-
PDF
- Split View
-
Views
-
Cite
Cite
M Shihabul Hassan, Sisith Ariyaratne, Christine Azzopardi, Karthikeyan P Iyengar, Arthur Mark Davies, Rajesh Botchu, The clinical significance of indeterminate pulmonary nodules in patients with primary bone sarcoma: a systematic review, British Journal of Radiology, Volume 97, Issue 1156, April 2024, Pages 747–756, https://doi.org/10.1093/bjr/tqae040
Close - Share Icon Share
Abstract
To report the incidence of indeterminate pulmonary nodules (IPN) and the rate of progression of IPNs to metastasis in patients with primary bone cancers. We also aimed to evaluate clinical or radiological parameters that may identify IPNs more likely to progress to metastatic disease and their effect on overall or event-free survival in patients with primary bone sarcoma.
A systematic search of the electronic databases Medline, Embase, and Cochrane Library was undertaken for eligible articles on IPNs in patients with primary bone sarcomas, published in the English language from inception of the databases to 2023. The Newcastle-Ottawa Quality Assessment Form for Cohort Studies was utilized to evaluate risk of bias in included studies.
Six studies, involving 1667 patients, were included in this systematic review. Pooled quantitative analysis found the rate of incidence of IPN to be 18.1% (302 out of 1667) and the rate of progression to metastasis to be 45.0% (136 out of 302). Nodule size (more than 5 mm diameter), number (more than or equal to 4), distribution (bilaterally distributed), incomplete calcification, and lobulated margins were associated with an increased likelihood of IPNs progressing to metastasis, however, their impact on overall or event-free survival remains unclear.
The risk of IPNs progressing to metastasis in patients with primary bone sarcoma is non-negligible. Large IPNs have a high risk to be an actual metastasis. We suggest that IPNs in these patients be followed up for a minimum of 2 years with CT imaging at 3, 6, and 12 month intervals, particularly for nodules measuring >5 mm in average diameter.
This is the first systematic review on IPNs in patients with primary bone sarcomas only and proposes viable management strategies for such patients.
Introduction
Primary bone sarcomas, including osteosarcoma, chondrosarcoma, and Ewing sarcoma, represent a group of rare but aggressive malignancies that are associated with significant morbidity and mortality.1 The lungs are the most common site of distant metastasis for these cancers and the presence of pulmonary metastases in patients serves as a negative prognostic factor.2,3 The 5-year overall survival rate for patients with pulmonary metastasis from osteosarcoma or Ewing sarcoma is around 30% and 40%, respectively, compared to 70% in those with non-metastatic disease.4,5 Thus, patients with primary bone sarcomas must undergo CT of the thorax to detect pulmonary metastases as part of routine staging.2 However, it is widely accepted that not all pulmonary nodules detected on CT imaging in patients with sarcoma represent metastatic lesions.6,7 Especially with the recent rise in use of thin-slice high resolution CT, sensitivity has dramatically increased for detection of small pulmonary nodules measuring less than 10 mm in diameter which cannot be accurately identified as benign or malignant on CT.8,9 Found in over 30% of patients,10 these nodules termed indeterminate pulmonary nodules (IPN), pose clinical dilemmas due to uncertainty over their clinical significance. They can represent a host of aetiologies including benign causes such as vascular malformations, granulomatosis, focal scars, infection or inflammation, and intrapulmonary lymph nodes or malignant causes such as tumour metastases.9,11 In patients with primary bone sarcoma, the inability to accurately identify IPNs as benign or malignant casts uncertainty over whether to classify their disease as metastatic or non-metastatic. This has significant implications on the management of the primary tumour, follow-up of IPNs and prognosis counselling as treatment decisions have to be tailored to patients’ prognosis and extent of disease.12,13
Currently, guidance on the follow up of IPNs in patients with primary bone sarcomas is also limited. The British Thoracic Society Guidelines for the Investigation and Management of Pulmonary Nodules and Fleischner Society recommendations do not apply to patients with a primary cancer.14,15 Furthermore, the Fleischner Society recommendations do not apply to patients younger than 35 years old,15 while the two commonest primary bone cancers, osteosarcoma and Ewing sarcoma, frequently occur between 15 and 20 years of age.15–17 Some studies have found that multiple, mineralized, round nodules larger than 5 mm wide have a higher likelihood of being malignant,18–20 however, others have found no such correlations.21 The challenges in managing IPNs in patients with bone malignancy and its’ treatment implications on the primary tumour warrant a thorough review of past literature on the topic. Although several articles have addressed the issue, they have either focused on patients with malignancies other than primary bone cancers11 or have grouped primary bone cancers with other sarcomas such as soft tissue sarcomas.22 Therefore, this systematic review has been undertaken to fulfil the following aims.
Aims/objectives
The objective of this systematic review of past literature is to answer the following questions: (1) What is the rate of incidence of IPNs and the rate of progression of IPNs to metastasis in patients with primary bone cancers? (2) What is the effect of IPNs on overall or event-free survival in patients with primary bone sarcomas? (3) Which clinical or radiological parameters exist that can identify IPNs that are more likely to be malignant or progress to metastasis?
Methods
Literature search
This systematic review was written according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.22 It was registered on PROSPERO, an international register of systematic reviews, and can be found using the registration number CRD42023461327. Search terms, outlined in Table 1, were used to search the electronic databases Medline, Embase and Cochrane Library for eligible articles published in the English language from inception of the databases (1946, 1974 and 1993) through to July 30, 2023. To maximize search output, both key-words and Medical Subject Headings (MeSH) were utilized. At the search screen, a preliminary title screening was performed to remove duplicates, results that were clearly outside the scope of the review question, and articles where the full text could not be accessed. The remaining full text articles were screened thoroughly and assessed against the inclusion/exclusion criteria detailed below. A manual cross-reference search was conducted from reference lists of included studies to identify articles not found in the original search.
