-
PDF
- Split View
-
Views
-
Cite
Cite
Robertina Giacconi, Francesco Piacenza, Fabrizio Maggi, Alexander Bürkle, María Moreno-Villanueva, Lucia Mancinelli, Pietro Giorgio Spezia, Federica Novazzi, Francesca Drago Ferrante, Claudia Minosse, Paolo Antonio Grossi, Nicasio Mancini, Monia Cecati, Martijn E T Dollé, Eugène Jansen, Tilman Grune, Efstathios S Gonos, Claudio Franceschi, Miriam Capri, Birgit Weinberger, Ewa Sikora, Florence Debacq-Chainiaux, Wolfgang Stuetz, Mikko Hurme, P Eline Slagboom, Jürgen Bernhardt, Davide Gentilini, Luciano Calzari, Mirko Di Rosa, Anna Rita Bonfigli, Roberta Galeazzi, Antonio Cherubini, Fabrizia Lattanzio, Mauro Provinciali, Marco Malavolta, Association Between TTV Viremia, Chronic Inflammation, and Ischemic Heart Disease Risk: Insights From MARK-AGE and Report-Age Projects, The Journals of Gerontology: Series A, Volume 79, Issue 11, November 2024, glae228, https://doi.org/10.1093/gerona/glae228
Close - Share Icon Share
Abstract
The implication of Torquetenovirus (TTV) in ischemic heart disease (IHD) has not been thoroughly explored. This study investigated the association between TTV viremia, pro-inflammatory cytokines, and IHD risk in an aging population. This cross-sectional study included 900 non-IHD subjects and 86 individuals with IHD (aged 55–75 years) selected from the MARK-AGE project. Results were verified in another independent Report-Age cohort, including 94 inpatients with chronic IHD and 111 inpatients with non-IHD (aged 65–96 years). Multivariable logistic regression in the MARK-AGE cohort revealed that male sex, TTV viremia ≥4log, Cu/Zn ratio, diabetes, hypertension, and smoking were significant IHD predictors. Notably, TTV viremia ≥4log independently increased the IHD risk (odds ratio [OR]: 2.51, 95% confidence interval [CI]: 1.42–4.43), confirmed in the Report-Age cohort (OR: 4.90, 95% CI: 2.32–10.39). In a RASIG subgroup, individuals with TTV viremia ≥4 log, both with and without IHD, exhibited increased plasma pro-inflammatory cytokine levels (IFN-γ, IL-1β, IL-6, IL-10, IL-12p70, TNF-α) compared to those with TTV viremia <4 log. No significant difference in cytokine production was observed between IHD patients and non-IHD with TTV viremia ≥4 log. A positive correlation between TTV viremia and DNA methylation estimator of leukocyte telomere length was observed in Report-Age patients. Additionally, IHD Report-Age patients with TTV viremia ≥4 log displayed higher NLR and SIRI index than those with TTV viremia <4 log.
In conclusion, a high TTV viremia is associated with an elevated IHD risk in the older population, potentially arising from an augmented pro-inflammatory response and immunosenescence.
Ischemic heart disease (IHD) is a chronic inflammatory disease characterized by atherosclerosis of the coronary arteries and a serious health threat worldwide, particularly in the older population (1). The severity of IHD is associated with cardiovascular death and all-cause mortality (2). Plentiful evidence establishes a link between chronic viral infections and the development of IHD. For instance, an elevated prevalence of coronary artery disease has been observed in patients with chronic hepatitis C or human immunodeficiency virus (3,4). Moreover, cytomegalovirus (CMV), which induces chronic adaptive immune activation and advances immunosenescence (5), might accelerate atherogenesis; CMV viremia has also been linked to acute coronary syndrome (6). One potential mechanism by which viruses may contribute to the development of atherosclerosis and cardiovascular disease is through the induction of cellular senescence (CS). CS is characterized by increased β-galactosidase activity and elevated expression of cell cycle arrest markers, such as p16/INK4, p21/WAF-1, and p53. This process is accompanied by the secretion of a senescence-associated secretory phenotype and the presence of shorter telomeres (7,8).
Population studies indicate that leukocyte telomere length is associated with a higher incidence of IHD, independent of other risk factors (9,10). Senescent endothelial cells, vascular smooth muscle cells, foam cells, and macrophages may all play a role in the pathophysiology of atherosclerosis trough increased levels of inflammatory cytokines and adhesion molecules and reduced nitric oxide production (8).
Multiple studies have demonstrated that various viral infections, including CMV (11,12) can trigger CS responses. Additionally, in HIV patients, the activation and senescence biomarkers of T cells have been found to correlate positively with the presence of subclinical atherosclerosis, independent of age, sex, and traditional cardiovascular risk factors (13).
Increasing evidence points to the involvement of Torquetenovirus (TTV) in immunosenescence among the older population (14,15). TTV is a small, non-enveloped, single-stranded DNA virus belonging to the Anelloviridae family with a high prevalence in the general population (14,16). TTV constitutes a major component of the human virome (9). Although its exact role and clinical significance are still unclear, TTV DNA levels can be used to determine the degree of immunosuppression (17,18). Previously, our group showed that TTV viremia higher than 4log was associated with immune dysfunction, mortality, and an increased risk of frailty in an aging population (14,15,19).
Chronic inflammation and the production of several cytokines are related to the pathophysiology of IHD (20–23), but it is still unclear whether high TTV replication may induce a dysregulation in cytokine production and promote the process of atherogenesis. Moreover, TTV has been found in atheromatous plaque from patients with coronary artery disease (24).
Hence, the goal of this study was to determine the potential relationship between TTV viremia and IHD, along with the production of pro-inflammatory cytokines and markers of systemic inflammation and DNA methylation estimator of leukocyte telomere length (DNAm-LTL) (25).
