-
PDF
- Split View
-
Views
-
Cite
Cite
Najwan Abu Al-Saad, Chris Skedgel, Jurgens Nortje, Principles of resource allocation in critical care, BJA Education, Volume 17, Issue 12, December 2017, Pages 390–395, https://doi.org/10.1093/bjaed/mkx029
Close - Share Icon Share
Key points
Critical care is a scarce resource facing increasing demand.
Rationing of health care is a reality of all health systems at some level.
Ethical and economic dimensions of resource allocation in critical care are inseparable.
Scarce resources need to be used as efficiently as possible to maximize achievable health gains and maintain the sustainability of critical care.
Well-conducted cost-effectiveness analyses can inform more explicit and transparent resource allocation decisions but need consideration of local context.
There is evidence that critical care in the UK can be cost-effective.
Although critical care is a high-cost, low-volume specialty,1 especially relative to preventative or primary care interventions, there is evidence that critical care provision in the UK NHS can be very cost-effective.3,4
Acting in individual patients’ best interests when presenting to critical care is the central tenet of clinical practice. Yet health care delivery in all settings occurs in the context of finite resources and ever-increasing demand, making it a scarce resource. Drivers for rising demand5 in critical care include:
an ageing population living longer with multiple long-term conditions;
increasing number and complexity of surgical interventions performed;
development of new therapies;
rising public expectations of the availability and effectiveness of healthcare based on improved outcomes over time. An observational study in Australia and New Zealand6 showed that in-hospital mortality secondary to severe sepsis and septic shock decreased year on year between 2000 and 2012 from 35% to 18.4%, unrelated to changes in case definitions or illness severity.
Scarcity and rationing
The relative scarcity of health care necessitates alignment of individual patient interests with sustainable models of providing health care. The NHS Commissioning Board7 (NHSCB) acknowledges that it ‘does not have the budget to fulfil all needs of all patients within its area of responsibility’. The US Task Force on Values, Ethics and Rationing in Critical Care8 defines rationing as ‘the allocation of healthcare resources in the face of limited availability, which necessarily means that beneficial interventions are withheld from some individuals’.
Rationing within health care occurs at three levels:9
Macro-level rationing at state or national government level when determining overall health budgets, with governments being accountable to their electorates.
Meso-level rationing regionally or locally, e.g. Clinical Commissioning Groups (CCGs) that are accountable to the Secretary of State for Health via NHS England, assessed broadly by their population health outcomes and spending.
Micro-level rationing, also referred to as bedside rationing, occurs at the level of individual clinicians and patients. The General Medical Council’s (GMC) leadership and management guidance10 states all doctors should be prepared to contribute to discussions about resource allocation, priority setting, and commissioning of services for the wider patient population. Decisions affecting patients should be ‘fair, based on clinical need and the likely effectiveness of treatments’, acknowledging that ‘treatment options that can be offered to patients may be affected by limits in resources’.
Who should have access to critical care?
There are various approaches to determining who needs access to health care.11 Clinical need is often defined in terms of capacity to benefit from available therapies. This is in keeping with the GMC’s leadership guidance10 and the NHSCB definition7 of a health care need as a health problem that ‘can be addressed by a clinically effective intervention’. Conversely, denying patients access to interventions where there is little or no expected health benefit is not rationing.11
In critical care, cost-effectiveness of admission is also related to capacity to benefit. The most critically ill patients have the most to gain (in terms of mortality reduction) from admission.12 As many intensive care unit (ICU) costs are fixed irrespective of illness severity, the cost-effectiveness of admitting patients with higher predicted mortality improves in comparison with those with lower predicted mortality. A multicentre prospective observational study12 (Table 1) showed that the effectiveness (relative risk reductions in 28-day mortality) for patients admitted to ICU compared with non-admitted referrals varied with predicted mortality. Similarly, the cost-effectiveness of ICU admission (cost per life saved and cost per life-year saved) was greatest for patients with predicted mortality ≥40%.
