-
PDF
- Split View
-
Views
-
Cite
Cite
Mansi Verma, Amit Ajit Deshpande, Niraj Nirmal Pandey, Sanjeev Kumar, Periaortic air in native and post-operative aorta on computed tomography, British Journal of Radiology, Volume 95, Issue 1129, 1 January 2022, 20210878, https://doi.org/10.1259/bjr.20210878
Close - Share Icon Share
Periaortic air can be seen in various conditions which can be a benign imaging finding or harbinger of a catastrophic event. The causes vary in native aorta and post-operative aorta. A radiologist has an important part in the management process of these patients, as the treatment varies from conservative to radical surgery based on the aetiology. The presence of periaortic air seen in the light of various clinical, laboratory and radiological findings can guide the radiologist towards a particular aetiology. Cross-sectional imaging, mainly computed tomography, is an indispensable tool in recognising ectopic periaortic air and to identify the associated findings and eventually make an accurate diagnosis. We present a pictorial review of various causes of the periaortic air in native and postoperative aorta, the salient features and management of the described conditions.
Introduction
Periaortic air can be seen in a wide gamut of conditions with aetiologies differing in native and post-operative aorta. This seemingly innocuous imaging abnormality can be a harbinger of a future catastrophic event. The current state-of-the-art imaging techniques like computed tomography (CT) can identify periaortic air, aid in characterising underlying imaging abnormality as well as provide a roadmap of the vascular tree to facilitate further management. CT provides these high-resolution images in a short acquisition time. Post-processing techniques like multiplanar reconstruction, maximum intensity projection and volume rendering allow presentation of the imaging findings in a more lucid fashion. In this pictorial essay, we emphasise on the various etiologies of periaortic air on CT and how imaging can help in making a definite diagnosis.
Aortic CT angiography protocol
The scan coverage is from lung apices till the common femoral artery bifurcation. Assessment of aorta typically involves multiphasic acquisition and consists of non-enhanced scanning through the volume of interest followed by scanning in the arterial phase. Additionally, a delayed scan (80 sec) may be performed to detect suspected leakage in cases postaortic stent graft implantation. Contrast timing techniques like test bolus or bolus tracking are preferred over fixed delays.1 The test bolus technique utilises a small bolus of contrast time of the contrast arrival and measure the transit time whereas the bolus triggering technique utilises multiple low-dose sequential “monitoring” scans at the level of ascending aorta and scan is automatically triggered when threshold of approximately 100–150 HU is reached. Non-ionic iodinated contrast is injected at a dose of 1–1.5 mL/Kg body weight and at a rate of 3–5 mL/s followed by saline chaser for optimal enhancement. Acquired images can be visualised in multiple planes utilising the various post-processing techniques available for evaluation of aortic pathology.
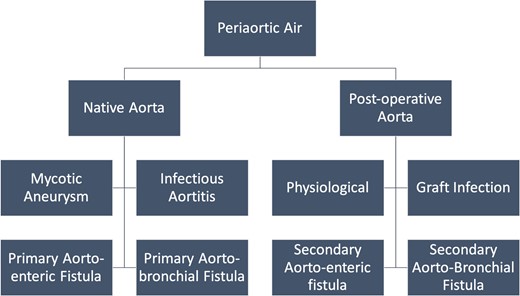
Aetiology of peri-aortic air
The aetiology of periaortic gas varies in native and peri-operative aorta. The various causes are enlisted in Figure 1.
Native aorta
The presence of air in native aorta can be caused by mycotic aneurysm, infectious aortitis, primary aortoenteric or aortobronchial fistula.
Mycotic aneurysm
Mycotic or infected aneurysm is characterised by a break in the arterial wall resulting in a saccular outpouching contiguous with arterial lumen.2 Staphylococci and Streptococci fare the most commonly implicated organisms. The varied protein manifestations of these aneurysms make early diagnosis challenging. Mycotic aneurysms comprise about 0.7–1% of all surgically managed aneurysms of the aorta.2 While individually the infrarenal abdominal aorta is most frequently involved, the combined incidence of descending thoracic, thoraco-abdominal and suprarenal aortic lesions is higher.3 Early changes occur before the formation of aneurysm and include irregular contour, periaortic soft tissue thickening, oedema and periaortic air (Figure 2).2 On CT angiography, periaortic oedema is seen as fat stranding and in early stages, the soft tissue thickening appears as homogeneously enhancing mass. Later with development of necrosis in the inflammatory mass, the enhancement becomes heterogeneous. The presence of periaortic lesion or fat stranding is the most frequently observed finding seen in 48% of the cases.4 Periaortic air is an infrequent feature of infected aneurysms.4 In an imaging series of 31 infected aortic aneurysms, only two patients (7%) had presence of periaortic air.4 Later, there is formation of focal contrast-enhanced outpouching, usually saccular, which can be uni- or multiloculated. Early treatment is of utmost importance to prevent fulminant sepsis, arterial rupture and death.
