-
PDF
- Split View
-
Views
-
Cite
Cite
Rochelle Buffenstein, Vince G Amoroso, The Untapped Potential of Comparative Biology in Aging Research: Insights From the Extraordinary-Long-Lived Naked Mole-Rat, The Journals of Gerontology: Series A, Volume 79, Issue 8, August 2024, glae110, https://doi.org/10.1093/gerona/glae110
Close - Share Icon Share
Abstract
The search for solutions to the vagaries of aging has, historically, been akin to searching at night in the bright light under street lamps by utilizing the few preexisting and well-established animal model systems. Throughout my career as a comparative biologist, I have ventured into the darkness across 4 continents and studied over 150 different animal species, many of which have evolved remarkable adaptations to survive on the harsh and rugged fitness landscape that exists outside of the laboratory setting. In this Fellows Forum, I will discuss the main focus of my research for the last 25 years and dig deeply into the biology of the preternaturally long-lived naked mole-rat that makes it an ideal model system for the characterization of successful strategies to combat aging.
Little did I know in 1980, as a student research assistant with Jenny Jarvis, that our field trip to the arid areas in Kenya to test and ultimately confirm a hunch that naked mole-rats, like bees and wasps, were eusocial (1) would ignite a passionate curiosity in this biology of this exceptional species that has continued on for more than 40 years. Over the years, despite conducting research on humans, kangaroos, bats, tenrecs, and a plethora of rodent species, I have found myself repeatedly drawn back to the unknown discoveries that lie deep in these “saber-toothed sausage-like animals.” At that time, none of us had any inkling then that these unusual animals would take center stage as a nonconventional animal model for several areas in biomedical research, nor did we have any idea that naked mole-rats would live 40 years in captivity. Indeed, naked mole-rats have become an attractive “off-the-beaten-track” animal model for aging research as well as a renowned biomedical model of resistance to cancer (2), cardiovascular disease (3), and neurodegeneration (4,5).
Conventional Models of Aging
The ultimate goal of using animal models in aging research is to find innovations that may delay or abrogate the structural wear and tear, and homeostatic dysfunction that characterizes human aging. The multiplicity of aging mechanisms and their diverse manifestations all pose a major challenge in the choice of a model organism. This complexity is not entirely intractable; distinct relationships exist between species body size, membrane composition, and the pace of life with maximum and median lifespan (6). The selection of an appropriate model organism, however, requires one to take into consideration both the biological relevance to the research question being asked and the species amenability to laboratory experimentation. For more than a century, biomedical research has primarily focused upon a few conventional model organisms, most notably—mice (Mus musculus), fruit flies (Drosophila melanogaster), and worms (Caenorhabditis elegans) under the premise that “below the skin we are all the same” (7). These evolutionarily distant animal models share an important feature for the study of aging in that they have very short lifespans, and as they get older, biological functions at all phenotypic levels diminish as illustrated by a decline in whole organism performance (eg, running speed), tissue structure and function (eg, muscle), organelle efficacy (eg, mitochondria), as well as macromolecule damage (eg, protein carbonyls) and concomitant instability. As a result of this disruption in homeostatic mechanisms the likelihood of dying increases, ultimately limiting both their potential longevity and their fitness. The short lifespans of these model organisms permit rapid examination of the mechanisms leading to their observed functional declines and also facilitate the discovery of compensatory interventions. These conventional models have led to spectacular advances in numerous areas of biogerontological research: they have enabled the investigation of molecular mechanisms, genomic manipulation, and drug interventions with both expediency and exactitude. Novel data continuously build upon the myriad of collated information regarding these organisms and the ever-expanding arsenal of novel experimental tools developed specifically for them. These findings have markedly advanced the field of aging research. Through the use of these conventional models, we now know that health span and lifespan can be extended through a number of genetic (8), dietary (9,10), and pharmacological interventions (11). Surprisingly, none of these life-extending interventions in mice prolong their lifespans beyond their expected maximum lifespan predicated on the basis of scaling to body size (12). Rather, these experimental manipulations simply allow mice to attain lifespans that come close to their expected lifespan but not exceed it. Moreover, the interventions that work in conventional models may not provide any additional advantage to humans whose longevity already far exceeds that predicted on the basis of body size-corrected, allometric expectation (13). The translational significance and relevance of these findings to human aging and pathologies have also been called into question, with numerous well-documented clinical failures (14–17). Indeed, to date, none of these interventions has yet been successfully applied to humans. It is therefore possible that aging processes during the course of evolution have been both dynamically and adaptively remodeled by natural selection and as a result may be systematically different between short- and long-lived species. It is likely that additional pivotal mechanisms may be employed in those species that are long-lived outliers of the traditional allometric maximum species lifespan scaling laws. Insights into these additional mechanisms may be obtained by studying unconventional species that during the course of their evolution developed traits to avoid senescence and age-associated pathologies.
