-
PDF
- Split View
-
Views
-
Cite
Cite
Anjum Ahmed-Nusrath, Anaesthesia for head and neck cancer surgery, BJA Education, Volume 17, Issue 12, December 2017, Pages 383–389, https://doi.org/10.1093/bjaed/mkx028
Close - Share Icon Share
Key points
Patients with head and neck cancers often have multiple co-morbidities.
A significant proportion of these patients present with a difficult airway requiring a range of advanced airway management techniques.
Airway difficulties can be minimized by agreeing a safe primary management strategy followed up with suitable backup plans.
Advanced planning and clear communication across the multidisciplinary teams minimize adverse outcomes.
Background
Head and neck cancers include cancer of the upper aerodigestive tract, paranasal sinuses, and thyroid and salivary glands. Patients presenting with head and neck cancer have a 10% risk of having a synchronous primary elsewhere within the aerodigestive tract. The principal risk factors for this type of cancer are smoking and alcohol, which appear to have a synergistic effect. Chewing tobacco, poor oral hygiene, and exposure to wood dust are additional risk factors. The incidence of head and neck cancers has increased in the past decade because of the emergence of human papilloma virus transmission linked to orogenital sex.1
Ideally, all head and neck cancer patients should be assessed by a multidisciplinary team of specialists. The overall clinical strategy is guided by the patient’s general medical condition, tolerance, and likely compliance with the proposed treatment plan.
Preoperative assessment
The aim of preoperative assessment is to identify patients with potentially difficult airways, stratify the risks, treat co-morbidities, and optimize their physiology before major surgery.
Airway assessment
The incidence of difficult airways in head and neck cancer is higher than in the general population. Evaluation of the airway should include an assessment of the difficulty in intubation and the feasibility of appropriate rescue plans in achieving oxygenation. Subtle changes to the voice, dysphagia, orthopnoea, and recent onset of snoring may indicate airway compromise. In slowly progressive cancer, with conditioning of the respiratory muscles, patients may have few signs or symptoms, despite significant narrowing of the airway. Prior treatment using radiotherapy results in a ‘fibrotic airway’ associated with woody, non-compliant tissue, which may make both face mask ventilation and laryngoscopy difficult.
Radiological imaging with computed tomography or magnetic resonance imaging (MRI) helps to determine the extent of the cancer and the impact of the pathology and potential obstruction. In experienced hands, ultrasonography is useful in identifying the cricothyroid membrane before induction of anaesthesia (Fig. 1). Awake nasal endoscopy can be carried out before induction of anaesthesia and is especially useful when no other radiological investigations are available. It gives a real-time view of the upper airway and the larynx and is useful in identifying patients in whom an awake technique is more appropriate.
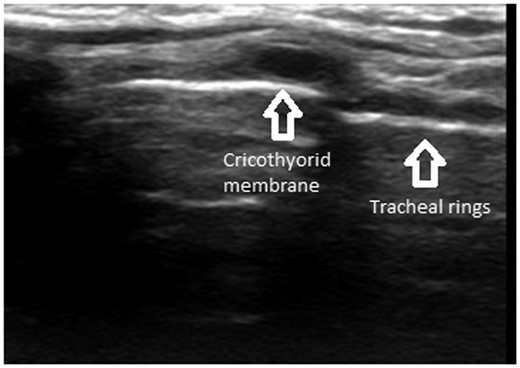
Parasagittal ultrasound of the neck showing tracheal rings (string of pearls) and the cricothyroid membrane.
Co-morbidity
Head and neck cancer patients often have significant cardiorespiratory disease and poor nutritional states linked with smoking and excessive alcohol consumption. The perioperative risk associated with major surgery increases with advancing age and the increasing number of co-morbidities. The potential benefit of the surgery must be weighed against its risks; this is where anaesthetists play a vital part in deciding the treatment plan as part of the multidisciplinary team.
Baseline investigations include full blood count, clotting screen, biochemical profile with urea and electrolytes, liver function test, blood sugar, and electrocardiography. Further investigations such as chest X-ray, pulmonary function tests, arterial blood gases, and echocardiogram should be requested based on the risk factors and symptoms at presentation.
Chronic obstructive pulmonary disease (COPD) is common in head and neck cancer patients and any reversible element should be optimised before surgery by modification of bronchodilator therapy, treatment of acute infection, and trial of steroids. Flow volume loops generated during pulmonary function testing can be used to differentiate dyspnoea from upper airway obstruction and chronic airways disease. For example, in comparison with the single plateau of the expiratory limb in COPD, plateaus can be seen in both the inspiratory and the expiratory limbs during fixed upper airway obstruction (Fig. 2). However, reproducible spirometry data depend on patient cooperation and coordination in addition to an encouraging, well-trained respiratory technician.