Examples of published critical care health economic evaluations. CEA, cost-effectiveness analysis; QALY, quality-adjusted life year; LoS, length of stay; NHS, National Health Service; NICE, National Institute for Health and Care Excellence; CUA, cost–utility analysis; ECMO, extracorporeal membrane oxygenation; CI, confidence interval; HRQoL, Health-Related Quality of Life; EQ5D, Euro Quality of Life 5 Dimension questionnaire; ICER, incremental cost-effectiveness ratio; OR, odds ratio; SAPS, simplified acute physiology score; RCT, randomized controlled trial
| Study . | Study type . | Intervention . | Perspective . | Outcome measures . | Results . | Assumptions/limitations . |
|---|---|---|---|---|---|---|
| Hutchings et al.3 | Retrospective CEA | Implementation of ‘comprehensive critical care’, including 35% increase in resourced critical care beds | Hospital wide | Incremental net monetary gain | Increase in mean annual net monetary benefit from £402 to £1096 | Life expectancy assumed 80% age and sex-matched population |
| Quality of life down-weighted by 20% | ||||||
| QALY gained valued at £20 000 | ||||||
| Net monetary gain by reduction in ICU LoS | ||||||
| Ridley and Morris4 | CEA | None | NHS (hospital and lifetime care costs) | Incremental cost per QALY | £7010 per QALY gained driven by 17.4% ARR between patients admitted to ICU or not | Incremental cost per QALY only exceeded NICE threshold of £30 000 if ARR <5%. |
| If admission practices changed to allow admission of patients less likely to survive ICU, cost-effectiveness of ICU would decrease | ||||||
| CESAR26 | RCT including prospective CEA and CUA | ECMO for severe adult respiratory failure vs conventional ventilation | Publically funded health and social care, patients, and carers | Lifetime cost per QALY for intervention with ECMO | Lifetime cost per QALY £19 252 (95% CI: £7622–£59 200) | HRQoL measured at 6 months using EQ 5D |
| Life expectation and health care utilization of survivors assumed equivalent to age/sex-matched general population at 24 months | ||||||
| Wide CI because of high degree of uncertainty surrounding some costs and effects | ||||||
| Edbrooke et al.12 | CEA of ICU admission. Prospective multicentre observational cohort study | Observational comparing patients accepted or rejected for admission after ICU triage/referral | Hospital wide | Primary measure 28-day mortality | OR for ICU admission 0.70 (95% CI: 0.51–0.97) | Predicted mortality based on SAPS II score |
| Cost per life saved (ICER) and cost per life-year saved | Average cost per life saved $103 771. This fell to $60 046 for patients with predicted mortality ≥40% | Life expectancy for patients admitted to ICU lower than general population for first 2 years, then reverts to normal | ||||
| Costs measured by cost-block methodology |
| Study . | Study type . | Intervention . | Perspective . | Outcome measures . | Results . | Assumptions/limitations . |
|---|---|---|---|---|---|---|
| Hutchings et al.3 | Retrospective CEA | Implementation of ‘comprehensive critical care’, including 35% increase in resourced critical care beds | Hospital wide | Incremental net monetary gain | Increase in mean annual net monetary benefit from £402 to £1096 | Life expectancy assumed 80% age and sex-matched population |
| Quality of life down-weighted by 20% | ||||||
| QALY gained valued at £20 000 | ||||||
| Net monetary gain by reduction in ICU LoS | ||||||
| Ridley and Morris4 | CEA | None | NHS (hospital and lifetime care costs) | Incremental cost per QALY | £7010 per QALY gained driven by 17.4% ARR between patients admitted to ICU or not | Incremental cost per QALY only exceeded NICE threshold of £30 000 if ARR <5%. |
| If admission practices changed to allow admission of patients less likely to survive ICU, cost-effectiveness of ICU would decrease | ||||||
| CESAR26 | RCT including prospective CEA and CUA | ECMO for severe adult respiratory failure vs conventional ventilation | Publically funded health and social care, patients, and carers | Lifetime cost per QALY for intervention with ECMO | Lifetime cost per QALY £19 252 (95% CI: £7622–£59 200) | HRQoL measured at 6 months using EQ 5D |
| Life expectation and health care utilization of survivors assumed equivalent to age/sex-matched general population at 24 months | ||||||
| Wide CI because of high degree of uncertainty surrounding some costs and effects | ||||||
| Edbrooke et al.12 | CEA of ICU admission. Prospective multicentre observational cohort study | Observational comparing patients accepted or rejected for admission after ICU triage/referral | Hospital wide | Primary measure 28-day mortality | OR for ICU admission 0.70 (95% CI: 0.51–0.97) | Predicted mortality based on SAPS II score |
| Cost per life saved (ICER) and cost per life-year saved | Average cost per life saved $103 771. This fell to $60 046 for patients with predicted mortality ≥40% | Life expectancy for patients admitted to ICU lower than general population for first 2 years, then reverts to normal | ||||
| Costs measured by cost-block methodology |
