) |
| Clavien–Dindo classification | |
| I | 89 (4.8) |
| II | 124 (6.7) |
| III | 36 (1.9) |
| IV | 5 (0.3) |
| V | 3 (0.2) |
| Time to first flatus* | 2 (2–3) |
| Time to first stool* | 4 (3–5) |
| Time to first diet* | 3 (2–4) |
| Length of hospital stay* | 13 (10–16) |
| Length of postoperative hospital stay* | 8 (6–10) |
| Reoperation | 16 (0.9) |
| Readmission | 23 (1.2) |
| Mortality | 3 (0.2) |
| Morbidity | 216 (11.7) |
| Postoperative complications | |
| Anastomotic leak | 26 (1.4) |
| Abdominal abscess | 10 (0.5) |
| Wound infection | 41 (2.2) |
| Chyle leak | 34 (3.4) |
| Intra-abdominal bleeding | 12 (0.6) |
| Intestinal bleeding | 9 (0.5) |
| Ileus | 47 (2.5) |
| Other | 78 (4.2) |
| Clavien–Dindo classification | |
| I | 89 (4.8) |
| II | 124 (6.7) |
| III | 36 (1.9) |
| IV | 5 (0.3) |
| V | 3 (0.2) |
| Time to first flatus* | 2 (2–3) |
| Time to first stool* | 4 (3–5) |
| Time to first diet* | 3 (2–4) |
| Length of hospital stay* | 13 (10–16) |
| Length of postoperative hospital stay* | 8 (6–10) |
Values are n (%) unless otherwise indicated. *Data are presented as median (range) number of days.
Through LASSO analysis, eight factors were selected as potential predictors of postoperative morbidity. At univariate and multivariate analysis, ASA grade > II (P < 0.001), D3 lymph node dissection (P = 0.008) and intraoperative blood loss > 50 ml (P = 0.044) were identified as independent risk factors for postoperative morbidity (Table 5, Fig. S2).
Predictive factors for postoperative morbidity after right hemicolectomy for colon cancer
| . | All patients (n = 1854) . | Patients with morbidity (n = 216) . | Patients without morbidity (n = 1638) . | P (univariate analysis) . | OR . | 95% c.i. . | P (multivariate analysis) . |
|---|---|---|---|---|---|---|---|
| ASA fitness grade | <0.001 | <0.001 | |||||
| I–II | 1637 | 170 (10.4) | 1467 (89.6) | 1 | – | ||
| III–IV | 217 | 46 (21.2) | 171 (78.8) | 2.194 | 1.470–3.274 | ||
| Lymph-node dissection | 0.008 | ||||||
| D2 | 871 | 79 (9.1) | 792 (90.9) | – | 1 | – | |
| D3 | 969 | 136 (14.0) | 833 (86.0) | 0.001 | 1.629 | 1.196–2.218 | 0.002 |
| D1 | 14 | 1 (7.1) | 14 (92.9) | 0.47 | 0.907 | 0.115–7.155 | 0.927 |
| Intraoperative blood loss (ml) | 0.109 | 0.044 | |||||
| 0–50 | 1238 | 131 (10.6) | 1107 (89.4) | – | 1 | – | |
| 50–200 | 567 | 77 (13.6) | 490 (86.4) | 0.065 | 1.440 | 1.051–1.974 | 0.023 |
| >200 | 49 | 8 (16.3) | 41 (83.6) | 0.208 | 1.767 | 0.790–3.956 | 0.183 |
| Anastomotic type | 0.016 | 0.160 | |||||
| End-to-side | 764 | 69 (9.0) | 695 (91.0) | – | 1 | – | |