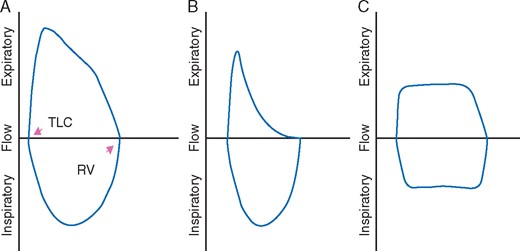
Flow volume loops: (A) normal inspiratory and expiratory limb, (B) the slowing and flattening of the expiratory limb in COPD, and (C) fixed upper airway obstruction showing plateaus in both the inspiratory and the expiratory limbs.
The calculation of metabolic equivalent based on self-reported functional capacity is subjective and high-risk patients cannot be identified by this method alone.1 Cardiopulmonary exercise testing (CPET) provides a dynamic measure of the cardiorespiratory function. Patients with anaerobic threshold less than 11 ml min−1 kg−1 are at a higher risk of cardiac complications.2 Although the full application of CPET in head and neck patients is currently being evaluated, any additional information can be used to guide preoperative discussion and determine the level of postoperative care required. Of the cardiovascular risk factors, poorly controlled heart failure (New York Heart Association Grade III–IV) has important prognostic significance. Cardiac biomarkers such as brain natriuretic peptide (BNP) and N-terminal proBNP are useful in screening for heart failure and are independently predictive of 30-day cardiac mortality.3 Right heart failure associated with pulmonary hypertension carries significant risk and non-surgical treatment options must be explored.4
Preoperative malnutrition independently correlates with poor wound healing, infection, and increased risk of postoperative complications. Patients may be malnourished from poor dietary habits (e.g. alcoholism), dysphagia, cancer cachexia, systemic effects of chemotherapy and radiation mucositis. The UK head and neck cancer guidelines recommend that all patients have nutritional screening by a clinician at presentation and specialist dietician input throughout their care.5 Nutritional therapy is indicated if the BMI is less than 18.5, weight loss greater than 10% of body weight, or if inadequate food intake is likely after surgery. The nutritional support depends on the extent of the tumour, planned surgical procedure, and social support. This ranges from oral supplements to percutaneous gastrostomy. The incidence of refeeding syndrome as a result of reintroduction of feeds is high in head and neck cancer patients.5 This is caused by hormonal and metabolic changes that lead to clinically significant hypophosphataemia, thiamine deficiency, and hypomagnesaemia. A management plan for refeeding syndrome is presented in Table 1.
| Check serum potassium, calcium, phosphate, and magnesium levels |
| Before feeding starts, administer thiamine orally, vitamin B (oral or intravenous), and supplement trace elements |
| Rehydrate and supplement serum potassium (give 2–4 mmol kg−1 day−1), phosphate (0.3–0.6 mmol kg−1 day−1), calcium, and magnesium (0.2 mmol kg−1 day−1 i.v. or 0.4 mmol kg−1 day−1 orally) as required |
| Start feeding 0.0418 MJ kg−1 day−1 |
| Slowly increase feeding for 4–7 days |
| Monitor serum potassium, phosphate, calcium, and magnesium levels for the first 2 weeks and replace as appropriate |
| Check serum potassium, calcium, phosphate, and magnesium levels |
| Before feeding starts, administer thiamine orally, vitamin B (oral or intravenous), and supplement trace elements |
| Rehydrate and supplement serum potassium (give 2–4 mmol kg−1 day−1), phosphate (0.3–0.6 mmol kg−1 day−1), calcium, and magnesium (0.2 mmol kg−1 day−1 i.v. or 0.4 mmol kg−1 day−1 orally) as required |
| Start feeding 0.0418 MJ kg−1 day−1 |
| Slowly increase feeding for 4–7 days |
| Monitor serum potassium, phosphate, calcium, and magnesium levels for the first 2 weeks and replace as appropriate |
| Check serum potassium, calcium, phosphate, and magnesium levels |
| Before feeding starts, administer thiamine orally, vitamin B (oral or intravenous), and supplement trace elements |
| Rehydrate and supplement serum potassium (give 2–4 mmol kg−1 day−1), phosphate (0.3–0.6 mmol kg−1 day−1), calcium, and magnesium (0.2 mmol kg−1 day−1 i.v. or 0.4 mmol kg−1 day−1 orally) as required |
| Start feeding 0.0418 MJ kg−1 day−1 |
| Slowly increase feeding for 4–7 days |
| Monitor serum potassium, phosphate, calcium, and magnesium levels for the first 2 weeks and replace as appropriate |
| Check serum potassium, calcium, phosphate, and magnesium levels |
| Before feeding starts, administer thiamine orally, vitamin B (oral or intravenous), and supplement trace elements |
| Rehydrate and supplement serum potassium (give 2–4 mmol kg−1 day−1), phosphate (0.3–0.6 mmol kg−1 day−1), calcium, and magnesium (0.2 mmol kg−1 day−1 i.v. or 0.4 mmol kg−1 day−1 orally) as required |
| Start feeding 0.0418 MJ kg−1 day−1 |
| Slowly increase feeding for 4–7 days |
| Monitor serum potassium, phosphate, calcium, and magnesium levels for the first 2 weeks and replace as appropriate |
Patients with a high level of alcohol dependency should be considered for active in-patient withdrawal treatment for at least 48 h and for optimization of nutritional, electrolyte, and haematological indices before surgery.