Examples of published critical care health economic evaluations. CEA, cost-effectiveness analysis; QALY, quality-adjusted life year; LoS, length of stay; NHS, National Health Service; NICE, National Institute for Health and Care Excellence; CUA, cost–utility analysis; ECMO, extracorporeal membrane oxygenation; CI, confidence interval; HRQoL, Health-Related Quality of Life; EQ5D, Euro Quality of Life 5 Dimension questionnaire; ICER, incremental cost-effectiveness ratio; OR, odds ratio; SAPS, simplified acute physiology score; RCT, randomized controlled trial
| Study . | Study type . | Intervention . | Perspective . | Outcome measures . | Results . | Assumptions/limitations . |
|---|---|---|---|---|---|---|
| Hutchings et al.3 | Retrospective CEA | Implementation of ‘comprehensive critical care’, including 35% increase in resourced critical care beds | Hospital wide | Incremental net monetary gain | Increase in mean annual net monetary benefit from £402 to £1096 | Life expectancy assumed 80% age and sex-matched population |
| Quality of life down-weighted by 20% | ||||||
| QALY gained valued at £20 000 | ||||||
| Net monetary gain by reduction in ICU LoS | ||||||
| Ridley and Morris4 | CEA | None | NHS (hospital and lifetime care costs) | Incremental cost per QALY | £7010 per QALY gained driven by 17.4% ARR between patients admitted to ICU or not | Incremental cost per QALY only exceeded NICE threshold of £30 000 if ARR <5%. |
| If admission practices changed to allow admission of patients less likely to survive ICU, cost-effectiveness of ICU would decrease | ||||||
| CESAR26 | RCT including prospective CEA and CUA | ECMO for severe adult respiratory failure vs conventional ventilation | Publically funded health and social care, patients, and carers | Lifetime cost per QALY for intervention with ECMO | Lifetime cost per QALY £19 252 (95% CI: £7622–£59 200) | HRQoL measured at 6 months using EQ 5D |
| Life expectation and health care utilization of survivors assumed equivalent to age/sex-matched general population at 24 months | ||||||
| Wide CI because of high degree of uncertainty surrounding some costs and effects | ||||||
| Edbrooke et al.12 | CEA of ICU admission. Prospective multicentre observational cohort study | Observational comparing patients accepted or rejected for admission after ICU triage/referral | Hospital wide | Primary measure 28-day mortality | OR for ICU admission 0.70 (95% CI: 0.51–0.97) | Predicted mortality based on SAPS II score |
| Cost per life saved (ICER) and cost per life-year saved | Average cost per life saved $103 771. This fell to $60 046 for patients with predicted mortality ≥40% | Life expectancy for patients admitted to ICU lower than general population for first 2 years, then reverts to normal | ||||
| Costs measured by cost-block methodology |
| Study . | Study type . | Intervention . | Perspective . | Outcome measures . | Results . | Assumptions/limitations . |
|---|---|---|---|---|---|---|
| Hutchings et al.3 | Retrospective CEA | Implementation of ‘comprehensive critical care’, including 35% increase in resourced critical care beds | Hospital wide | Incremental net monetary gain | Increase in mean annual net monetary benefit from £402 to £1096 | Life expectancy assumed 80% age and sex-matched population |
| Quality of life down-weighted by 20% | ||||||
| QALY gained valued at £20 000 | ||||||
| Net monetary gain by reduction in ICU LoS | ||||||
| Ridley and Morris4 | CEA | None | NHS (hospital and lifetime care costs) | Incremental cost per QALY | £7010 per QALY gained driven by 17.4% ARR between patients admitted to ICU or not | Incremental cost per QALY only exceeded NICE threshold of £30 000 if ARR <5%. |
| If admission practices changed to allow admission of patients less likely to survive ICU, cost-effectiveness of ICU would decrease | ||||||
| CESAR26 | RCT including prospective CEA and CUA | ECMO for severe adult respiratory failure vs conventional ventilation | Publically funded health and social care, patients, and carers | Lifetime cost per QALY for intervention with ECMO | Lifetime cost per QALY £19 252 (95% CI: £7622–£59 200) | HRQoL measured at 6 months using EQ 5D |
| Life expectation and health care utilization of survivors assumed equivalent to age/sex-matched general population at 24 months | ||||||
| Wide CI because of high degree of uncertainty surrounding some costs and effects | ||||||
| Edbrooke et al.12 | CEA of ICU admission. Prospective multicentre observational cohort study | Observational comparing patients accepted or rejected for admission after ICU triage/referral | Hospital wide | Primary measure 28-day mortality | OR for ICU admission 0.70 (95% CI: 0.51–0.97) | Predicted mortality based on SAPS II score |
| Cost per life saved (ICER) and cost per life-year saved | Average cost per life saved $103 771. This fell to $60 046 for patients with predicted mortality ≥40% | Life expectancy for patients admitted to ICU lower than general population for first 2 years, then reverts to normal | ||||
| Costs measured by cost-block methodology |
The strategic aims of NHS England regarding provision of critical care13 are to:
Prevent avoidable mortality and morbidity as a result of patients requiring critical care not accessing the appropriate level of care or organ support.