Evolutionarily Designed Animal Models of Aging
An alternative approach for gleaning novel insights into successfully slowing aging may be to exploit “evolutionarily designed” solutions that other long-lived species have adopted due to an unusual or idiosyncratic location on the fitness landscape. This “biomimetic approach” (18), integrating insights from comparative biology rather than from inbred conventional animal models focuses primarily on those organisms living in harsh environments (eg, deserts) that cannot easily migrate when environmental conditions become untenable such as when they are challenged by extreme temperatures, lack of oxygen, nutrient insecurity, or restricted water availability (19). Rather, these species may have to hunker down and protect their soma at the expense of reproductive fitness, with the long-term goal that they will live long enough to once again encounter more optimal conditions to reproduce and ensure the survival of their species. Although this is a common feature of many small mammals (eg, bats and rodents) that hibernate or estivate when conditions are extreme (20,21), even larger mammals (eg, oryx and elephant) alter both their behavior and physiology when resources are limiting (22–24) and employ protective mechanisms that may also facilitate greater resilience when faced with chronic age-associated ailments (eg, cancer) (18,25). Indeed, many pathological states share the same physicochemical challenges (eg, hypoxia, oxidation stress, starvation, and dehydration) to those that extremophilic animals may encounter in the various hostile environments (eg, deserts, high altitude, subterranean) that they inhabit. Animals living in such extreme environments find themselves navigating a drastically different fitness landscape than conventional model systems and have likely evolutionarily optimized themselves to these conditions using altered biochemical, informational, and regulatory mechanisms to survive and thrive in such hostile habitats. These evolved mechanisms are likely to be distinct from those employed by humans in response to similar, acute, physicochemical pathological stressors, and potentially may be more effective in overcoming the challenges associated with chronic age-associated diseases. This opens the door for therapeutic translational opportunities to lengthen normal aging by abrogating the onset of a myriad of chronic age-associated diseases, thereby extending health span and, ultimately, longevity.
The Naked Mole-Rat: A Nontraditional Extremophilic Animal Model
The naked mole-rat (Heterocephalus glaber, Figure 1), is one such extremophilic hystricomorph rodent species found in the arid and semi-arid regions of equatorial Africa. Here, it leads an eusocial, subterranean lifestyle living in an extensive maze of poorly ventilated, dark, dank, hot, and humid sealed burrows. The bulbs, corms, and tubers they consume are sparse and patchily distributed and finding these food sources is an energetically costly activity. Animals forage below ground by blindly excavating more superficial tunnels from the main hub of the burrow system, in which they rest together in crowded deep underground nests. As such, it is likely that naked mole-rats commonly encounter environmental conditions associated with hypoxia, hypercapnia, limited food availability, and no access to free water. The naked mole-rat is highly specialized for this inhospitable habitat with numerous sensory and ecophysiological adaptations to optimize mechanisms for survival under the adverse conditions it routinely may encounter (26,27) (Figure 2). The naked mole-rat is also exceptionally long-lived on the basis of body size, with the oldest animal in my care living 40 years, an order of magnitude longer than laboratory mice. This species likely possesses relevant biologies that are lacking in conventional models.

Photograph of an adult naked mole-rat. Picture taken by Megan Smith.
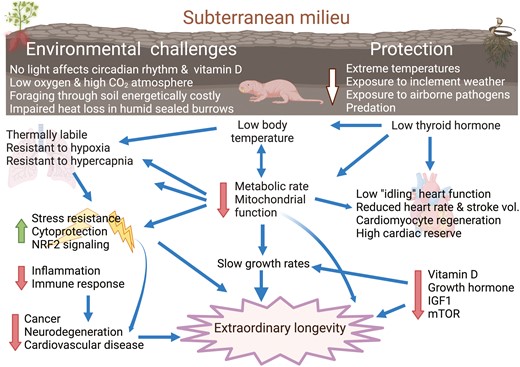
Naked mole-rats exhibit numerous physiological adaptations to a subterranean lifestyle including low body temperatures and low metabolic rates that contribute to enhanced stress resistance, slower growth rates, diminished chronic age-associated diseases, and extreme longevity. Figure modified from Buffenstein and Craft (28) created by biorender.com.