Materials and Methods
Study Population, Recruitment, Data, and Blood Collection
This cross-sectional study included 900 healthy control subjects and 86 IHD patients, all of whom were selected from the RASIG (Randomly recruited Age-Stratified Individuals from the General population) participants within the age bracket of 55–75 years. These individuals were recruited as part of the European MARK-AGE project (26).
Details of the recruitment procedures and the collection of anthropometric, clinical, and demographic data have been previously published (27,28). Health status and IHD diagnosis were evaluated on the basis of detailed questionnaires administered to participants. The self-reported data were supplemented with medical history of previous acute myocardial infarction, myocardial revascularization, or angina pectoris (28). Plasma isolation procedure from blood as well as the shipment and distribution of biological samples have been described (28). Briefly, lithium heparin plasma was prepared from whole blood, obtained by phlebotomy after overnight fasting, and subsequently stored at −80°C. Samples were then shipped from the various recruitment centers to the MARK-AGE Biobank located at the University of Hohenheim, Stuttgart, Germany. From the Biobank, coded samples were subsequently sent to the IRCCS INRCA on dry ice, where they were stored at −80°C until use.
A second cohort of 205 patients, chosen for data validation, was identified from the Report-Age project, adhering to specific criteria such as the availability of blood samples and complete clinical information. The patients were categorized into 2 groups: 94 individuals with chronic IHD and 111 with other diseases and no evidence of IHD (NIHD). Exclusion criteria encompassed a diagnosis of cancer, acute stroke, sepsis, and acute pancreatitis. The Report-AGE project, a large-scale observational study of older patients (age >65 years) hospitalized at IRCCS INRCA between June 16, 2012, and November 3, 2017 (29), is registered under Trial Registration no. NCT01397682. Blood samples, collected in EDTA tubes (Becton, Dickinson and Company, NJ) within the initial 24 hours following hospital admission, were stored in the INRCA BioGer Biobank. Comprehensive data on the medical history of all participants in this study were retrieved from medical records. Participating physicians and nurses were specifically trained before starting recruitment, as previously described (30,31). Diagnoses were coded in accordance with the International Classification of Diseases, 9th revision (http://www.icd9data.com/). The inclusion criterion for chronic IHD was confirmed based on the presence of at least one diagnosis (at admission and/or earlier) with one of the following ICD-9 codes: 412 (previous myocardial infarction) 413.x (angina pectoris), 414.0, 414.8, 414.9 (IHD). No patients diagnosed with an acute heart attack were present in this cohort (ICD-9: 410).
Upon hospital admission, laboratory parameters such as blood cell counts, creatinine, albumin, hemoglobin, and aspartate aminotransferase/alanine aminotransferase (AST/ALT) were measured using standardized procedures. The SIRI index was calculated as the formula of neutrophil count * monocyte count/lymphocyte count. GFR was estimated according to Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation (32).
TTV DNA Detection and Quantification
Viral DNA was extracted from whole blood samples using QIAamp DNA Blood mini kit (Qiagen GmbH, Germany) according to the manufacturer’s instructions. The presence and load of TTV DNA were determined in a single-step in-house TaqMan PCR assay as described elsewhere (14,33). This assay is called “universal PCR” because it uses forward and reverse primers designed on a highly conserved segment of the untranslated region of the viral genome, and it quantifies the total load of TTV DNA amplifying, without discrimination between the TTV species that circulate in the blood of a single subject.
The lower limit of detection was 10 copies of TTV DNA per mL of blood. The procedures used for copy number quantification and the assessment of specificity, sensitivity, intra- and interassay precision, and reproducibility have already been described (33). TTV viremia equal to or exceeding 10 000 copies/mL (equivalent to ≥4 log copies) was categorized as high-level viremia.
Hs-cTnT and NT-proBNP Assays
Serum HS-cTnT was dosed by Elecsys Troponin T hs STAT assay. Age-specific 99th percentile, normal troponin range levels for HS-cTnT in our laboratory is ≤40 pg/mL for over 85 years patients (34). Serum NT-proBNP was assessed on a Dimension Vista 1500 automated chemistry analyzer using NT-proBNP (PBNP) assays (Siemens Healthineers, Germany) based on LOCI technology.
Zinc and Copper Plasma Level Determinations
Cu/Zn ratio is a relevant biomarker of chronic inflammation associated with aging and a predictor of all-cause mortality in older population (35–37). Moreover, elevated serum Cu/Zn ratio is associated with an increased risk of heart failure and cardiovascular disease (38,39). The plasma zinc (Zn) and copper (Cu) were determined by a Thermo XII Series ICP-MS (Thermo Electron Corporation, Waltham, MA) by adapting methods used for the measurement of trace elements in human plasma with slight modifications (37).
Cytokine Assay
BD CBA Human Flex set” and the “BD FACS Array” (BD Biosciences) were used to quantify specifically and simultaneously concentration of the plasma cytokines (IL-1β, IL-6, IL-10, IL-12p70, TNF-α, and IFN-γ).
Estimation of surrogate DNAm-based telomere length in leukocytes (DNAm-LTL)
Genomic DNA was extracted from whole blood using the QIAamp DNA Blood Kit (Qiagen) and bisulfite-converted using the EZ DNA Methylation Kit (Zymo Research). Genome-wide DNA methylation was assessed with the Infinium Human Methylation EPIC BeadChip (Illumina), following the manufacturer’s instructions (40). DNAm-LTL Leukocyte telomere length (LTL) estimation (25) was calculated using the R package dnaMethyAge A user-friendly R package to predict epigenetic age and calculate age acceleration from DNA methylation data (https://github.com/yiluyucheng/dnaMethyAge).