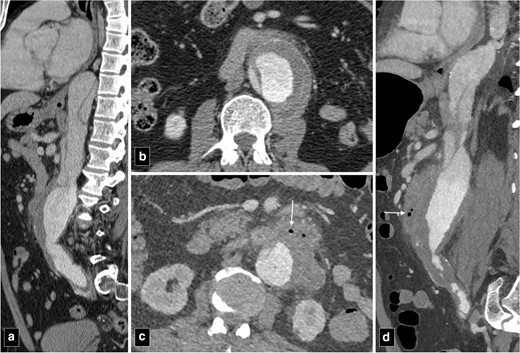
CT angiogram images of type B aortic dissection at presentation (a, b) and a year later (c, d) showing secondary infection of the abdominal aorta. Note the periaortic air (marked by arrows), fat stranding and irregularity of the aortic contour (c, d).
Infectious aortitis
It is characterised by inflammation of the aortic wall. Mycotic (infected aneurysms) and infectious aortitis represent different entities on an imaging spectrum. Aorta has normal caliber in cases of infectious aortitis; on the other hand, in infected aneurysms, the aorta is grossly dilated and at a risk of rupture. On CT, aortic wall thickening along with intramural air is a characteristic feature.5
Primary aortoenteric fistula
Aortoenteric fistula can cause life-threatening gastrointestinal bleeding and is characterised by an abnormal communication between bowel lumen and aortic lumen. They are classified as primary if they occur between native aorta and bowel in the absence of any previous history of aortic surgery/ trauma.5 Predisposing factors include the presence of an atherosclerotic aortic aneurysm (most common), tuberculosis, collagen vascular disease and mycotic infection. Enteric stent-related erosion of aorta can result from perforation of the stent through normal mucosa into the aorta leading to the development of aorto-enteric fistula.6 Primary aorto-enteric fistulas can also be observed as a complication of diverticulitis in duodenum and colon as the penetrating abscesses can cause fistulisation to adjacent structures including the aorta.7
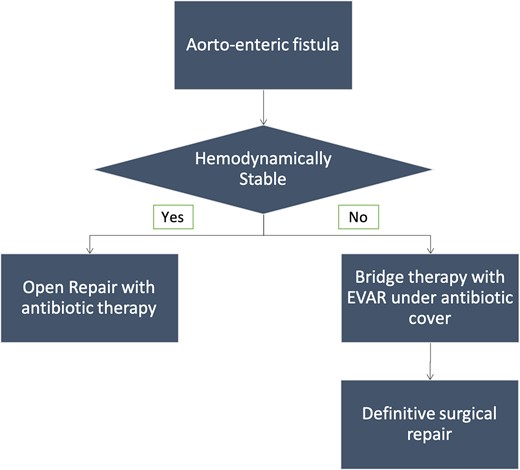
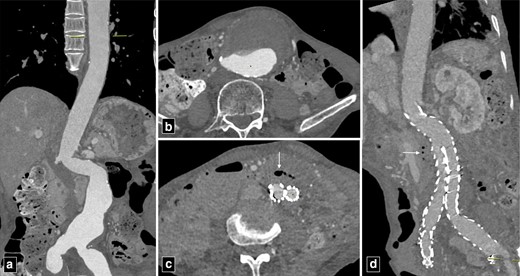
The predisposing factors for aortoesophageal fistula, apart from thoracic aortic aneurysms, include foreign body ingestion, malignant lesions of the oesophagus, oesophageal reflux, tuberculosis, corrosive esophagitis and congenital anomalies. Chiari’s triad including the presence of mid-thoracic pain, a sentinel arterial haemorrhage and bleeding after an asymptomatic interval is observed in one-third of the cases.8 The “arterial” or bright blood distinguishes aortoesophageal from other causes of massive haematemesis.9 Most fistulas occur in descending thoracic aorta where there is close apposition of two structures.9 Pathologically, there is inflammatory destruction of wall of aorta or oesophagus depending upon the aetiology with pressure necrosis of interposed soft tissue. The surrounding soft tissue may try to contain the inflammation but ultimately the weakest point gives rise to formation of fistulous tract. Upper gastrointestinal endoscopy is a useful modality to visualise the oesophageal end of the fistula and characterise various intrinsic and extrinsic causes. However, endoscopy is an invasive modality and fails to detect most of the cases with sensitivity around 25%.10 CT angiography can detect most of these cases and can demonstrate active contrast extravasation into oesophagus in arterial phase images. Ectopic air adjacent or within the aorta is a common finding on CT (Figure 3). Thickening of the esophageal wall can be observed along with obliteration of the intervening fat plane between aorta and oesophagus. The management of primary aortoenteric fistula is primarily surgical with in-situ aortic repair or an extra anatomic bypass with bowel repair. Endovascular management can be performed as a bridge therapy in patients not suitable for surgery (Figure 4).11