Signs of Successful Aging of the Naked Mole-Rat
Unlike most mammalian species, where lifespan is limited by an exponential increase in the per-day risk of death (ie, mortality hazard) with age (29,30) the demographic aging profile of the naked mole-rat demonstrates that this increased mortality hazard with advancing age is not a fixed property of mammalian biology. Rather the naked mole-rat appears to be the only species known to date to defy these Gompertzian laws of mortality (31). Instead, this small rodent species shows constant mortality hazard across the full spectrum of observed lifespans, with no hazard increase evident even at ages many-fold beyond their allometrically predicted maximum lifespan (32–34). This atypical demographic aging pattern makes the naked mole-rat stand out as an exemplary mammalian model of successfully aging. In defiance of the disposable soma theory of aging, there is a notable survival difference between those of dominant breeding status versus those with nonbreeding subordinate status, with reproductively active individuals exhibiting a mortality hazard of ~20% of that of subordinates and thereby enjoying longer survival. This extended longevity in breeders is accompanied by a phenoplastic epigenetic clock in which breeding females, regardless of when they changed their dominance status, have significantly lower levels of DNA methylation than those of subordinates (35).
This lack of demographic aging in naked mole-rats is accompanied by seemingly indefinite ability to maintain physiological function of major organ systems as they get older (36), compressing morbidity to a very small fraction of their long lifespan. Although to date we have little conclusive data as to what naked mole-rats predominantly naturally die from, pathological assessments upon necropsy have shown some evidence of degenerative pathology manifest in chronic renal, gastrointestinal, and periodontal disease (37). Also, hepatic hemosiderosis has been observed as has adrenal cortical hyperplasia, fibrosis in multiple tissues, and cataracts. These degenerative pathologies are not restricted to old animals but rather have been seen, in all age cohorts (37–39). Unlike mice that show pronounced age-dependent changes in body composition by 2 years of age, naked mole-rats maintain lean tissue, fat mass, and bone mineral density for more than 3 decades (3). Moreover, naked mole-rats maintain metabolic rate, fertility, blood pressure, and vascular stiffness with advancing years (36). In contrast to the data obtained after 18 months of age in mice, electrocardiograms (ECGs) revealed no age-associated changes in the cardiac conduction system with no sign of any arrhythmias in young and old animals, suggestive of a well-maintained sinus rhythm and cardiomyocyte properties (3). Furthermore, although cardiac hypertrophy and diastolic dysfunction were evident in mice by 2 years of age, naked mole-rats resist age-associated left ventricular hypertrophy even when 34 years old. Similarly, early/atrial left ventricular filling velocity (E/A) ratio is unchanged suggesting well-maintained ventricular compliance (3,40,41). Large age-associated declines in cardiac reserve capacity are a common occurrence in both middle-aged humans and mice, in sharp contrast MRI revealed that beta-adrenergic stimulation of heart rate, stroke volume, and cardiac output was unchanged in young and old mole-rats, with the latter maintaining similar functional cardiac reserve capacity in their fourth decade to that exhibited by young adults in the prime of their life (3). Clearly, the naked mole-rat embodies a successful conquest of mitigating age-related declines in physiological function and provides a counter-example to the inevitability of age-associated decline in health (36).
Naked mole-rats maintain numerous youthful pedomorphic or neotenic features throughout their long lives (42). For example, throughout their lives, they retain fetal hemoglobin that can more avidly bind oxygen than adult hemoglobin and maintain expression of the N-methyl-D-aspartate (NMDA) receptor subunit GluN2D, which is normally highly expressed in the neonatal period. Many of these retained juvenile traits may contribute to exceptional tolerance of prolonged hypoxia and even anoxia (28,43). They also rely predominantly upon innate rather than adaptive immunity (44). Moreover, naked mole-rats show an enhanced ability to regenerate damaged tissues (eg, brain and heart) and even produce within the ovary primordial follicles postnatally (45–47). Maintenance of oogenesis postnatally contributes to a large ovarian reserve and the lack of menopause observed even in well-established breeders that are in their third decade of life. Similarly, maintenance of a large stem cell population, numerous growth factors, and organ-specific regenerative capacity help to maintain tissue homeostasis with advancing years and contribute to their negligible senescence phenotype (47).