| Side-to-side (extracorporeal) | 769 | 110 (14.3) | 659 (87.1) | 0.001 | 1.451. | 1.045–2.014 | 0.026 |
| Side-to-side (intracorporeal) | 292 | 33 (11.3) | 259 (88.7) | 0.265 | 1.263 | 0.804–1.984 | 0.310 |
| End-to-end | 29 | 4 (13.8) | 25 (86.2) | 0.388 | 1.648 | 0.541–5.024 | 0.380 |
| Mesenteric defect closure | 0.053 | 0.102 | |||||
| Yes | 783 | 78 (10.0) | 705 (90.0) | 1 | – | ||
| No | 1071 | 138 (12.9) | 933 (87.1) | 1.287 | 0.951–1.742 | ||
| ECOG score | 0.027 | 0.776 | |||||
| 0–1 | 1696 | 189 (11.1) | 1507 (88.9) | 1 | – | – | |
| 2–4 | 158 | 27 (17.1) | 131 (82.9) | 1.074 | 0.659–1.749 | ||
| Albumin (g/l) | 0.063 | 0.360 | |||||
| >35 | 1383 | 148 (10.7) | 1235 (89.3) | – | 1 | – | |
| 30–35 | 369 | 49 (13.3) | 320 (86.7) | 0.165 | 1.187 | 0.833–1.690 | 0.343 |
| 25–30 | 90 | 16 (17.8) | 74 (82.2) | 0.041 | 1.501 | 0.837–2.693 | 0.173 |
| <25 | 12 | 3 (25.0) | 9 (75.0) | 0.128 | 1.976 | 0.512–7.634 | 0.323 |
| Surgical duration (min) | 183.1 (63.9) | 184.9 (61.0) | 182.8 (64.2) | 0.661 |
| . | All patients (n = 1854) . | Patients with morbidity (n = 216) . | Patients without morbidity (n = 1638) . | P (univariate analysis) . | OR . | 95% c.i. . | P (multivariate analysis) . |
|---|---|---|---|---|---|---|---|
| ASA fitness grade | <0.001 | <0.001 | |||||
| I–II | 1637 | 170 (10.4) | 1467 (89.6) | 1 | – | ||
| III–IV | 217 | 46 (21.2) | 171 (78.8) | 2.194 | 1.470–3.274 | ||
| Lymph-node dissection | 0.008 | ||||||
| D2 | 871 | 79 (9.1) | 792 (90.9) | – | 1 | – | |
| D3 | 969 | 136 (14.0) | 833 (86.0) | 0.001 | 1.629 | 1.196–2.218 | 0.002 |
| D1 | 14 | 1 (7.1) | 14 (92.9) | 0.47 | 0.907 | 0.115–7.155 | 0.927 |
| Intraoperative blood loss (ml) | 0.109 | 0.044 | |||||
| 0–50 | 1238 | 131 (10.6) | 1107 (89.4) | – | 1 | – | |
| 50–200 | 567 | 77 (13.6) | 490 (86.4) | 0.065 | 1.440 | 1.051–1.974 | 0.023 |
| >200 | 49 | 8 (16.3) | 41 (83.6) | 0.208 | 1.767 | 0.790–3.956 | 0.183 |
| Anastomotic type | 0.016 | 0.160 | |||||
| End-to-side | 764 | 69 (9.0) | 695 (91.0) | – | 1 | – | |
| Side-to-side (extracorporeal) | 769 | 110 (14.3) | 659 (87.1) | 0.001 | 1.451. | 1.045–2.014 | 0.026 |
| Side-to-side (intracorporeal) | 292 | 33 (11.3) | 259 (88.7) | 0.265 | 1.263 | 0.804–1.984 | 0.310 |
| End-to-end | 29 | 4 (13.8) | 25 (86.2) | 0.388 | 1.648 | 0.541–5.024 | 0.380 |
| Mesenteric defect closure | 0.053 | 0.102 | |||||
| Yes | 783 | 78 (10.0) | 705 (90.0) | 1 | – | ||
| No | 1071 | 138 (12.9) | 933 (87.1) | 1.287 | 0.951–1.742 | ||
| ECOG score | 0.027 | 0.776 | |||||
| 0–1 | 1696 | 189 (11.1) | 1507 (88.9) | 1 | – | – | |
| 2–4 | 158 | 27 (17.1) | 131 (82.9) | 1.074 | 0.659–1.749 | ||