| Database . | Search terms . | Results . |
|---|---|---|
| Embase | 1 (pulmonary nodule* or lung nodule*).mp | 34 685 |
| 2 lung nodule/ | 28 106 | |
| 3 indeterminate* pulmonary nodule*.mp. | 471 | |
| 4 indeterminate* lung nodule*.mp. | 141 | |
| 5 1 or 2 or 3 or 4 | 34 685 | |
| 6 Bone Neoplasms.mp. or bone tumor/ | 26 850 | |
| 7 ewing sarcoma.mp. or Ewing sarcoma/ | 19 198 | |
| 8 chondrosarcoma/or chondrosarcoma.mp. | 15 105 | |
| 9 osteosarcoma/or osteosarcoma.mp. | 52 690 | |
| 10 (bone cancer or bone malignancy).mp. | 9193 | |
| 11 6 or 7 or 8 or 9 or 10 | 101 429 | |
| 12 5 and 11 | 677 | |
| Medline | 1 Multiple Pulmonary Nodules/or Solitary Pulmonary Nodule/ | 5980 |
| 2 indeterminate* pulmonary nodule*.mp. | 267 | |
| 3 indeterminate* lung nodule*.mp. | 70 | |
| 4 lung nodules*.mp. | 3753 | |
| 5 1 or 2 or 3 or 4 | 8888 | |
| 6 Bone Neoplasms/ | 69 623 | |
| 7 bone neoplasm*.mp. | 70 149 | |
| 8 ewing sarcoma.mp. or Sarcoma, Ewing/ | 9772 | |
| 9 Chondrosarcoma, Clear Cell/or chondrosarcoma.mp. or Chondrosarcoma/or Chondrosarcoma, Mesenchymal/ | 10 514 | |
| 10 osteosarcoma.mp. or Osteosarcoma, Juxtacortical/or Osteosarcoma/ | 36 354 | |
| 11 bone tumour.mp. | 1126 | |
| 12 bone cancer.mp. | 2228 | |
| 13 bone malignancy.mp. | 554 | |
| 14 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 | 101 159 | |
| 15 5 and 14 | 132 | |
| Cochrane | 1 MeSH descriptor: [Multiple Pulmonary Nodules] | 94 |
| 2 MeSH descriptor: [Solitary Pulmonary Nodule] | 121 | |
| 3 (Indeterminate* NEXT pulmonary nodules*) | 17 | |
| 4 (Indeterminate* NEXT lung nodules*) | 6 | |
| 5 (lung nodules*) | 717 | |
| 6 (pulmonary nodules) | 509 | |
| 7 #1 OR #2 OR #3 OR #4 OR #5 OR #6 | 800 | |
| 8 MeSH descriptor: [Bone Neoplasms] | 1591 | |
| 9 MeSH descriptor: [Sarcoma, Ewing] | 126 | |
| 10 MeSH descriptor: [Chondrosarcoma] | 46 | |
| 11 MeSH descriptor: [Osteosarcoma] | 404 | |
| 12 MeSH descriptor: [Bone Neoplasms] | 1591 | |
| 13 (Bone neoplasm* OR bone malignanc* OR bone cancer* OR bone tumour*) | 15 365 | |
| 14 (Chondrosarcoma* OR Ewing Sarcoma* OR Osteosarcoma*) | 994 | |
| 15 #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 | 16 117 | |
| 16 #7 AND #15 | 20 |
| Database . | Search terms . | Results . |
|---|---|---|
| Embase | 1 (pulmonary nodule* or lung nodule*).mp | 34 685 |
| 2 lung nodule/ | 28 106 | |
| 3 indeterminate* pulmonary nodule*.mp. | 471 | |
| 4 indeterminate* lung nodule*.mp. | 141 | |
| 5 1 or 2 or 3 or 4 | 34 685 | |
| 6 Bone Neoplasms.mp. or bone tumor/ | 26 850 | |
| 7 ewing sarcoma.mp. or Ewing sarcoma/ | 19 198 | |
| 8 chondrosarcoma/or chondrosarcoma.mp. | 15 105 | |
| 9 osteosarcoma/or osteosarcoma.mp. | 52 690 | |
| 10 (bone cancer or bone malignancy).mp. | 9193 | |
| 11 6 or 7 or 8 or 9 or 10 | 101 429 | |
| 12 5 and 11 | 677 | |
| Medline | 1 Multiple Pulmonary Nodules/or Solitary Pulmonary Nodule/ | 5980 |
| 2 indeterminate* pulmonary nodule*.mp. | 267 | |
| 3 indeterminate* lung nodule*.mp. | 70 | |
| 4 lung nodules*.mp. | 3753 | |
| 5 1 or 2 or 3 or 4 | 8888 | |
| 6 Bone Neoplasms/ | 69 623 | |
| 7 bone neoplasm*.mp. | 70 149 | |
| 8 ewing sarcoma.mp. or Sarcoma, Ewing/ | 9772 | |
| 9 Chondrosarcoma, Clear Cell/or chondrosarcoma.mp. or Chondrosarcoma/or Chondrosarcoma, Mesenchymal/ | 10 514 | |
| 10 osteosarcoma.mp. or Osteosarcoma, Juxtacortical/or Osteosarcoma/ | 36 354 | |
| 11 bone tumour.mp. | 1126 | |
| 12 bone cancer.mp. | 2228 | |
| 13 bone malignancy.mp. | 554 | |
| 14 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 | 101 159 | |
| 15 5 and 14 | 132 | |
| Cochrane | 1 MeSH descriptor: [Multiple Pulmonary Nodules] | 94 |
| 2 MeSH descriptor: [Solitary Pulmonary Nodule] | 121 | |
| 3 (Indeterminate* NEXT pulmonary nodules*) | 17 | |
| 4 (Indeterminate* NEXT lung nodules*) | 6 | |
| 5 (lung nodules*) | 717 | |
| 6 (pulmonary nodules) | 509 | |
| 7 #1 OR #2 OR #3 OR #4 OR #5 OR #6 | 800 | |
| 8 MeSH descriptor: [Bone Neoplasms] | 1591 | |
| 9 MeSH descriptor: [Sarcoma, Ewing] | 126 | |
| 10 MeSH descriptor: [Chondrosarcoma] | 46 | |
| 11 MeSH descriptor: [Osteosarcoma] | 404 | |
| 12 MeSH descriptor: [Bone Neoplasms] | 1591 | |
| 13 (Bone neoplasm* OR bone malignanc* OR bone cancer* OR bone tumour*) | 15 365 | |
| 14 (Chondrosarcoma* OR Ewing Sarcoma* OR Osteosarcoma*) | 994 | |
| 15 #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 | 16 117 | |
| 16 #7 AND #15 | 20 |
| Database . | Search terms . | Results . |
|---|---|---|
| Embase | 1 (pulmonary nodule* or lung nodule*).mp | 34 685 |
| 2 lung nodule/ | 28 106 | |
| 3 indeterminate* pulmonary nodule*.mp. | 471 | |
| 4 indeterminate* lung nodule*.mp. | 141 | |
| 5 1 or 2 or 3 or 4 | 34 685 | |
| 6 Bone Neoplasms.mp. or bone tumor/ | 26 850 | |
| 7 ewing sarcoma.mp. or Ewing sarcoma/ | 19 198 | |
| 8 chondrosarcoma/or chondrosarcoma.mp. | 15 105 | |
| 9 osteosarcoma/or osteosarcoma.mp. | 52 690 | |
| 10 (bone cancer or bone malignancy).mp. | 9193 | |