Statistical Analysis
Subject characteristics were reported as mean ± standard error of the mean (SEM) or percentages for continuous and categorical variables, respectively. For continuous variables, the normal distribution was verified by the 1-sample Kolmogorov–Smirnov test. All the variables not normally distributed were log-transformed. Differences among groups were checked by the One-way Analysis of Variance for continuous variables and Pearson’s χ2 test for categorical variables. ANOVA, after correction for age, sex (Report-Age cohort) or age, sex, and country (RASIG group) was used to evaluate differences in inflammatory markers between TTV DNA viremia categories among NIHD, IHD, and NIHD groups. Multilogistic regression analysis was carried out to explore the association between the TTV and the presence or absence of IHD in RASIG participants. The variables included were: age, sex, country, body mass index (BMI), C-reactive protein (CRP), TTV copies ≥4 log (binary variable), Cu/Zn ratio, CMV-IgG antibody levels, diabetes, hypertension, hypercholesterolemia, and smoking. Additionally, a second Multinomial Logistic Regression Analysis was conducted in the Report-Age cohort, incorporating the variables of age, sex, TTV copies ≥4 log (as a binary variable), Charlson Comorbidity Index, chronic heart failure, and the number of medications at discharge.
All the analyses were performed using the SPSS/Win program (version 27.0; SPSS Inc., Chicago, IL).
Results
Characteristics of the Study Population
Characteristics of the study population are summarized in Table 1.
| . | NIHD, N = 900 . | IHD Patients, N = 86 . | p Value . |
|---|---|---|---|
| Age (years) | 64.3 ± 6.7 | 65.5 ± 6.8 | NS |
| Males (%) | 504 (56.6) | 61 (70.9) | .010 |
| BMI (kg/m2) | 26.8 ± 0.1 | 28.0 ± 0.5 | .005 |
| Diabetes, n (%) | 61 (6.7) | 15 (17.4) | .001 |
| Hypertension, n (%) | 286 (31.7) | 47 (54.6) | <.001 |
| AF, n (%) | 70 (7.7) | 20 (23.2) | <.001 |
| Previous MI (%) | 0 (0) | 37 (43.0) | <.001 |
| CRP (μg/L) | 2.1 ± 0.1 | 2.8 ± 0.2 | .035 |
| NLR | 1.89 ± 0.03 | 1.90 ± 0.10 | NS |
| Neutrophils (×103/μL) | 3.4 ± 0.09 | 4.0 ± 0.10 | NS |
| Lymphocytes (×103/μL) | 1.99 ± 0.07 | 2.38 ± 0.22 | NS |
| Cu/Zn ratio | 1.57 ± 0.01 | 1.69 ± 0.03 | .001 |
| Total cholesterol (mmol/L) | 5.68 ± 0.03 | 5.13 ± 0.11 | <.001 |
| LDL cholesterol (mmol/L) | 3.42 ± 0.03 | 2.96 ± 0.10 | <.001 |
| Triglycerides (mmol/L) | 1.29 ± 0.03 | 1.40 ± 0.08 | NS |
| CMV-IgG | 40.8 ± 1.5 | 54.7 ± 4.8 | .006 |
| . | NIHD, N = 900 . | IHD Patients, N = 86 . | p Value . |
|---|---|---|---|
| Age (years) | 64.3 ± 6.7 | 65.5 ± 6.8 | NS |
| Males (%) | 504 (56.6) | 61 (70.9) | .010 |
| BMI (kg/m2) | 26.8 ± 0.1 | 28.0 ± 0.5 | .005 |
| Diabetes, n (%) | 61 (6.7) | 15 (17.4) | .001 |
| Hypertension, n (%) | 286 (31.7) | 47 (54.6) | <.001 |
| AF, n (%) | 70 (7.7) | 20 (23.2) | <.001 |
| Previous MI (%) | 0 (0) | 37 (43.0) | <.001 |
| CRP (μg/L) | 2.1 ± 0.1 | 2.8 ± 0.2 | .035 |
| NLR | 1.89 ± 0.03 | 1.90 ± 0.10 | NS |
| Neutrophils (×103/μL) | 3.4 ± 0.09 | 4.0 ± 0.10 | NS |
| Lymphocytes (×103/μL) | 1.99 ± 0.07 | 2.38 ± 0.22 | NS |
| Cu/Zn ratio | 1.57 ± 0.01 | 1.69 ± 0.03 | .001 |
| Total cholesterol (mmol/L) | 5.68 ± 0.03 | 5.13 ± 0.11 | <.001 |
| LDL cholesterol (mmol/L) | 3.42 ± 0.03 | 2.96 ± 0.10 | <.001 |
| Triglycerides (mmol/L) | 1.29 ± 0.03 | 1.40 ± 0.08 | NS |
| CMV-IgG | 40.8 ± 1.5 | 54.7 ± 4.8 | .006 |
Notes: Data are reported as mean ± standard error of the mean (SEM) or percentages. The laboratory parameter analysis was adjusted for age, sex, countries. AF = atrial fibrillation; BMI = body mass index; CRP = C-reactive protein, Cu/Zn = copper/zinc ratio; CMV = cytomegalovirus; IHD = ischemic heart disease; LDL = low-density lipoprotein; MI = myocardial infarction; NIHD = non-ischemic heart disease; NLR = neutrophil-to-lymphocyte ratio; NS = not significant.