| Albumin (g/l) | 0.063 | 0.360 | |||||
| >35 | 1383 | 148 (10.7) | 1235 (89.3) | – | 1 | – | |
| 30–35 | 369 | 49 (13.3) | 320 (86.7) | 0.165 | 1.187 | 0.833–1.690 | 0.343 |
| 25–30 | 90 | 16 (17.8) | 74 (82.2) | 0.041 | 1.501 | 0.837–2.693 | 0.173 |
| <25 | 12 | 3 (25.0) | 9 (75.0) | 0.128 | 1.976 | 0.512–7.634 | 0.323 |
| Surgical duration (min) | 183.1 (63.9) | 184.9 (61.0) | 182.8 (64.2) | 0.661 |
Predictive factors for postoperative morbidity after right hemicolectomy for colon cancer
| . | All patients (n = 1854) . | Patients with morbidity (n = 216) . | Patients without morbidity (n = 1638) . | P (univariate analysis) . | OR . | 95% c.i. . | P (multivariate analysis) . |
|---|---|---|---|---|---|---|---|
| ASA fitness grade | <0.001 | <0.001 | |||||
| I–II | 1637 | 170 (10.4) | 1467 (89.6) | 1 | – | ||
| III–IV | 217 | 46 (21.2) | 171 (78.8) | 2.194 | 1.470–3.274 | ||
| Lymph-node dissection | 0.008 | ||||||
| D2 | 871 | 79 (9.1) | 792 (90.9) | – | 1 | – | |
| D3 | 969 | 136 (14.0) | 833 (86.0) | 0.001 | 1.629 | 1.196–2.218 | 0.002 |
| D1 | 14 | 1 (7.1) | 14 (92.9) | 0.47 | 0.907 | 0.115–7.155 | 0.927 |
| Intraoperative blood loss (ml) | 0.109 | 0.044 | |||||
| 0–50 | 1238 | 131 (10.6) | 1107 (89.4) | – | 1 | – | |
| 50–200 | 567 | 77 (13.6) | 490 (86.4) | 0.065 | 1.440 | 1.051–1.974 | 0.023 |
| >200 | 49 | 8 (16.3) | 41 (83.6) | 0.208 | 1.767 | 0.790–3.956 | 0.183 |
| Anastomotic type | 0.016 | 0.160 | |||||
| End-to-side | 764 | 69 (9.0) | 695 (91.0) | – | 1 | – | |
| Side-to-side (extracorporeal) | 769 | 110 (14.3) | 659 (87.1) | 0.001 | 1.451. | 1.045–2.014 | 0.026 |
| Side-to-side (intracorporeal) | 292 | 33 (11.3) | 259 (88.7) | 0.265 | 1.263 | 0.804–1.984 | 0.310 |
| End-to-end | 29 | 4 (13.8) | 25 (86.2) | 0.388 | 1.648 | 0.541–5.024 | 0.380 |
| Mesenteric defect closure | 0.053 | 0.102 | |||||
| Yes | 783 | 78 (10.0) | 705 (90.0) | 1 | – | ||
| No | 1071 | 138 (12.9) | 933 (87.1) | 1.287 | 0.951–1.742 | ||
| ECOG score | 0.027 | 0.776 | |||||
| 0–1 | 1696 | 189 (11.1) | 1507 (88.9) | 1 | – | – | |
| 2–4 | 158 | 27 (17.1) | 131 (82.9) | 1.074 | 0.659–1.749 | ||
| Albumin (g/l) | 0.063 | 0.360 | |||||
| >35 | 1383 | 148 (10.7) | 1235 (89.3) | – | 1 | – | |
| 30–35 | 369 | 49 (13.3) | 320 (86.7) | 0.165 | 1.187 | 0.833–1.690 | 0.343 |
| 25–30 | 90 | 16 (17.8) | 74 (82.2) | 0.041 | 1.501 | 0.837–2.693 | 0.173 |
| <25 | 12 | 3 (25.0) | 9 (75.0) | 0.128 | 1.976 | 0.512–7.634 | 0.323 |
| Surgical duration (min) | 183.1 (63.9) | 184.9 (61.0) | 182.8 (64.2) | 0.661 |