Quantitative prediction of risk
Major head and neck surgery is classified as intermediate-risk surgery with a 1–5% risk of a 30-day cardiac event. Scoring systems, such as P-POSSUM (Portsmouth–Physiological and Operative Severity Score for the enUmeration of Mortality and morbidity), which have been extensively validated in colorectal and vascular surgery, do not accurately predict risk in head and neck cancer patients. Similarly the ACS-NSQIP (American College of Surgeons National Surgical Quality Improvement Program) score is not useful in the prediction of complications or length of stay. The 2016 UK multidisciplinary head and neck cancer guidelines recommend using the Revised (Lee) Cardiac Risk Index to predict cardiac risk in the perioperative period.4
Anaesthetic management
Airway management
Management should start with all the available imaging being reviewed jointly by the surgeon and the anaesthetist. The airway that was initially straightforward during a previous anaesthetic may have deteriorated because of the spread of the disease or as a result of treatment (Table 2).
| Radiotherapy |
| Limited neck extension |
| Temperomandibular joint ankylosis |
| Osteoradionecrosis of mandible |
| Hypothyroidism |
| Baroreceptor damage |
| Carotid artery stenosis |
| Poor wound healing |
| Maxillectomy and craniofacial resection |
| Difficult mask seal |
| Nasal access difficult |
| Temporalis contracture |
| Temperomandibular joint pseudoankylosis |
| Tongue, floor of mouth surgery |
| Trismus |
| Fixed immobile tongue |
| Limited mandibular space |
| Increased tongue:oral cavity ratio with flap reconstruction |
| Laryngeal surgery |
| Laryngeal stenosis |
| Impaired swallowing |
| Aspiration risk |
| Neck dissection |
| Damage to IX, X, XII nerves |
| Impaired swallowing |
| Aspiration risk |
| Vocal cord palsy |
| Radiotherapy |
| Limited neck extension |
| Temperomandibular joint ankylosis |
| Osteoradionecrosis of mandible |
| Hypothyroidism |
| Baroreceptor damage |
| Carotid artery stenosis |
| Poor wound healing |
| Maxillectomy and craniofacial resection |
| Difficult mask seal |
| Nasal access difficult |
| Temporalis contracture |
| Temperomandibular joint pseudoankylosis |
| Tongue, floor of mouth surgery |
| Trismus |
| Fixed immobile tongue |
| Limited mandibular space |
| Increased tongue:oral cavity ratio with flap reconstruction |
| Laryngeal surgery |
| Laryngeal stenosis |
| Impaired swallowing |
| Aspiration risk |
| Neck dissection |
| Damage to IX, X, XII nerves |
| Impaired swallowing |
| Aspiration risk |
| Vocal cord palsy |
| Radiotherapy |
| Limited neck extension |
| Temperomandibular joint ankylosis |
| Osteoradionecrosis of mandible |
| Hypothyroidism |
| Baroreceptor damage |
| Carotid artery stenosis |
| Poor wound healing |
| Maxillectomy and craniofacial resection |
| Difficult mask seal |
| Nasal access difficult |
| Temporalis contracture |
| Temperomandibular joint pseudoankylosis |
| Tongue, floor of mouth surgery |
| Trismus |
| Fixed immobile tongue |
| Limited mandibular space |
| Increased tongue:oral cavity ratio with flap reconstruction |
| Laryngeal surgery |
| Laryngeal stenosis |
| Impaired swallowing |
| Aspiration risk |
| Neck dissection |
| Damage to IX, X, XII nerves |
| Impaired swallowing |
| Aspiration risk |
| Vocal cord palsy |
| Radiotherapy |
| Limited neck extension |
| Temperomandibular joint ankylosis |
| Osteoradionecrosis of mandible |
| Hypothyroidism |
| Baroreceptor damage |
| Carotid artery stenosis |
| Poor wound healing |
| Maxillectomy and craniofacial resection |
| Difficult mask seal |
| Nasal access difficult |
| Temporalis contracture |
| Temperomandibular joint pseudoankylosis |
| Tongue, floor of mouth surgery |
| Trismus |
| Fixed immobile tongue |
| Limited mandibular space |
| Increased tongue:oral cavity ratio with flap reconstruction |
| Laryngeal surgery |
| Laryngeal stenosis |
| Impaired swallowing |
| Aspiration risk |
| Neck dissection |
| Damage to IX, X, XII nerves |
| Impaired swallowing |
| Aspiration risk |
| Vocal cord palsy |
Preoperative evaluation should establish:
If face mask ventilation is likely following induction of general anaesthesia?
