-
PDF
- Split View
-
Views
-
Cite
Cite
J Vidal Fortuny, V Belfontali, S M Sadowski, W Karenovics, S Guigard, F Triponez, Parathyroid gland angiography with indocyanine green fluorescence to predict parathyroid function after thyroid surgery, British Journal of Surgery, Volume 103, Issue 5, April 2016, Pages 537–543, https://doi.org/10.1002/bjs.10101
Close - Share Icon Share
Abstract
Postoperative hypoparathyroidism remains the most common complication following thyroidectomy. The aim of this pilot study was to evaluate the use of intraoperative parathyroid gland angiography in predicting normal parathyroid gland function after thyroid surgery.
Angiography with the fluorescent dye indocyanine green (ICG) was performed in patients undergoing total thyroidectomy, to visualize vascularization of identified parathyroid glands.
Some 36 patients underwent ICG angiography during thyroidectomy. All patients received standard calcium and vitamin D supplementation. At least one well vascularized parathyroid gland was demonstrated by ICG angiography in 30 patients. All 30 patients had parathyroid hormone (PTH) levels in the normal range on postoperative day (POD) 1 and 10, and only one patient exhibited asymptomatic hypocalcaemia on POD 1. Mean(s.d.) PTH and calcium levels in these patients were 3·3(1·4) pmol/l and 2·27(0·10) mmol/l respectively on POD 1, and 4·0(1.6) pmol/l and 2·32(0·08) mmol/l on POD 10. Two of the six patients in whom no well vascularized parathyroid gland could be demonstrated developed transient hypoparathyroidism. None of the 36 patients presented symptomatic hypocalcaemia, and none received treatment for hypoparathyroidism.
PTH levels on POD 1 were normal in all patients who had at least one well vascularized parathyroid gland demonstrated during surgery by ICG angiography, and none required treatment for hypoparathyroidism.
Introduction
Postoperative hypocalcaemia following total thyroidectomy is common, and may have a significant effect on quality of life. Transient hypocalcaemia is frequent and has been described in 15–30 per cent of patients, depending on the technical difficulty of the procedure and expertise of the surgeon. Permanent hypocalcaemia, defined as hypocalcaemia present for more than 6 months after thyroidectomy, has been reported in 1–3 per cent of patients1. Some authors have described rates of up to 10 per cent1,2, which suggests a possible underestimation of the true prevalence of permanent hypocalcaemia after thyroid surgery.
The main cause of hypocalcaemia after total thyroidectomy is hypoparathyroidism due to intraoperative damage to the parathyroid glands by trauma, inadvertent parathyroid gland removal or devascularization. The extent of damage to the parathyroid glands is difficult to predict during surgery. It has been generally accepted that half of one normal parathyroid gland can produce sufficient parathyroid hormone (PTH)2,3. To avoid postoperative hypocalcaemia, parathyroid autotransplantation can be performed, although the results have been controversial4,5.
Accurate prediction of post-thyroidectomy hypocalcaemia has the potential to influence management strategies and could possibly reduce the incidence of hypoparathyroidism if the precise mechanisms of this condition were to be elucidated. Among the newer techniques2,6,7, intraoperative parathyroid gland angiography during thyroidectomy might be used to evaluate parathyroid gland perfusion and function.
Initially used in the detection of macular degeneration8, the technique of angiography using indocyanine green (ICG) has been used to identify sentinel lymph nodes9, to determine the extent of oncological resections10 and to study hepatic function11. Recent studies have also demonstrated its usefulness in evaluating the vascular blood flow of intestinal anastomoses12.
The aim of this pilot study was to evaluate the use of ICG angiography in predicting parathyroid gland function and the absence of postoperative hypoparathyroidism in patients in whom good vascularization of at least one parathyroid gland could be demonstrated by the technique.
Methods
The study was approved by the Ethics Review Board of the University Hospitals of Geneva, and written informed consent was obtained from all the study participants. Patients who had a total thyroidectomy and consented to the study underwent ICG angiography when the equipment was available.