Naked mole-rats are also resistant to a myriad of age-related diseases such as cancer, neurodegeneration, and cardiovascular disease (3,37). The biological adjustments required to prevent age-associated decline and chronic age-associated diseases in naked mole-rats are likely complex, emerging from multi-million-year evolutionary adaptation to their underground niche in which the mole-rat tends to “idle on low with their shields up” in multiple organs (Figure 2) (28). This is manifest by thyroid deficiency and energy saving, low metabolic rates as well as enhanced translational fidelity, high levels of molecular chaperones, and sustained proteostasis with more stable proteins (Figure 3). Also noteworthy is the constitutive maintenance at high expression levels of various cytoprotective pathways such as nuclear factor erythroid 2–related factor 2 (Nrf2), the tumor protein p53, and hypoxia-inducible factor 1-alpha (HIF-1a) (48). Molecular adaptation to these underground conditions may not only confer survival when faced with rapid changes in oxygen levels but may also protect against pathological states (eg, cardiovascular disease and stroke) in which ischemia/reperfusion injury plays a pivotal role.
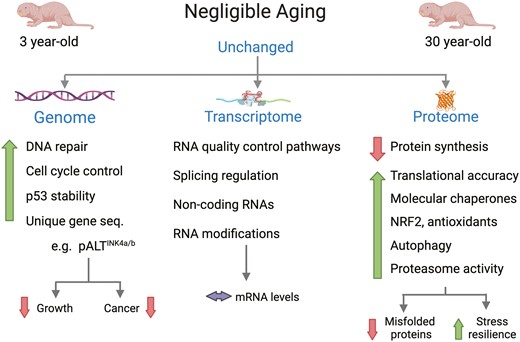
Features of genome, transcriptome, and proteome stability in naked mole-rats that may contribute to their extreme longevity. Figure modified from Narayan et al. (48) and created with BioRender.com.
Molecular Insights of Successful Aging in Naked Mole-Rats
To date, genomic sequencing analysis has identified several unusual features and unique polymorphisms that may contribute to delayed aging and their resistance to cancer (49,50). Yang et al., in a comparative study with other subterranean rodents and rats revealed that several cancer-associated proto-oncogenes (eg, Hras1, Smad3, Fas, Bcl2, BRCA1, Wnt3, and Myc) were mutated or missing possibly affecting upon their functionality (51,52). Collectively, these alterations may negatively influence cancer progression and apoptosis. Several genes appear to be under positive selection such that the expression of advantageous genes shows increased prevalence during the course of evolution. Positive selection signatures are evident in telomere-associated genes as well as in pathways associated with cellular respiration, oxidative metabolism, and stress response, and positive selection also appears to downregulate mTOR and insulin/insulin-like growth factor 1 [IGF1] signaling (27,53). This area of exploration is, however, still in its infancy and no doubt there are many other genomic polymorphisms or loss/gain of function changes that remain to be identified.
Considerable evidence has emerged since the first genome sequencing of the naked mole-rat in 2011 (49) of increased genomic stability (Figure 3) with a lower (a) proportion of transposable elements than humans, (b) intraspecies nucleotide diversity, (c) CpG diversity and thus methylation rates, and (d) susceptibility to mutation (54). Moreover, naked mole-rats reportedly have a tightly regulated genome and epigenome with more closed chromatin structures and more repressive H3K27 methylation on gene promoter regions (54). Naked mole-rats appear surprisingly resilient when faced with a wide variety of stressors (55,56). When irradiated with equivalent doses of radiation, mole-rats acquire less damage accruing significantly lower mutation rates than observed in mice. It is not known if this is only due to more efficient DNA repair and/or cell death pathways, or if they have other mechanisms in place to suppress DNA mutation (57). Transcriptomic assessments reveal that DNA-repair pathways are more highly expressed in naked mole-rats when compared to mice. Naked mole-rats have more efficient nonhomologous end joining, nucleotide excision repair, as well as that of base excision repair. This is attributed to more efficient (poly ADP-ribosylation) (PARylation) catalyzed by DNA-damage sensing orchestrated by poly(ADP-ribose) polymerases and the higher activity of SIRT6 in naked mole-rats (48). Also, several specific DNA repair genes are upregulated or expanded in naked mole-rats when compared to mice, likely contributing to a more stable genome (58). Naked mole-rats appear to strictly regulate their cell cycle, slowing cell proliferation when conditions are suboptimal (56). The rapid removal of DNA damage may also reflect rapid eradication of damaged or senescent cells. However, naked mole-rats show a loss of function mutation in genes RIPK3 (receptor-interacting serine/threonine-protein kinase 3) and MLKL (mixed-lineage kinase domain-like), which are known master regulators of necroptosis. Rather they rely on INK4a-RB-induced cell death and a resulting dampened inflammatory response with reduced immune cell infiltration. This robust anti-inflammatory response when faced with various stressors likely also contributes to the attenuation of inflammaging and the abrogated acquisition of oncogenic driver mutations in damaged cells (57).