Avoid triage by resource as opposed to triage by outcome. Patients should have access to care based on anticipated capacity to benefit from treatment rather than on what facilities are available (namely availability of staffed ICU beds).
Achieve equity of access to treatment. Patients with equivalent clinical need (in terms of severity of illness and capacity to benefit) should have equal access to optimal care irrespective of, for example, location or time of presentation.
Value, cost-effectiveness, and efficiency in critical care
Value for money in health care can be defined as achieving best health outcomes relative to cost.14 The objective of health economics is to maximize the value obtained from a given set of resources by prioritizing interventions delivering greatest health benefits for cost. Cost-effectiveness is synonymous with value for money, although it tends to imply value for money determined by means of a cost-effectiveness analysis (CEA).
Measurement of value for money in critical care includes the use of the Intensive Care National Audit and Research Centre Case Mix Programme15 (ICNARC CMP) to aid ‘decision-making, resource allocation and local quality improvement’.
There are two types of efficiency in health economics.9 First, allocative efficiency is concerned with ensuring that the value derived from a service outweighs the costs of its production. The greater the value relative to cost, the more allocatively efficient the service. The second type of efficiency is technical efficiency, which is concerned with maximizing the outcomes available with a given level of resources. The more outcomes that can be produced for a given budget, the more technically efficient the service.
Questions around what the best proportion of health care spending that should be allocated to critical care is (budget setting) and which groups of patients should have access to critical care (case selection) address allocative efficiency. Technical efficiency considers how a given budget can best be used to maximize delivery or minimize cost of a service. Clinical governance and quality improvement projects in critical care largely focus on the technical efficiency of service provision through improving processes and cost-containment at a local or regional level. Critical care admission practices could affect both local allocative efficiency and technical efficiency. It is possible for a service to be technically efficient while being allocatively inefficient.
Economic evaluation in critical care
Economic evaluations in critical care can provide evidence to improve efficiency of resource allocation.16 Comparisons between interventions within critical care can be made to prioritize how resources are best utilized within the specialty as well as against very different uses of scarce health care resources. The main types of health economic evaluation9,16,17 are:
Cost–benefit analysis (CBA): Total costs and total benefits of interventions (measured purely in monetary terms) are compared. This requires a monetary value to be assigned to all outcomes. For example, if comparing methods of weaning sedation and ventilatory support, a monetary cost would need to be assigned to outcomes such as an episode of delirium or pain and distress experienced by patients. CBA has the advantage of being able to assess allocative efficiency, but the information requirements are onerous and monetizing health outcomes can be viewed as morally objectionable.
Cost-minimization analysis (CMA): In circumstances where the effects of the interventions, are therapeutically identical, the objective is to identify the lowest cost option. That is, outcomes, including side-effect profiles and duration of treatment between compared interventions are equivalent. Such evaluations have been used to compare drug treatments, but it is rare for therapies to have identical outcomes so their role is increasingly narrow.
- CEA: An economic evaluation whose outcome measure is one of incremental cost-effectiveness, i.e. the difference in cost between two interventions divided by the difference in their effect. This involves estimating or measuring the total costs of an intervention against the health benefits measured in natural units such as premature deaths avoided, cases prevented, or changes in measures such as ICU or hospital length of stay. Table 1 lists some examples of CEAs within critical care. CEA is relatively straightforward but can only be used to compare interventions with the same outcome measures, e.g. 28-day mortality. Cost-effectiveness is expressed in terms of the additional cost per unit of effect, such as cost per life saved. This is also known as the incremental cost-effectiveness ratio (ICER). The denominator, difference in effect between intervention and control, is equivalent to the absolute risk reduction (ARR) between groups.