If laryngoscopy and intubation are likely to be difficult?
If an awake technique is more appropriate?
If emergency surgical airway and front of neck access (FONA) is feasible?
Management depends on the clinical presentation, individual expertise, and the available equipment. It is essential that anaesthetists are aware of human factors, maintain situational awareness, avoid task fixation, and do not resort to unfamiliar techniques. A coordinated team approach with clear communication is essential.
In head and neck cancers, airway difficulty is expected and should be planned for. The NAP 4 report emphasised the importance of having an ‘airway management strategy’ as apposed to a single plan and the timely and sequential implementation of these back-up plans for head and neck cancer.6 The principles discussed below help in formulating the primary and rescue plans.
The first step is to determine whether intubation is possible after induction of general anaesthesia or whether it would be achieved more safely with an awake technique. When difficulty in tracheal intubation and bag-mask ventilation is predicted or has been experienced previously, awake intubation should be considered. An awake approach potentially offers advantages of maintenance of airway patency, gas exchange, and protection against aspiration during the intubation process. General anaesthesia worsens airway obstruction, making identification of landmarks difficult on endoscopy. Fibreoptic intubation after induction of general anaesthesia will not always be successful in patients in whom an awake intubation is indicated. Awake tracheostomy under local anaesthesia should be strongly considered as a primary plan in patients with significant obstruction where awake fibreoptic intubation is not feasible.
Inhalation induction as a primary plan for cases involving head and neck pathology has been shown to frequently fail and suitable ‘rescue’ plans must be in place before induction of anaesthesia. The theoretical advantage of gas induction is that it is a slow induction that preserves spontaneous ventilation, and if at any point airway obstruction does occur, then the delivery of the inhaled anaesthetic ceases and the patient can theoretically wake up. The NAP-4 report highlighted that, in practice, when total airway obstruction occurs, patients do not exhale the anaesthetic gases and hypoxia rapidly ensues.6
It is essential that when direct laryngoscopy is performed, it is under optimum conditions with appropriate positioning, using an ‘ideal’ laryngoscope blade, after adequate preoxygenation and with sufficient neuromuscular block. If direct laryngoscopy fails, then this should be accepted, clearly communicated to the team, and the predetermined backup plan put in place. Repeated attempts at direct laryngoscopy risks bleeding and trauma in necrotic, friable tumours and may lead to complete airway obstruction.
Limited evidence exists on the use of video laryngoscopes in head and neck cancers. There is an overlap in predictive markers of difficulty with direct laryngoscopy with previous radiotherapy, malignancy, and previous surgery also leading to difficulty in intubation using video laryngoscopes.7 Consequently, there is no evidence to indicate that video laryngoscopes reduce the number of intubation attempts or the incidence of hypoxia especially after direct laryngoscopy has failed.8
Rescue oxygenation techniques must also be discussed at the start with the theatre team, in case the primary plan fails. The rescue plans may include face mask ventilation, supraglottic ventilation, or a surgical airway. It is essential to bear in mind that insertion and placement of supraglottic airway devices is difficult in patients with trismus, oropharyngeal lesions, and after radiotherapy.9 In these cases, rescue ventilation or fibreoptic intubation using a supraglottic airway as a conduit is difficult or even impossible.
Trans-nasal high-flow rapid insufflation ventilatory exchange (THRIVE) is useful in maintaining oxygen saturation and prolonging the apnoeic window in attempts to secure the airway.10 THRIVE involves apnoeic oxygenation, continuous positive airway pressure, and flow-dependent dead space flushing, and its role in difficult airway management is currently being evaluated.