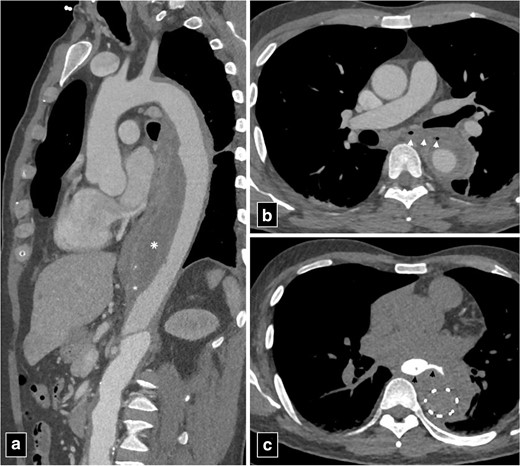
CT angiogram images of type B aortic dissection with thrombosed false lumen (marked by asterisk in a) with aorto-esophageal fistula (arrowheads in b). The patient was managed with bridging thoracic endovascular repair. Note the extravasation of column of oral contrast (arrowheads in c) communicating with the excluded false lumen of the aortic dissection post-endovascular repair.
Primary aortobronchial fistula
An aortobronchial fistula is a rare condition characterised by an anomalous communication between the aorta and the tracheobronchial tree. It commonly presents with haemoptysis with the most common site of fistula being between the descending thoracic aorta and the left tracheobronchial tree.12 However, involvement of right lobe of lung and the left main stem bronchus has also been reported previously.12 These fistulas usually arise secondary to an aortic aneurysm or pseudoaneurysm which on expansion, lead to adherence and pressure necrosis of the tracheobronchial system. The detection rate of this condition on CT is about 50%.13 On CT angiography, the presence of aortic aneurysm and ground glass opacities in the surrounding lung parenchyma can suggest its occurrence. Sometimes, intrabronchial soft-tissue densities representing a clot can also be visualised. CT can also detect vascular leaks and contrast media within the bronchial tree.
Post-operative aorta
Physiological- Post-endovascular or open surgical repair of aortic aneurysms
Perigraft soft tissue oedema, fluid collection and adjacent air can be normally visualised on CT after an open surgical aortic repair. The presence of air post-procedurally can occur due to direct delivery of atmospheric air bubbles after surgical procedure or can be percutaneously introduced after endovascular procedure (Figures 5 and 6). However, the presence of periaortic air after 3–4 week is abnormal and should raise suspicion for perigraft infection or bowel fistulation in appropriate clinical setting.14 In patients presenting post-operatively with clinical symptoms, delineating between normal post-procedural air and perigraft infection is challenging. Even in the presence of clinical findings as fever and leukocytosis, the presence of perigraft infection is far from certain as it can also occur due to “early post-implantation syndrome”. Post implantation syndrome (PIS) is observed in one-third patients after endovascular aneurysm repair (EVAR). It is triggered by sheath and catheter manipulations within aortic lumen causing the release of inflammatory mediators like tumor necrosis factor α, interleukin-6 and other cytokines. Some authors speculate endothelial injury with protein C activation and coagulopathy to be causative of PIS.15 In addition, the use of gelfoam for hemostasis has also been documented to result in perigraft air after open surgical repair.16 On CT, the air associated with gelfoam is arranged linearly in a fixed position in contrast to a random arrangement of air foci seen in cases due to infection.
CT angiogram images of abdominal aortic aneurysm at presentation (a, b). CT angiogram post-endovascular repair of abdominal aorta showing air foci in the thrombosed segment of the aneurysm (arrow in c, d). Note the absence of periaortic thickening, lymphadenopathy or fat stranding to suggest infection.