Cost–utility analysis (CUA): This is a special case of CEA, where the effects are measured in terms of a utility (a measure of preference or value that an individual or society assigns a health state). The most widely used utility measure is the quality-adjusted life year (QALY). By standardizing the outcome measure, CUAs allow direct comparison of very different health interventions with different health benefits. This facilitates explicit resource allocation decisions such as those made by the UK National Institute for Health and Care Excellence (NICE), where treatments under £20 000 per QALY gained are generally considered cost-effective and those above £30 0003,4 per QALY are less frequently approved.
QALYs and measuring health utility
QALYs are a composite measure of the state of health of a person or group in which benefits, in terms of length of life, are adjusted to reflect the quality of life. Using QALYs as a composite outcome measure allows morbidity and mortality to be considered together.18,19 QALYs are calculated by weighting each expected year of life by the quality, or utility, of that year on a scale of 0 (dead) to 1 (perfect health). For example, 1 year of life in perfect health would count as 1.0 QALYs, while 1 year at 60% of full health would count as 0.6 QALYs. For health states seen as worse than death, a negative utility value can be assigned.
A US study of predictors of health utilities in acute respiratory distress syndrome survivors using the Euro Quality of Life 5 Dimension (EQ5D) Health-Related Quality of Life (HRQoL) questionnaire reported that extreme problems in all five domains corresponded with a calculated health utility of −0.11 compared with utility of 1.0 with no problems in any of the domains.20
Health state utilities can be elicited directly, using techniques such as the standard gamble (SG) or time trade-off (TTO), or indirectly (Fig. 1), using generic HRQoL questionnaires such as the Short Form (36) Health Survey (SF-36) or EQ5D. With direct approaches, individuals are asked to rate their health across a series of dimensions. These ratings are assigned utility scores derived using TTO methods in a different sample of the general population. Indirect methods are more straightforward to administer and are much more common in health economic evaluations than direct methods, but it is important to recognize the potential for divergence between the preferences of the patients rating the health states and the public sample involved in eliciting the utility weights. Such differences have implications for the estimation of QALYs and cost-effectiveness of interventions.
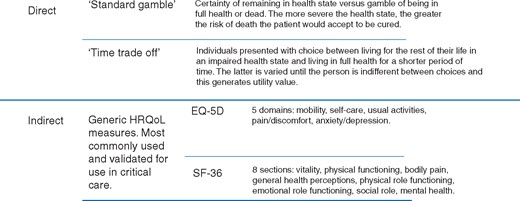
In the same way that clinical evidence needs to be critically appraised to understand how transferable the evidence is to a local population or case-mix, health economic evaluations also need to be understood in a local context8,12,16 and are perhaps even more context sensitive as costs vary greatly between health systems and over time.18
One cost that should ideally be included in estimates of total costs of interventions or models of care is opportunity cost.9,21 When choices are made regarding how best to use scarce resources, there is always a next-best alternative that cannot be chosen. This is the opportunity cost: the value of the best, mutually exclusive alternative foregone to pursue a certain action. For example, in critical care, part of the total cost of buying specific equipment or funding interventions is the value that could otherwise be gained by the next-best use of the same resources, either within critical care or within a different treatment area. Similarly, the impact of micro-level resource allocation decisions that lead to an ICU operating at full capacity (even when such decision-making is optimal) occurs at the cost of being able to admit new referrals in a timely manner, with potentially harmful consequences.21,22
Challenges in measuring cost-effectiveness
Maximizing the value14 of critical care by improving clinical outcomes is key to efficient resource allocation. If acting in patients’ best interests is at the heart of good practice, then the outcomes measured need to be of importance and relevance to patients. Outcomes measured in much critical care research have often focused on short-term mortality (at best), changes in physiological parameters, or process measures (such as length of stay) used as proxies for improved clinical outcomes. As short-term mortality has improved, so research is increasingly aimed at longer-term mortality, morbidity, HRQoL,23 and socio-economic impact of critical illness on patients24 and carers.
Apart from the practical difficulties in measuring longer-term patient-centred outcomes, critical care outcomes research faces many challenges. First, the heterogeneity of the patient population within critical care, in terms of both the pre-existing health status of patients and the diversity of critical illness presentations such as sepsis, trauma, and respiratory failure. Second, outcomes in critical care are heavily dependent on performance in many other clinical areas such as the emergency department, operating theatres, and pre-hospital and diagnostic services. Therefore, measuring outcomes directly attributable solely to critical care is difficult. Third, outcomes research attempting to predict patient outcome, such as illness severity scoring systems, provide estimates of outcome probability at a population level which are not transferable to individual patients.