Planning for postoperative airway
Surgical tracheostomy is essential for the postoperative period when swelling and oedema can lead to airway compromise. The decision to perform planned tracheostomy is based on the extent of surgery, likelihood of further postoperative swelling, and the ability to rescue the airway. Preceding difficult intubation and preoperative respiratory reserve also influence this decision. In patients with advanced cancers presenting for debulking, the airway could potentially worsen in the immediate postoperative period.
Tracheostomy has several advantages. In particular, the airway is secure with more effective bronchial toilet and the reduction in dead space assists in weaning from mechanical ventilation. However, tracheostomy carries its own risks—haemorrhage, obstruction, displacement, stomal recurrence, and poor healing after radiotherapy. Tracheostomy is not necessary after every major cancer resection and reconstruction—postoperative ventilation with an endotracheal tube is an option in selected cases.11
Practical anaesthetic management
The majority of head and neck operations need supine positioning with 15–20° head-up tilt to improve venous drainage. As access to the head and neck is limited, long ventilator tubing and vascular access lines are required. The eyes should be protected by tapes and eye shields or moistened eye pads for laser procedures. All pressure points must be padded and graduated compression stockings and intermittent pneumatic calf compression are recommended.
For major resections, large-bore venous access is essential when significant blood loss is anticipated. In addition to standard monitoring, the use of invasive techniques is determined by the patient’s co-morbidities and the nature of surgery. Central venous lines can be useful for longer-term perioperative intravenous access, measurement of central venous pressure, and assessment of response to fluid therapy. Femoral vein catheters or peripherally placed central catheters are preferred if surgical access to the neck and chest is needed.
Some studies indicate that goal-directed fluid therapy based on cardiac output monitoring is useful in guiding intraoperative fluid therapy and avoiding fluid overload in free flap transfer.12 Cardiac output monitoring based on pulse contour analysis such as LiDCO® or FloTrac® is preferred as nasal oesophageal Doppler probes are easily dislodged during surgery, requiring frequent repositioning.
During prolonged procedures, temperature measurement is essential to avoid overheating when only the head and neck is exposed. Peripheral or rectal temperature measurement avoids the difficulty of placing the probe in the nasal or oral cavity and interfering with the surgical field. Bladder temperature measurement using urinary catheters with temperature sensors is ideal as rectal temperature lags behind core body temperature. In free flap transfer, both the skin and the core bladder temperatures are measured to ensure that the core–periphery gradient is less than 1.5°.
Anaesthesia may be maintained with total intravenous anaesthesia (TIVA) or a combination of inhalation anaesthetic with remifentanil. Remifentanil enables rapid titration and blunting of the haemodynamic response during phases of intense surgical stimulation. Moderate hypotension with remifentanil may reduce bleeding and improve the operative field in craniofacial resections. However, induced hypotension should be avoided in patients with autonomic neuropathy, chronic hypertension, and in those susceptible to ischaemia, e.g. coronary and carotid atherosclerosis.
Postoperative analgesic requirements are usually managed using longer-acting opiates, e.g. morphine 0.15 mg kg−1 given incrementally towards the end of the surgery. Dexamethasone is often given to reduce airway oedema, and antiemetics, such as ondansetron or cyclizine, help to reduce postoperative nausea and vomiting.
Specific anaesthetic considerations
The management of specific cancers in the head and neck region are discussed in the following sections:
Oral and maxillofacial cancers
Oral intubation facilitates access to lesions within the maxilla, nose, and paranasal sinuses, whereas nasal tracheal intubation is often the surgical preference for oral cancers. Submental intubation for improved access to the oral cavity is an absolute contraindication in cancer surgery because of the risk of creating an orocutaneous fistula.
In craniofacial resection for skull base cancer, surgical access is through a bicoronal or transnasal route. The principal goals of anaesthetic management are similar to that of a neurosurgical procedure, i.e. preservation of adequate cerebral perfusion pressure and oxygen delivery while providing optimal operative conditions. In the author’s experience, anaesthesia is best maintained using an inhalation anaesthetic supplemented with remifentanil or using a TIVA technique aimed at maintaining normocapnia and normotension to preserve cerebral blood flow. The complications of skull base resection are cerebrospinal fluid leak, vascular injury, and visual deficits from injury or ischaemia of optic nerve; therefore, regular neurological observations are essential in the immediate postoperative period. Broad-spectrum antibiotic cover is recommended as the dura is breached and risk of contamination is high.