Surgical procedure and indocyanine green angiography protocol
Thyroid surgery was performed by three surgeons, or a surgical resident under their direct supervision. Magnifying loupes were always used. The recurrent laryngeal nerve was sought systematically and identified using intraoperative neuromonitoring (NIM 3·0®; Medtronic, Dublin, Ireland). During dissection, special care was taken to visualize the parathyroid glands and to preserve the vascular pedicles, but without searching for the parathyroid glands when they were not apparent during the initial perithyroidal dissection. The surgical technique was no different for the patients in this study compared with that performed for other patients having surgery in the authors' unit. The thyroid specimens were examined ex vivo for parathyroid glands before sending the specimens for histopathological examination.
ICG was prepared according to protocols used for abdominal surgery10; the dye was mixed with 10 ml sterile water, and 3·5 ml was injected intravenously. The injection could be repeated until a maximum dose of 5 mg per kg per day was reached. The catheter was purged after each injection for a rapid image gain. After approximately 1–2 min, images were acquired with a laparoscopic PINPOINT® camera (Novadaq, Ontario, Canada). ICG is a 775-Da molecule with a maximum absorption spectrum of 805 nm and re-emission at 835 nm. It becomes completely and permanently fixed to plasma proteins once in the bloodstream, and circulates in the intravascular compartment only. ICG has a mean(s.d.) half-life of 3·4(0.7) min; it is taken up from plasma almost exclusively by the hepatic parenchymal cells, and secreted entirely into the bile.
After thyroid resection, all identified parathyroid glands were scored visually for viability from grade 0 (no vascularity) to 2 (excellent vascularity), and angiography with ICG was then performed. The parathyroid glands appeared in shades of grey depending on the amount of ICG flowing through the parathyroid tissue, thereby reflecting the degree of vascular blood flow. An imaging score for ICG was established: ICG 0, the parathyroid is black after the injection of ICG, indicating that the gland is not vascularized; ICG 2, the parathyroid is white, indicating that the gland is well vascularized; or ICG 1, the parathyroid is grey or heterogeneous, suggesting that the gland is partially vascularized13 (Fig. 1).
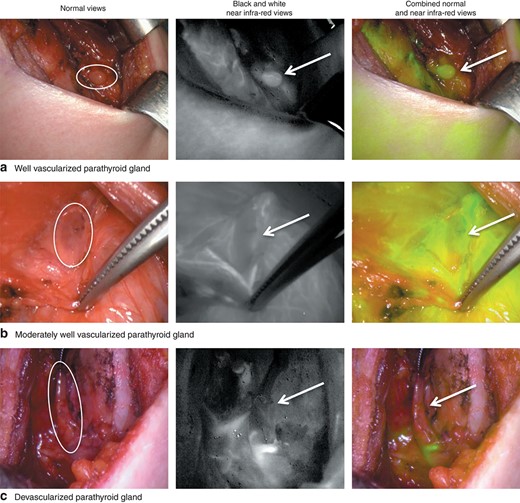
Representative parathyroid indocyanine green (ICG) angiography images. a A well vascularized parathyroid gland (ICG score 2). b A moderately well vascularized parathyroid gland (ICG score 1). c A devascularized parathyroid gland (ICG score 0). Circles and arrows indicate the parathyroid gland
In the case of discordance between the visual evaluation of the gland and the angiography results (for example, visually the gland appeared well vascularized, but on angiography the gland was black), a parenchymal incision of the parathyroid gland was performed. If no active bleeding was found after the incision, the gland was autotransplanted into the sternocleidomastoid muscle.
The standard postoperative protocol of the institution was followed for all patients. This consisted of one night of surveillance with calcium and PTH levels measured on postoperative day (POD) 1, and oral systematic supplementation with Calcimagon D3 Forte® (1 g calcium and 800 units 25-hydroxyvitamin D; Takeda Pharma, Freienbach, Switzerland) twice daily until the first follow-up appointment between POD 10 and POD 15. Calcium, albumin and PTH levels were measured in the clinical laboratories of the University Hospitals of Geneva. Serum levels of calcium were adjusted to the albumin levels. Normal values for the assays used in the authors' institution are 2·20–2·52 mmol/l and 1·1–6·8 pmol/l for calcium and PTH respectively. Hypocalcaemia was defined as an adjusted calcium level of less than 2·00 mmol/l, which corresponds to the levels cited in a recent literature review by Lorente-Poch and colleagues2. Hypoparathyroidism was defined as a PTH level below 1·1 pmol/l.