Posttranscriptional control of gene expression is essential for maintaining homeostasis. Unlike that observed in other mammals, transcript levels in both young (4 years old) and older (20 years old) naked mole-rats were largely similar (49). Moreover, unlike mice that show a decline in splicing factor levels, naked mole-rats maintain throughout their long lives twofold higher levels of a broad range of splicing factors relative to those observed in young mice (59). Alternative splicing facilitates the maintenance of transcriptome plasticity, particularly in response to cell damage. Clearly, naked mole-rats better maintain transcriptional control of gene expression. This likely contributes to the maintenance of a stable proteome well into old age. Naked mole-rats harbor a distinct ribosomal cleavage pattern that following the transcription of its precursor rRNA is processed to yield 18S, 28S, and 5.8S rRNA in mature ribosomes. It is not known what cellular machinery regulates this unusual splicing and if these mature ribosomes have altered functions or, more specifically, influence the rates of translational fidelity and accuracy. Gene enrichment analysis showed that both ribosome and peptide elongation pathways are decreased in naked mole-rats giving rise to the observed lower rates of protein synthesis and turnover (48). Collectively, these features likely contribute to the prolonged maintenance of a stable and functional proteome (Figure 3).
Transcriptomic data suggest that under nonstressed conditions naked mole-rats have very low levels of expression of components in the insulin, growth hormone, and mTOR pathways; indeed IGF1 is not detectable in young healthy naked mole-rats (27). Comparative genomics studies support the premise of growth hormone insensitivity with divergent sequences seen in the extracellular ligand-binding domain of GHR, suggesting impaired downstream signaling in this endocrine cascade. Rather, transcripts of the fetal form, insulin-like growth factor 2 (IGF2), and its binding protein IGF2BP2, are high (49). In contrast, constitutively high expression levels of 3 cytoprotective transcription factors are evident. These are heat shock factor 1, the master regulator of molecular chaperone expression, NRF2—a transcription factor that regulates a myriad of cytoprotective pathways, and HIF1a that facilitates tolerance to hypoxia. Although all 3 are primed to respond rapidly if stressors are encountered, they nevertheless can also be ramped up if needed to neutralize specific stressors or eradicate any signs of cell damage and thereby maintain homeostasis (48).
Proteomic data confirms that the proteostasis system is more robust than that of mice. There is no age-dependent decline in the abundance nor function of components of the proteostasis network. Rather, molecular chaperones, most notably HSP70, HSPA12A/B, and HSP27 are maintained at high levels throughout life and likely play a pivotal role in the observed resistance of proteins to oxidation, heat, and urea stressors. These, likely also contribute to the efficient maintenance of protein tertiary structure and prevention of the accrual of misfolded aggregated proteins. Similarly, protein degradation and cellular recycling machinery maintain a low level of constitutive activity in the naked mole-rat, but can be ramped up to high levels if needed and these dynamic changes to environmental challenge are unchanged with age. Both proteasome activity and autophagic flux are maintained at high levels throughout their long lives and likely play critical roles in preventing the accrual of damaged organelles or protein aggregates (48).
Conclusion
The combination of the above alterations in biological design has allowed the naked mole-rat to present a phenotype that is a living counter-example to the inevitability of aging. It’s only through experimentation that, as a research community, we can discover the mechanisms and solutions, which can prevent the adverse effects of human aging. In the case of the naked mole-rat, nature has run a successful experiment, more than 30 million years in the making, and as documented above, has provided us a blueprint for how to hold at bay the many adverse effects of aging commonly observed in mammals and experienced by all humanity. The task left in front of us is to firstly remain steadfast in our receptivity to finding solutions wherever they may exist, exploiting more than 8 million extant eukaryotic species that have successfully survived through various environmentally stressful periods during their course of evolution and that may be worthy of exploration. Furthermore, we must remain at the vanguard of our field and master not just the quickly evolving advances in genomics, transcriptomics, proteomics, and metabolomics, but work in an integrative manner to layer and combine such analysis to synergistically yield insights into systems biology that can be understood and reverse-engineered. And, finally, the goal of this knowledge must be the translation of these evolutionary optimizations to conventional models and the betterment of human health. Ultimately, the high risk and technological challenges of working with idiosyncratic species, such as the naked mole-rat, are abated by the greater reward of deciphering their code to avoid the inevitability of aging.
Funding
In writing this manuscript, the authors were supported by the University of Illinois, Chicago, and from a generous gift from Calico Life Sciences LLC.
Conflict of Interest
None.
Acknowledgments
The authors want to thank Dr. Roz Anderson for the invitation to contribute to the Fellows Forum.
Author Contributions
R.B. and V.A. drafted the text of this manuscript. Both authors contributed equally in the creation of the accompanying figures.