| . | All patients (n = 1854) . | Patients with morbidity (n = 216) . | Patients without morbidity (n = 1638) . | P (univariate analysis) . | OR . | 95% c.i. . | P (multivariate analysis) . |
|---|---|---|---|---|---|---|---|
| ASA fitness grade | <0.001 | <0.001 | |||||
| I–II | 1637 | 170 (10.4) | 1467 (89.6) | 1 | – | ||
| III–IV | 217 | 46 (21.2) | 171 (78.8) | 2.194 | 1.470–3.274 | ||
| Lymph-node dissection | 0.008 | ||||||
| D2 | 871 | 79 (9.1) | 792 (90.9) | – | 1 | – | |
| D3 | 969 | 136 (14.0) | 833 (86.0) | 0.001 | 1.629 | 1.196–2.218 | 0.002 |
| D1 | 14 | 1 (7.1) | 14 (92.9) | 0.47 | 0.907 | 0.115–7.155 | 0.927 |
| Intraoperative blood loss (ml) | 0.109 | 0.044 | |||||
| 0–50 | 1238 | 131 (10.6) | 1107 (89.4) | – | 1 | – | |
| 50–200 | 567 | 77 (13.6) | 490 (86.4) | 0.065 | 1.440 | 1.051–1.974 | 0.023 |
| >200 | 49 | 8 (16.3) | 41 (83.6) | 0.208 | 1.767 | 0.790–3.956 | 0.183 |
| Anastomotic type | 0.016 | 0.160 | |||||
| End-to-side | 764 | 69 (9.0) | 695 (91.0) | – | 1 | – | |
| Side-to-side (extracorporeal) | 769 | 110 (14.3) | 659 (87.1) | 0.001 | 1.451. | 1.045–2.014 | 0.026 |
| Side-to-side (intracorporeal) | 292 | 33 (11.3) | 259 (88.7) | 0.265 | 1.263 | 0.804–1.984 | 0.310 |
| End-to-end | 29 | 4 (13.8) | 25 (86.2) | 0.388 | 1.648 | 0.541–5.024 | 0.380 |
| Mesenteric defect closure | 0.053 | 0.102 | |||||
| Yes | 783 | 78 (10.0) | 705 (90.0) | 1 | – | ||
| No | 1071 | 138 (12.9) | 933 (87.1) | 1.287 | 0.951–1.742 | ||
| ECOG score | 0.027 | 0.776 | |||||
| 0–1 | 1696 | 189 (11.1) | 1507 (88.9) | 1 | – | – | |
| 2–4 | 158 | 27 (17.1) | 131 (82.9) | 1.074 | 0.659–1.749 | ||
| Albumin (g/l) | 0.063 | 0.360 | |||||
| >35 | 1383 | 148 (10.7) | 1235 (89.3) | – | 1 | – | |
| 30–35 | 369 | 49 (13.3) | 320 (86.7) | 0.165 | 1.187 | 0.833–1.690 | 0.343 |
| 25–30 | 90 | 16 (17.8) | 74 (82.2) | 0.041 | 1.501 | 0.837–2.693 | 0.173 |
| <25 | 12 | 3 (25.0) | 9 (75.0) | 0.128 | 1.976 | 0.512–7.634 | 0.323 |
| Surgical duration (min) | 183.1 (63.9) | 184.9 (61.0) | 182.8 (64.2) | 0.661 |
Anastomotic leak
A total of 26 patients (1.4 per cent) had an anastomotic leak within 30 days of surgery (12 Clavien–Dindo II, and 14 Clavien–Dindo III). Twelve patients were treated with antibiotics alone, five with radiologically guided drainage and nine with laparotomy. Through LASSO analysis, three factors were selected as potential predictors of anastomotic leak. At univariate and multivariate analysis, extracorporeal side-to-side anastomosis (P = 0.031), intraoperative blood loss > 50 ml (P = 0.004) and neoadjuvant chemotherapy (P = 0.004) were identified as independent risk factors for anastomotic leak (Table 6, Fig. S3).