| . | NIHD, N = 900 . | IHD Patients, N = 86 . | p Value . |
|---|---|---|---|
| Age (years) | 64.3 ± 6.7 | 65.5 ± 6.8 | NS |
| Males (%) | 504 (56.6) | 61 (70.9) | .010 |
| BMI (kg/m2) | 26.8 ± 0.1 | 28.0 ± 0.5 | .005 |
| Diabetes, n (%) | 61 (6.7) | 15 (17.4) | .001 |
| Hypertension, n (%) | 286 (31.7) | 47 (54.6) | <.001 |
| AF, n (%) | 70 (7.7) | 20 (23.2) | <.001 |
| Previous MI (%) | 0 (0) | 37 (43.0) | <.001 |
| CRP (μg/L) | 2.1 ± 0.1 | 2.8 ± 0.2 | .035 |
| NLR | 1.89 ± 0.03 | 1.90 ± 0.10 | NS |
| Neutrophils (×103/μL) | 3.4 ± 0.09 | 4.0 ± 0.10 | NS |
| Lymphocytes (×103/μL) | 1.99 ± 0.07 | 2.38 ± 0.22 | NS |
| Cu/Zn ratio | 1.57 ± 0.01 | 1.69 ± 0.03 | .001 |
| Total cholesterol (mmol/L) | 5.68 ± 0.03 | 5.13 ± 0.11 | <.001 |
| LDL cholesterol (mmol/L) | 3.42 ± 0.03 | 2.96 ± 0.10 | <.001 |
| Triglycerides (mmol/L) | 1.29 ± 0.03 | 1.40 ± 0.08 | NS |
| CMV-IgG | 40.8 ± 1.5 | 54.7 ± 4.8 | .006 |
| . | NIHD, N = 900 . | IHD Patients, N = 86 . | p Value . |
|---|---|---|---|
| Age (years) | 64.3 ± 6.7 | 65.5 ± 6.8 | NS |
| Males (%) | 504 (56.6) | 61 (70.9) | .010 |
| BMI (kg/m2) | 26.8 ± 0.1 | 28.0 ± 0.5 | .005 |
| Diabetes, n (%) | 61 (6.7) | 15 (17.4) | .001 |
| Hypertension, n (%) | 286 (31.7) | 47 (54.6) | <.001 |
| AF, n (%) | 70 (7.7) | 20 (23.2) | <.001 |
| Previous MI (%) | 0 (0) | 37 (43.0) | <.001 |
| CRP (μg/L) | 2.1 ± 0.1 | 2.8 ± 0.2 | .035 |
| NLR | 1.89 ± 0.03 | 1.90 ± 0.10 | NS |
| Neutrophils (×103/μL) | 3.4 ± 0.09 | 4.0 ± 0.10 | NS |
| Lymphocytes (×103/μL) | 1.99 ± 0.07 | 2.38 ± 0.22 | NS |
| Cu/Zn ratio | 1.57 ± 0.01 | 1.69 ± 0.03 | .001 |
| Total cholesterol (mmol/L) | 5.68 ± 0.03 | 5.13 ± 0.11 | <.001 |
| LDL cholesterol (mmol/L) | 3.42 ± 0.03 | 2.96 ± 0.10 | <.001 |
| Triglycerides (mmol/L) | 1.29 ± 0.03 | 1.40 ± 0.08 | NS |
| CMV-IgG | 40.8 ± 1.5 | 54.7 ± 4.8 | .006 |
Notes: Data are reported as mean ± standard error of the mean (SEM) or percentages. The laboratory parameter analysis was adjusted for age, sex, countries. AF = atrial fibrillation; BMI = body mass index; CRP = C-reactive protein, Cu/Zn = copper/zinc ratio; CMV = cytomegalovirus; IHD = ischemic heart disease; LDL = low-density lipoprotein; MI = myocardial infarction; NIHD = non-ischemic heart disease; NLR = neutrophil-to-lymphocyte ratio; NS = not significant.
IHD patients share a comparable age with the NIHD but had a higher proportion of males. IHD subjects also displayed elevated BMI, and some inflammatory markers (CRP, Cu/Zn ratio), increased anti-CMV-IgG antibody levels, along with reduced levels of total and LDL cholesterol due to the administration of hypolipidemic drugs, compared to the healthy donors (p < .0001).
The medications taken by IHD MARK-AGE participants are listed in Supplementary Table 2. Furthermore, the prevalence of diabetes mellitus, hypertension, and atrial fibrillation was significantly greater in the patient group compared to the NIHD group (p < .001). 43.0% of IHD subjects had undergone a myocardial infarction in the past. The characteristics of the Report-Age cohort are summarized in Supplementary Table 1. There is no disparity in age, sex distribution, or prevalence of the main comorbidities between IHD and NIHD patients, except for a higher incidence of congestive heart failure in those with IHD and an increased use of beta-blockers, vasodilators, statins, and antithrombotic agents among IHD patients compared to NIHD patients (Supplementary Table 1). Additionally, IHD patients exhibited lower eGFR values than NIHD (Supplementary Table 1) and were predominantly characterized by advanced CKD (G4: 63.7%) and moderate stage CKD (G3b: 36.3%), whereas NIHD patients were more commonly affected by mild to moderate CKD (G3a: 33.3%; G3b: 66.7%).
Multilogistic Regression Analysis to Determine the Association Between the TTV and the Presence or Absence of IHD in the MARK-AGE and the Report-Age Cohort
Table 2 shows the results of the association between the participants’ sociodemographic and clinical characteristics, TTV, inflammatory markers, and IHD, in the adjusted multinomial regression models in the MARK-AGE participants. The main predictors of IHD included male gender, TTV viremia ≥4 log, Cu/Zn ratio, diabetes, hypertension, and smoking. TTV viremia ≥4 log (odds ratio [OR]: 2.51, 95% confidence interval [CI]: 1.42–4.43) was an independent risk factor of IHD in RASIG participants. The association of TTV viremia ≥4 log (OR: 4.90, 95% CI: 2.32–10.39) with chronic IHD has been confirmed in the Report-Age cohort adjusting for age, sex, Charlson Comorbidity Index, chronic heart failure and number of medications at discharge (Table 3) even after including the major comorbidities (OR: 5.71 95% CI: 2.72–11.97; Supplementary Table 3). TTV viremia was compared among NIHD and IHD patients. IHD patients exhibited significantly higher levels of TTV viremia than NIHD patients (Supplementary Figure 1).