Free flap transfer
The aim of anaesthetic management is to maintain a full, hyperdynamic circulation with increased cardiac output, peripheral vasodilation, and normothermia to maximize flap perfusion. The haematocrit is maintained at 30–35% to improve oxygen transfer and red cell velocity within the microcirculation. The consequences of systemically administered inotropes for flap microcirculation remain unclear. In general, norepinephrine with its predominant vasoconstrictive effect is avoided, whereas dobutamine is preferred because of its inodilator action. However, the resultant tachycardia seen with dobutamine often limits its use. There is some evidence that exposure and handling of the flap results in vasoconstriction; therefore, an increase in mean arterial blood pressure using an epinephrine infusion may assist with perfusion dynamics.13
The absolute contraindications for free flap transfer are sickle cell disease and untreated polycythaemia rubra vera, because flap failure rate is high from microcirculatory ‘sludging’ and hypercoagulability. The incidence of anastomotic thrombosis is high in patients with active vasculitis associated with collagen vascular disease; therefore, specialist referral and treatment is indicated before surgery. With peripheral vascular disease, magnetic resonanace angiography is indicated to determine patency of donor vessels in the fibular flap for mandibular reconstruction.
Salivary gland cancer
In parotid surgery where the facial nerve is to be preserved, nerve monitoring is used to prevent iatrogenic injury. This should be discussed with the surgeon and neuromuscular blocking agents given accordingly. In one study evaluating neuromuscular monitoring, the mean time from induction to incision and identification of the nerve was 31.6 min and 61.2 min, respectively, indicating that if used in appropriate doses, neuromuscular blocking drugs are not contraindicated for this type of surgery.14 For example, neuromuscular blockers can be administered to facilitate intubation, and then remifentanil may be used to avoid the need for any further doses of muscle relaxant.
Thyroid cancer
For these cases, the history and assessment is focused on establishing the patient’s thyroid function as well as the difficulty in managing the airway. Patients with thyroxine-secreting tumours should be medically optimized to achieve euthyroid status before surgery. In patients where the airway is not compromised, standard i.v. induction incorporating neuromuscular block and intubation with reinforced tube is recommended. It is important to note that FONA to rescue the airway may not be possible in patients with large thyroid lesions.
In retrosternal goitres, with mid-to-low tracheal compression, tracheostomy will not relieve the obstruction. Management of retrosternal goitre remains controversial. Cook et al.15 showed that there is no consensus even among international airway experts on the primary management plan to secure the airway. However, all agreed that rigid bronchoscopy should be part of the backup plan. The rigid bronchoscope stents the airway open and allows ventilation using both high-pressure (jet) sources and conventional anaesthetic machines. A suitable ‘Plan A’ involves fibreoptic inspection of the awake airway, followed by tracheal intubation beyond the stenosis or intravenous induction with neuromuscular block and intubation with either a small tracheal tube or ventilation using a rigid bronchoscope (Plan B). With lesions extending below the aortic arch or tracheal carina, sternotomy may be needed, and these cases are best managed in a cardiothoracic centre.
Neural integrity monitor tubes (Fig. 3) with embedded electrodes designed to measure intralaryngeal surface EMG are recommended to identify the recurrent laryngeal nerve in malignant adhesions and in repeat operations.16 A unilateral or partial injury results in hoarseness of voice; however, bilateral nerve injury leads to stridor requiring tracheostomy. Other complications of thyroid surgery include postoperative hypocalcaemia and tracheomalacia in long-standing tracheal compression.
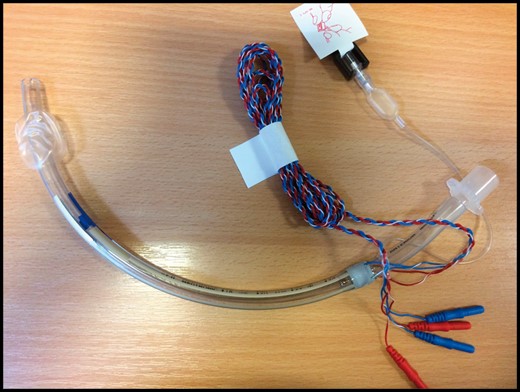
Neural integrity monitor tubes with embedded electrodes for intralaryngeal electromyography.