| 11 6 or 7 or 8 or 9 or 10 | 101 429 | |
| 12 5 and 11 | 677 | |
| Medline | 1 Multiple Pulmonary Nodules/or Solitary Pulmonary Nodule/ | 5980 |
| 2 indeterminate* pulmonary nodule*.mp. | 267 | |
| 3 indeterminate* lung nodule*.mp. | 70 | |
| 4 lung nodules*.mp. | 3753 | |
| 5 1 or 2 or 3 or 4 | 8888 | |
| 6 Bone Neoplasms/ | 69 623 | |
| 7 bone neoplasm*.mp. | 70 149 | |
| 8 ewing sarcoma.mp. or Sarcoma, Ewing/ | 9772 | |
| 9 Chondrosarcoma, Clear Cell/or chondrosarcoma.mp. or Chondrosarcoma/or Chondrosarcoma, Mesenchymal/ | 10 514 | |
| 10 osteosarcoma.mp. or Osteosarcoma, Juxtacortical/or Osteosarcoma/ | 36 354 | |
| 11 bone tumour.mp. | 1126 | |
| 12 bone cancer.mp. | 2228 | |
| 13 bone malignancy.mp. | 554 | |
| 14 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 | 101 159 | |
| 15 5 and 14 | 132 | |
| Cochrane | 1 MeSH descriptor: [Multiple Pulmonary Nodules] | 94 |
| 2 MeSH descriptor: [Solitary Pulmonary Nodule] | 121 | |
| 3 (Indeterminate* NEXT pulmonary nodules*) | 17 | |
| 4 (Indeterminate* NEXT lung nodules*) | 6 | |
| 5 (lung nodules*) | 717 | |
| 6 (pulmonary nodules) | 509 | |
| 7 #1 OR #2 OR #3 OR #4 OR #5 OR #6 | 800 | |
| 8 MeSH descriptor: [Bone Neoplasms] | 1591 | |
| 9 MeSH descriptor: [Sarcoma, Ewing] | 126 | |
| 10 MeSH descriptor: [Chondrosarcoma] | 46 | |
| 11 MeSH descriptor: [Osteosarcoma] | 404 | |
| 12 MeSH descriptor: [Bone Neoplasms] | 1591 | |
| 13 (Bone neoplasm* OR bone malignanc* OR bone cancer* OR bone tumour*) | 15 365 | |
| 14 (Chondrosarcoma* OR Ewing Sarcoma* OR Osteosarcoma*) | 994 | |
| 15 #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 | 16 117 | |
| 16 #7 AND #15 | 20 |
| Database . | Search terms . | Results . |
|---|---|---|
| Embase | 1 (pulmonary nodule* or lung nodule*).mp | 34 685 |
| 2 lung nodule/ | 28 106 | |
| 3 indeterminate* pulmonary nodule*.mp. | 471 | |
| 4 indeterminate* lung nodule*.mp. | 141 | |
| 5 1 or 2 or 3 or 4 | 34 685 | |
| 6 Bone Neoplasms.mp. or bone tumor/ | 26 850 | |
| 7 ewing sarcoma.mp. or Ewing sarcoma/ | 19 198 | |
| 8 chondrosarcoma/or chondrosarcoma.mp. | 15 105 | |
| 9 osteosarcoma/or osteosarcoma.mp. | 52 690 | |
| 10 (bone cancer or bone malignancy).mp. | 9193 | |
| 11 6 or 7 or 8 or 9 or 10 | 101 429 | |
| 12 5 and 11 | 677 | |
| Medline | 1 Multiple Pulmonary Nodules/or Solitary Pulmonary Nodule/ | 5980 |
| 2 indeterminate* pulmonary nodule*.mp. | 267 | |
| 3 indeterminate* lung nodule*.mp. | 70 | |
| 4 lung nodules*.mp. | 3753 | |
| 5 1 or 2 or 3 or 4 | 8888 | |
| 6 Bone Neoplasms/ | 69 623 | |
| 7 bone neoplasm*.mp. | 70 149 | |
| 8 ewing sarcoma.mp. or Sarcoma, Ewing/ | 9772 | |
| 9 Chondrosarcoma, Clear Cell/or chondrosarcoma.mp. or Chondrosarcoma/or Chondrosarcoma, Mesenchymal/ | 10 514 | |
| 10 osteosarcoma.mp. or Osteosarcoma, Juxtacortical/or Osteosarcoma/ | 36 354 | |
| 11 bone tumour.mp. | 1126 | |
| 12 bone cancer.mp. | 2228 | |
| 13 bone malignancy.mp. | 554 | |
| 14 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 | 101 159 | |
| 15 5 and 14 | 132 | |
| Cochrane | 1 MeSH descriptor: [Multiple Pulmonary Nodules] | 94 |
| 2 MeSH descriptor: [Solitary Pulmonary Nodule] | 121 | |
| 3 (Indeterminate* NEXT pulmonary nodules*) | 17 | |
| 4 (Indeterminate* NEXT lung nodules*) | 6 | |
| 5 (lung nodules*) | 717 | |
| 6 (pulmonary nodules) | 509 | |
| 7 #1 OR #2 OR #3 OR #4 OR #5 OR #6 | 800 | |
| 8 MeSH descriptor: [Bone Neoplasms] | 1591 | |
| 9 MeSH descriptor: [Sarcoma, Ewing] | 126 | |
| 10 MeSH descriptor: [Chondrosarcoma] | 46 | |
| 11 MeSH descriptor: [Osteosarcoma] | 404 | |
| 12 MeSH descriptor: [Bone Neoplasms] | 1591 | |
| 13 (Bone neoplasm* OR bone malignanc* OR bone cancer* OR bone tumour*) | 15 365 | |
| 14 (Chondrosarcoma* OR Ewing Sarcoma* OR Osteosarcoma*) | 994 | |
| 15 #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 | 16 117 | |
| 16 #7 AND #15 | 20 |
Inclusion/exclusion criteria
Randomized controlled trials and observational cohort studies which reported findings on IPNs in patients with osteosarcoma, chondrosarcoma, or Ewing sarcoma were considered for inclusion. Studies on patients with other malignancies including soft tissue sarcomas were excluded unless if they reported separate findings for primary bone sarcomas. Case reports, letters to the editor, and conference abstracts were also excluded. There were no restrictions placed on the definition of IPNs and the variations in definitions used in different studies are discussed later in this article.
Study outcomes, data extraction, and quality assessment
The primary outcome measures of interest in this review were the incidence of IPNs in patients with primary bone sarcomas and the rate of IPNs progressing to metastatic disease, diagnosed in follow-up. Secondary outcomes included differences in overall or event-free survival in patients with IPNs compared to those with normal staging CT and clinical or radiological parameters predicting IPN progression to metastasis.