Multinomial Logistic Regression Analysis of Ischemic Heart Disease (IHD) in the MARK-AGE Population
| Variables . | Categories . | IHD . | p Value . |
|---|---|---|---|
| OR (95% CI) . | |||
| Age | 1.0 (0.97–1.05) | NS | |
| Sex | Males | 3.45 (1.81–6.59) | <.0001 |
| Females | 1 (reference) | ||
| Countries | 0.91 (0.80–1.03) | NS | |
| BMI | 1.01 (0.96–1.08) | NS | |
| Cu/Zn | 3.71 (1.63–8.42) | .002 | |
| CMV-IgG | 1.00 (0.99–1.01) | NS | |
| CRP | 1.00 (0.94–1.07) | NS | |
| TTV viremia | <4 log | 1 (reference) | |
| ≥4 log | 2.51 (1.42–4.43) | .002 | |
| Diabetes | Yes | 2.54 (1.22–5.26) | .012 |
| No | 1 (reference) | ||
| Hypertension | Yes | 2.34 (1.37–3.99) | .002 |
| No | 1 (reference) | ||
| Hypercholesterolemia | Yes | 0.68 (0.39–1.16) | NS |
| No | 1 (reference) | ||
| Smoking | Current | 2.08 (1.13–3.84) | .019 |
| No | 1 (reference) |
| Variables . | Categories . | IHD . | p Value . |
|---|---|---|---|
| OR (95% CI) . | |||
| Age | 1.0 (0.97–1.05) | NS | |
| Sex | Males | 3.45 (1.81–6.59) | <.0001 |
| Females | 1 (reference) | ||
| Countries | 0.91 (0.80–1.03) | NS | |
| BMI | 1.01 (0.96–1.08) | NS | |
| Cu/Zn | 3.71 (1.63–8.42) | .002 | |
| CMV-IgG | 1.00 (0.99–1.01) | NS | |
| CRP | 1.00 (0.94–1.07) | NS | |
| TTV viremia | <4 log | 1 (reference) | |
| ≥4 log | 2.51 (1.42–4.43) | .002 | |
| Diabetes | Yes | 2.54 (1.22–5.26) | .012 |
| No | 1 (reference) | ||
| Hypertension | Yes | 2.34 (1.37–3.99) | .002 |
| No | 1 (reference) | ||
| Hypercholesterolemia | Yes | 0.68 (0.39–1.16) | NS |
| No | 1 (reference) | ||
| Smoking | Current | 2.08 (1.13–3.84) | .019 |
| No | 1 (reference) |
Notes: Gender was categorized as follows: 1 = males and 0 = females. BMI = body mass index; CI = confidence interval; CMV-IgG = cytomegalovirus IgG; CRP = C-reactive protein; Cu/Zn = copper/zinc ratio; IgG = immunoglobulin G; NS = not significant; OR = odds ratio; TTV = Torquetenovirus.
Multinomial Logistic Regression Analysis of Ischemic Heart Disease (IHD) in the MARK-AGE Population
| Variables . | Categories . | IHD . | p Value . |
|---|---|---|---|
| OR (95% CI) . | |||
| Age | 1.0 (0.97–1.05) | NS | |
| Sex | Males | 3.45 (1.81–6.59) | <.0001 |
| Females | 1 (reference) | ||
| Countries | 0.91 (0.80–1.03) | NS | |
| BMI | 1.01 (0.96–1.08) | NS | |
| Cu/Zn | 3.71 (1.63–8.42) | .002 | |
| CMV-IgG | 1.00 (0.99–1.01) | NS | |
| CRP | 1.00 (0.94–1.07) | NS | |
| TTV viremia | <4 log | 1 (reference) | |
| ≥4 log | 2.51 (1.42–4.43) | .002 | |
| Diabetes | Yes | 2.54 (1.22–5.26) | .012 |
| No | 1 (reference) | ||
| Hypertension | Yes | 2.34 (1.37–3.99) | .002 |
| No | 1 (reference) | ||
| Hypercholesterolemia | Yes | 0.68 (0.39–1.16) | NS |
| No | 1 (reference) | ||
| Smoking | Current | 2.08 (1.13–3.84) | .019 |
| No | 1 (reference) |
| Variables . | Categories . | IHD . | p Value . |
|---|---|---|---|
| OR (95% CI) . | |||
| Age | 1.0 (0.97–1.05) | NS | |
| Sex | Males | 3.45 (1.81–6.59) | <.0001 |
| Females | 1 (reference) | ||
| Countries | 0.91 (0.80–1.03) | NS | |
| BMI | 1.01 (0.96–1.08) | NS | |
| Cu/Zn | 3.71 (1.63–8.42) | .002 | |
| CMV-IgG | 1.00 (0.99–1.01) | NS | |
| CRP | 1.00 (0.94–1.07) | NS | |
| TTV viremia | <4 log | 1 (reference) | |
| ≥4 log | 2.51 (1.42–4.43) | .002 | |
| Diabetes | Yes | 2.54 (1.22–5.26) | .012 |
| No | 1 (reference) | ||
| Hypertension | Yes | 2.34 (1.37–3.99) | .002 |
| No | 1 (reference) | ||
| Hypercholesterolemia | Yes | 0.68 (0.39–1.16) | NS |
| No | 1 (reference) | ||
| Smoking | Current | 2.08 (1.13–3.84) | .019 |
| No | 1 (reference) |
Notes: Gender was categorized as follows: 1 = males and 0 = females. BMI = body mass index; CI = confidence interval; CMV-IgG = cytomegalovirus IgG; CRP = C-reactive protein; Cu/Zn = copper/zinc ratio; IgG = immunoglobulin G; NS = not significant; OR = odds ratio; TTV = Torquetenovirus.