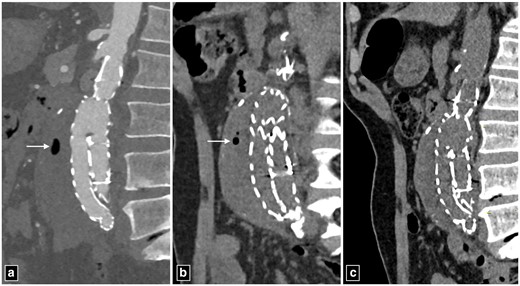
Post-operative images of abdominal aortic aneurysm managed with endovascular aortic repair. The periaortic air foci seen on day 10 (a) showing significant reduction on day 20 (b) and the absence of air foci on day 30. Note the absence periaortic thickening, lymphadenopathy or fat stranding to suggest infection.
Graft infection
It is a feared complication with high mortality that can occur after either open or endovascular repair of aortic aneurysms. The reported incidence is 0.6–3% with mortality as high as 20–40% in untreated cases.16 Early endograft infection occurs because of procedural contamination while late infection occurs due to remote site infection leading to graft colonisation. The diagnosis of perigraft infection is challenging due to vague signs and symptoms. Patients present with leukocytosis, fever and back pain.
On CT, the findings include perigraft fluid, soft tissue thickening, presence of air foci and pseudoaneurysm formation (Figure 7).17 The presence of an air-fluid level, rim enhancement and adjacent lymphadenopathy are also indicative of infection. If air foci are seen in an imaging study while not seen in previous studies or if the amount of air increases on serial imaging studies, it is suspicious for infection. Management include surgical debridement, removal of infected graft and administration of i.v. antibiotics.18
CT angiogram maximum intensity projection image of aorto bi-iliac occlusion (a), managed with surgical aorto bi-iliac graft. The infection of the surgical graft with complete occlusion, intraluminal and periaortic air (arrows in b, c) along with periaortic stranding and luminal irregularities (arrows in d).
Secondary aortoenteric fistula
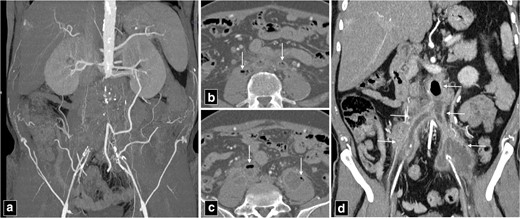
Secondary aortoenteric fistula is characterised by communication between the aortic lumen and lumen of adjacent bowel/oesophagus as a complication of aortic repair, with/without prosthetic graft placement. Approximately 80% of these fistulae occur in duodenum. Secondary aortoenteric fistula usually occur in the setting of advanced perigraft infection. Chronic low-grade perigraft infection coupled with continuous pressure from aortic pulsations has been postulated to cause these fistulae.19 Thus, aortoenteric fistula and perigraft infection can frequently demonstrate overlapping imaging appearances (Table 1). Clinically, patients most commonly present with gastrointestinal bleeding, features of sepsis and abdominal pain. Most CT findings are non-specific and can be observed in perigraft infection even without fistula formation or infectious aortitis. Hence, appropriate clinical setting must be considered in making a diagnosis. The presence of contrast extravasation into bowel lumen, although rare, is a direct and specific sign of aortoenteric fistula. The specific CT findings that strongly suggest aortoenteric fistula are the presence of ectopic air, aortic wall discontinuity and focal bowel wall thickening especially in the setting of gastrointestinal bleeding (Figure 8).20,21 The other non-specific findings include loss of perigraft fat plane, the presence of perigraft soft tissue, perigraft fluid and pseudoaneurysm formation. It is a catastrophic complication with one-year mortality of almost 100% if managed conservatively. Radical surgery with aortic reconstruction and bowel repair offers the best possible outcome similar to primary aorto-esophageal fistula with one-year survival rate of approximately 46%.22
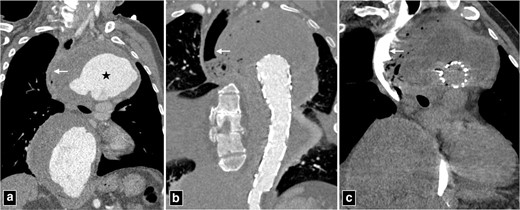
CT angiogram of thoracic aortic aneurysm (marked by star in a) with peripheral thrombosis causing indentation and displacement of oesophagus (arrow in a). The patient was managed with thoracic endovascular repair and developed secondary aorto-oesophageal fistula (b, c). Note the rupture of wall of oesophagus and continuous column of air communicating with excluded portion of the aneurysm.