Predictive factors for anastomotic leak after right hemicolectomy for colon cancer
| . | All patients (n = 1854) . | Patients with anastomotic leak (n = 26) . | Patients without anastomotic leak (n = 1828) . | P (univariate analysis) . | OR . | 95% c.i. . | P (multivariate analysis) . |
|---|---|---|---|---|---|---|---|
| Anastomotic type | 0.026 | 0.031 | |||||
| End-to-side | 764 | 3 (0.4) | 761 (99.6) | – | 1 | – | |
| Side-to-side (extracorporeal) | 769 | 19 (2.5) | 750 (97.5) | 0.003 | 6.415 | 1.879–21.898 | 0.003 |
| Side-to-side (intracorporeal) | 292 | 4 (1.4) | 288 (98.6) | 0.101 | 4.332 | 0.95–19.748 | 0.058 |
| End-to-end | 29 | 0 (0.0) | 29 (100) | 0.998 | – | – | – |
| Intraoperative blood loss | 0.003 | 0.004 | |||||
| 0–50 | 1238 | 10 (0.8) | 1228 (99.2) | – | |||
| 50–200 | 567 | 13 (2.3) | 554 (97.7) | 0.012 | 2.977 | 1.282–6.917 | 0.011 |
| >200 | 49 | 3 (6.5) | 46 (93.5) | 0.002 | 7.355 | 1.900–28.477 | 0.004 |
| Neoadjuvant chemotherapy | 0.005 | 0.004 | |||||
| No | 1784 | 22 (1.2) | 1762 (98.8) | 1 | – | ||
| Yes | 70 | 4 (5.7) | 66 (94.3) | 5.174 | 1.676–15.977 |
| . | All patients (n = 1854) . | Patients with anastomotic leak (n = 26) . | Patients without anastomotic leak (n = 1828) . | P (univariate analysis) . | OR . | 95% c.i. . | P (multivariate analysis) . |
|---|---|---|---|---|---|---|---|
| Anastomotic type | 0.026 | 0.031 | |||||
| End-to-side | 764 | 3 (0.4) | 761 (99.6) | – | 1 | – | |
| Side-to-side (extracorporeal) | 769 | 19 (2.5) | 750 (97.5) | 0.003 | 6.415 | 1.879–21.898 | 0.003 |
| Side-to-side (intracorporeal) | 292 | 4 (1.4) | 288 (98.6) | 0.101 | 4.332 | 0.95–19.748 | 0.058 |
| End-to-end | 29 | 0 (0.0) | 29 (100) | 0.998 | – | – | – |
| Intraoperative blood loss | 0.003 | 0.004 | |||||
| 0–50 | 1238 | 10 (0.8) | 1228 (99.2) | – | |||
| 50–200 | 567 | 13 (2.3) | 554 (97.7) | 0.012 | 2.977 | 1.282–6.917 | 0.011 |
| >200 | 49 | 3 (6.5) | 46 (93.5) | 0.002 | 7.355 | 1.900–28.477 | 0.004 |
| Neoadjuvant chemotherapy | 0.005 | 0.004 | |||||
| No | 1784 | 22 (1.2) | 1762 (98.8) | 1 | – | ||
| Yes | 70 | 4 (5.7) | 66 (94.3) | 5.174 | 1.676–15.977 |
Predictive factors for anastomotic leak after right hemicolectomy for colon cancer
| . | All patients (n = 1854) . | Patients with anastomotic leak (n = 26) . | Patients without anastomotic leak (n = 1828) . | P (univariate analysis) . | OR . | 95% c.i. . | P (multivariate analysis) . |
|---|---|---|---|---|---|---|---|
| Anastomotic type | 0.026 | 0.031 | |||||
| End-to-side | 764 | 3 (0.4) | 761 (99.6) | – | 1 | – | |
| Side-to-side (extracorporeal) | 769 | 19 (2.5) | 750 (97.5) | 0.003 | 6.415 | 1.879–21.898 | 0.003 |
| Side-to-side (intracorporeal) | 292 | 4 (1.4) | 288 (98.6) | 0.101 | 4.332 | 0.95–19.748 | 0.058 |
| End-to-end | 29 | 0 (0.0) | 29 (100) | 0.998 | – | – | – |
| Intraoperative blood loss | 0.003 | 0.004 | |||||