Multinomial Logistic Regression Analysis of Ischemic Heart Disease (IHD) in the Report-Age Cohort
| Variables . | Categories . | IHD . | p Value . |
|---|---|---|---|
| OR (95% CI) . | |||
| Age | 1.03 (0.98–1.08) | NS | |
| Sex | Males | 1.12 (0.59–2.12) | NS |
| Females | 1 (reference) | ||
| TTV viremia | <4 log | 1 (reference) | |
| ≥4 log | 4.90 (2.32–1.39) | <.0001 | |
| CCI | 1.14 (0.73–1.76) | NS | |
| CHF | 1.27 (0.55–2.95) | NS | |
| Number of medications at discharge | 1.13 (1.12–1.43) | <.0001 | |
| Variables . | Categories . | IHD . | p Value . |
|---|---|---|---|
| OR (95% CI) . | |||
| Age | 1.03 (0.98–1.08) | NS | |
| Sex | Males | 1.12 (0.59–2.12) | NS |
| Females | 1 (reference) | ||
| TTV viremia | <4 log | 1 (reference) | |
| ≥4 log | 4.90 (2.32–1.39) | <.0001 | |
| CCI | 1.14 (0.73–1.76) | NS | |
| CHF | 1.27 (0.55–2.95) | NS | |
| Number of medications at discharge | 1.13 (1.12–1.43) | <.0001 | |
Notes: Gender was categorized as follows: 1 = males and 0 = females. CCI = Charlson Comorbidity Index; CHF = chronic heart failure; CI = confidence interval; NS = not significant; OR = odds ratio; TTV = Torquetenovirus.
Multinomial Logistic Regression Analysis of Ischemic Heart Disease (IHD) in the Report-Age Cohort
| Variables . | Categories . | IHD . | p Value . |
|---|---|---|---|
| OR (95% CI) . | |||
| Age | 1.03 (0.98–1.08) | NS | |
| Sex | Males | 1.12 (0.59–2.12) | NS |
| Females | 1 (reference) | ||
| TTV viremia | <4 log | 1 (reference) | |
| ≥4 log | 4.90 (2.32–1.39) | <.0001 | |
| CCI | 1.14 (0.73–1.76) | NS | |
| CHF | 1.27 (0.55–2.95) | NS | |
| Number of medications at discharge | 1.13 (1.12–1.43) | <.0001 | |
| Variables . | Categories . | IHD . | p Value . |
|---|---|---|---|
| OR (95% CI) . | |||
| Age | 1.03 (0.98–1.08) | NS | |
| Sex | Males | 1.12 (0.59–2.12) | NS |
| Females | 1 (reference) | ||
| TTV viremia | <4 log | 1 (reference) | |
| ≥4 log | 4.90 (2.32–1.39) | <.0001 | |
| CCI | 1.14 (0.73–1.76) | NS | |
| CHF | 1.27 (0.55–2.95) | NS | |
| Number of medications at discharge | 1.13 (1.12–1.43) | <.0001 | |
Notes: Gender was categorized as follows: 1 = males and 0 = females. CCI = Charlson Comorbidity Index; CHF = chronic heart failure; CI = confidence interval; NS = not significant; OR = odds ratio; TTV = Torquetenovirus.
Plasma Cytokine Concentrations in IHD and NIHD Subjects From MARK-AGE Study
Plasma pro-inflammatory cytokine levels were analyzed in a subgroup of 20 IHD patients (7 females and 13 males; mean age 66.4 ± 5.8) and 182 NIHD (110 females and 72 males; mean age 64.6 ± 6.0) from the RASIG cohort. IFN-γ, IL-1β, IL-6, IL-10, IL-12p70, and TNF-α increased in IHD patients and NIHD with TTV viremia ≥4 log as compared to those with TTV viremia <4 log (Figure 1). Furthermore, IHD who exhibited TTV viremia ≥4 log had significantly higher plasma cytokine concentrations than NIHD with TTV viremia <4 log. However, their cytokine levels were comparable to those of NIHD with TTV viremia ≥4 log.
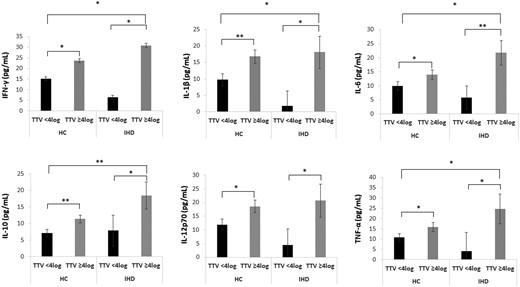
Plasma cytokine concentrations in ischemic heart disease (IHD) patients and non-ischemic heart disease (NIHD) subjects according to TTV viremia in the MARK-AGE study. IFN-γ, IL-1β, IL-6, IL-10, IL-12p70, and TNF-α increased in IHD patients and NIHD with TTV viremia ≥4 log compared to those with TTV viremia <4 log. IHD with TTV viremia ≥4 log exhibited heightened cytokine production compared to NIHD with TTV viremia <4 log, but this effect was not observed in comparison to NIHD with TTV viremia >4 log. ANCOVA analysis correcting for age, sex and country. HC = healthy controls; TTV = Torquetenovirus. *p < .05. **p < .01.