Statistical analysis
Data for continuous variables are presented as mean(s.d.) values. The χ2 test was used for comparisons between groups for discrete variables. P < 0·050 was considered statistically significant. Statistical analyses were performed using XLStat® 2015.5.01 (Addinsoft, Paris, France).
Results
Between May and October 2014, 181 consecutive patients underwent thyroid surgery at the University Hospitals of Geneva. A flow chart of the study is shown in Fig. 2. Table 1 provides clinical data and biochemical results of the 36 patients who underwent total thyroidectomy followed by angiography with ICG.
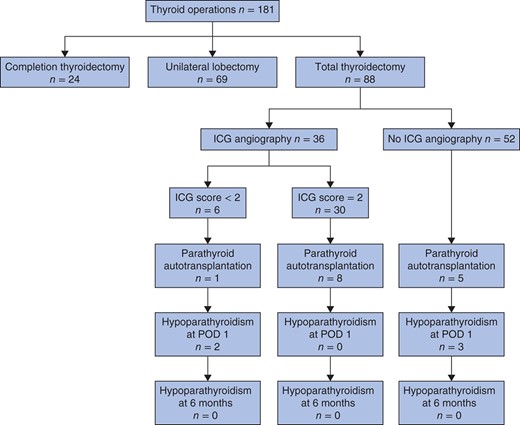
Flow chart for the study of intraoperative parathyroid angiography with the fluorescent dye indocyanine green (ICG); an ICG score of 2 indicates a well vascularized parathyroid gland whereas a score of less than 2 indicates no perfectly well vascularized parathyroid gland (see text for details). Hypoparathyroidism was defined by a parathyroid hormone level below 1·1 pmol/l. POD, postoperative day
| . | No. of patients* (n = 36) . |
|---|---|
| Age (years)† | 49.8(15.7) |
| Sex ratio (M : F) | 4 : 32 |
| Indication for surgery | |
| Multinodular goitre | 20 |
| Thyroid cancer | 8 |
| Graves' disease | 8 |
| Extent of surgery | |
| Total thyroidectomy | 32 |
| Total thyroidectomy + central neck dissection | 3 |
| Total thyroidectomy + central + lateral neck dissection | 1 |
| Biochemical values for patients with ICG score 2 (n = 30)† | |
| Corrected calcium level (mmol/l) | |
| POD 1 | 2·27(0·10) |
| POD 10 | 2·32(0·08) |
| PTH level (pmol/l) | |
| POD 1 | 3·3(1·4) |
| POD 10 | 4·0(1.6) |
| Biochemical values for patients with ICG score < 2 (n = 6)† | |
| Corrected calcium level (mmol/l) | |
| POD 1 | 2·26(0·11) |
| POD 10 | 2·37(0·09) |
| PTH level (pmol/l) | |
| POD 1 | 2.6(1·5) |
| POD 10 | 4·4(2·3) |
| . | No. of patients* (n = 36) . |
|---|---|
| Age (years)† | 49.8(15.7) |
| Sex ratio (M : F) | 4 : 32 |
| Indication for surgery | |
| Multinodular goitre | 20 |
| Thyroid cancer | 8 |
| Graves' disease | 8 |
| Extent of surgery | |
| Total thyroidectomy | 32 |
| Total thyroidectomy + central neck dissection | 3 |
| Total thyroidectomy + central + lateral neck dissection | 1 |
| Biochemical values for patients with ICG score 2 (n = 30)† | |
| Corrected calcium level (mmol/l) | |
| POD 1 | 2·27(0·10) |
| POD 10 | 2·32(0·08) |
| PTH level (pmol/l) | |
| POD 1 | 3·3(1·4) |
| POD 10 | 4·0(1.6) |
| Biochemical values for patients with ICG score < 2 (n = 6)† | |
| Corrected calcium level (mmol/l) | |
| POD 1 | 2·26(0·11) |
| POD 10 | 2·37(0·09) |
| PTH level (pmol/l) | |
| POD 1 | 2.6(1·5) |
| POD 10 | 4·4(2·3) |
Unless indicated otherwise;
values are mean(s.d.). ICG, indocyanine green; POD, postoperative day; PTH, parathyroid hormone.