A pre-established data extraction table was used to collate data on study characteristics, type of bone cancer studied, total number of patients, patients with IPNs, patients with pulmonary metastases at baseline, patients with IPNs progressing to metastasis, median time to metastasis, overall or event-free survival of different groups and clinical or radiological features predicting IPNs likely to be metastatic. The risk of bias present in studies was evaluated by one author using the Newcastle-Ottawa Quality Assessment Form for Cohort Studies.23 Studies were assigned a maximum total score of 9 across the domains of cohort selection, cohort comparability and assessment of outcomes. A higher score indicated a lower risk of bias.
Results
Study selection
The initial search resulted in 829 articles being identified, as depicted in Figure 1. Following removal of duplicates and articles not relevant to the review question, 52 full-text articles were reviewed and evaluated against the inclusion/exclusion criteria. Studies that pooled pulmonary nodules of various sizes together, without explicitly reporting findings on IPNs were excluded. Two studies on pulmonary micronodules measuring less than 5 mm in diameter, which initially appeared to meet the inclusion criteria, were excluded as they pooled diagnostically challenging or “doubtful” nodules with clearly benign CT findings. Several articles which grouped primary bone cancers along with other malignancies such as soft tissue sarcomas were also excluded. A total of 6 articles met inclusion criteria,24–29 comprising of 4 studies on patients with osteosarcoma,24–27 1 on patients with chondrosarcoma28 and 1 on patients with Ewing sarcoma.29 A pooled quantitative analysis was conducted for IPN incidence, rate of IPN progression to metastasis, and the median time to metastasis. However, a meta-analysis was not possible due to high grade of heterogeneity between studies.
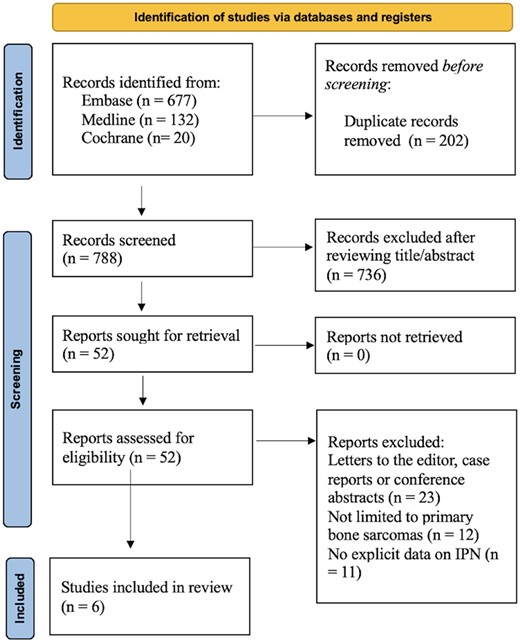
This PRISMA 2020 flow diagram details the full search strategy used to identify studies for this systematic review.
Study characteristics
All studies included were retrospective observational cohort studies and no randomized controlled trials were found. Study authors retrospectively classified patients with primary bone cancer who underwent routine staging CT into at least two groups—either having a normal CT scan or having IPN detected on CT imaging at baseline. Four studies also included a third group of patients having pulmonary metastases at baseline.24,25,28,29 The clinical course for each patient was studied to determine overall or event-free survival, with events being defined as local recurrence, lung metastasis, extrapulmonary metastasis, second primary malignancy or death. Patients’ follow-up CT scans or their CT reports were reviewed to identify IPN progression to metastasis. Only one study explicitly mentioned using Maximum Intensity Projection images and multiplanar formatting to detect IPNs.29 The reported median follow-up times ranged from 27 to 60 months.25–28
Quality assessment
The average total score assigned to the studies included in this review was 7.2 out of 9 and ranged from 6 to 8, indicating that the overall risk of bias was low. Cohorts were deemed to be only somewhat representative, rather than truly representative, as recruitment of participants from single institutions in most studies24,26–29 introduced selection bias. A few concerns arose regarding comparability of cohorts as differing treatments could not be controlled for. All patients were treated with standard chemotherapeutic regimens according to regional/national guidelines, but treatments differed beyond first line chemotherapy, and patients with metastases at baseline were treated non-operatively while those with IPN or normal CT may have received surgery. Furthermore, in most studies, study authors who reviewed CT scans were not blind to patients’ clinical outcomes.26–29 Scores for individual items for each study can be found in Table 2.
| Study . | Item and score . | |||||||
|---|---|---|---|---|---|---|---|---|
| Representativeness of the exposed cohort (1) . | Selection of the non-exposed cohort (1) . | Ascertainment of exposure (1) . | Demonstration that outcome of interest was not present at start of study (1) . | Comparability of cohorts on the basis of the design or analysis (2) . | Assessment of outcome (1) . | Was follow up long enough for outcomes to occur (1) . | Adequacy of follow up of cohorts (1) . | |
| Tsoi et al24 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Ghosh et al25 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 |
| Zhou et al26 | 1 | 1 | 1 | 0 | 2 | 1 | 1 | 1 |
| Seher et al27 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 |
| Tsoi et al28 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 |
| McLoughin et al29 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 |
| Study . | Item and score . | |||||||
|---|---|---|---|---|---|---|---|---|
| Representativeness of the exposed cohort (1) . | Selection of the non-exposed cohort (1) . | Ascertainment of exposure (1) . | Demonstration that outcome of interest was not present at start of study (1) . | Comparability of cohorts on the basis of the design or analysis (2) . | Assessment of outcome (1) . | Was follow up long enough for outcomes to occur (1) . | Adequacy of follow up of cohorts (1) . | |
| Tsoi et al24 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Ghosh et al25 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 |
| Zhou et al26 | 1 | 1 | 1 | 0 | 2 | 1 | 1 | 1 |
| Seher et al27 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 |
| Tsoi et al28 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 |
| McLoughin et al29 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 |
| Study . | Item and score . | |||||||
|---|---|---|---|---|---|---|---|---|