Similarly, measuring costs of critical care are equally challenging. Current measures focus largely on generating an average daily cost for critical care multiplied by the length of patient stay to give an estimate of the cost for length of ICU stay. First, as with outcomes, costs of critical care are heavily dependent on areas outside the critical care unit. Second, cost of critical care is not constant throughout patient stay.1 Patient stay tends to be particularly resource intensive early in the ICU admission and less so as length of stay increases. Third, the heterogeneous case-mix means patient costs can be very variable. Fourth, despite difficulties in accurate measurement of short-term ICU costs, an idea of resource utilization by survivors of ICU in the community is needed to estimate the true cost-effectiveness of providing critical care.
Appraising CEAs in critical care
The reliability of any CEA is only as good as the quality of the data it is based on. For an intervention to be cost-effective, it must first be shown to be clinically effective. The more robust the clinical effectiveness data, the less uncertainty surrounds the cost-effectiveness estimates. Defining effective critical care interventions as those which lead to improved survival and health of those exposed to critical illness requires evidence of meaningful improvements in patient-centred outcomes, namely sustained reductions in mortality with satisfactory functional recovery and HRQoL. The short-term outcome measures most commonly used in ICU studies are not well suited to robust CEAs.
In addition, the costs and effects considered depend on the perspective chosen for the evaluation and this needs to be stated in any health economic analysis. This could be at the level of an individual ICU, hospital, national health system, or a societal perspective. The latter is preferred because it is the broadest perspective, considering all costs and benefits, including those incurred in sectors outside health and the impact of interventions on caregivers. NICE guidelines, however, currently recommend a payer perspective, as it is more relevant to funding decisions. In the NHS, this would be the commissioning board or the local CCG. With the drive towards integration of health and social care, a societal perspective may be increasingly needed to compare different interventions or models of care.
A systematic review of CEAs in critical care16 found that two of the 14 studies assessed used a societal perspective when evaluating cost-effectiveness. These were both assessing the economic impact of activated protein C in sepsis, a treatment where considerable prospective cost-effectiveness data were published. Although this evidence is now largely obsolete, as the drug was withdrawn because of doubts around the evidence of its clinical effectiveness and safety, the methodology is still noteworthy.
Sensitivity analysis is a technique used in economic evaluations to vary the underlying assumptions of costs or effects used in the analysis and around which there is uncertainty. It allows multiple ‘what if?’ scenarios to be performed,16 testing the robustness of the conclusions and is recommended when conducting CEAs.
Discounting25 is performed to account for ‘time preference’. This is the idea that people weigh costs and benefits that occur now more strongly than costs and benefits that will occur in the future. For example, most individuals would prefer to receive £100 today to £100 a year from today. Similarly, a QALY gained in the present is valued more highly than one in the future. The discount rate represents the degree to which future events are less heavily weighted or discounted. The discount rate recommended and used by NICE is 3.5%.
Analysing the cost-effectiveness of specific interventions within critical care from the perspective of the individual unit or hospital trust may sometimes be sufficient to guide resource allocation decisions. Minimizing costs at the level of a hospital trust or individual unit is important, provided the effectiveness of care is not compromised. However, cost minimization within critical care may simply serve to displace costs to a different part of the health service or the community. Although this may benefit fiscal management within a hospital trust or directorate, it merely represents cost shifting, as there is no gain in overall efficiency or cost-effectiveness of the system.
Conclusion
The excess demand for health care relative to supply means not all beneficial health interventions can be funded. Rationing is, therefore, an unavoidable feature of health systems.
There is evidence that critical care can be a very cost-effective use of health care resources compared with other interventions. However, demand for critical care is continually rising. As short-term survival following intensive care continues to improve, measuring longer-term patient-centred outcomes is increasingly important to guide resource allocation decisions. Estimating both the direct and the indirect costs of critical care provision to the health system, patients, carers, and wider society needs to be included in health economic evaluations of critical care. Although significant challenges exist in measurement of both outcomes and costs, doing so would produce increased understanding of how to sustainably deliver valuable critical care.
Declaration of interest
None declared.
MCQs
The associated MCQs (to support CME/CPD activity) can be accessed at http://www.oxforde-learning.com/journals/ by subscribers to BJA Education.
References
Available from http://www.nuffieldtrust.org.uk/nhs-numbers-0 (accessed 17 September 2016)
Available from https://www.icnarc.org/Our-Audit/Audits/Cmp/About (accessed 20 September 2016)