Panendoscopy
The ideal technique for panendoscopy provides a clear unobstructed view with protection from aspiration. This depends on the site of lesion, surgical preference, and the available equipment. The advantages and limitations of the various techniques used for panendoscopy are summarized in Table 3. Spontaneous ventilation is rarely used in adults, because the deep plane of anaesthesia required to maintain vocal cord relaxation without neuromuscular paralysis often results in apnoea and cardiovascular instability; however, high-flow oxygen therapy (THRIVE) can be useful for ‘apnoeic’ oxygenation during endoscopic procedures.17
| Microlaryngoscopy tube and IPPV |
| Advantages |
| Airway protection with cuffed tube |
| Specialist equipment for jet ventilation not required |
| Unobstructed view of anterior two-thirds of larynx |
| Disadvantages |
| Limited access to posterior commissure |
| Risk of airway fire with laser |
| High airway resistance of small diameter tracheal tube |
| Supraglottic jet ventilation |
| Advanatges |
| Optimal surgical access especially for posterior commissure lesions |
| Disadvantages |
| Gastric distension with entrained air |
| Poor ventilation with misalignment of suspension laryngoscope or jetting needle |
| No airway protection |
| Inability to monitor end-tidal carbon dioxide concentration |
| Vocal cord flutter with ventilation |
| Barotrauma and aspiration risk |
| Potential for tumour seeding |
| TIVA technique required |
| Subglottic jet ventilation |
| Advantages |
| Minimal vocal cord movement |
| More efficient than supraglottic ventilation |
| Disadvantages |
| TIVA technique required |
| Risk of barotrauma greater than supraglottic ventilation |
| Trans-tracheal ventilation |
| Advantages |
| Can be used in managing difficult airway |
| Disadvantages |
| Barotrauma |
| Seeding of the tumour |
| Problems with trans-tracheal catheter: kink, block, and dislodgement |
| TIVA technique required |
| No airway protection |
| Microlaryngoscopy tube and IPPV |
| Advantages |
| Airway protection with cuffed tube |
| Specialist equipment for jet ventilation not required |
| Unobstructed view of anterior two-thirds of larynx |
| Disadvantages |
| Limited access to posterior commissure |
| Risk of airway fire with laser |
| High airway resistance of small diameter tracheal tube |
| Supraglottic jet ventilation |
| Advanatges |
| Optimal surgical access especially for posterior commissure lesions |
| Disadvantages |
| Gastric distension with entrained air |
| Poor ventilation with misalignment of suspension laryngoscope or jetting needle |
| No airway protection |
| Inability to monitor end-tidal carbon dioxide concentration |
| Vocal cord flutter with ventilation |
| Barotrauma and aspiration risk |
| Potential for tumour seeding |
| TIVA technique required |
| Subglottic jet ventilation |
| Advantages |
| Minimal vocal cord movement |
| More efficient than supraglottic ventilation |
| Disadvantages |
| TIVA technique required |
| Risk of barotrauma greater than supraglottic ventilation |
| Trans-tracheal ventilation |
| Advantages |
| Can be used in managing difficult airway |
| Disadvantages |
| Barotrauma |
| Seeding of the tumour |
| Problems with trans-tracheal catheter: kink, block, and dislodgement |
| TIVA technique required |
| No airway protection |
| Microlaryngoscopy tube and IPPV |
| Advantages |
| Airway protection with cuffed tube |
| Specialist equipment for jet ventilation not required |
| Unobstructed view of anterior two-thirds of larynx |
| Disadvantages |
| Limited access to posterior commissure |
| Risk of airway fire with laser |
| High airway resistance of small diameter tracheal tube |
| Supraglottic jet ventilation |
| Advanatges |
| Optimal surgical access especially for posterior commissure lesions |
| Disadvantages |
| Gastric distension with entrained air |
| Poor ventilation with misalignment of suspension laryngoscope or jetting needle |
| No airway protection |
| Inability to monitor end-tidal carbon dioxide concentration |
| Vocal cord flutter with ventilation |
| Barotrauma and aspiration risk |
| Potential for tumour seeding |
| TIVA technique required |
| Subglottic jet ventilation |
| Advantages |
| Minimal vocal cord movement |
| More efficient than supraglottic ventilation |
| Disadvantages |
| TIVA technique required |
| Risk of barotrauma greater than supraglottic ventilation |
| Trans-tracheal ventilation |
| Advantages |
| Can be used in managing difficult airway |
| Disadvantages |
| Barotrauma |
| Seeding of the tumour |
| Problems with trans-tracheal catheter: kink, block, and dislodgement |
| TIVA technique required |
| No airway protection |
| Microlaryngoscopy tube and IPPV |
| Advantages |
| Airway protection with cuffed tube |
| Specialist equipment for jet ventilation not required |
| Unobstructed view of anterior two-thirds of larynx |
| Disadvantages |
| Limited access to posterior commissure |
| Risk of airway fire with laser |
| High airway resistance of small diameter tracheal tube |