| Representativeness of the exposed cohort (1) . | Selection of the non-exposed cohort (1) . | Ascertainment of exposure (1) . | Demonstration that outcome of interest was not present at start of study (1) . | Comparability of cohorts on the basis of the design or analysis (2) . | Assessment of outcome (1) . | Was follow up long enough for outcomes to occur (1) . | Adequacy of follow up of cohorts (1) . | |
| Tsoi et al24 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Ghosh et al25 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 |
| Zhou et al26 | 1 | 1 | 1 | 0 | 2 | 1 | 1 | 1 |
| Seher et al27 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 |
| Tsoi et al28 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 |
| McLoughin et al29 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 |
| Study . | Item and score . | |||||||
|---|---|---|---|---|---|---|---|---|
| Representativeness of the exposed cohort (1) . | Selection of the non-exposed cohort (1) . | Ascertainment of exposure (1) . | Demonstration that outcome of interest was not present at start of study (1) . | Comparability of cohorts on the basis of the design or analysis (2) . | Assessment of outcome (1) . | Was follow up long enough for outcomes to occur (1) . | Adequacy of follow up of cohorts (1) . | |
| Tsoi et al24 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Ghosh et al25 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 |
| Zhou et al26 | 1 | 1 | 1 | 0 | 2 | 1 | 1 | 1 |
| Seher et al27 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 |
| Tsoi et al28 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 |
| McLoughin et al29 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 |
IPN definitions
Two studies defined IPN as lung parenchymal nodules measuring less than 10 mm in diameter, with or without calcification, which could not be identified as benign or malignant by the reporting radiologist.24,28 Ghosh et al considered only non-calcified indeterminate lung nodules less than 10 mm in diameter as IPNs.25 Tsoi et al classified lung nodules with a maximum of 9 mm diameter as IPNs.29 Zhou et al defined IPNs as (1) less than five pulmonary nodules measuring less than 5 mm or (2) a single nodule measuring between 5 and 10 mm.26
Four studies defined IPN progression to metastasis as an increase in nodule size on subsequent imaging,24–26,28 with two studies specifying an increase by at least 25% for the nodule to be considered metastatic.24,28 At baseline, pulmonary metastasis was defined as the presence of pulmonary nodules greater than 10 mm in diameter in three studies.24,25,28 Zhou et al defined pulmonary metastasis as (1) one or more pulmonary nodule measuring 10 mm or wider, (2) two or more well-defined pulmonary nodules measuring 5-10 mm in diameter or (3) five or more well defined nodules measuring less than 5 mm in diameter.26 Seher et al defined pulmonary metastasis as a single nodule measuring more than 10 mm in diameter or three or more nodules measuring 5-9 mm in diameter and classified any nodule not meeting these criteria as IPNs.27
IPN incidence
A pooled quantitative analysis of all included articles was undertaken as part of this systematic review, which identified 1667 patients with primary bone sarcomas, comprising of 1054 patients with osteosarcoma, 173 patients with Ewing sarcoma and 440 patients with chondrosarcoma. IPNs were identified on staging CT in 302 out of these 1667 patients, resulting in a pooled IPN incidence of 18.1%. Sub-group analyses revealed an incidence rate of 22.3% (235 out of 1054) in patients with osteosarcoma,24–27 8.7% (15 out of 173) in patients with Ewing sarcoma29 and 12% (52 out of 440) in patients with chondrosarcoma.28 Incidence rates for IPNs reported by individual studies ranged between 8.7% and 29% across all types of primary bone sarcomas (Table 3).
| Study author(s) . | Study type . | Type of primary bone sarcoma . | Total number of patients . | Patients with IPN (%) . | Patients with normal staging CT . | Patients with pulmonary metastasis at baseline . | Patients with IPN progression to metastasis (%) . | Median time to metastasis in months (range) . | CT slice thickness used to detect IPN (mm) . |
|---|---|---|---|---|---|---|---|---|---|
| Tsoi et al24 | Retrospective observational cohort study | Osteosarcoma | 431 | 68 (16) | 302 | 61 | 50 (74) | 5.3 (1.8-56.9) | NA |
| Ghosh et al25 | Retrospective cohort study | Osteosarcoma | 104 | 30 (29) | 55 | 19 | 6 (20) | 6.2 (2.1-68.4) | 3.0 |
| Zhou et al26 | Retrospective observational cohort study | Osteosarcoma | 364 | 88 (24) | 276 | 0 | 41 (47) | NA | NA |
| Seher et al27 | Retrospective observational cohort study | Osteosarcoma | 155 | 49 (32) | 106 | 0 | 21 (49) | 14 (NA-45) | 0.5 |
| McLoughin et al28a | Retrospective observational cohort study | Chondrosarcoma | 440 | 52 (12) | 362 | 26 | 12 (23) | NA | 1.0 and 4.0 |
| Tsoi et al29 | Retrospective observational cohort study | Ewing sarcoma | 173 | 15 (8.7) | 108 | 50 | 6 (40) | 9.6 (1.2-15.2) | 1.0 |
| Study author(s) . | Study type . | Type of primary bone sarcoma . | Total number of patients . | Patients with IPN (%) . | Patients with normal staging CT . | Patients with pulmonary metastasis at baseline . | Patients with IPN progression to metastasis (%) . | Median time to metastasis in months (range) . | CT slice thickness used to detect IPN (mm) . |
|---|---|---|---|---|---|---|---|---|---|
| Tsoi et al24 | Retrospective observational cohort study | Osteosarcoma | 431 | 68 (16) | 302 | 61 | 50 (74) | 5.3 (1.8-56.9) | NA |
| Ghosh et al25 | Retrospective cohort study | Osteosarcoma | 104 | 30 (29) | 55 | 19 | 6 (20) | 6.2 (2.1-68.4) | 3.0 |
| Zhou et al26 | Retrospective observational cohort study | Osteosarcoma | 364 | 88 (24) | 276 | 0 | 41 (47) | NA | NA |
| Seher et al27 | Retrospective observational cohort study | Osteosarcoma | 155 | 49 (32) | 106 | 0 | 21 (49) | 14 (NA-45) | 0.5 |
| McLoughin et al28a | Retrospective observational cohort study | Chondrosarcoma | 440 | 52 (12) | 362 | 26 | 12 (23) | NA | 1.0 and 4.0 |
| Tsoi et al29 | Retrospective observational cohort study | Ewing sarcoma | 173 | 15 (8.7) | 108 | 50 | 6 (40) | 9.6 (1.2-15.2) | 1.0 |
NA: Not Available.