NLR and SIRI Index in the Report-Age and MARK-AGE Cohorts Based on TTV Viremia and Correlation Between DNAm-LTL, Age and TTV DNA Loads in Report-Age Participants
To assess the systemic inflammatory response within our cohorts and explore potential modulation in relation to TTV viremia, NLR and SIRI index have been analyzed. In the Report-Age cohort, IHD patients with TTV viremia ≥4 log exhibited significantly elevated NLR and SIRI indices compared to those with TTV viremia <4 log (Figure 2A and B). However, no significant differences were observed in the NLR and SIRI parameters among NIHD patients from the Report-Age group or NIHD and IHD subjects from the MARK-AGE cohort based on TTV viremia levels (Figure 2C and D).
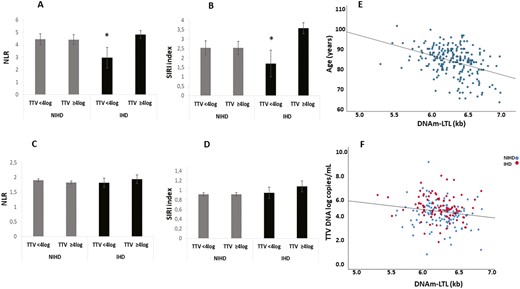
NLR and SIRI index in the Report-Age and MARK-AGE cohorts according to TTV viremia and correlation between DNAm-LTL, age and TTV DNA loads in Report-Age participants. IHD Report-Age patients with TTV viremia ≥4 log showed significantly higher NLR and SIRI index compared to those with TTV viremia <4 log in (A, B). No significant differences were found in the NLR and SIRI index among subjects from the MARK-AGE non-ischemic heart disease (NIHD) group or those with ischemic heart disease (IHD), based on TTV viremia (C, D). ANCOVA analysis correcting for age and sex (A, B) or age, sex and countries (C, D) was applied; data are reported mean ± Standard Error of the Mean (SEM) *p < .05 compared to IHD patients with TTV viremia ≥4 log. (E, F) Scatter plots and Sperman correlation between DNAm-LTL, age and TTV DNA loads in Report-Age cohort. A negative correlation between DNAm-LTL and age was observed (r = −0.366, p < .001) in Report-Age cohort (E). TTV DNA loads (log-transformed data) were negatively correlated with DNAm-LTL in both NIHD (r = −0.172, p < .05) and IHD subjects (r = −0.228, p < .05) (F). DNAmTL values were expressed in kilobases (Kb) and viral loads were reported as number of viral genomic copies per mL of whole blood. NLR = neutrophil-to-lymphocyte ratio; SIRI = standardized inflammatory response index; TTV = Torquetenovirus.
A negative correlation between DNAm-LTL and age was observed (r = −0.366, p < .001) in Report-Age cohort (Figure 2E). TTV viremia was negatively correlated with DNAm-LTL in both NIHD (r = −0.172, p < .05) and IHD subjects (r = −0.228, p < .05; Figure 2F).
Discussion
IHD is a leading cause of morbidity and mortality worldwide, with noteworthy implications for both medical and public health systems (41). In addition to traditional cardiovascular risk factors (hypertension, hyperlipidemia, smoking, obesity, and diabetes) causing IHD, emerging evidence shows a role of viral infections in the disease etiology through chronic, repeated, and persistent inflammation (6,42). In our study we found, for the first time, that the risk of IHD is closely associated with TTV viremia ≥4 log, observed both in a younger population like RASIG participants of the MARK-AGE study and in a geriatric population with multiple comorbidities, as seen in the Report-Age cohort. Although it is not possible to establish a causal role of TTV in the development of coronary syndromes, many indications suggest a link between TTV and inflammation. Indeed, TTV has also been associated with immunosenescence and frailty syndrome (14,15,19), and inflammation has been involved in the pathogenesis of both conditions (43). An elevated TTV DNA load has also been linked to reduced CD4 T-cell counts (14), a condition that is associated with an increased risk of developing cardiovascular events (44). While some evidence suggests a potential role in coronary artery disease for other viruses associated with immunosenescence, such as CMV (6,45), conflicting data also exist (46). In our study, individuals with IHD exhibit higher levels of anti-CMV-IgG levels compared to controls in the MARK-AGE population. However, a Multinomial Logistic Regression Analysis reveals no significant association with IHD after adjusting for various confounding factors.
Despite not being classified as a pathogenic virus, TTV has been clinically linked to several chronic immune diseases, including Crohn’s disease, where TTV species are more abundant in the relapse rather than remission phase (47). Increased TTV DNA loads have also been associated with pulmonary function and the severity of chronic obstructive pulmonary disease, in which TTV is highly prevalent and multiple TTV genotype combinations are frequent (48).
We explored the potential association between markers of systemic inflammation, such as the SIRI index and NLR, within our cohorts. No significant differences were noted in the NLR and SIRI parameters or Cu/Zn ratio (Supplementary Figure 2) among NIHD group, and IHD individuals in the MARK-AGE cohort based on TTV viremia levels. In contrast, IHD patients from the Report-Age cohort exhibited increases in both systemic inflammatory parameters in relation to TTV viremia levels. Although a systemic inflammation, characterized by elevated CRP, Cu/Zn ratio (49), NLR, and SIRI index parameters, may not be evident in younger individuals with IHD (MARK-AGE cohort) and high TTV viremia, an increased pro-inflammatory response was observed. Indeed, individuals within the RASIG group with TTV viremia exceeding 4 logs, showed a notable upregulation of several pro-inflammatory cytokines such as IFN-γ, IL-1β, IL-6, IL-10, IL-12p70, and TNF-α. Previous in vitro experiments showed that TTV can induce gene expression of IFNγ, IL-6, and IL-10 cytokines (50).
All these cytokines may have a significant role in the development and progression of IHD. Interleukins (IL-1β and IL-6), along with interferon-γ (IFN-γ), and tumor necrosis factor-alpha (TNF-α), are cytokines commonly associated with endothelial dysfunction, vascular inflammation, and the progression of atherosclerosis (51).