| . | Aortoenteric fistula . | Perigraft infection without fistula formation . |
|---|---|---|
| Contrast extravasation from aortic lumen into bowel or leakage of enteric contrast into aorta | + | − |
| Presence of ectopic air >4 weeks after aortic repair | ++ | + |
| Aortic wall disruption | ++ | + |
| Focal bowel wall thickening | ++ | + |
| Loss of fat planes between bowel and aorta | + | + |
| Pseudoaneurysm formation | + | + |
| . | Aortoenteric fistula . | Perigraft infection without fistula formation . |
|---|---|---|
| Contrast extravasation from aortic lumen into bowel or leakage of enteric contrast into aorta | + | − |
| Presence of ectopic air >4 weeks after aortic repair | ++ | + |
| Aortic wall disruption | ++ | + |
| Focal bowel wall thickening | ++ | + |
| Loss of fat planes between bowel and aorta | + | + |
| Pseudoaneurysm formation | + | + |
| . | Aortoenteric fistula . | Perigraft infection without fistula formation . |
|---|---|---|
| Contrast extravasation from aortic lumen into bowel or leakage of enteric contrast into aorta | + | − |
| Presence of ectopic air >4 weeks after aortic repair | ++ | + |
| Aortic wall disruption | ++ | + |
| Focal bowel wall thickening | ++ | + |
| Loss of fat planes between bowel and aorta | + | + |
| Pseudoaneurysm formation | + | + |
| . | Aortoenteric fistula . | Perigraft infection without fistula formation . |
|---|---|---|
| Contrast extravasation from aortic lumen into bowel or leakage of enteric contrast into aorta | + | − |
| Presence of ectopic air >4 weeks after aortic repair | ++ | + |
| Aortic wall disruption | ++ | + |
| Focal bowel wall thickening | ++ | + |
| Loss of fat planes between bowel and aorta | + | + |
| Pseudoaneurysm formation | + | + |
Secondary aortobronchial fistula
Secondary aortobronchial fistula is characterised by the presence of fistula between postoperative aorta and airways that can occur after thoracic aortic procedures or cardiac surgery. In a survey, the incidence of aortobronchial fistula was reported as 1.7% after thoracic endovascular repair of aorta (TEVAR) and the higher rate of occurrence was associated with pseudoaneurysm repair, emergent and complex procedures.23 In another registry, the incidence after TEVAR was 0.56% and the mechanisms postulated for formation were extrinsic compression of airways, endoleak and aortic ischemia.24 The main presenting symptom is haemoptysis, which can be massive or intermittent. CT angiography is often unable to visualise fistula directly. However, can depict lung consolidation due to haemorrhage and unlikely the contrast passage from aorta into the tracheobronchial tree.25
The diagnostic algorithm of periaortic gas in post-operative aorta is provided in Figure 9.
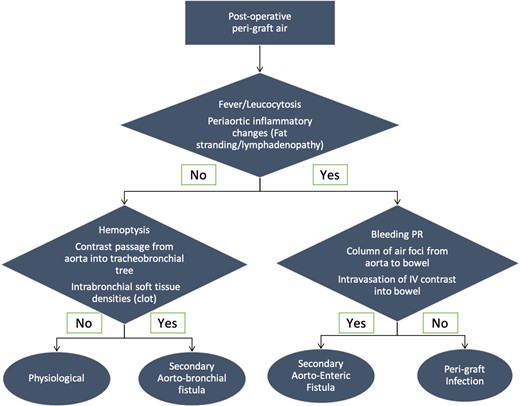
Diagnostic algorithm of periaortic air in post-operative aorta.
Adjunct imaging modalities
The use of adjunct imaging modalities such as leukocyte scintigraphy is especially useful in low-grade infections and acts as a complementary tool in confirming the diagnosis of infection in native as well as post-operative aorta. Indium-111- and technetium-99-labelled leukocytes can mark the hot spots in case of suspected infection with the sensitivity and specificity ranging from 50 to 100%.26 Fluorodeoxyglucose (FDG)-PET can also be used to detect focus of infection when clinical suspicion is high and other techniques are negative.27 To further increase the diagnostic accuracy, FDG-PET can be combined with CT. The exact anatomical location of the site of infection can be depicted by CT where as FDG-PET can provide metabolic information. In the presence of focal intense FDG uptake along with suspected lesion on CT, the probability of graft infection is around 97%.27
Conclusion
Periaortic air can be visualised in multiple conditions. It can be a benign postoperative finding or a harbinger of a catastrophic event. Vigilance and early recognition of this imaging finding can enable prompt diagnosis and prove lifesaving. A thorough knowledge of potential causes of periaortic air and accurate radiological imaging is of utmost importance to establish early diagnosis and institute appropriate treatment. The imaging features must always be interpreted keeping in mind the clinical background to establish correct diagnosis.
REFERENCES