McLoughin et al analysed CT reports rather than images in detecting IPNs.
| Study author(s) . | Study type . | Type of primary bone sarcoma . | Total number of patients . | Patients with IPN (%) . | Patients with normal staging CT . | Patients with pulmonary metastasis at baseline . | Patients with IPN progression to metastasis (%) . | Median time to metastasis in months (range) . | CT slice thickness used to detect IPN (mm) . |
|---|---|---|---|---|---|---|---|---|---|
| Tsoi et al24 | Retrospective observational cohort study | Osteosarcoma | 431 | 68 (16) | 302 | 61 | 50 (74) | 5.3 (1.8-56.9) | NA |
| Ghosh et al25 | Retrospective cohort study | Osteosarcoma | 104 | 30 (29) | 55 | 19 | 6 (20) | 6.2 (2.1-68.4) | 3.0 |
| Zhou et al26 | Retrospective observational cohort study | Osteosarcoma | 364 | 88 (24) | 276 | 0 | 41 (47) | NA | NA |
| Seher et al27 | Retrospective observational cohort study | Osteosarcoma | 155 | 49 (32) | 106 | 0 | 21 (49) | 14 (NA-45) | 0.5 |
| McLoughin et al28a | Retrospective observational cohort study | Chondrosarcoma | 440 | 52 (12) | 362 | 26 | 12 (23) | NA | 1.0 and 4.0 |
| Tsoi et al29 | Retrospective observational cohort study | Ewing sarcoma | 173 | 15 (8.7) | 108 | 50 | 6 (40) | 9.6 (1.2-15.2) | 1.0 |
| Study author(s) . | Study type . | Type of primary bone sarcoma . | Total number of patients . | Patients with IPN (%) . | Patients with normal staging CT . | Patients with pulmonary metastasis at baseline . | Patients with IPN progression to metastasis (%) . | Median time to metastasis in months (range) . | CT slice thickness used to detect IPN (mm) . |
|---|---|---|---|---|---|---|---|---|---|
| Tsoi et al24 | Retrospective observational cohort study | Osteosarcoma | 431 | 68 (16) | 302 | 61 | 50 (74) | 5.3 (1.8-56.9) | NA |
| Ghosh et al25 | Retrospective cohort study | Osteosarcoma | 104 | 30 (29) | 55 | 19 | 6 (20) | 6.2 (2.1-68.4) | 3.0 |
| Zhou et al26 | Retrospective observational cohort study | Osteosarcoma | 364 | 88 (24) | 276 | 0 | 41 (47) | NA | NA |
| Seher et al27 | Retrospective observational cohort study | Osteosarcoma | 155 | 49 (32) | 106 | 0 | 21 (49) | 14 (NA-45) | 0.5 |
| McLoughin et al28a | Retrospective observational cohort study | Chondrosarcoma | 440 | 52 (12) | 362 | 26 | 12 (23) | NA | 1.0 and 4.0 |
| Tsoi et al29 | Retrospective observational cohort study | Ewing sarcoma | 173 | 15 (8.7) | 108 | 50 | 6 (40) | 9.6 (1.2-15.2) | 1.0 |
NA: Not Available.
McLoughin et al analysed CT reports rather than images in detecting IPNs.
IPN risk of progression to metastasis
Our pooled analysis also revealed that IPN progression to pulmonary metastasis was seen in 45.0% (136 out of 302) of patients with IPNs and ranged from 20% to 74% in individual studies. Despite most included studies considering lung nodules measuring any size less than 10 mm as IPNs, a heightened level of clinical suspicion for metastasis emerges with IPNs that are bigger in size. Two studies explicitly stated the number of IPNs measuring ≤ 5 mm in diameter progressing to metastasis compared to the number of IPNs measuring ≥ 6 mm progressing to metastasis.25,29 A sub-group analysis was conducted and their pooled results revealed only 17.1% (6 out of 35) of IPNs measuring ≤ 5 mm progressed to metastasis whereas 60% (6 out of 10) IPNs 6 mm or more in diameter progressed to metastasis. Sub-group analyses revealed IPNs progressed to metastatic disease in 50.2% (118 out of 235) of patients with osteosarcoma,24–27 40% (6 out of 15) of patients with Ewing sarcoma29 and 23% (12 out of 52) of patients with chondrosarcoma.29 Two studies compared the risk of progression to metastasis between patients with IPNs and those with a normal staging CT, concluding that patients with IPNs were significantly more likely to develop metastatic disease.24,27 Among them, Tsoi et al24 reported that presence of IPNs was associated with higher incidence of pulmonary metastases (HR 3.6 [95% CI, 2.5-5.2]; P < .001) and Seher et al27 reported that patients with IPNs at baseline had a relative risk of 1.6 of developing pulmonary metastases compared to those without IPNs [95% CI, 1.031-2.553].
Median time for IPN progression to metastasis
Four studies reported the median time from baseline staging to metastasis development which was 7.9 months and ranged from 1.2 to 68.4 months.24,25,27,29 In patients with osteosarcoma, this median time (range) was 6.2 months (1.8-56.9) whereas it was 9.6 months (1.2-15.2) in patients with Ewing sarcoma. In their study on patients with chondrosarcoma, McLoughin et al found that the mean time for metastasis occurrence in patients with high grade (2/3 or dedifferentiated) chondrosarcoma and IPN at baseline was 30.5 months.28 Ghosh et al found that 80% of patients with IPN progression to metastatic disease were diagnosed within 1 year,25 while Tsoi et al reported that disease progression was seen in 87% of these patients within 2 years.24
Effect of IPN on overall or event free survival
Five out of the six included studies addressed the effect of IPNs on overall or event free survival in patients.24–27,29 Three of these found no significant difference between overall or event free survival in osteosarcoma or Ewing sarcoma patients with IPNs compared to those with a normal staging CT.25,26,29 The studies include Zhou et al who demonstrated that overall survival was comparable in both groups (HR 1.687 [0.910-3.125] P-value .097),26 and Tsoi et al who found no difference in 2-year overall survival (73.3% [95% CI, 43.6-89] in Ewing sarcoma patients with IPNs compared to 89.4% [95% CI, 81.6-94] (P = .34) in patients with no nodules.29 They concluded that IPNs behaved more like benign nodules than metastatic disease in these patients. In their study on patients with chondrosarcoma, McLaughin et al did not address survival outcomes in patients with IPN.28
However, in a study on 431 osteosarcoma patients, Tsoi et al found overall survival for patients with IPN to be significantly worse than those with a normal staging CT (HR 1.9 [95% CI, 1.3-2.8], P < .001).24 Patients with IPNs were also found to have worse metastasis free survival than those with normal staging CT (HR 3.6 [95% CI, 2.5-5.2]; P < .001). At the 2-year point, patients with IPNs had a metastasis-free survival of 35% (95% CI, 24-46) while patients with no IPNs detected on staging CT had a metastasis-free survival of 77% (95% CI, 71-81; P < .001). In the study on paediatric patients with osteosarcoma by Seher et al, patients with IPNs were also found to have lower overall and event-free survival compared with those who had normal CT scans at baseline. However, the result was not statistically significant (P-value = .572 for Overall Survival and 0.397 for Event Free Survival).27
When comparing patients with IPNs to those with pulmonary metastasis at baseline, all three studies reporting this association found that overall survival for patients with IPNs were significantly better than for patients with lung metastases on presentation.24,25,29 In the study on osteosarcoma patients by Tsoi et al, two-year overall survival for patients with IPNs was 65% [95% CI, 52-75] compared to 45% [95% CI, 32-57] for patients with metastases (P = .001).24 In a separate study on patients with Ewing sarcoma, Tsoi et al found overall survival to be 73.3% [95% CI, 43.6-89] in the IPN group compared to 46.0% [95% CI, 31.9-59]; P < .0001 in the metastases group.29