TNF-α is a key pro-inflammatory mediator implicated in various immunopathogenic processes, triggering acute vascular inflammation and facilitating the transition from stable coronary artery disease to acute coronary syndrome by upregulating ICAM-1 (22,51). IL-6 serves as an independent risk factor for cardiovascular mortality and could be a prognostic indicator of cardiovascular events (52). IL-10, typically protective, shows elevated levels in patients with severe coronary stenosis (53,54). Pro-inflammatory IFN-γ plays a crucial role in regulating the Th1/Th2 ratio and is significantly associated with increased risk and severity of CAD (55,56). IL-12, produced by activated classical antigen-presenting cells, is elevated in human atherosclerotic plaques and significantly associated with a higher risk of cardiovascular events and CAD severity (23).
This study is the first to exhibit an association between elevated TTV viremia and increased cytokine production among patients with IHD. Another study, carried out in children, who underwent bone marrow transplantation and experienced liver injury, revealed an upregulation of IFN-γ, TNF-α, FGF-basic, and MCP-3 in TTV-positive patients when compared to both TTV-negative individuals and the control subjects (57). Further research is necessary to establish the role of TTV in the inflammatory response and its implication in IHD pathogenesis. Another interesting result that might explain the immunosenescent and pro-inflammatory phenotype of subjects with a high TTV DNA load is the negative association found between DNAm-LTL and TTV viremia. DNAm-LTL has been associated with chronological age, sex, ethnicity, lifestyle factors, clinical biomarkers of cardiovascular disease, and results to have better predictive power than LTL for time-to-death and time-to-coronary heart disease (25). The correlation between DNAm-LTL and TTV viremia in our study may support the role of viral infection in inducing CS and aligns with other studies that have demonstrated the contribution of virus to accelerated CS (58,59), suggesting another mechanism through which high TTV viremia may be a risk factor for IHD (9,10).
Our study has several limitations that should be acknowledged. In the MARK-AGE cohort, IHD was defined based on self-reported disease diagnosis rather than standardized diagnostic guidelines, which might introduce misclassification bias. In the Report-AGE cohort, the diagnosis of IHD was coded according to the International Classification of Diseases, 9th revision, which might not be accurate.
However, measurements of high-sensitivity cardiac troponin and NT-proBNP in a subgroup of Report-Age patients showed higher values of both biomarkers in IHD patients compared to NIHD (Supplementary Figure 3). Other limitations include the small sample size of IHD patients, the absence of IFN type I and III assays, which are implicated in controlling viremia, the cross-sectional design, that prevents to establish the causal relationship between TTV and IHD development, the absence of an assessment to determine whether the drugs taken by study participants could impact TTV viremia. Moreover, the lack of a follow-up period to monitor the progression of IHD over time and to evaluate the association of increased TTV viremia and future cardiovascular events. Larger prospective studies should be conducted to examine the diagnostic significance of high TTV viral load in IHD patients.
In conclusion, TTV viral load ≥4 log copies/mL of blood was identified as an independent IHD risk factor and associated with increased pro-inflammatory response and reduced DNAm-LTL values.
Funding
This work was funded by the European Union – Next Generation EU – PNRR M6C2 – Investimento 2.1 Valorizzazione e potenziamento della Ricerca biomedica del SSN PNRR-MAD-2022-12376334 VIROMA project.
Conflict of Interest
None.
Data Availability
The data sets analyzed in this study are available from the corresponding author upon reasonable request.
Acknowledgments
The authors thank the MARK-AGE consortium, the subjects who participated in the study, and the BioGer Institutional Biobank at IRCCS INRCA for providing the samples. A special thanks goes to the late Oliver Toussaint, who dedicated his life to research with great energy, creativity, and enthusiasm. We remember him with deep admiration and affection.
Author Contributions
Conceptualization: R.G. Methodology: P.G.S., C.M., F.P., F.N., F.D.F. Software: R.G., M.M.V. BioBank. T.G, Recruitment of Subjects: B.W., (Austrian cohort), F.D.C. (Belgian cohort) J.B. (German cohort), E.S.G. (Greek cohort), C.F., M.C., A.C. (Italian cohort), and E.S. (Polish cohort). M.H. (Finnish cohort); Validation, P.G.S., C.M.; Formal Analysis, M.P., A.B., M.M.V., W.S., T.G. M.E.T.D, M.H., P.A.G., N.M., P.E.S, A.R.B; Investigation, R.G., F.P.; Resources, A.B, M.P., F.M.; Data Curation, R.G., P.G.S., F.P., D.G., L.C., M.M., M.E.T.D. M.M.V., E.J., F.N., F.D.F; M.D.R; R.Ga; A.R.B; Writing—Original Draft Preparation, R.G., Writing—Review & Editing, R.G., L.M., F.M., M.Ce., M.P., A.B., M.M.V., M.M. F.P., A.C.; Supervision, M.M., F.M.; Project Administration, A.B. F.L.; Funding Acquisition, A.B., R.G; All authors have read and agreed to the published version of the manuscript.
Ethics Approval and Consent to Participate
All information was accessed in accordance with the applicable laws and ethical requirements for the study period concerned and was compliant with the Declaration of Helsinki. The Local Research Ethics Committees of the respective recruiting centers provided ethical approval for the MARK-AGE project, which was registered retrospectively at the German Clinical Trials Register (DRKS00007713; Ethics Committee No.: 2008-075-f, Ethik-Kommission bei der Landesärztekammer Baden-Württemberg). The Ethical Committee of the IRCCS INRCA has approved the study protocol of Report-Age project, in accordance with Helsinki Declaration. The trial registration number is NCT01397682. Informed consent was obtained from all subjects involved in the study.