| 0–50 | 1238 | 10 (0.8) | 1228 (99.2) | – | |||
| 50–200 | 567 | 13 (2.3) | 554 (97.7) | 0.012 | 2.977 | 1.282–6.917 | 0.011 |
| >200 | 49 | 3 (6.5) | 46 (93.5) | 0.002 | 7.355 | 1.900–28.477 | 0.004 |
| Neoadjuvant chemotherapy | 0.005 | 0.004 | |||||
| No | 1784 | 22 (1.2) | 1762 (98.8) | 1 | – | ||
| Yes | 70 | 4 (5.7) | 66 (94.3) | 5.174 | 1.676–15.977 |
| . | All patients (n = 1854) . | Patients with anastomotic leak (n = 26) . | Patients without anastomotic leak (n = 1828) . | P (univariate analysis) . | OR . | 95% c.i. . | P (multivariate analysis) . |
|---|---|---|---|---|---|---|---|
| Anastomotic type | 0.026 | 0.031 | |||||
| End-to-side | 764 | 3 (0.4) | 761 (99.6) | – | 1 | – | |
| Side-to-side (extracorporeal) | 769 | 19 (2.5) | 750 (97.5) | 0.003 | 6.415 | 1.879–21.898 | 0.003 |
| Side-to-side (intracorporeal) | 292 | 4 (1.4) | 288 (98.6) | 0.101 | 4.332 | 0.95–19.748 | 0.058 |
| End-to-end | 29 | 0 (0.0) | 29 (100) | 0.998 | – | – | – |
| Intraoperative blood loss | 0.003 | 0.004 | |||||
| 0–50 | 1238 | 10 (0.8) | 1228 (99.2) | – | |||
| 50–200 | 567 | 13 (2.3) | 554 (97.7) | 0.012 | 2.977 | 1.282–6.917 | 0.011 |
| >200 | 49 | 3 (6.5) | 46 (93.5) | 0.002 | 7.355 | 1.900–28.477 | 0.004 |
| Neoadjuvant chemotherapy | 0.005 | 0.004 | |||||
| No | 1784 | 22 (1.2) | 1762 (98.8) | 1 | – | ||
| Yes | 70 | 4 (5.7) | 66 (94.3) | 5.174 | 1.676–15.977 |
Discussion
Our study prospectively enrolled patients with right colon cancer who underwent RHC in 52 tertiary hospitals, providing valuable insights into the current clinical practice of RHC in high-volume expert centres in China. Additionally, this study reported low rates of conversion, morbidity, and mortality10,11,22,23, which differs significantly from previous studies24,25. This difference can be attributed to the screening of enrolment centres and patients, which helped to identify and implement effective clinical practices. The patients included in our study in China were relatively younger than those in previous European Society of Coloproctology Collaborating Group (ESCP) studies10, with 88.3 per cent of patients being ASA I–II and exclusion of emergency surgery, indicating better baseline conditions. The lower BMI most probably also made the operations easier to perform. Furthermore, over 95 per cent of operations were performed by chief or associate chief surgeons with extensive experience in laparoscopic surgery, contributing to the excellent clinical outcomes reported in this study. The length of postoperative hospital stay was 8 (6–10) days, which is consistent with the findings of the REAL study conducted in China26. Although this might be related to cultural factors, it also indicates that enhanced recovery after surgery (ERAS) protocols are not being implemented effectively in most Chinese centres.