Clinical and radiological factors predicting IPN progression to metastasis
In all studies included, staging CT images or reports were analysed to identify patient or nodule characteristics associated with increased risk of IPN progression to metastatic disease. In patients with osteosarcoma, Ghosh et al noted that the size of IPNs in half (3 out of 6) of the patients whose IPNs progressed to metastatic disease was more than 5 mm, whereas in 19 out of 21 patients with stable IPNs, the size of IPNs was less than 5 mm wide.25 Seher et al observed that having more than 3 nodules was associated with a 75% risk of IPNs progressing to pulmonary metastasis and that patients with bilateral nodules (n = 11) were more likely to develop metastatic disease (63.6% vs 36.8%) compared to those with unilateral nodules (n = 38) although the latter was not found to be a statistically significant result.27
In patients with Ewing sarcoma, Tsoi et al also found that 85.7% (6 out of 7) of patients with more four or more IPNs went on to develop metastasis. IPNs were also found bilaterally in all patients who developed metastatic disease.29
In patients with chondrosarcoma, McLoughin et al observed associations between nodule size, location, distribution, and calcification with risk of IPN progression. About 87% of IPNs (35 out of 40) less than 5 mm in diameter showed no progression on subsequent CT scanning, thus making them more likely to be benign than those measuring greater than 5 mm. Nodules with lobulate margins appeared only in patients with IPNs that progressed to metastasis. Completely calcified nodules less than 10 mm in diameter were more likely to be benign granulomas whereas those with partial or subtle calcification and greater than 10 mm diameter were likely to represent metastases (Figure 2). Bilateral nodules and nodules seen either centrally or both centrally and peripherally were also more likely to be metastatic.28
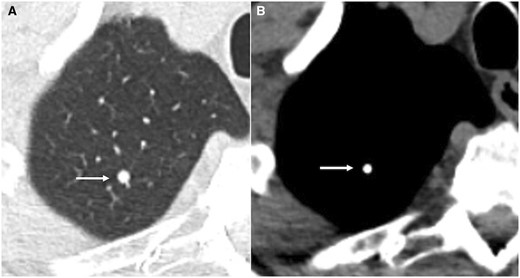
Axial CT lung window (A) and soft tissue window (B) showing calcified granuloma (arrow).
Patient and nodule characteristics associated with overall or event free survival
Five out of the six included studies analysed differences in overall or event free survival between patients, with IPNs, from different demographic groups and with different nodule characteristics.24–27,29 Only Zhou et al found that a greater number of paediatric patients, younger than 18 years old, with IPNs developed lung metastases and hence had significantly poorer event-free survival compared to adults with IPNs.26 Tsoi et al found that the presence of nodule mineralization was associated with improved overall survival (87% [95% CI, 39-98] versus 57% [95% CI, 43-69]; P = .008) but were unable to confirm this association through a multivariable analysis.24 Apart from these findings, no other study was able to determine any prognostic indicator for survival such as IPN number, size, site or shape or patient demographics.
Discussion
Recent advancements in CT technology and the rise in use of thin-slice CT have led to IPNs being detected with increasing frequency.30 The vast majority of these are found to be benign in patients without history of a primary cancer, but the same cannot be confidently said for patients with primary bone sarcoma—which frequently disseminates to the lungs. An accurate evaluation of the prognostic impact of IPN holds paramount importance as it can significantly influence treatment strategies for the primary tumour.
Our pooled analysis revealed that IPNs are detected in nearly 1 in 5 patients with primary bone cancer. The true incidence rate may have been even higher as some studies did not re-review imaging for patients with normal CT at baseline.24,29 Furthermore, a few studies either did not report the CT scan thickness applied,24,26 or used 3 mm CT slices25 which are associated with lower detection rates for small pulmonary nodules.8 At least one study only analysed CT reports, rather than images28—raising concerns about the potential under detection of IPNs and uncertainty over meaningful conclusions being drawn from textual reports alone. In a systematic review on IPNs in patients with colorectal cancer, van den Broek et al found that the incidence of IPNs was significantly higher in studies where CT images, rather than their reports, were re-evaluated (23% vs 7%, P < .0001).11 In our review, incidence of IPNs was also found to be higher in patients with osteosarcoma (22.3%) compared to those with Ewing sarcoma (8.7%) or chondrosarcoma (12%).
In prior literature, the incidence rate of IPNs has been reported as being between 21% and 45%9,10,21,31,32 and the rate of progression to metastasis was reported to be 31% and 28% in two articles.10,31 However, these studies pooled primary bone cancer patients with those having other sarcomas and used a broad range of definitions for pulmonary nodules, which makes it difficult to draw comparisons with our findings. Our systematic review isolated only patients with primary bone cancer which showed that the rate of IPN progression to metastasis was 45%, with IPNs progressing in 50% of patients with osteosarcoma. This could indicate that IPNs may be more likely to progress to metastases in patients with bone sarcomas, in particular—osteosarcoma, compared to other types of sarcomas.
We also reviewed clinical or radiological features of IPNs more likely to progress to metastases and found that nodule size (>5 mm diameter), number of nodules (≥4), nodule distribution (bilaterally distributed), incomplete calcification and lobulated margins were associated with an increased likelihood of IPNs progressing to metastasis (Figures 3-5). There is considerable overlap between our findings with those of other reviews. Saifuddin et al reviewed literature published from 2000 onwards to conclude that in patients with osteosarcoma, multiple nodules (especially more than 7), a diameter of 5 mm or more (90% specificity if more than 6 mm, 100% if more than 13 mm), partial nodule calcification and response to chemotherapy through change in number or size of nodules were likely to indicate metastatic lesions.33 For patients with chondrosarcoma, they identified high grade and nodule size greater than 10 mm to be associated with increased likelihood of metastasis (Table 4). Although Saifuddin et al did not investigate the association between these features and overall survival in patients, multiple studies included in our review did and were unable to find any prognostic indicator for survival among the various clinical or radiological features studied.
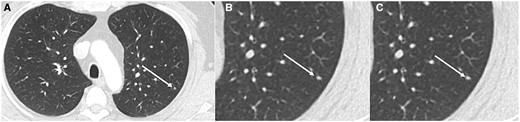
Axial CT (lung window) (A) and magnified view (B) in 19-year-old patient with Ewing sarcoma showing indeterminate lung nodule (arrow—3 mm) in left lower lobe (A, B) which was unchanged on 3 months follow up CT (C).