| Supraglottic jet ventilation |
| Advanatges |
| Optimal surgical access especially for posterior commissure lesions |
| Disadvantages |
| Gastric distension with entrained air |
| Poor ventilation with misalignment of suspension laryngoscope or jetting needle |
| No airway protection |
| Inability to monitor end-tidal carbon dioxide concentration |
| Vocal cord flutter with ventilation |
| Barotrauma and aspiration risk |
| Potential for tumour seeding |
| TIVA technique required |
| Subglottic jet ventilation |
| Advantages |
| Minimal vocal cord movement |
| More efficient than supraglottic ventilation |
| Disadvantages |
| TIVA technique required |
| Risk of barotrauma greater than supraglottic ventilation |
| Trans-tracheal ventilation |
| Advantages |
| Can be used in managing difficult airway |
| Disadvantages |
| Barotrauma |
| Seeding of the tumour |
| Problems with trans-tracheal catheter: kink, block, and dislodgement |
| TIVA technique required |
| No airway protection |
Laryngectomy
A detailed review was published by Stevens etal. in BJA Education (2017).18
Extubation
A systematic approach with upright positioning, full reversal of residual neuromuscular blocking drugs, and preoxygenation is essential before extubation. The diffficult airway society (DAS) extubation guidelines serve as a framework to identify ‘low-risk’ or ‘at-risk’ extubation.19 For patients identified as being ‘at risk’ of airway obstruction, the safer option is a planned tracheostomy or delayed extubation. In the author’s experience, a low-dose remifentanil infusion with an effect site target of 1–1.5 ng ml−1 assists with smooth emergence, obtunds cardiovascular reflexes, and reduces agitation and coughing. Extubation over an airway exchange catheter (AEC) is only appropriate for carefully selected head and neck cancer patients.20 The AEC can be used for emergency reintubation or oxygenation with standard anaesthetic circuits or high-pressure source (jet) ventilation. The catheters can get easily kinked or displaced, and meticulous care must be taken to ensure that the distal tip is positioned in the mid-trachea at all times.
Postoperative care
Pain in the head and neck region is moderate and is managed by a combination of regular paracetamol and opiates administered by subcutaneous injection, or orally if swallowing is not impaired. Patient-controlled analgesia is reserved for patients with extensive resection. The analgesic requirement for flap donor areas such as the ischium in deep circumflex iliac artery bone flap is higher. Local anaesthetic infusions using wound catheters can help reduce opioid requirements.
The presence of a new tracheostomy often produces coughing and irritation in the recovery; humidified oxygen or nebulized 4% lignocaine with judicious use of opiates alleviates these symptoms. Extrapolation of concepts of enhanced recovery to patients with head and neck cancer has been shown to improve patient satisfaction and reduce length of stay.
Postoperative care should be in specialist wards or critical care where the staff is suitably trained in tracheostomy care. Regular antiemetics, a liberal fluid regime, and establishing oral intake all help to reduce postoperative nausea and vomiting. Intravenous steroids, continued for 48–72 h, help to reduce oedema. Low-molecular-weight heparin is administered for thromoboprophylaxis. The aims of postoperative care in free flap surgery are maintenance of normotension, normothermia with adequate filling, and regular monitoring of the flap. The haematocrit should be maintained at 30–35% (dextran and aspirin are no longer given because of lack of proof of efficacy). Nutritional support should be commenced as soon as possible after surgery. In total pharyngolaryngectomy and oesophaeal reconstruction with jejunal conduit, swallowing is assessed clinically and with video fluoroscopy (modified barium swallow) at Day 5 and oral intake started under supervision of a specialist dietician.
Respiratory distress in the postoperative period is especially challenging because of swelling, intraoral flap, or blood in the airway. Head elevation, nebulized epinephrine, and high-flow nasal oxygen serve as temporizing measures. Careful nasal endoscopy may be attempted to determine whether fibreoptic intubation is feasible. In all these circumstances, the primary plan for intubation may fail and a ‘double airway intervention set-up’ with personnel and equipment for emergency surgical airway is essential.9 Management of a displaced tracheostomy tube in the postoperative period is based on the national tracheostomy project guidelines and this depends on whether the ‘native’ upper airway is patent.21
Declaration of interest
None declared.
MCQs
The associated MCQs (to support CME/CPD activity) can be accessed at http://www.oxforde-learning.com/journals/ by subscribers to BJA Education.
Podcasts
This article has an associated podcast which can be accessed at https://dbpia.nl.go.kr/bjaed/pages/Podcasts.