This study also found that laparoscopic surgery has been widely implemented in high-volume expert centres, with D3 lymph node dissection rates exceeding 50 per cent. However, the surgical time was longer than that reported in the ESCP study, possibly due to the complexity of the surgery. Comparing anastomotic techniques to the ESCP study, stapled anastomosis (97.8 per cent versus 59.8 per cent) and end-to-side anastomosis (41.2 per cent versus 11.3 per cent) were significantly higher, while intracorporeal anastomosis was comparable (15.7 per cent versus 17.4 per cent)10.
Anastomotic leak is a major complication after RHC and has a negative impact on short- and long-term outcomes10–14. The incidence of anastomotic leak varies widely from 1.0 per cent to 8.4 per cent6,10,11,27,28. Anastomotic leak rates in retrospective studies ranged from 4 to 8 per cent10,11,22, and 1–3 per cent in RCTs6,27,28. Our study reported an anastomotic leak rate of only 1.4 per cent. In addition to excluding high-risk emergency surgery and inflammatory bowel disease, and selecting high-volume expert centres, the definition and diagnosis of anastomotic leak may impact upon this reported rate. There is currently no uniform definition of anastomotic leak after RHC. The ESCP defines both clinically suspected anastomotic leaks confirmed radiologically or intraoperatively (type 1) and the presence of an intraperitoneal fluid collection (type 2), reporting 4.6 per cent (type 1 only) and 7.4 per cent (types 1 and 2 combined)10. This revealed that a broad definition may contribute to high reporting of anastomotic leak. Because regular postoperative imaging was not necessary, this study required clinical suspicion of anastomotic leak to then undertake radiological examination.
In the present analysis, extracorporeal side-to-side anastomosis, intraoperative bleeding > 50 ml and neoadjuvant chemotherapy were identified as independent risk factors for anastomotic leak after RHC. Compared with end-to-side anastomosis, side-to-side anastomosis is wider, and cross-stapling in an isoperistaltic fashion (more commonly used in extracorporeal anastomoses) may increase the risk of anastomotic leak. Previous studies have shown that the leak rate of a stapled end-to-side anastomosis was similar to that of a hand-sewn anastomosis, but much higher for stapled side-to-side anastomoses29. A study from ESCP also revealed that the anastomotic leak rate was higher in stapled anastomosis, in which side-to-side anastomosis accounted for 87.8 per cent10. Interestingly, side-to-side anastomosis was performed in all intracorporeal anastomoses, and although no significant difference in leak rate was shown compared with extracorporeal anastomosis (0.4 per cent versus 1.4 per cent, P = 0.058), this may be due to the fact that an isoperistaltic anastomosis, which had no cross-stapling, was more commonly used in the intracorporeal setting. In addition, reduced mobilization and torsion of the mesentery may improve the blood supply of the anastomosis, although further studies are required to investigate this. Considering that intracorporeal anastomosis was used infrequently, there is a learning curve that needs to be overcome.
Intraoperative bleeding was another risk factor for anastomotic leak, which was consistent with previous studies30,31. Intraoperative bleeding was considered a sign of surgical damage and may slow wound healing. Neoadjuvant chemotherapy was also identified as an independent risk factor for anastomotic leak in our study, but this conclusion is not consistently reported32–34. Nevertheless, attention must be given to the optimal timing for surgery and perioperative management in patients receiving neoadjuvant chemotherapy. Our study found no significant negative impact of ASA classification, malnutrition, open surgery, or duration of surgery on anastomotic leak.