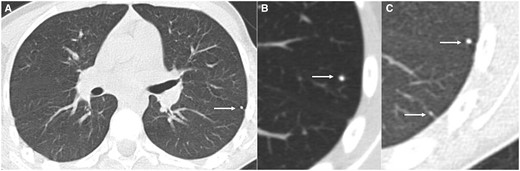
Axial CT (lung window) (A) and magnified view (B, C) in 20 year old patient with osteosarcoma showing multiple indeterminate lung nodules (arrow—4 and 2 mm) in left lower lobe.
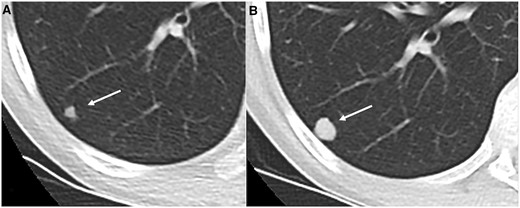
Axial CT (lung window) magnified view (A, B) in 19 year old patient with Ewing sarcoma showing indeterminate lung nodule (arrow—6 mm) in right lower lobe (A) which increased in size (14 mm) on 3 months follow up CT (B).
Types of primary bone sarcoma and corresponding clinical or radiological features indicative of IPN that are likely to progress to metastasis.
| Type of primary bone sarcoma . | Clinical or radiological features indicative of IPN likely to progress to metastasis . |
|---|---|
| Osteosarcoma |
|
| Ewing sarcoma |
|
| Chondrosarcoma |
|
| Type of primary bone sarcoma . | Clinical or radiological features indicative of IPN likely to progress to metastasis . |
|---|---|
| Osteosarcoma |
|
| Ewing sarcoma |
|
| Chondrosarcoma |
|
Types of primary bone sarcoma and corresponding clinical or radiological features indicative of IPN that are likely to progress to metastasis.
| Type of primary bone sarcoma . | Clinical or radiological features indicative of IPN likely to progress to metastasis . |
|---|---|
| Osteosarcoma |
|
| Ewing sarcoma |
|
| Chondrosarcoma |
|
| Type of primary bone sarcoma . | Clinical or radiological features indicative of IPN likely to progress to metastasis . |
|---|---|
| Osteosarcoma |
|
| Ewing sarcoma |
|
| Chondrosarcoma |
|
The prognostic impact of IPNs continues to remain unclear, owing to inconsistency in literature on the matter. While Tsoi et al found that patients with IPNs had significantly worse overall survival compared to those with normal CT, no other study in our review observed a statistically significant difference, despite patients with IPNs being significantly more likely to develop metastatic disease. Both groups of patients (those with IPNs and those with normal staging CT) also had significantly better overall survival than patients with pulmonary metastasis at baseline—indicating that in these patients, IPNs behaved more like localized than metastatic disease. Such patients could be ideal candidates for more aggressive treatment, however, there currently does not exist a validated risk-stratification tool to identify this sub-set of patients at baseline. Therefore, multi-centre studies with larger sample sizes and longer follow-up times are warranted to investigate true associations between IPN characteristics and survival.
At present, it is difficult to recommend stringent management guidelines for IPNs due to limited evidence. According to Tsoi et al, negative survival implications for patients with IPNs must be discussed with patients or their families, especially when considering treatment strategies with high risk of complications or long post-operative periods.24 Regarding the follow-up of IPNs, there is consensus among study authors to closely monitor IPNs through routine chest CT for a minimum of two years. This duration aligns well with our pooled analysis which revealed the median time for IPN progression to metastasis in the studies included to be 7.9 months. Although metastasis occurred even at 68 months from baseline staging in one case, 80% of patients with metastases from IPNs were diagnosed within a year in the study by Ghosh et al25 and disease progression was seen in 87% of patients with metastasis by two years in the study by Tsoi et al.24 Prospective studies are required to evaluate the natural history and progression of IPNs in primary bone cancer patients and accurately determine optimum follow-up times.
It also important to follow standardised technical parameters when assessing and following up IPNs. The Fleischner society recommends the scans be acquired with thin slices (ideally 1 mm) and on full inspiration.15 The measurements should be performed on lung windows and usually in the axial plane, although coronal and sagittal planes can be used if the greatest dimension lies in these planes. Nodules measuring 3-10 mm should be expressed as the average of the short-axis and long-axis diameters (measured on the same slice) and rounded to nearest whole millimetre. Nodules >10 mm should be described in both short and long axis measurements.15 We suggest using a similar approach to measuring IPNs in primary bone malignancies to ensure consistency of measurements. Additionally, the complete resection of lung nodules is another option which has been shown to benefit patients with bone sarcoma.34 However, intra-operative and post-operative complications are common in osteosarcoma patients undergoing lung surgery,35 which must be taken into account. Future studies are needed to evaluate the prognostic benefit of thoracotomy in patients with IPNs as well as identify clinically insignificant nodules which would not require surgery.9
There are several limitations to this systematic review and the studies included. Firstly, there is considerable heterogeneity between the studies, stemming from the broad range of definitions used for IPNs and pulmonary metastasis. In some studies, patients with IPNs may have also received different treatment from those with normal chest CT, which could not be controlled for. Treatments also varied between patients with IPNs and those with metastasis at baseline, with patients with metastatic disease being treated non-operatively in most cases. All studies were conducted retrospectively, with patients being selected from single institutions in the majority of studies, which inevitably introduced selection bias. Furthermore, the long study durations in some studies means that there may have been considerable chronological variance between the start and end. During this time, CT protocols may have changed considerably, and patients may have been treated differently due to the advancements in surgical techniques or due to changes in clinicians’ preferences. Finally, two studies did not re-review imaging for patients with normal staging CT at baseline, which could have led to an under-estimation of IPN incidence.
IPNs, in the absence of prior comparison studies to assess for stability, pose a clinical and diagnostic dilemma in patients with primary bone malignancies. Based on the evidence gathered, we suggest that IPNs in patients with primary bone tumours be followed up for a minimum of 2 years, given that current evidence suggests that the vast majority of these that progressed to metastases did so within this timeframe. We suggest follow up at 3, 6, and 12-month intervals. The follow up is particularly important with nodules measuring >5 mm in average diameter.
Author contributions
M. Shihabul Hassan and Rajesh Botchu (conception and design, or acquisition of data, or analysis and interpretation of data), M. Shihabul Hassan, Sisith Ariyaratne, and Rajesh Botchu (design, or acquisition of data, or analysis and interpretation of data), M Shihabul Hassan, Sisith Ariyaratne, Christine Azzopardi, Karthikeyan P. Iyengar, Arthur Mark Davies , Rajesh Botchu (drafting the article or revising it critically for important intellectual content and final approval of the version to be published)
Funding
This research received no external funding or support from any agency in the public, commercial, or not-for-profit sectors.
Conflicts of interest
The authors declare that they have no conflict of interest.