| . | No. of patients* (n = 36) . |
|---|---|
| Age (years)† | 49.8(15.7) |
| Sex ratio (M : F) | 4 : 32 |
| Indication for surgery | |
| Multinodular goitre | 20 |
| Thyroid cancer | 8 |
| Graves' disease | 8 |
| Extent of surgery | |
| Total thyroidectomy | 32 |
| Total thyroidectomy + central neck dissection | 3 |
| Total thyroidectomy + central + lateral neck dissection | 1 |
| Biochemical values for patients with ICG score 2 (n = 30)† | |
| Corrected calcium level (mmol/l) | |
| POD 1 | 2·27(0·10) |
| POD 10 | 2·32(0·08) |
| PTH level (pmol/l) | |
| POD 1 | 3·3(1·4) |
| POD 10 | 4·0(1.6) |
| Biochemical values for patients with ICG score < 2 (n = 6)† | |
| Corrected calcium level (mmol/l) | |
| POD 1 | 2·26(0·11) |
| POD 10 | 2·37(0·09) |
| PTH level (pmol/l) | |
| POD 1 | 2.6(1·5) |
| POD 10 | 4·4(2·3) |
| . | No. of patients* (n = 36) . |
|---|---|
| Age (years)† | 49.8(15.7) |
| Sex ratio (M : F) | 4 : 32 |
| Indication for surgery | |
| Multinodular goitre | 20 |
| Thyroid cancer | 8 |
| Graves' disease | 8 |
| Extent of surgery | |
| Total thyroidectomy | 32 |
| Total thyroidectomy + central neck dissection | 3 |
| Total thyroidectomy + central + lateral neck dissection | 1 |
| Biochemical values for patients with ICG score 2 (n = 30)† | |
| Corrected calcium level (mmol/l) | |
| POD 1 | 2·27(0·10) |
| POD 10 | 2·32(0·08) |
| PTH level (pmol/l) | |
| POD 1 | 3·3(1·4) |
| POD 10 | 4·0(1.6) |
| Biochemical values for patients with ICG score < 2 (n = 6)† | |
| Corrected calcium level (mmol/l) | |
| POD 1 | 2·26(0·11) |
| POD 10 | 2·37(0·09) |
| PTH level (pmol/l) | |
| POD 1 | 2.6(1·5) |
| POD 10 | 4·4(2·3) |
Unless indicated otherwise;
values are mean(s.d.). ICG, indocyanine green; POD, postoperative day; PTH, parathyroid hormone.
One parathyroid gland was identified in one patient, two in 11 patients, three in 18 patients and four in six patients. The mean(s.d.) duration of the ICG angiography procedure was 6(2) min. Visual and angiography scores are presented in Table 2. The visual scores were significantly higher than the ICG angiography scores (P = 0·005). There were no adverse reactions.
Angiographic and visual classification of parathyroid glands in 36 patients who underwent thyroidectomy and intraoperative angiography with indocyanine green
| . | Right superior parathyroid . | Right inferior parathyroid . | Left superior parathyroid . | Left inferior parathyroid . |
|---|---|---|---|---|
| Total no. of parathyroid glands indentified | 28 | 17 | 29 | 25 |
| Score 2 | ||||
| ICG angiography | 15 | 7 | 18 | 11 |
| Visual evaluation | 21 | 9 | 23 | 18 |
| Score 1 | ||||
| ICG angiography | 10 | 5 | 10 | 11 |
| Visual evaluation | 5 | 6 | 6 | 5 |
| Score 0 | ||||
| ICG angiography | 3 | 3 | 1 | 1 |
| Visual evaluation | 2 | 2* | 0 | 2* |
| . | Right superior parathyroid . | Right inferior parathyroid . | Left superior parathyroid . | Left inferior parathyroid . |
|---|---|---|---|---|
| Total no. of parathyroid glands indentified | 28 | 17 | 29 | 25 |
| Score 2 | ||||
| ICG angiography | 15 | 7 | 18 | 11 |
| Visual evaluation | 21 | 9 | 23 | 18 |
| Score 1 | ||||
| ICG angiography | 10 | 5 | 10 | 11 |
| Visual evaluation | 5 | 6 | 6 | 5 |
| Score 0 | ||||
| ICG angiography | 3 | 3 | 1 | 1 |
| Visual evaluation | 2 | 2* | 0 | 2* |
One parathyroid gland found in thyroid specimen. Indocyanine green (ICG) score: ICG 0, the parathyroid remains black after injection of ICG solution, indicating the gland is not vascularized; ICG 2; the parathyroid is white, indicating the gland is well vascularized; ICG 1, the parathyroid is grey or heterogeneous. Visual score: grade 0, the parathyroid gland is visually devascularized; grade 2, the parathyroid is visually well vascularized; grade 1, the parathyroid gland is visually not well vascularized but deemed not completely devascularized.