ASA category and intraoperative bleeding are well known to be predictors of postoperative morbidity35,36. In this study, D3 lymph node dissection was an independent risk factor for morbidity, although it has not been consistently reported as such2,4,37. The recent RELARC study reported a similar complication rate in CME compared with D2 lymph node dissection but increased risk of vascular injury6. The number of harvested lymph nodes were significantly higher in the CME group, and 3 per cent of central lymph nodes were detected to be metastatic. Data on whether these pathological benefits will improve prognosis is highly anticipated. Although D3 lymph node dissection has been performed in many centres, attention consideration must be given to the potential for increased morbidity, and appropriate patient selection and perioperative management are necessary.
The main strength of this study is that the data from a large sample of high-volume expert centres in China were prospectively collected. This ensures the homogeneity of data as much as possible by excluding patient and surgeon factors, and more accurately predicting those factors related to surgical technique and postoperative outcomes. The limitations of this study are as follows: this is an observational study, and selection bias needs to be considered; the generalizability of the study findings may be affected as we have screened both surgical centres and patients; there is currently no uniform definition for anastomotic leak, only clinically diagnosed anastomotic leak is identified here, and some radiological anastomotic leaks may have been undetected; due to the limited collection of variables, complications other than those related to surgery, such as respiratory or cardiac complications, were not collected in sufficient detail; due to the low incidence of target events, there may be a risk of bias during statistical analysis. However, the LASSO methodology is capable of producing a model that only includes a subset of the predictor variables to reduce the bias from other confounding factors.
Conclusion
In high-volume expert centres in China, laparoscopic RHC and D3 lymph node dissection were performed in most patients, and overall postoperative morbidity and mortality were low. An ASA fitness grade > II, intraoperative blood loss > 50 ml and D3 lymph node dissection were identified as independent risk factors for morbidity. Extracorporeal side-to-side anastomosis, intraoperative blood loss > 50 ml and neoadjuvant chemotherapy were identified as independent risk factors for anastomotic leak. Further high-quality studies should focus on the optimal techniques for radical tumour resection and anastomosis, for which the results of this study will provide a basis.
Collaborators
Jurriaan Tuynman, Department of Surgery, VU University Medical Center, Amsterdam, The Netherlands
Email: [email protected]
Hendrik Bonjer, Department of Surgery, VU University Medical Center, Amsterdam, The Netherlands
Email: [email protected]
Hongwei Yao, Department of General Surgery, Beijing Friendship Hospital, Capital Medical University, National Clinical Research Center of Digestive Diseases, Beijing 100050, China
Email: [email protected]
Zhongtao Zhang, Department of General Surgery, Beijing Friendship Hospital, Capital Medical University, National Clinical Research Center of Digestive Diseases, Beijing 100050, China
Email: [email protected]
Funding
This work was supported by grants from the National Key Technologies R&D Program (No. 2015BAI13B09), National Key Technologies R&D Program of China (No. 2017YFC0110904), Clinical Center for Colorectal Cancer, Capital Medical University (No. 1192070313).
Acknowledgements
The authors acknowledge the all the RHC-SNAPSHOT investigators (Appendix 2). The authors also thank Yuanyuan Kong (Department of Clinical Epidemiology and EBM Unit, Beijing Friendship Hospital) for guidance on methodology, and Yishan Liu (National Clinical Research Center of Digestive Diseases) for her data monitoring assistance.
Disclosure
No potential conflict of interest was reported by the authors.
Supplementary material
Supplementary material is available at BJS online.
Data availability
Data used and analysed during this study are available from the corresponding author on reasonable request.
References
Author notes
Jiale Gao, Xiaodong Gu, Minghui Pang, Hong Zhang, Yugui Lian, Lei Zhou, Bo Feng, Guiying Wang, Zhicao Zhang, He Huang, Gang Xiao, Fanghai Han, Xinxiang Li shared first authorship and contributed equally to this work.