Angiographic and visual classification of parathyroid glands in 36 patients who underwent thyroidectomy and intraoperative angiography with indocyanine green
| . | Right superior parathyroid . | Right inferior parathyroid . | Left superior parathyroid . | Left inferior parathyroid . |
|---|---|---|---|---|
| Total no. of parathyroid glands indentified | 28 | 17 | 29 | 25 |
| Score 2 | ||||
| ICG angiography | 15 | 7 | 18 | 11 |
| Visual evaluation | 21 | 9 | 23 | 18 |
| Score 1 | ||||
| ICG angiography | 10 | 5 | 10 | 11 |
| Visual evaluation | 5 | 6 | 6 | 5 |
| Score 0 | ||||
| ICG angiography | 3 | 3 | 1 | 1 |
| Visual evaluation | 2 | 2* | 0 | 2* |
| . | Right superior parathyroid . | Right inferior parathyroid . | Left superior parathyroid . | Left inferior parathyroid . |
|---|---|---|---|---|
| Total no. of parathyroid glands indentified | 28 | 17 | 29 | 25 |
| Score 2 | ||||
| ICG angiography | 15 | 7 | 18 | 11 |
| Visual evaluation | 21 | 9 | 23 | 18 |
| Score 1 | ||||
| ICG angiography | 10 | 5 | 10 | 11 |
| Visual evaluation | 5 | 6 | 6 | 5 |
| Score 0 | ||||
| ICG angiography | 3 | 3 | 1 | 1 |
| Visual evaluation | 2 | 2* | 0 | 2* |
One parathyroid gland found in thyroid specimen. Indocyanine green (ICG) score: ICG 0, the parathyroid remains black after injection of ICG solution, indicating the gland is not vascularized; ICG 2; the parathyroid is white, indicating the gland is well vascularized; ICG 1, the parathyroid is grey or heterogeneous. Visual score: grade 0, the parathyroid gland is visually devascularized; grade 2, the parathyroid is visually well vascularized; grade 1, the parathyroid gland is visually not well vascularized but deemed not completely devascularized.
In five patients there was discordance between the visual and ICG scores (ICG score 0, whereas visual score was 1 or 2). In these patients, as predicted by the ICG score, no active bleeding was found by incision of the gland parenchyma, and the glands were autotransplanted.
Of the 36 patients who underwent ICG angiography, 30 had an ICG score of 2 for at least one parathyroid gland. In four of these patients, a parathyroid gland was discovered in the thyroid specimen, including one with an incidental hyperplastic parathyroid gland. However, no information on the preoperative PTH level was available for this patient, so a diagnosis of primary hyperparathyroidism could not be established.
In the 30 patients with at least one parathyroid gland with an ICG score of 2, postoperative PTH levels were in the normal range. The postoperative adjusted calcium levels were within the normal range in 29 patients. The patient in whom a hyperplastic parathyroid gland was found in the surgical specimen presented with asymptomatic hypocalcaemia of 1.94 mmol/l and a PTH level of 1·3 pmol/l. The patient's levels normalized at POD 10 to 2·16 mmol/l and 3·1 pmol/l respectively.
In six patients ICG angiography did not demonstrate a well vascularized parathyroid gland. Two of these patients developed transient hypoparathyroidism (PTH level 0.9 and 1·0 pmol/l on POD 1), with recovery on POD 10 for the first patient (PTH 4·2 pmol/l) and after 2 months for the second (PTH 3·3 pmol/l). Neither of these patients developed hypocalcaemia at the time of measurements.
None of the 36 patients presented symptomatic hypocalcaemia, and none required treatment with active vitamin D analogue.
Discussion
This study has shown that ICG angiography in patients undergoing total thyroidectomy is safe, and the results suggest an excellent correlation between parathyroid perfusion and function. PTH levels on POD 1 were normal in all patients who had at least one well vascularized parathyroid gland, according to the ICG scores.
Postoperative hypoparathyroidism is currently the most common complication of total thyroidectomy1, and there is a need for a reliable tool that can accurately predict whether a patient will develop hypocalcaemia1,14,15. Currently available methods to evaluate parathyroid function are based on measurements of calcium16,17 and PTH2,6,7,18–20 levels at different time points after thyroidectomy. Published studies suggest that early (from a few minutes to 12 h after thyroid resection) measurement of PTH levels is a reliable tool to predict the absence of hypoparathyroidism, with a positive predictive value up to 97 per cent in some studies2,18,19, although this has been challenged by some authors21,22. Unlike angiography with ICG, none of these protocols is able to predict the absence of hypoparathyroidism only a few minutes after thyroid resection.
Because of the time needed to obtain results, measurements of calcium and PTH levels are usually not able to guide intraoperative decision-making. Some authors6,23,24 have suggested that the demonstration of parathyroid insufficiency using quick PTH measurements could help the surgeon decide whether or not to autotransplant one parathyroid gland. ICG angiography enables an early, direct evaluation of the parathyroid glands, and could assist in selecting patients who require parathyroid autotransplantation when a non-vascularized parathyroid gland is identified.
In the present study, the surgical procedure was modified in five patients in whom the angiography results showed a devascularized parathyroid gland, although the gland was evaluated visually as well vascularized. As suggested by other authors5,25, visual evaluation of the parathyroid gland is not a reliable predictor of good postoperative parathyroid function after thyroid surgery.
Over the long term, definitive hypoparathyroidism may lead to cerebral, vascular, ocular and renal damage, and to a significant reduction in quality of life26–29. Knowing the anatomical position and vascular supply of the parathyroid glands is essential to avoid hypoparathyroidism after thyroid surgery30,31. According to a study of 100 cadaveric thyroid glands32, 38.2 per cent of the parathyroid vessels were considered at risk of damage during standard thyroidectomy; the authors reported that the four parathyroid glands were at risk in 5 per cent of the subjects. These results32 provide an anatomical explanation for the consistent reporting of a small proportion of patients with definitive hypoparathyroidism in most registry-based or multicentre studies1,33,34. Hypothetically, ICG angiography could identify the vascular supply of parathyroid glands at risk of damage during dissection.
This study has shown that intraoperative angiography with ICG is simple and reproducible. The cost of ICG is €66.50 per 25-mg vial of the powder. Although the laparoscopic imaging camera system is currently expensive, it produces high-quality images and can also be used in other surgical procedures, making the equipment more cost-effective.
One of the difficulties of this technique is the identification of parathyroid glands with high accuracy. It has been shown35 that even the most experienced thyroid surgeons can misinterpret other anatomical structures, such as thyroid and thymus nodules or lymph nodes, as a parathyroid gland. If good vascularization of non-parathyroid tissue were demonstrated by ICG angiography, this would lead to a false reassurance that the patient will not develop hypoparathyroidism. The magnitude of this problem remains to be elucidated.
One limitation of the present study is that all four parathyroid glands were not identified in all patients, which is not unusual during standard thyroidectomy. It is therefore inherent that, in the group of six patients with no well vascularized parathyroid gland at ICG angiography, vascularization of the unidentified parathyroid glands was not known. A further limitation is the definition of visual and ICG scores. Most thyroid surgeons rely on visual inspection of a parathyroid gland to decide whether it is well vascularized, or whether it should be autotransplanted. At the University Hospitals of Geneva, a three-grade scoring system was used as, with the fluorescence equipment used during the present study, it was not possible to quantify the intensity of fluorescence. Vascularization was evaluated by differentiating shades of grey and the increased fluorescence of the parathyroid gland compared with that of surrounding vessels and tissues during ICG angiography13.
Within the context of these limitations, this pilot study demonstrated that when at least one parathyroid gland was well vascularized after thyroidectomy, the PTH level on POD 1 was within the normal range in all patients. These findings suggest an excellent correlation between parathyroid perfusion and postoperative parathyroid function. ICG angiography could be a tool for assessing the function of parathyroid glands following thyroid resection in real time.
Disclosure
The authors declare no conflict of interest.
References
Author notes
Presented to the Annual Meeting of the American Association of Endocrine Surgeons, Nashville, Tennessee, USA, May 2015, and to the Swiss Surgical Congress, Berne, Switzerland, June 2015



